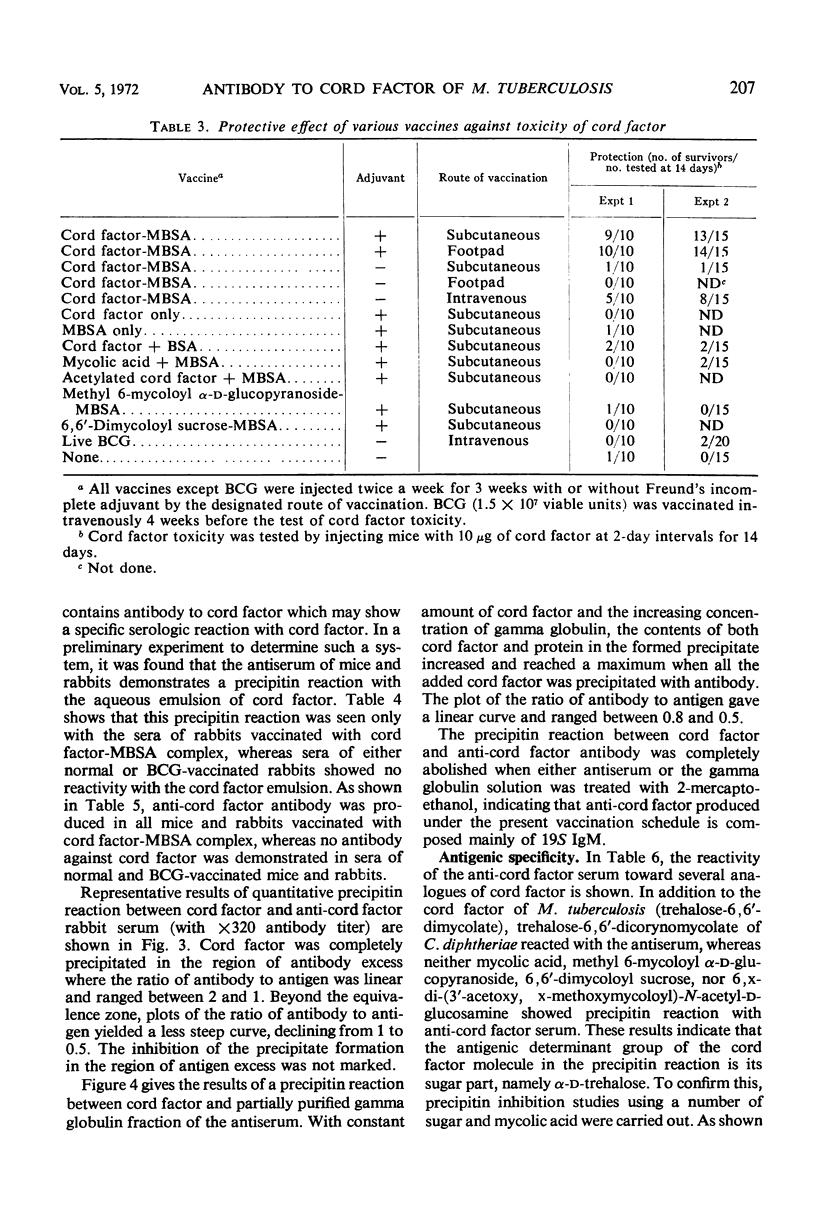
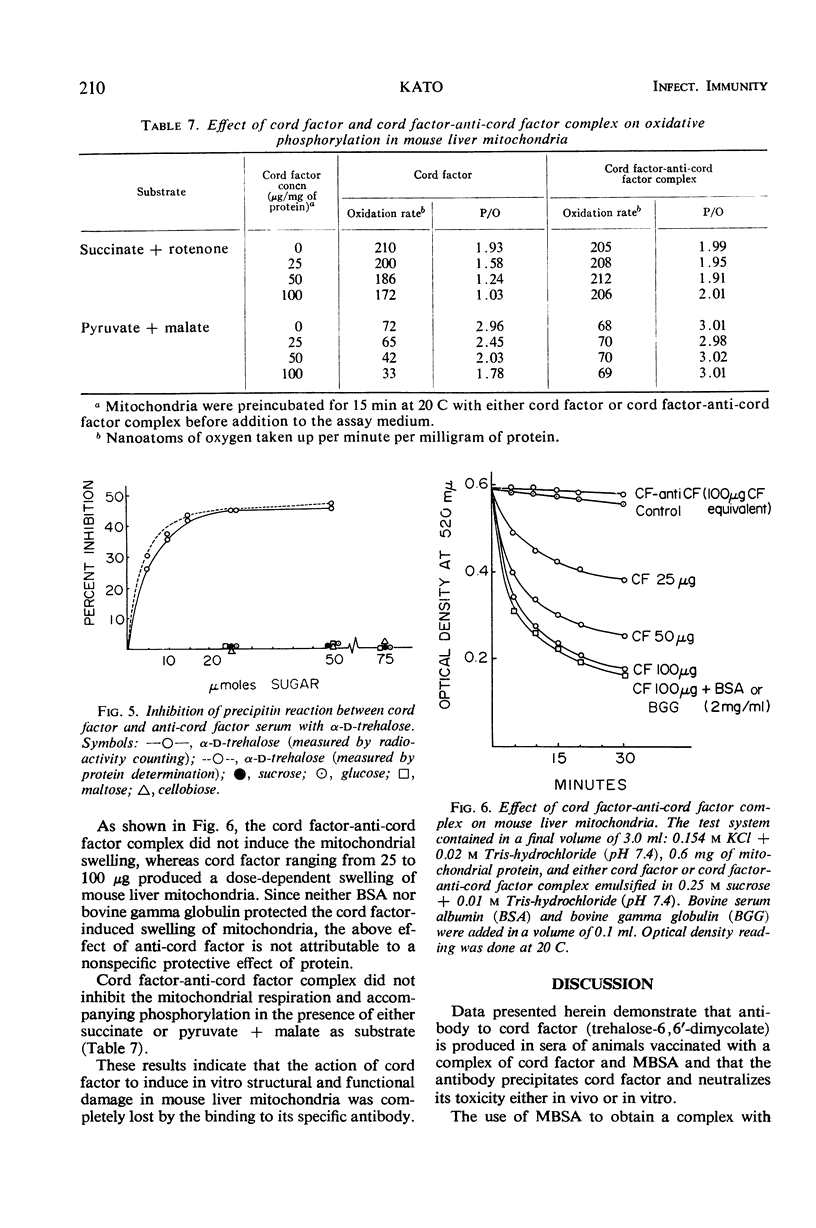
Abstract
A complex of trehalose-6, 6′-dimycolate (cord factor) and methylated bovine serum albumin was prepared, and its effect on the toxicity of cord factor in mice was studied. Either the active immunization with cord factor-methylated bovine serum albumin complex or the passive transfer of sera of rabbits vaccinated with the complex protected mice against the toxic action of cord factor. The antisera of mice and rabbits and gamma globulin fraction of rabbit antiserum demonstrated a precipitin reaction with an aqueous emulsion of cord factor. The reactivity of anticord factor was totally lost by treatment with 2-mercaptoethanol. The antigenic determinant group of cord factor was α-d-trehalose. Cord factor-anticord factor complex was devoid of activity to induce in vitro swelling and inhibition of respiratory and phosphorylative activity of mouse liver mitochondria. These results suggest that vaccination with cord factor-methylated bovine serum albumin complex induced the production of antibody to cord factor in mouse and rabbit serum which binds to cord factor and neutralizes its toxic activity either in vivo or in vitro.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASSELINEAU J., LEDERER E. Sur la constitution chimique des acides mycoliques de deux souches humaines virulentes de Mycobacterium tuberculosis. Biochim Biophys Acta. 1951 May;7(1):126–145. doi: 10.1016/0006-3002(51)90011-x. [DOI] [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Nojima S. Immunochemical studies of phospholipids. 3. Production of antibody to cardiolipin. Biochim Biophys Acta. 1967 Oct 2;144(2):409–414. [PubMed] [Google Scholar]
- Kato M., Fukushi K. Studies of a biochemical lesion in experimental tuberculosis in mice. X. Mitochondrial swelling induced by cord factor in vivo and accompanying biochemical change. Am Rev Respir Dis. 1969 Jul;100(1):42–46. doi: 10.1164/arrd.1969.100.1.42. [DOI] [PubMed] [Google Scholar]
- Kato M. Site II-specific inhibition of mitochondria oxidative phosphorylation by trehalose-6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis. Arch Biochem Biophys. 1970 Oct;140(2):379–390. doi: 10.1016/0003-9861(70)90079-2. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. 8. Effect of derivatives and chemical analogues of cord factor on structure and function of mouse liver mitochondria. Am Rev Respir Dis. 1968 Oct;98(4):668–676. doi: 10.1164/arrd.1968.98.4.668. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. IX. Response of respiratory enzymes in immune mouse liver to cord factor and tuberculin PPD. Am Rev Respir Dis. 1969 Jan;99(1):112–115. doi: 10.1164/arrd.1969.99.1.112. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. VII. Structural and functional damage in mouse liver mitochondria under the toxic action of cord factor. Am Rev Respir Dis. 1968 Aug;98(2):260–269. doi: 10.1164/arrd.1968.98.2.260. [DOI] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. XI. Mitochondrial swelling induced by cord factor in vitro. Am Rev Respir Dis. 1969 Jul;100(1):47–53. doi: 10.1164/arrd.1969.100.1.47. [DOI] [PubMed] [Google Scholar]
- Kato M., Tanaka A. Studies of a biochemical lesion in experimental tuberculosis in mice. V. Further study on a toxic lipid fraction in firmly bound lipids. Am Rev Respir Dis. 1967 Sep;96(3):460–468. doi: 10.1164/arrd.1967.96.3.460. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- NOLL H., BLOCH H. Studies on the chemistry of the cord factor of Mycobacterium tuberculosis. J Biol Chem. 1955 May;214(1):251–265. [PubMed] [Google Scholar]
- Senn M., Ioneda T., Pudles J., Lederer E. Spectrométrie de masse de glycolipides. I. Structure du "cord factor" de Corynebacterium diphtheriae. Eur J Biochem. 1967 May;1(3):353–356. doi: 10.1111/j.1432-1033.1967.tb00081.x. [DOI] [PubMed] [Google Scholar]



