Figure 6.
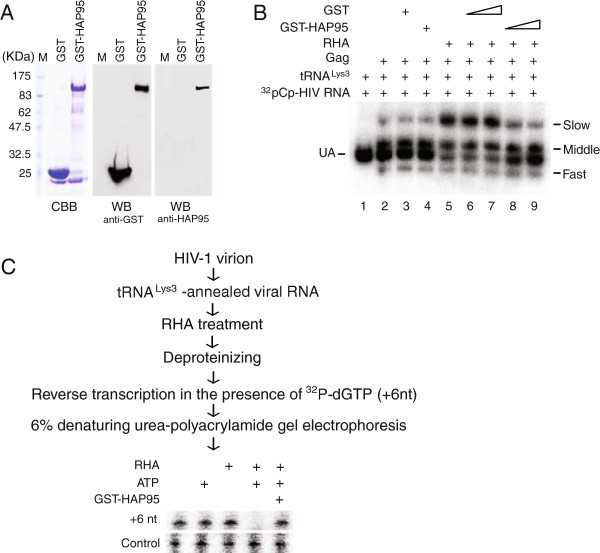
HAP95 inhibits activity of RHA in vitro . (A) Purification of GST-tagged HAP95. CBB, The proteins in SDS-PAGE were stained with Commassie brilliant blue R250. WB, Western blots of proteins purified from 293E cells were probed with anti-GST or anti-HAP95. M, protein size marker shown in kDa. (B) Annealing of tRNALys3 to viral RNA in vitro. 100 fmoles of tRNALys3 were annealed to 30 fmoles of 32pCp-labeled synthetic viral RNA by Gag in the presence of RHA, GST-HAP95, or both RHA and GST-HAP95. Purified GST was used in place of GST-HAP95 as a negative control. The annealing reaction mixture was incubated at 25°C for 40 min, separated in 5% native polyacrylamide gel, and visualized using a PhosphorImager instrument. Unannealed (UA) viral RNA and the slow-, middle-, and fast-migrating tRNALys3-annealed viral RNA bands are indicated. (C) RHA disrupts tRNALys3-viral RNA complex isolated from HIV-1 virion. tRNALys3-annealed viral RNA was isolated from HIV-1 particles, exposed to RHA in the presence or absence of ATP and/or GST-HAP95, extended by reverse transcription in the presence of 32P-dGTP, and separated in denaturing 6% polyacrylamide gel. The control panel is the same as described in Figure 4 legend.
