Abstract
Context:
In a previous microarray analysis, GRB2-associated binding protein 1 (GAB1), a docking protein closely related to the insulin receptor substrate, was down-regulated in endometrium of women with polycystic ovary syndrome (PCOS).
Objective:
The objective of the study was to characterize the cyclic expression of endometrial GAB1 in vivo in normal women and those with PCOS as well as investigate the possible mechanisms of endometrial regulation of GAB1 expression and action in vitro.
Design:
This was an experimental and case-control study.
Setting:
The study was conducted at a tertiary university hospital.
Patients:
Normal proven fertile women (controls; n = 31) and women with PCOS (cases; n = 26) participated in the study.
Interventions:
Interventions included timed endometrial biopsies at different phases of the menstrual cycle. Ishikawa cells were cultured with β-estradiol (E2), medroxyprogesterone acetate, and E2 + medroxyprogesterone acetate. Transfection of small interfering RNA for GAB1 in Ishikawa cells incubated with or without insulin.
Main Outcome Measures:
GAB1 mRNA expression in Ishikawa cells and in endometrium of cases and controls was measured. Protein expression of phosphorylated MAPK by Western blot was also measured. Immunohistochemical localization and expression of phosphorylated GAB1 in endometrium was also measured, using a digital histological score.
Results:
In endometrial tissue, GAB1 mRNA was reduced in the proliferative phase of PCOS women, compared with controls (P = .003; ANOVA). When all the phases of the menstrual cycle were grouped, GAB1 protein expression was reduced in endometrium of PCOS women (P < .0001; Student t test). E2 increases GAB1 mRNA expression in Ishikawa cells (P = .001; ANOVA). Phosphorylated MAPK is reduced in cells transfected with small interfering RNA for GAB1 (P = .008; ANOVA) and incubated with insulin.
Conclusions:
GAB1 mRNA expression is positively modulated by E2. Endometrial GAB1 protein and mRNA expression are reduced in women with PCOS, suggesting that the endometrium of PCOS women have a defect in insulin signaling due to GAB1 down-regulation.
Insulin action in human endometrium is poorly understood, and the effects of hyperinsulinemia and insulin resistance in women with polycystic ovary syndrome (PCOS) remain unclear. The resistance to insulin action seen in women with PCOS is thought to occur in the insulin-signaling pathway downstream from the receptor (1) because insulin receptor expression does not differ between the endometrium of women with or without PCOS (2). Ormazabal et al (3) reported that the downstream molecule of insulin receptor, phosphor-Y14 caveolin-1, a plasma membrane protein that regulates cell signaling, is reduced in women with PCOS, suggesting a diminished endometrial sensitivity to insulin. We have recently reported decreased expression of growth factor receptor-bound protein 2 (GRB2)-associated binding protein 1 gene (GAB1), a molecule involved in insulin signaling, in PCOS women in use of clomiphene citrate (4). Differential splicing of the GAB1 gene results in two highly similar protein isoforms, belonging to the insulin receptor substrate (IRS) protein family (5, 6). As docking proteins, the IRS family proteins amplify and integrate signals from multiple receptors involved in cell growth and metabolism, serving a common substrate to incorporate multiple inputs (7). Structurally, the IRS family proteins have three common domains: a conserved N-terminal pleckstrin homology domain, a central proline-rich domain, and multiple tyrosine residues (8). The phosphorylation of these tyrosine residues creates potential docking sites for SH2 domain-containing proteins such as SHP2, p85 subunit of phosphatidylinositol-3 kinase, phospholipase C-γ, and v-crk avian sarcoma virus CT10 oncogene homolog (8).
Because GAB1 is a substrate for the insulin receptor, is involved with insulin receptor signaling and shows reduced expression in midsecretory endometrium of women with PCOS undergoing ovulation induction, we hypothesized that decreased GAB1 might result in endometrial dysfunction by causing insulin resistance. To understand the role of GAB1, we have examined the expression and localization of GAB1 across the menstrual cycle in normal women and those with PCOS with spontaneous ovulation. We have also performed, in vitro, mechanistic experiments to demonstrate a role for GAB1 in endometrial signaling.
Materials and Methods
Modulation of GAB1 with steroids in Ishikawa cells
Ishikawa cells, an endometrial adenocarcinoma cell line, were obtained from Valley Biomedical and used for in vitro experiments using the same methods as previously reported (9). Briefly, cells were plated at a density of 1.3 × 104 cells/mL in a T-75 flask (Corning Life Sciences) and propagated as monolayer culture in 10 mL of DMEM/F-12 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (Valley Biomedical), 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 250 ng/mL of Fungizone. Cells were incubated in humid chamber at 37°C, with 5% CO2, and treated for 3 days with the β-estradiol (10−8 M), medroxyprogesterone acetate (MPA; 10−6 M), estradiol + MPA (10−8 and 10−6 M, respectively). All reagents were from Sigma. For control medium, 0.1% dimethyl sulfoxide (Sigma) in DMEM/F12, phenol-red free medium (GIBCO), supplemented with 5% charcoal/dextran-treated fetal bovine serum (Atlanta Biological) was used. Each experimental group was made in triplicate. After treatments, medium was removed and cells were lysed in 350 μL of RA1 buffer (CLONTECH) containing 1% (vol/vol) 2-mercaptoethanol.
Small interfering RNA (siRNA) transfection and protein extraction
Ishikawa cells were seeded in six-well plates at a concentration of 5 × 105 cells/well in DMEM/F12, 10% fetal bovine serum with streptomycin and penicillin. After reaching 80% confluence, cells were transfected with siRNA target to GAB1 or to nonspecific RNA (negative control). Transfection with predesigned siRNA constructs targets to mRNAs corresponding GAB1 (sequence CCACGTAAGCAAAAGAGCA; S5463; Ambion) and to negative siRNA (4390843; Ambion) were used according to the manufacture's protocol. Lipofectamine RNAiMAX transfection reagent (Invitrogen, Life Technologies) was diluted (1:17) with Opti-MEMI medium (Invitrogen) and incubated with Ishikawa cells for 5 minutes at room temperature. A scramble siRNA (4390843; Ambion) at 5 nM was used as the negative control. After 48 hours, the cells were incubated with 100 ng/mL of insulin for 10 minutes, as suggested by Lathi et al (10). Next, cells were washed with cold PBS and harvested. The protein was extracted using radioimmunoprecipitation assay lysis buffer (Millipore) and phosphorylated MAPK was measured by Western blot as previously described (10).
Western blot
Total protein lysates from primary cell cultures were subjected to Western blot analysis as previously described (11). The phospho-p44/42 MAPK (ERK 1/2) rabbit monoclonal antibody (4370S; Cell Signaling Technology Inc) against human was diluted at 1:1000. Normalization was done with pan-actin (D18C11; Cell Signaling) with a dilution of 1:1000. Multiple experiments performed in our laboratory have shown that pan-actin has been stably expressed in Ishikawa cells. A second antibody was used at a dilution of 1:30 000 (horseradish peroxidase conjugated antirabbit IgG; R5506; Sigma-Aldrich). Protein expression was measured using Image Studio Lite software (LI-COR, Inc; version 3.1.4). Experiments were done in triplicates and repeated in two independent experiments.
Endometrial biopsies
Normal endometrial samples were prospectively obtained from volunteers. Normal endometrial samples were stored in our laboratory archives, either as frozen biopsies at −80°C or in formalin-fixed, paraffin-embedded blocks. Inclusion criteria were as follows: 1) healthy women with proven fertility, 19–34 y old, 2) with regular menstrual cycles (25–35 d), and 3) without use of an oral contraceptive for at least 2 months. Those with a body mass index (BMI) greater than 29.9 kg/m2 or the use of hormones within 60 days prior to enrollment were excluded. Endometrial biopsies from women with PCOS were also obtained. PCOS was defined according to the Rotterdam criteria (12). Those who used oral contraceptives in the previous 2 months, had history of endometriosis, had no spontaneous cycles, or had elevated levels of thyroid-stimulating hormone were excluded. No restrictions for age or BMI were observed. Endometrial biopsies were obtained at different phases of spontaneous menstrual cycle: proliferative, early, mid-, and late secretory phases. Midsecretory phase was LH+9 and LH+10 of natural menstrual cycles. LH surge day was determined using once daily urinary LH measurement (Clearblue Easy Digital; Unipath Ltd). Endometrial tissue was snap frozen in liquid nitrogen and kept at −80°C for RNA analysis and in formaldehyde 10% for immunohistochemical analysis.
Reverse transcriptase-polymerase chain reaction
Total RNA was extracted and purified from endometrial biopsies as previously reported (13). RT-PCRs were performed in a Stratagene MX3000P machine (Stratagene) in duplicates using predesigned TaqMan probe-primer sets for GAB1 (Hs00157646_m1; Applied Biosystems, Life Technologies). In Ishikawa cells, glyceraldehyde-3-phosphate dehydrogenase (Hs02758991_g1; Applied Biosystems) was used as a housekeeping gene. In human endometrium, expression was normalized with constitutively expressed gene, peptidylprolyl isomerase A (PPIA; Hs99999904-ml; Applied Biosystems). PPIA was chosen after a validation of 12 housekeeping genes in endometrium. Confirmation was performed with RefFinder (http://www.leonxie.com/referencegene.php?type=reference), in which major computational programs (geNorm, Normfinder, BestKeeper, and the comparative methods) were used. Final ranking of PPIA was 1.9, in a scale from 0 to 12, in which a lower number reflects better stability of gene expression. Total reaction volume for all RT-PCR experiments was 20 μL, using 40 two-step cycles (95°C for 25 sec, followed by 60°C for 1 min). The RT-PCR data were analyzed using the 2−ΔCt method, as described (14).
Immunohistochemical analysis
Paraffin blocks were cut at 8 μm, put onto silane-coated slides, deparaffinized, and rehydrated in a graded ethanol series. Endogenous peroxidases were quenched with 0.3% H2O2 in absolute methanol for 30 minutes at room temperature. After three 2-minute rinses in PBS, nonspecific epitopes were blocked with 2% normal goat serum for 20 minutes. After removing excess serum, sections were incubated at 4°C overnight with primary antibody [1:50 anti-GAB1/phosphorylated (GAB1-phospho-Tyr659, number A0788; Assay Biotechnology Co) in a 2% goat serum in PBS. Sections were washed in PBS and incubated with secondary antibody for 30 minutes at room temperature. The stain was detected using the Vectastain Elite ABC kit (Vector Laboratories) for 30 minutes followed with 3,3′-diaminobenzidine substrate kit for peroxidase (SK-4100; Vector Laboratories) for 10 minutes. The slides were counterstained with hematoxylin for 45 seconds and mounted. The specificity of GAB1 primary antibody was verified by the manufacturer using the two different following methods: 1) confirmation of the correct molecular weight of GAB1 in Western blot analysis and 2) by immunofluorescence analysis of HepG2 cells. Breast tissue was used as a positive external control. Secondary antibody control was performed by eliminating the primary antibody in the reaction, as suggested in the literature (15). Digital pictures were taken from the slides, and diaminobenzidine intensity on glands was analyzed with ImageJ software (National Institutes of Health, Bethesda, Maryland) as previously reported (16).
Statistical analysis, ethical aspects, and human subjects
All subjects gave written informed consent. This study was submitted and approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. GraphPad Prism version 6 for Macintosh (GraphPad Software, Inc) was used for statistical analysis, using ANOVA with Tukey as post hoc test to compare the expression of GAB1 across the different groups. Sample size for immunohistochemical analysis was calculated according to the literature (17), using the following parameters: an α error (zα) of .05, power (zβ) of .8, an estimate SD of GAB1 of 40 [thus variance (s)2 = 1600], and a difference (d) to be found of 75 points in a scale ranging from 0 to 255 (ie, a difference of 30%). The variance was obtained from a sample of 10 normal cases in the midsecretory phase. These figures yielded a sample size of at least six cases in each group. An analysis of covariance (ANCOVA) was used for adjusting the histological score between groups if age in controls and cases was significantly different. An ANCOVA was calculated using an online tool (http://vassarstats.net/ancova2L.html).
The mean age of the normal subjects was 32.2 years with a SD of 2.1 and was significantly higher than those with PCOS at 20.9 with an SD of 4.6 (P = .001 using a Student t test).
Results
GAB1 mRNA expression in the endometrium women with PCOS vs controls
In normal women, GAB1 mRNA expression was higher in the proliferative phase than the luteal phase (Figure 1A); however, in women with PCOS, GAB1 expression did not vary significantly across the menstrual cycle (data not shown). GAB1 mRNA was significantly higher in normal proliferative endometrium (Figure 1B) than in proliferative endometrium from women with PCOS.
Figure 1.
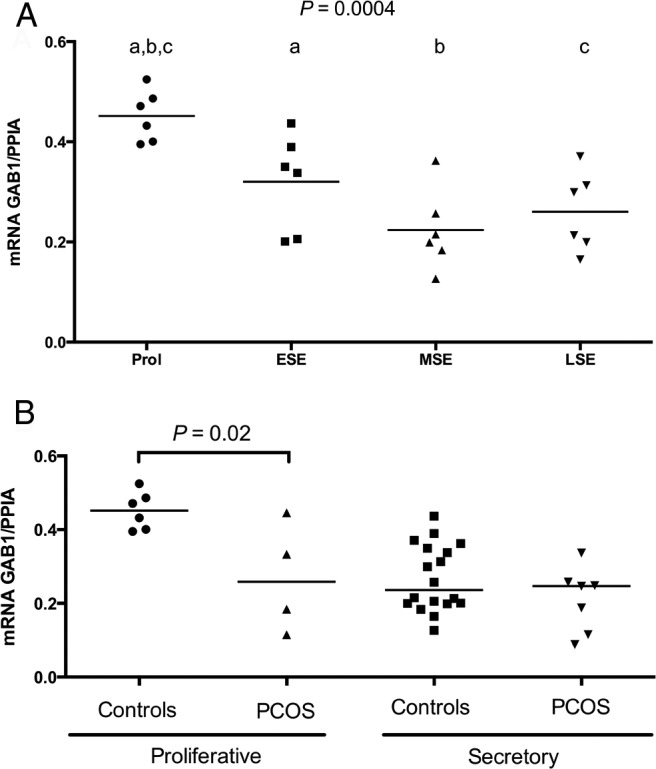
A, Comparison of endometrial GAB1 mRNA levels over the menstrual cycle in normal women. A significant difference was found among different phases of the menstrual cycle (P = .0004l; ANOVA). Tukey's post hoc test identified that GAB1 mRNA was significantly higher in the proliferative phase compared with the following: 1) early secretory endometrium (P = .04), 2) midsecretory (P = .004), and 3) late secretory endometrium (P = .002). B, Comparison between endometrial GAB1 mRNA expression in endometrium of women with PCOS and normal women (controls). Samples were grouped as proliferative and secretory phases because a distinct difference was found only between the proliferative and secretory phases in normal women. GAB1 mRNA levels were reduced in the proliferative phase of women with PCOS compared with controls (P = .02; ANOVA; Tukey post hoc test). Bar represents mean values. ESE, early secretory endometrium; LSE, late secretory endometrium; MSE, midsecretory endometrium; Prol, proliferative phase.
Immunohistochemical expression of phosphorylated GAB1 over the cycle
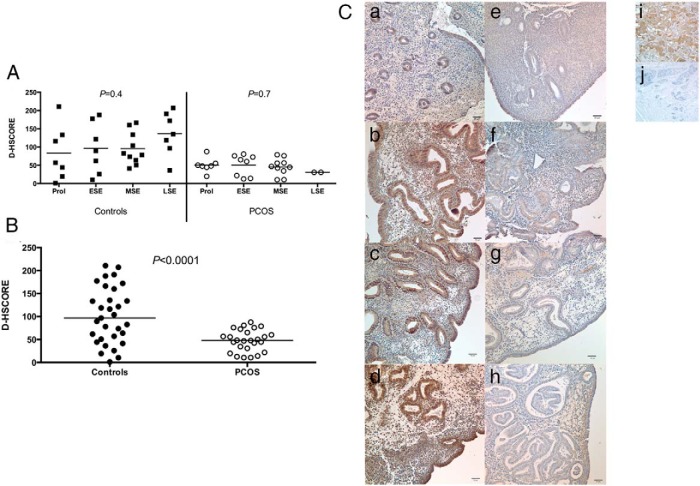
At the protein level, no difference was observed in phosphorylated GAB1 staining over the cycle, either in normal or PCOS women (Figure 2A). However, a significant reduction of the endometrial immunostaining was observed in women with PCOS vs that in normal subjects (Figure 2B). The immunostaining for phosphorylated GAB1 was largely cytoplasmic and predominantly in glandular and luminal epithelium (Figure 2C). An ANCOVA analysis, adjusting for age differences, confirmed the significant difference in phosphorylated GAB1 immunostaining of endometrium from women with PCOS and controls (P = .01).
Figure 2.
A, Immunohistochemistry expression of phosphorylated GAB1 over the menstrual cycle in normal women (controls) and in women with PCOS. No significant difference was observed among different phases of menstrual either in controls (P = .4; ANOVA), or in women with PCOS (P = 0.7; ANOVA). B, Immunohistochemistry of endometrial expression of phosphorylated GAB1 between normal women (controls) and women with PCOS. A significant reduction was observed in endometrium of women with PCOS (P < .0001; unpaired Student t test). All phases of the menstrual cycle were grouped because no difference was found among phases within individual groups. Bars represent means. C, Representative images of immunostaining for phosphorylated GAB1 in endometrium of normal women (a, proliferative; b, early secretory; c, midsecretory; d, late secretory) and in women with PCOS (e, proliferative; f, early secretory; g, midsecretory; h, late secretory). Breast tissue was used as positive (i) and negative (j) external controls. Magnification is ×200. Bars represent 50 μm. ESE, early secretory endometrium; LSE, late secretory endometrium; MSE, midsecretory endometrium; Prol, proliferative phase.
Sex steroids can modulate GAB1 expression by Ishikawa cells
Given the increased GAB1 expression in the proliferative phase, we conducted in vitro studies to determine a possible role of estrogen in regulating GAB1 expression. Ishikawa cells were treated with 10−8 M estradiol for 3 days, resulting in significantly increased GAB1 mRNA expression (Figure 3, P < .01; ANOVA with Tukey's post hoc test). However, treatment with MPA (10−6 M), alone or in combination with estradiol, did not significantly change GAB1 mRNA expression (Figure 3). Treatment for 24 hours showed no significant effect (data not shown) of estradiol or MPA.
Figure 3.
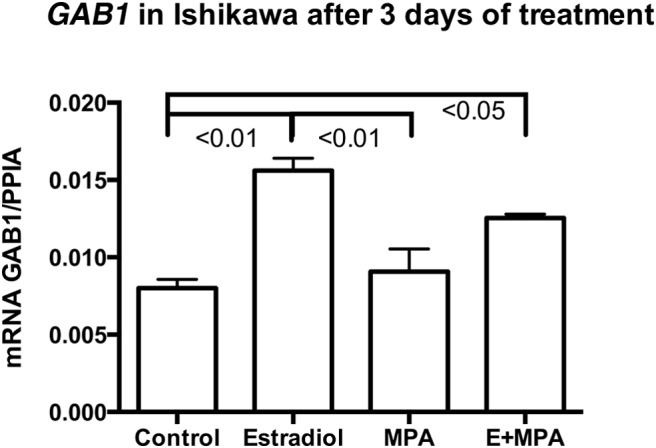
Expression of GAB1 mRNA in Ishikawa cells cultured with β-estradiol (E; 10−8 M), MPA (10−6 M), or E+MPA for 3 days. The experiment was done in triplicate, and an ANOVA with a Tukey post hoc test was used for statistical analysis.
Silencing of GAB1 expression results in reduced insulin signaling in Ishikawa cells
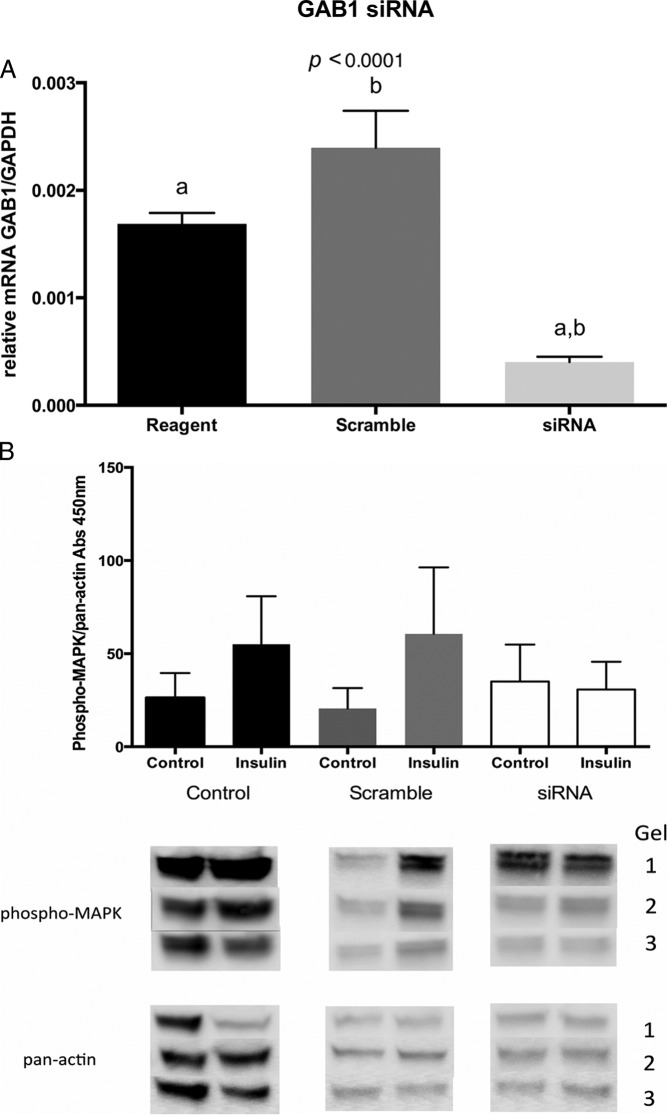
We next asked whether GAB1 could play a role in endometrial cell insulin action by silencing GAB1 expression using siRNA. Figure 4A demonstrates the specificity and efficacy of the GAB1 siRNA, and Figure 4B demonstrates that GAB1 silencing consistently reduced insulin-induced p42/44 MAPK phosphorylation (Figure 4B), suggesting a role for GAB1 in insulin signaling in Ishikawa cells.
Figure 4.
A, Relative expression of GAB1 mRNA in Ishikawa cells transfected with siRNA for GAB1 (siRNA), nonspecific siRNA (Scramble), or reagents without transfection (Reagent). After specific transfection, GAB1 mRNA levels were significantly reduced (ANOVA, P < .0001). B, Western blot of phosphorylated p44/42 MAPK (ERK 1/2) (phospho-MAPK) mediated activity by insulin in Ishikawa cells transfected with silencing RNA for GAB1. Normalization of protein expression was done with pan-actin. Ishikawa cells without siRNA for GAB1 (Scramble) or without siRNA (Control) showed an increased response of phosphorylated MAPK after incubation with 100 ng/mL of insulin for 10 minutes, compared with cells transfected with siRNA for GAB1 (siRNA).
Discussion
Insulin resistance has been defined as a decreased ability of insulin to stimulate peripheral glucose uptake and inhibit lipolysis, resulting in an increased insulin requirement for a given metabolic action (18). Current data suggest that insulin resistance contributes to the pathophysiology of PCOS. The fundamental defect that causes PCOS remains elusive, and it is likely to be multifactorial in origin. Our previous finding that GAB1 expression was reduced in midsecretory endometrium of women with PCOS who used clomiphene citrate, and the fact that GAB1 is a docking protein related to insulin receptor action led us to further investigate the expression of this protein in the endometrium of the women with and without PCOS.
Our data revealed that GAB1 mRNA is up-regulated by estrogen but not by a potent progestin. The data on the up-regulation of GAB1 by estradiol is novel because we can find no evidence in the literature of GAB1 regulation by any sex steroid. Nevertheless, it has been shown that there is a cross talk between estrogen receptor and epidermal growth factor receptor (19), and epidermal growth factor activates Gab1 in a murine model (20).
The in vitro study silencing GAB1 mRNA in the presence of insulin revealed that GAB1 is important for MAPK activity, as others have shown (21). We were able to identify that by silencing GAB1 mRNA, MAPK activity was reduced in Ishikawa cells cultured with insulin (Figure 3). This GAB1 defect may be the postbinding defect in insulin signaling in PCOS women suggested by Diamanti-Kandarakis and Papavassiliou (22). This postbinding defect would result in a marked decrease in insulin sensitivity. Interestingly, decreased levels of theca cell MAPK phosphorylation are correlated with increased androgen production from polycystic ovaries, suggesting a possible mechanism by which the hyperandrogenism of PCOS could be involved in insulin resistance (23).
The estradiol up-regulation of GAB1 mRNA expression in Ishikawa cells (Figure 3) was mirrored by the increased expression of GAB1 mRNA observed in proliferative endometrium of normal women as compared with the secretory phase (Figure 1A). In PCOS women, a reduction of GAB1 mRNA expression was observed in proliferative phase, compared with normal endometrium (Figure 1B). The data on GAB1 mRNA expression in endometrium expands on our data published previously (4). In our previous study, our data was derived from midsecretory endometrium of women with PCOS who underwent ovulation induction with clomiphene citrate; in this study, we provide data on PCOS women with spontaneous ovulation. This difference may explain why no difference was found in GAB1 mRNA expression in midsecretory endometrium between both groups. However, the immunohistochemical analysis revealed the same results (Figure 2).
Phosphorylated GAB1 was mainly located in luminal and glandular epithelium (Figure 2C), although no difference in abundance was observed among the different phases of the menstrual cycle in normal women or in women with PCOS (Figure 2A). When all the phases of the menstrual cycle were put together and GAB1 phosphorylation was compared between controls and women with PCOS, a reduction in phosphorylated GAB1 was seen in the endometrium of women with PCOS (Figure 2B). This global reduction over the menstrual cycle is consistent with our previous data, in which endometrial phosphorylated GAB1 protein expression was reduced in PCOS women after ovulation induction with clomiphene citrate (4). In addition, by silencing GAB1 mRNA, we were able to demonstrate that MAPK response to insulin is reduced (Figure 4B), revealing the key role of GAB1 to the insulin pathway. With these results we hypothesize that the reduced expression of GAB1 may confers insulin resistance in endometrium but does not halt endometrial proliferation/hyperplasia, a common occurrence in women with PCOS (24).
The strengths of the study include a proper sample size calculation for the immunohistochemical study and the mechanistic experiment with the endometrial cell line.
Unfortunately, we were not able to use primary epithelial endometrial cells derived from normal women or with PCOS for the in vitro study with siRNA. The reagents for the transfection were too toxic for primary endometrial epithelial cells, hindering cell culture. Therefore, Ishikawa cells were used as model for endometrial epithelium. Another potential weakness was that insulin receptor quantification was not done in either group. However, Fornes et al (2) performed this investigation and did not find a difference in the expression of insulin receptor in endometrium of women with or without PCOS, which supports our hypothesis that endometrial insulin resistance in women with PCOS is related to a second messenger defect. This finding in human endometrium may be tissue specific because the deletion of Gab1 in mouse liver improves glucose uptake (25). Of note, our results should be interpreted with care because BMI may be a confounder. Thus, we are not able to conclude the findings are related to PCOS or to obesity itself.
In conclusion, we were able to verify that GAB1 protein expression is not cycle modulated, but estradiol up-regulates the mRNA expression of GAB1. In women with PCOS, the mean expression of GAB1 is reduced compared with normal fertile women.
Altogether our results shed some light in the complex and multifactorial pathophysiology of PCOS, more specifically the insulin resistance in the endometrial compartment. In addition to exploring the role of GAB1 in the pathophysiology of PCOS, we have presented the first description of the regulation of GAB1 in normal endometrium. These results provide a rationale for future studies to investigate GAB1 as a potential target for PCOS treatment.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54 HD-30476 (to S.L.Y.) as part of the Specialized Cooperative Centers Program in Reproduction Research and Infertility; by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Grant R01 HD067721 (to S.L.Y.); and by Conselho Nacional de Desenvolvimento Científico e Tecnológico Grant 240239/2012-1 (to R.F.S.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ANCOVA
- analysis of covariance
- BMI
- body mass index
- GAB1
- GRB2-associated binding protein 1
- IRS
- insulin receptor substrate
- MPA
- medroxyprogesterone acetate
- PCOS
- polycystic ovary syndrome
- PPIA
- peptidylprolyl isomerase A
- siRNA
- small interfering RNA.
References
- 1. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fornes R, Ormazabal P, Rosas C, et al. Changes in the expression of insulin signaling pathway molecules in endometria from polycystic ovary syndrome women with or without hyperinsulinemia. Mol Med. 2010;16:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ormazabal P, Romero C, Gabler F, Quest AF, Vega M. Decreased phosphorylation of Y(1)(4)caveolin-1 in endometrial tissue of polycystic ovary syndrome patients may be related with an insulin resistant state in this tissue. Horm Metab Res. 2013;45:291–296. [DOI] [PubMed] [Google Scholar]
- 4. Savaris RF, Groll JM, Young SL, et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab. 2011;96:1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. [DOI] [PubMed] [Google Scholar]
- 6. Wohrle FU, Daly RJ, Brummer T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal. 2009;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White MF. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 8. Gu H, Neel BG. The “Gab” in signal transduction. Trends Cell Biol. 2003;13:122–130. [DOI] [PubMed] [Google Scholar]
- 9. Plante BJ, Lessey BA, Taylor RN, et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci. 2012;19:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC. Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90:1599–1606. [DOI] [PubMed] [Google Scholar]
- 11. Pohnke Y, Schneider-Merck T, Fahnenstich J, et al. Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells. J Clin Endocrinol Metab. 2004;89:5233–5244. [DOI] [PubMed] [Google Scholar]
- 12. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- 13. Usadi RS, Groll JM, Lessey BA, et al. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93:4058–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 15. Burry RW. Controls for immunocytochemistry: an update. J Histochem Cytochem. 2011;59:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuhrich DG, Lessey BA, Savaris RE. Comparison of HSCORE assessment of endometrial β3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anal Quant Cytol Histol. 2013;35:210–216. [PMC free article] [PubMed] [Google Scholar]
- 17. Jekel JF, Elmore JG, Katz DL. Sample size, randomization, and probability theory. In: Jekel JF, Elmore JG, Katz DL, eds. Epidemiology, Biostatistics, Preventive Medicine. Philadelphia: W. B. Saunders Co; 1996:160–171. [Google Scholar]
- 18. Kahn CR. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. [DOI] [PubMed] [Google Scholar]
- 19. Egloff AM, Rothstein ME, Seethala R, Siegfried JM, Grandis JR, Stabile LP. Cross-talk between estrogen receptor and epidermal growth factor receptor in head and neck squamous cell carcinoma. Clin Cancer Res. 2009;15:6529–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayakawa-Yano Y, Nishida K, Fukami S, et al. Epidermal growth factor signaling mediated by grb2 associated binder1 is required for the spatiotemporally regulated proliferation of olig2-expressing progenitors in the embryonic spinal cord. Stem Cells. 2007;25:1410–1422. [DOI] [PubMed] [Google Scholar]
- 21. Lehr S, Kotzka J, Avci H, et al. Identification of major ERK-related phosphorylation sites in Gab1. Biochemistry. 2004;43:12133–12140. [DOI] [PubMed] [Google Scholar]
- 22. Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12:324–332. [DOI] [PubMed] [Google Scholar]
- 23. Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, et al. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–390. [DOI] [PubMed] [Google Scholar]
- 24. Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–244. [DOI] [PubMed] [Google Scholar]
- 25. Bard-Chapeau EA, Hevener AL, Long S, Zhang EE, Olefsky JM, Feng GS. Deletion of Gab1 in the liver leads to enhanced glucose tolerance and improved hepatic insulin action. Nat Med. 2005;11:567–571. [DOI] [PubMed] [Google Scholar]