Abstract
Context:
Macrosomic infants are at increased risk for adverse metabolic outcomes. Improving prediction of large-for-gestational-age (LGA) birth may help prevent these outcomes.
Objective:
This study sought to determine whether genes associated with obesity-related traits in adults are associated with newborn size, and whether a genetic risk score (GRS) predicts LGA birth.
Setting and Design:
Single nucleotide polymorphisms (SNPs) in 40 regions associated with adult obesity-related traits were tested for association with newborn size. GRS's for birth weight and sum of skinfolds (SSF) specific to ancestry were calculated using the most highly associated SNP for each ancestry in genomic regions with one or more SNPs associated with birth weight and/or SSF in at least one ancestry group or meta-analyses.
Participants:
Newborns from the Hyperglycemia Adverse Pregnancy Outcomes Study were studied (942 Afro-Caribbean, 1294 Northern European, 573 Mexican-American, and 1182 Thai).
Outcome Measures:
Birth weight >90th percentile (LGA) and newborn SSF >90th percentile were primary outcomes.
Results:
After adjustment for ancestry, sex, gestational age at delivery, parity, maternal genotype, maternal smoking/alcohol intake, age, body mass index, height, blood pressure and glucose, 25 and 23 SNPs were associated (P < .001) with birth weight and newborn SSF, respectively. The GRS was highly associated with both phenotypes as continuous variables across all ancestries (P ≤ 1.6 × 10−19) and improved prediction of birth weight and SSF >90th percentile when added to a baseline model incorporating the covariates listed above.
Conclusions:
A GRS comprised of SNPs associated with adult obesity-related traits may provide an approach for predicting LGA birth and newborn adiposity beyond established risk factors.
Infants born large for gestational age (LGA) (birth weight for gestational age >90th percentile) are at increased risk of adverse perinatal outcomes and higher mortality during the first year of life (1–3). High birth weight is also associated with adverse metabolic outcomes during childhood and adulthood, including higher risk of metabolic diseases such as obesity, dyslipidemia, hypertension, and type 2 diabetes (4–6). In addition, former LGA mothers themselves are at increased risk for having LGA offspring, perpetuating the cycle of adverse metabolic health outcomes (4). The incidence of LGA birth has increased over the last three decades (6), likely due, in part, to environmental changes in the setting of an underlying genetic predisposition.
Fetal growth and adiposity are influenced by complex interactions between fetal genes and the intrauterine environment with maternal glucose having a significant effect on birth weight and adiposity (7–9). Limited information is available on fetal genetic variation that influences size at birth, although recent genome-wide association data have identified several loci associated with birth weight or newborn adiposity (10–12). These loci have generally not demonstrated associations with adult obesity (10–12).
The present study tested whether genetic variation within genes associated with adult obesity-related traits is associated with newborn size. These studies were performed using genome-wide single nucleotide polymorphism (SNP) data collected from four different ancestry groups by the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, an observational study that examined associations of levels of glucose intolerance during pregnancy and risks of adverse newborn outcomes (7). Genetic risk scores (GRSs) comprised of SNPs with ancestry-specific associations were created to ascertain whether a greater burden of adult obesity susceptibility alleles is associated with higher birth weight and greater newborn adiposity and whether application of the GRS improves prediction of LGA birth and greater newborn adiposity beyond traditional risk factors.
Materials and Methods
HAPO Study
The HAPO Study was an international epidemiologic study conducted at 15 centers in nine countries. Study methods have been described (13). Briefly, eligible women underwent a 75-g oral glucose tolerance test (OGTT) between 24 and 32 weeks' gestation. Using standardized methods and procedures, maternal height, weight, and blood pressure were measured, and maternal demographic information was collected. Cord blood was collected for measurement of glucose and C-peptide and DNA extraction. Using standardized methods and equipment, neonatal anthropometric measurements were obtained within 72 hours of birth, including head circumference, birth length, birth weight, and skinfold thickness measured at the flank, triceps, and subscapular regions.
Genotyping and imputation
HAPO newborn and maternal genotype data were derived from four ancestry groups: Northern European (EU), Afro-Caribbean (AC), Mexican-American (MA), and Thai (TH). Genotyping was performed using Illumina genome-wide arrays at the Broad Institute or Johns Hopkins Center for Inherited Disease Research. Quality control of genotype data was performed as described previously (12, 14). Imputation was performed using a HapMap 3 cosmopolitan reference panel and BEAGLE (15). Details of genotyping platforms, genotype calling algorithms, quality control and imputation procedures, and population substructure estimates were previously reported (12).
Candidate gene analyses
SNPs in 40 gene regions with replicated genome-wide significant (P < 5 × 10−8) associations with adult body mass index (BMI) or waist-to-hip ratio were collated from the literature and referred to as the index SNP (Supplemental Tables 1 and 2) (16–19). For index SNPs located within genes, SNPs within the gene region and 15 kb 5′ and 3′ of the gene were tested for association with birth weight and newborn sum of skinfolds (SSF) after square root transformation (12) as continuous outcomes. For index SNPs located outside a gene region, SNPs in the region 5 kb 5′ and 3′ to the index SNP were also tested for association with birth weight and SSF. A similar approach was used to identify the ancestry-specific SNP with the lowest P value within seven additional genomic regions containing SNPs previously reported to be associated with birth weight at genome-wide significance (LEKR1/CCNL1, ADCY5, LCORL, 5q11.1, CDKAL1, ADRB1, HMGA2) (11). Linear regression analyses were conducted within ancestry group with an additive genotype variable, adjusting for population substructure within ancestry, sex, gestational age at delivery, parity, maternal smoking status, and alcohol intake during pregnancy, along with maternal age, BMI, height, and mean arterial blood pressure at the time of OGTT, and maternal fasting, 1-hour and 2-hour glucose levels during the OGTT. Maternal genotype was included in regression models after mean-centering and Gram-Schmidt orthogonalization to the fetal genotype variable. This approach alleviated high correlation of fetal and maternal genotypes (roughly 50%) while still covering the same covariate vector space (20). Importantly, orthogonalization of maternal genotype to fetal genotype allowed for evaluation of associations strongly attributable to maternal genotype beyond contributions captured by fetal genotype. For each fetal SNP and phenotype, meta-analysis across ancestry groups was performed under a fixed-effects model weighting each stratum by standard error. SNPs with P < .001 were considered significant, corresponding to Bonferroni-corrected P = .00125 to maintain overall 0.05 Type I error for the 40 index SNPs located in 40 independent linkage disequilibrium (LD) blocks.
Genetic risk score
Genomic regions in which SNPs were associated with birth weight and/or SSF among newborns in at least one ancestry group or meta-analyses after covariate adjustment were selected for inclusion in the genetic risk score (GRS). For each ancestry group, the SNP most highly associated with the phenotype (birth weight or SSF) within each genomic region was chosen for inclusion in the GRS specific to that ancestry group. The GRS was calculated by summing for each offspring the number of risk alleles (0,1,2) for all SNPs included in the GRS specific to that ancestry group. A weighted GRS (wGRS) was calculated by multiplying the jointly estimated β-coefficients by the number of risk alleles for each SNP (0,1,2) and then summing the resulting values for each offspring. In addition, a GRS was constructed using the seven previously reported birth weight loci (7-GRS) by summing for each offspring the number of risk alleles (0,1,2) for the most significant ancestry-specific SNPs within these regions.
Statistical analysis
Direction of the associations with the continuous outcomes for the GRS combining all selected SNPs was confirmed using linear regression with GRS as the main predictor of interest, adjusted for all covariates previously described. Values for the 90th percentiles of birth weight (LGA) and SSF were determined specifically by sex and race, adjusting for gestational age, study center, and parity using quantile regression (21). Logistic regression was used to investigate the birth weight and SSF > 90th percentile outcomes, with primary models of interest including GRS alone, all baseline clinical covariates, and GRS plus baseline clinical covariates. Additional logistic regression analyses were conducted to investigate associations and potential increases in predictive accuracy for models including 7-GRS, GRS + 7-GRS, and wGRS with and without maternal genotype, all adjusted for baseline clinical covariates. Logistic regression model fit for all models was assessed using Akaike Information Criterion (AIC) and Hosmer-Lemeshow tests. Receiver Operating Characteristic (ROC) curves were calculated for all models. Predictive accuracy was summarized using area under the ROC curve (AUC) including leave-one-out cross-validation, and specificity estimates were reported at sensitivity of 0.75. DeLong tests were used to compare AUCs for non-nested models. For nested model comparisons, logistic regression P values for each predictor of interest were used (22). All analyses were conducted in R (3.0.2) and SAS 9.3 (SAS Institute).
Power
Sample sizes afforded 90% power at two-sided Type I error 0.001 to detect linear regression birth weight (g) effect sizes in the range of 80.0–314.9 (EU), 65.9–255.9 (TH), 107.3–420.1 (AC), and 101.9–398.9 (MA) and square root–transformed SSF (in mm) effect sizes in the range of 0.06–0.23 (EU), 0.06–0.23 (TH), 0.06–0.24 (AC) and 0.09–0.37 (MA), depending on the SD of the additive risk allele predictor. In logistic regression analyses, there was 90% power at two-sided Type I error 0.05 to detect odds ratios of 1.14 (EU, TH), 1.18 (AC), and 1.22 (MA) or greater for GRS higher by one risk allele.
Results
Given that the index SNPs associated with adult BMI and/or waist-to-hip ratio were largely identified in European ancestry populations, we initially examined association of the 40 index SNPs with birth weight or newborn SSF among 1294 genotyped European HAPO newborns. There was no evidence for association of the index SNPs with these phenotypes (Supplemental Table 3). However, analyses examining association of SNPs within the 40 genomic regions containing the index SNPs in 3991 genotyped European, Afro-Caribbean, Mexican-American, and Thai newborns (Table 1) demonstrated significant associations for 142 SNPs with birth weight and/or SSF in at least one of the four ancestry groups or meta-analysis (P < .001). After trimming for LD (D′ = 1, r2 ≥ 0.5), 25 and 23 SNPs in 48 independent LD blocks were associated with birth weight and SSF, respectively, in at least one ancestry group (Supplemental Tables 4 and 5). None of these identified loci were in LD with the index SNP in the corresponding gene region. Linearity of the additive model was confirmed for all significant SNPs by modeling risk allele count (0, 1, or 2) as a categorical variable with 0 as the referent group and confirming consistency of the beta estimates with additive association. Meta-analyses demonstrated significant association of four SNPs in independent LD blocks within TBX15, ETV5, and NFE2L3 with birth weight and five SNPs within ETV5, ZNF608, and LRP1B with SSF (Supplemental Tables 4 and 5). Twelve different genomic regions had one or more SNPs associated with birth weight in at least one ancestry group and/or the meta-analysis, whereas 15 regions contained one or more SNPs associated with SSF. Loci with SNPs associated with both phenotypes in the same ancestry group included LRP1B, NRXN3, and NEGR1 (Supplemental Tables 4 and 5).
Table 1.
Population Characteristics
| AC | EU | MA | TH | |
|---|---|---|---|---|
| Pregnant women, n | 942 | 1294 | 573 | 1182 |
| Age at OGTT, y | 25.5 ± 0.18 | 31.3 ± 0.15 | 28.8 ± 0.23 | 27.7 ± 0.16 |
| BMI at OGTT, kg/m2 | 27.7 ± 0.20 | 28.5 ± 0.13 | 30.2 ± 0.24 | 25.7 ± 0.11 |
| MAP at OGTT, mm Hg | 79.2 ± 0.25 | 83.8 ± 0.21 | 84.1 ± 0.33 | 79.7 ± 0.22 |
| Fasting PG, mg/dL | 80.5 ± 0.25 | 82.2 ± 0.19 | 83.9 ± 0.32 | 79.9 ± 0.19 |
| 1-hr PG, mg/dL | 122.8 ± 0.90 | 132.3 ± 0.82 | 137.3 ± 1.46 | 148.0 ± 0.91 |
| 2-hr PG, mg/dL | 109.0 ± 0.73 | 109.3 ± 0.61 | 111.4 ± 1.03 | 119.4 ± 0.73 |
| Gestational age at delivery, wk | 39.8 ± 0.04 | 40.0 ± 0.03 | 39.7 ± 0.05 | 39.4 ± 0.04 |
| Maternal smoking, % | 0.53 | 13.52 | 0.00 | 0.68 |
| Male offspring, % | 51.9 | 49.9 | 50.6 | 49.0 |
| Primiparous births, % | 46.3 | 56.9 | 26.4 | 53.1 |
| Offspring birth weight, gm | 3219 ± 14.57 | 3426 ± 13.90 | 3445 ± 17.73 | 3102 ± 11.38 |
| SSF, mm | 11.4 ± 0.06 | 13.0 ± 0.07 | 14.2 ± 0.12 | 11.7 ± 0.07 |
Abbreviations: OGTT, oral glucose tolerance test; BMI, body mass index; MAP, mean arterial pressure; PG, plasma glucose.
Except where indicated, values are means ± SE.
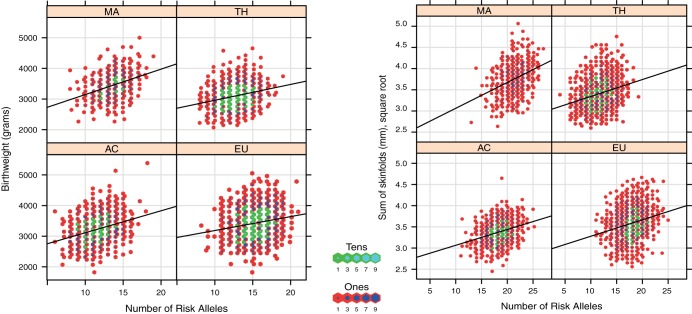
A GRS for birth weight specific to each ancestry group was calculated using the most highly associated SNP in each ancestry group from 12 genomic regions: NEGR1, TBX15, LRP1B, CADM2 TMEM18, ETV5, SLC39A8, NFE2L3, ITPR2, PRKD1, NRXN3, and ZRNF3. The same approach was used to calculate a GRS for SSF from 15 genomic regions: NEGR1, TBX15, FANCL, LRP1B, GRB14, ETV5, ZNF608, LY86, VEGFA, LRRN6C, ITPR2, NRXN3, MAP2K5, FTO, and TMEM160. SNPs used in the GRS specific to each ancestry group differed and, with a few exceptions, were not in LD (Supplemental Tables 6 and 7). For birth weight, Afro-Caribbean newborns carried, on average, the fewest number of risk alleles, 11, with a range of 6–18, due, in part, to lower allele frequencies for the selected risk SNPs in Afro-Caribbean newborns. European, Mexican-American, and Thai newborns carried, on average 13–15 risk alleles. For newborn SSF, Thai newborns carried, on average, the fewest number of risk alleles, 11, with a range of 4–19 due again, in part, to lower allele frequencies for the selected risk SNPs among Thai newborns. Afro-Caribbean, European, and Mexican-American newborns carried, on average, 18–21 risk alleles (Table 2). The GRS for each ancestry group was highly associated with birth weight and SSF as continuous variables in linear regression models across all HAPO ancestry groups (Figure 1, Table 2).
Table 2.
Association of GRS With Continuous Outcomes
| N | Mean Risk Alleles | SD | Risk Allele Range | β | SE | P Value | |
|---|---|---|---|---|---|---|---|
| Birth weight | |||||||
| AC | 941 | 11.49 | 1.96 | 6–18 | 71.9 | 6.47 | 6.0 × 10−27 |
| EU | 1294 | 15.29 | 1.94 | 8–21 | 52.3 | 5.79 | 6.4 × 10−19 |
| MA | 573 | 13.61 | 1.78 | 8–19 | 79.9 | 7.81 | 1.2 × 10−22 |
| TH | 1162 | 12.75 | 1.85 | 8–19 | 48.7 | 5.14 | 1.7 × 10−20 |
| SSF | |||||||
| AC | 941 | 18.25 | 2.16 | 11–25 | 0.04 | 0.0037 | 4.2 × 10−25 |
| EU | 1294 | 17.83 | 2.24 | 11–25 | 0.04 | 0.0041 | 8.2 × 10−24 |
| MA | 570 | 21.29 | 2.23 | 13–27 | 0.06 | 0.0064 | 7.2 × 10−22 |
| TH | 1161 | 11.49 | 2.33 | 4–19 | 0.04 | 0.0038 | 1.1 × 10−25 |
Baseline model adjusted for population substructure within each ancestry, sex, gestational age at delivery, parity, maternal genotype, maternal smoking and alcohol intake during pregnancy, maternal age, BMI, mean arterial blood pressure, and height at the time of oral glucose tolerance test; and maternal fasting, 1-hour and 2-hour glucose levels observed during the oral glucose tolerance test.
Figure 1.
Association of GRS with birth weight and SSF. The x-axis indicates number of risk alleles. The y-axis represents birth weight in grams (left panel) and skinfold thickness in mm (right panel). The solid black lines indicate the regression line.
We next examined association of the GRS with LGA and SSF > 90th percentile (Table 3). The GRS was significantly associated with birth weight and SSF >90th percentile in a model on its own and after controlling for known risk factors for LGA birth (Table 3). Odds ratio estimates for each GRS changed only slightly in all ancestries after inclusion of baseline covariates, suggesting that the GRS is an independent risk factor for the two outcomes. The high P values obtained using Hosmer-Lemeshow test (Supplemental Table 10) indicated consistency of the observed outcome frequencies compared with the expected logistic regression model outcome frequencies, indicating adequate model fit for models including the GRS.
Table 3.
GRS Associations and AUC for Birth Weight >90th Percentile and SSF >90th Percentile
| Ancestry Group | Model | Birth Weight |
SSF |
||||
|---|---|---|---|---|---|---|---|
| AUC (CV-AUC) | GRS OR (95% CI) | P Value | AUC (CV-AUC) | GRS OR (95% CI) | P Value | ||
| AC | Baseline | 0.65 (0.55) | — | — | 0.65 (0.55) | — | — |
| GRS | 0.69 (0.61) | 1.39 (1.24–1.57) | <.0001 | 0.68 (0.61) | 1.41 (1.24–1.60) | <.0001 | |
| Baseline + GRS | 0.74 (0.68) | 1.41 (1.24–1.60) | <.0001 | 0.74 (0.68) | 1.38 (1.22–1.55) | <.0001 | |
| EU | Baseline | 0.74 (0.71) | — | — | 0.69 (0.64) | — | — |
| GRS | 0.60 (0.53) | 1.22 (1.11–1.33) | <.0001 | 0.62 (0.56) | 1.23 (1.13–1.34) | <.0001 | |
| Baseline + GRS | 0.77 (0.74) | 1.29 (1.17–1.42) | <.0001 | 0.73 (0.69) | 1.26 (1.15–1.38) | <.0001 | |
| MA | Baseline | 0.78 (0.71) | — | — | 0.73 (0.64) | — | — |
| GRS | 0.70 (0.62) | 1.59 (1.32–1.92) | <.0001 | 0.66 (0.60) | 1.34 (1.15–1.55) | <.0001 | |
| Baseline + GRS | 0.84 (0.78) | 1.64 (1.33–2.01) | <.0001 | 0.77 (0.70) | 1.39 (1.19–1.63) | <.0001 | |
| TH | Baseline | 0.75 (0.71) | — | — | 0.74 (0.69) | — | — |
| GRS | 0.60 (0.53) | 1.23 (1.10–1.38) | .00038 | 0.66 (0.60) | 1.28 (1.17–1.40) | <.0001 | |
| Baseline + GRS | 0.77 (0.73) | 1.23 (1.08–1.38) | .0011 | 0.79 (0.75) | 1.31 (1.19–1.45) | <.0001 | |
Abbreviation: AUC, area under the ROC curve; CV-AUC, AUC under cross-validation; OR, odds ratio.
Baseline model adjusted for population substructure within each ancestry, sex, gestational age at delivery, parity, maternal smoking/alcohol intake during pregnancy, maternal age, BMI, mean arterial blood pressure, and height at the time of OGTT, and maternal fasting, 1-h and 2-h glucose levels observed during the OGTT.
OR is reported for GRS higher by one risk allele.
P value corresponds to OR.
AUC is reported for the full data set for all models.
CV-AUC is reported using leave-one-out cross-validation.
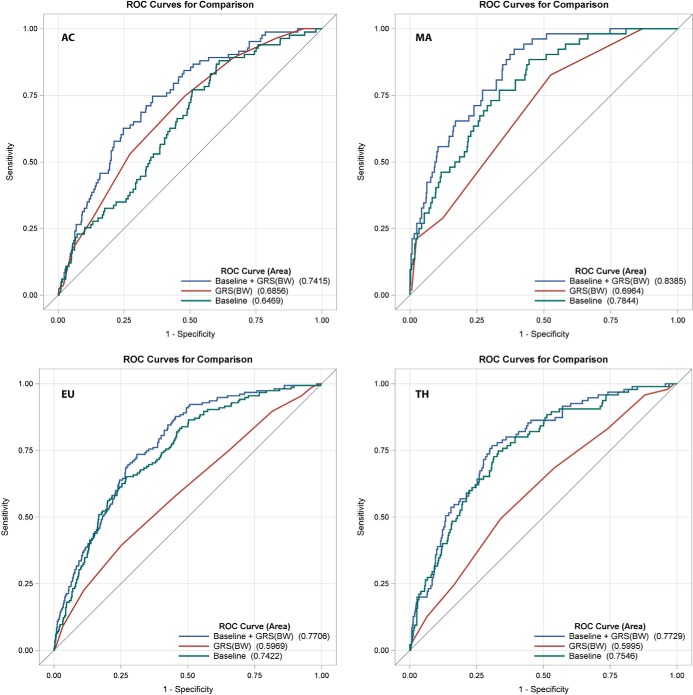
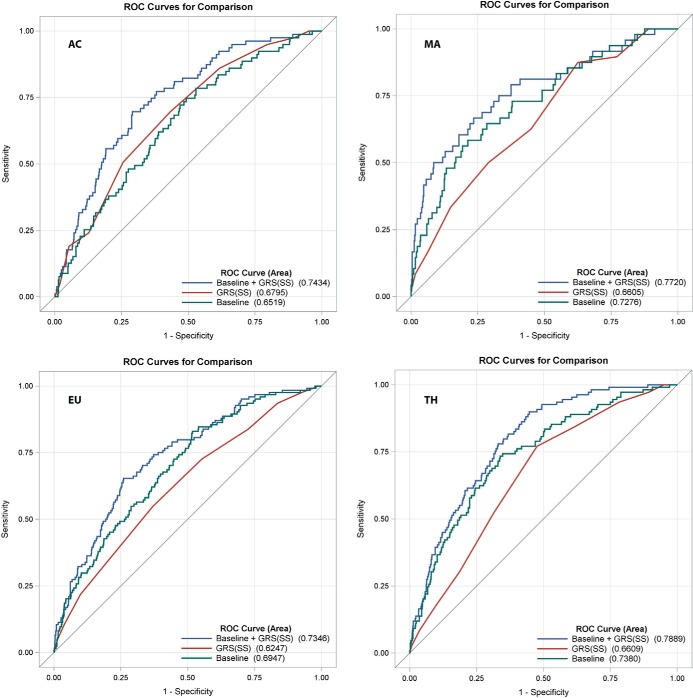
Predictive accuracy for LGA birth and SSF >90th percentile was estimated for models including the GRS alone, all baseline clinical covariates, and the GRS plus baseline clinical covariates using AUC for the whole data set and using leave-one-out cross-validation (Table 3 and Figures 2 and 3). A predictive model including the GRS and baseline clinical covariates significantly increased AUC for birth weight and SSF >90th percentile across all ancestries when compared with models using either the GRS or baseline clinical covariates alone for both the whole data set and after cross validation (Figure 2, 3, Table 3). These data suggest that the GRS contributes independently to the prediction of either outcome above traditional risk factors including maternal glucose, and that both components are strong contributors to outcome prediction. For the models including GRS and baseline covariates, at a sensitivity of 0.75, specificities for LGA were 0.64 (AC), 0.65 (EU), 0.73 (MA), and 0.70 (TH), and specificities for SSF >90th percentile were 0.64 (AC), 0.61 (EU), 0.67 (MA), and 0.68 (TH).
Figure 2.
ROC curves for birth weight > 90th percentile. The baseline model (includes maternal glucose), GRS, combined models, and calculation of the AUCs are described in the Methods.
Figure 3.
ROC curves for SSF > 90th percentile. The baseline model (includes maternal glucose), GRS, combined models, and calculation of the AUCs are described in the Methods.
Previous genome wide association studies reported association of seven loci with birth weight (11). Given their potential for predicting LGA and SSF >90th percentile, we developed a 7-GRS by summing the risk allele counts in these loci. We evaluated predictive accuracy of the 7-GRS in the whole data set and after cross-validation using AUC and model fit using AIC for models including 7-GRS or 7-GRS + GRS in addition to baseline clinical covariates. Although addition of 7-GRS to the baseline model increased AUC for all ancestries for both birth weight and SSF > 90th percentile, addition of the GRS to the 7-GRS increased predictive accuracy even further (Supplemental Figures 3 and 4, Supplemental Table 10). AUC for models including 7-GRS + GRS and baseline covariates were comparable and often equivalent to those including only GRS and baseline covariates both for the whole data set and after cross-validation with differences in AUC for models with and without 7-GRS ranging from 0.00–0.02 (Supplemental Table 10). Confirming this observation, logistic regression models including the GRS had similar AIC values with and without 7-GRS for all ancestry groups (Supplemental Table 10), suggesting modest gains at best in terms of model fit when adding 7-GRS to the GRS.
To investigate possible refinements to GRS models, we evaluated models including wGRS with and without maternal genotype, in addition to baseline clinical covariates. Given the approach used to select SNPs for the GRS, as expected, the wGRS was significantly associated with continuous birth weight and SSF outcomes (Supplemental Figure 1), as well as birth weight and SSF > 90th percentile in logistic regression analyses after control for baseline covariates (Supplemental Table 10, Supplemental Figure 2). Although the wGRS allows for the possibility that SNPs are associated with outcomes with variable degrees of risk, we found no statistically significant difference in AUC between the wGRS vs GRS models for predicting birth weight or SSF > 90th percentile using DeLong tests (Supplemental Figure 2). In linear regression models, maternal genotypes for the individual SNPs used in the GRS were not significantly associated with continuous birth weight and SSF outcomes after orthogonalization to fetal genotype. Corroborating this observation, AIC values were all higher for logistic regression models including maternal genotype, suggesting that increased model complexity by adding maternal genotype did not improve model fit (Supplemental Table 10). If maternal genotype was the primary driver of association with neonatal adiposity beyond that attributable to fetal genotype, for example through raised maternal glucose, the additional contribution would be quantified in regression model coefficients with low P values and lower AIC values even after orthogonalization of the maternal genotype to the fetal genotype. Furthermore, for prediction purposes, whereas inclusion of maternal genotype in logistic regression models increased AUC for the whole data set, these increases were not evident upon cross-validation, suggesting potential model overfitting (Supplemental Figure 2, Supplemental Table 10).
In summary, GRS models including baseline covariates demonstrate significant associations of the GRS with continuous birth weight and SSF outcomes. For both birth weight and SSF > 90th percentile, the GRS demonstrated statistically significant odds ratios and gains in AUC after baseline covariate adjustment with comparable increases in magnitude both for the full data set and under cross validation. AIC values were in general lowest for the GRS and baseline covariate models, with 7-GRS resulting in a modest improvement in fit for some ancestries. The more-easily applied GRS performs comparably to wGRS for prediction purposes, and maternal genotype modeling does not improve cross-validated AUC estimates or model fit in terms of AIC (Supplemental Table 10).
Discussion
LGA newborns are at increased risk for adverse metabolic outcomes including obesity, type 2 diabetes, and metabolic syndrome (3–6). Prediction of LGA birth has been challenging, although a number of maternal risk factors have been identified, including height, parity, ethnicity, age, previous delivery of an LGA infant, and fetal sex (3, 23, 24), as well as modifiable risk factors such as maternal BMI and weight gain during pregnancy (3, 7, 21, 23). Clinical and sonographic estimates of fetal weight have been tested as predictors, although these are most useful late in pregnancy when intervention may be less effective (3, 25, 26). We report here that a GRS is associated with birth weight and newborn SSF after adjustment for known risk factors, including maternal BMI and glucose. When added to a model that includes known risk factors, the GRS improved prediction of birth weight > 90th percentile (LGA) and SSF > 90th percentile in four ancestry groups. Using a GRS in combination with known predictors of LGA birth may be an alternative approach to identifying fetuses at risk for LGA birth.
Prediction of size at birth has focused on birth weight and, together with maternal factors, largely relies on fetal ultrasonography or clinical examination. Sensitivity and specificity of ultrasonographic approaches to estimate fetal weight at 28 to at least 41 weeks range from 12–75% and 68–98%, respectively, whereas sensitivity and specificity for clinical examination at 37 to at least 41 weeks range from 34–82% and 87–98%, respectively (27–29). A more recent report suggested improved sensitivity (88%) and specificity (73%) for LGA birth based on ultrasonographic measurements of fetal weight at 32–34 weeks of gestation (30). In this case, addition of maternal clinical factors only minimally improved prediction. Fetal ultrasonography is subject to limitations, eg, imaging quality, maternal adiposity, and ultrasonographer bias (3).
Birth weight is a function of both fat and lean body mass (23). Compared with appropriate-for-gestational-age newborns, LGA newborns typically have increased absolute body fat (31). The adverse metabolic consequences associated with LGA are likely secondary to excess fat as opposed to lean body mass (32, 33), thus, specifically identifying newborn adiposity is a key challenge. Methods to reliably determine fetal fat mass in utero have not been developed. Although ultrasonography can help detect excess birth weight in utero, fetal weight estimated between 33 and 38 weeks' gestation using 2-D ultrasound did not correlate with newborn adiposity as measured by air displacement plethysmography (34). Our data demonstrate that a GRS not only helped to predict LGA birth (sensitivity, 75%; specificities, 64–70%) but also improved prediction of SSF > 90th percentile, a direct measure of newborn adiposity, even after accounting for maternal genotype, glucose levels, and BMI (sensitivity 75%, specificities 61–68%). Thus, our data suggest that a GRS may assist with identifying fetuses at risk for excess adiposity at birth and its long-term consequences.
We and others have previously identified genes through genome wide association studies associated with newborn birth weight and/or SSF (10–12). Whether genetic variants associated with adult obesity-related traits are also associated with newborn size at birth is less clear. Previous studies addressing this question have been inconsistent (35, 36). We found no association of the index SNPs associated with adult obesity-related traits with birth weight or newborn adiposity. However, we did find that SNPs within or near some genetic loci containing the index SNPs demonstrated some evidence for association with newborn birth weight and/or SSF in individual ancestry groups or after meta-analysis across ancestries. Differences in patterns of LD and/or allele frequencies across ancestries may account, in part, for these findings (37–40).
Although the size of each of our ancestry groups was limited, power analyses suggested that the sample sizes afforded 90% power or more to detect the reported effect sizes. Moreover, because of the availability of maternal genotypes, we were able to investigate potential contribution of maternal genotype beyond that of fetal phenotype, an approach which has not been previously reported. One limitation of our study was the lack of data on gestational weight gain and maternal nutrition, both of which can affect fetal size at birth. A second limitation was that all mothers and newborns were from the HAPO Study, although multiple ancestry groups from different locations were studied.
In summary, a goal of this study was to examine associations of a GRS with fetal outcomes above and beyond traditional risk factors for birth weight and fetal adiposity and, for prediction purposes, to consider traditional risk factors together with the GRS. To ensure that our GRS model was robust, we examined the effect of adding in seven additional known birth weight loci (7-GRS), using a wGRS and considering the effect of maternal genotype on the observed associations. Neither model fit statistics (AIC) nor predictive accuracy (cross-validated AUC) suggested that these modifications added substantially to model performance, supporting use of a simpler GRS. As might be expected, adding the known birth weight loci modestly improved the accuracy of prediction in some ancestry groups, so future evaluation of the usefulness of these or other loci in the GRS will be important. Our results have provided proof of principle that a GRS may be effective in helping to predict LGA birth or fetal adiposity in conjunction with traditional risk factors. This approach will require validation in additional cohorts as well as further work to optimize the GRS and approaches for its use.
Acknowledgments
We thank the participants and research personnel of the HAPO study at each of the following field centers: Newcastle and Brisbane, Australia; Bridgetown, Barbados; Toronto, Canada; Bangkok, Thailand; Belfast, United Kingdom; and Bellflower, California.
This work was supported by National Institutes of Health (NIH) Grants HD34242, HD34243, HG004415, and DK099820, and by the American Diabetes Association.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- Afro-Caribbean
- AIC
- Akaike Information Criterion
- AUC
- area under the ROC curve
- BMI
- body mass index
- EU
- Northern European
- GRS
- genetic risk score
- HAPO
- Hyperglycemia and Adverse Pregnancy Outcome
- LD
- linkage disequilibrium
- LGA
- large for gestational age
- MA
- Mexican-American
- OGTT
- oral glucose tolerance test
- ROC
- Receiver Operating Characteristic
- SNP
- Single nucleotide polymorphism
- SSF
- sum of skinfolds
- TH
- Thai
- wGRS
- weighted genetic risk score.
References
- 1. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birth weight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672 e1–4. [DOI] [PubMed] [Google Scholar]
- 2. Zhang X, Decker A, Platt RW, Kramer MS. How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol. 2008;198(5):517 e1–6. [DOI] [PubMed] [Google Scholar]
- 3. Walsh JM, McAuliffe FM. Prediction and prevention of the macrosomic fetus. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):125–30. [DOI] [PubMed] [Google Scholar]
- 4. Ahlsson F, Gustafsson J, Tuvemo T, Lundgren M. Females born large for gestational age have a doubled risk of giving birth to large for gestational age infants. Acta Paediatr. 2007;96(3):358–62. [DOI] [PubMed] [Google Scholar]
- 5. Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308(5):242–5. [DOI] [PubMed] [Google Scholar]
- 6. Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity–a vicious circle across generations. Int J Obes (Lond). 2012;36(10):1320–1324. [DOI] [PubMed] [Google Scholar]
- 7. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 8. Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–69. [DOI] [PubMed] [Google Scholar]
- 9. Sacks DA. Determinants of fetal growth. Curr Diab Rep. 2004;4(4):281–7. [DOI] [PubMed] [Google Scholar]
- 10. Freathy RM, Mook-Kanamori DO, Sovio U, et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat Genet. 2010;42(5):430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horikoshi M, Yaghootkar H, Mook-Kanamori DO, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Urbanek M, Hayes MG, Armstrong LL, et al. The chromosome 3q25 genomic region is associated with measures of adiposity in newborns in a multi-ethnic genome-wide association study. Hum Mol Genet. 2013;22(17):3583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet. 2002;78(1):69–77. [DOI] [PubMed] [Google Scholar]
- 14. Laurie CC, Doheny KF, Mirel DB, et al. Quality control and quality assurance in genotypic data for genome-wide association studies. Genet Epidemiol. 2010;34(6):591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84(2):210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heid IM, Jackson AU, Randall JC, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42(11):949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panagiotou OA, Ioannidis JP. What should the genome-wide significance threshold be? Empirical replication of borderline genetic associations. Int J Epidemiol. 2012;41(1):273–86. [DOI] [PubMed] [Google Scholar]
- 20. Rodgers JL, Nicewander WA, Toothaker L. Linearly Independent, Orthogonal, and Uncorrelated Variables. Am Stat. 1984;38(2):133–4. [Google Scholar]
- 21. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes. 2009;58(2):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Demler OV, Pencina MJ, D'Agostino RB., Sr. Misuse of DeLong test to compare AUCs for nested models. Stat Med. 2012;31(23):2577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–33. [DOI] [PubMed] [Google Scholar]
- 24. Lingwood BE, Henry AM, d'Emden MC, et al. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care. 2011;34(12):2581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrikovsky BM, Oleschuk C, Lesser M, Gelertner N, Gross B. Prediction of fetal macrosomia using sonographically measured abdominal subcutaneous tissue thickness. J Clin Ultrasound. 1997;25(7):378–82. [DOI] [PubMed] [Google Scholar]
- 26. Khoury FR, Stetzer B, Myers SA, Mercer B. Comparison of estimated fetal weights using volume and 2-dimensional sonography and their relationship to neonatal markers of fat. J Ultrasound Med. 2009;28(3):309–315. [DOI] [PubMed] [Google Scholar]
- 27. Chauhan SP, Grobman WA, Gherman RA, et al. Suspicion and treatment of the macrosomic fetus: A review. Am J Obstet Gynecol. 2005;193(2):332–46. [DOI] [PubMed] [Google Scholar]
- 28. Kernaghan D, Ola B, Fraser RB, Farrell T, Owen P. Fetal size and growth velocity in the prediction of the large for gestational age (LGA) infant in a glucose impaired population. Eur J Obstet Gynecol Reprod Biol. 2007;132(2):189–92. [DOI] [PubMed] [Google Scholar]
- 29. Nelson L, Wharton B, Grobman WA. Prediction of large for gestational age birth weights in diabetic mothers based on early third-trimester sonography. J Ultrasound Med. 2011;30(12):1625–8. [DOI] [PubMed] [Google Scholar]
- 30. Lindell G, Maršál K, Källén K. Predicting risk for large-for-gestational age neonates at term: A population-based Bayesian theorem study. Ultrasound Obstet Gynecol. 2013;41(4):398–405. [DOI] [PubMed] [Google Scholar]
- 31. Hammami M, Walters JC, Hockman EM, Koo WW. Disproportionate alterations in body composition of large for gestational age neonates. J Pediatr. 2001;138(6):817–21. [DOI] [PubMed] [Google Scholar]
- 32. Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189(6):1698–704. [DOI] [PubMed] [Google Scholar]
- 33. Moore TR. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am J Obstet Gynecol. 2010;202(6):643–9. [DOI] [PubMed] [Google Scholar]
- 34. Moyer-Mileur LJ, Slater H, Thomson JA, Mihalopoulos N, Byrne J, Varner MW. Newborn adiposity measured by plethysmography is not predicted by late gestation two-dimensional ultrasound measures of fetal growth. J Nutr. 2009;139(9):1772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kilpeläinen TO, den Hoed M, Ong KK, et al. Obesity-susceptibility loci have a limited influence on birth weight: A meta-analysis of up to 28,219 individuals. Am J Clin Nutr. 2011;93(4):851–60. [DOI] [PubMed] [Google Scholar]
- 36. Elks CE, Loos RJ, Sharp SJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 2010;7(5):e1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cooper RS, Tayo B, Zhu X. Genome-wide association studies: Implications for multiethnic samples. Hum Mol Genet. 2008;17(R2):R151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morris AP. Transethnic meta-analysis of genomewide association studies. Genet Epidemiol. 2011;35(8):809–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zaitlen N, Paaniuc B, Gur T, Ziv E, Halperin E. Leveraging genetic variability across populations for the identification of causal variants. Am J Hum Genet. 2010;86(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andersen V, Ernst A, Sventoraityte J, et al. Assessment of heterogeneity between European Populations: A Baltic and Danish replication case-control study of SNPs from a recent European ulcerative colitis genome wide association study. BMC Med Genet. 2011;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]