Abstract
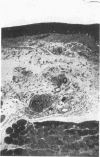
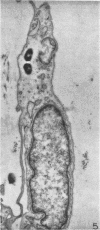
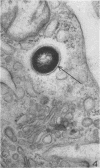
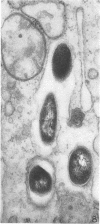
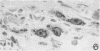
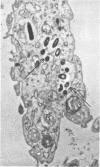
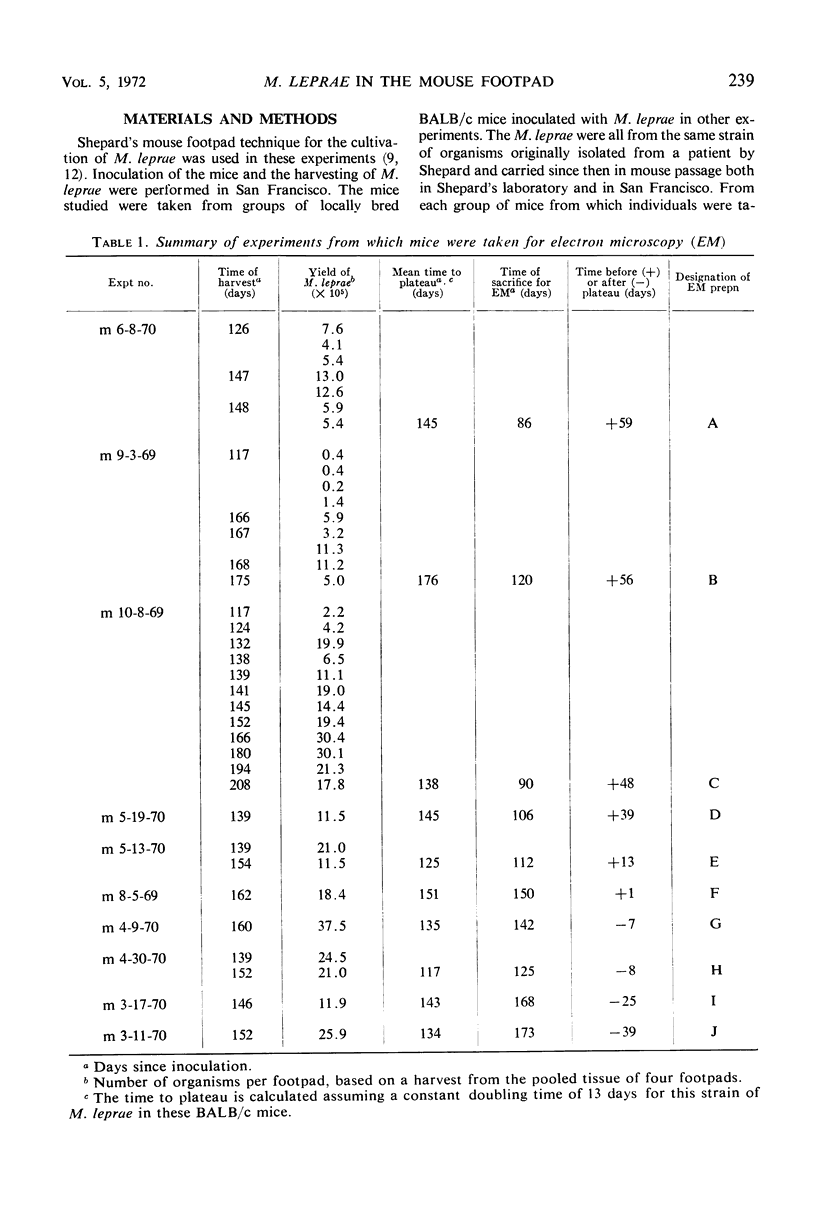
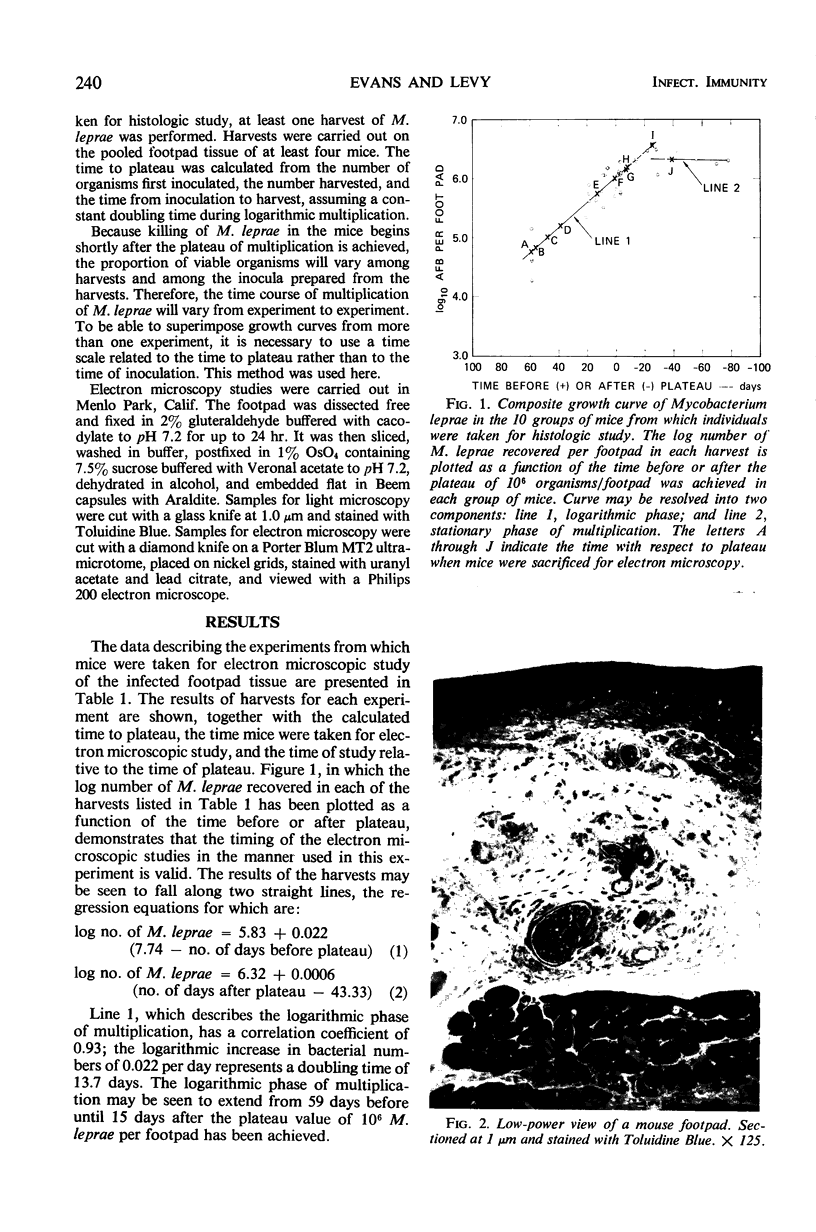
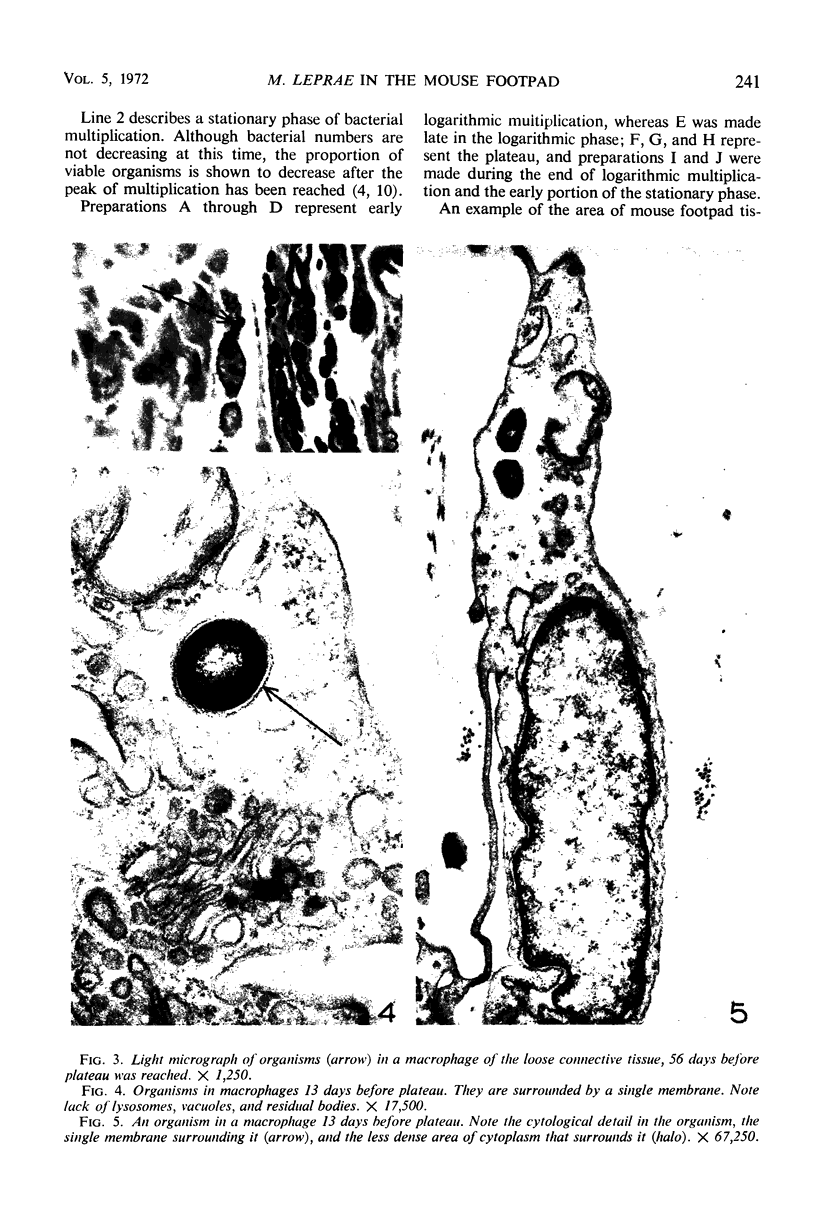
Ultrastructural changes in cells of the mouse footpad are described which occurred during the log phase of multiplication, the plateau, and the stationary phase of growth of Mycobacterium leprae.BALB/c mice were inoculated in the right hind footpad with 5 × 103 organisms and sacrificed in pairs at 86 to 173 days after inoculation. Tissue samples were prepared for electron microscopy by standard techniques. During the early growth phase of M. leprae in the mouse footpad, few organisms can be detected. Those present are in macrophages and are bound by a single membrane. The cytoplasm of the macrophage is less dense around the organism. There are few lysosomes, and the bacteria do not appear to be degenerating. At the peak of the growth phase, the organisms within a macrophage are bound by either a single or double membrane. There is an increased number of vacuoles, which are also bound by a double membrane, and lysosomes. During the stationary phase, most of the macrophages have taken on a vacuolar appearance and contain lysosomes. The vacuoles are bound by a double membrane, as are most of the organisms within the macrophage. Many of these organisms appear to be degenerating. Occasionally, organisms are encountered in the sarcoplasm of striated muscle. They are usually bound by a single membrane and do not appear to be degenerating.
Full text
PDF
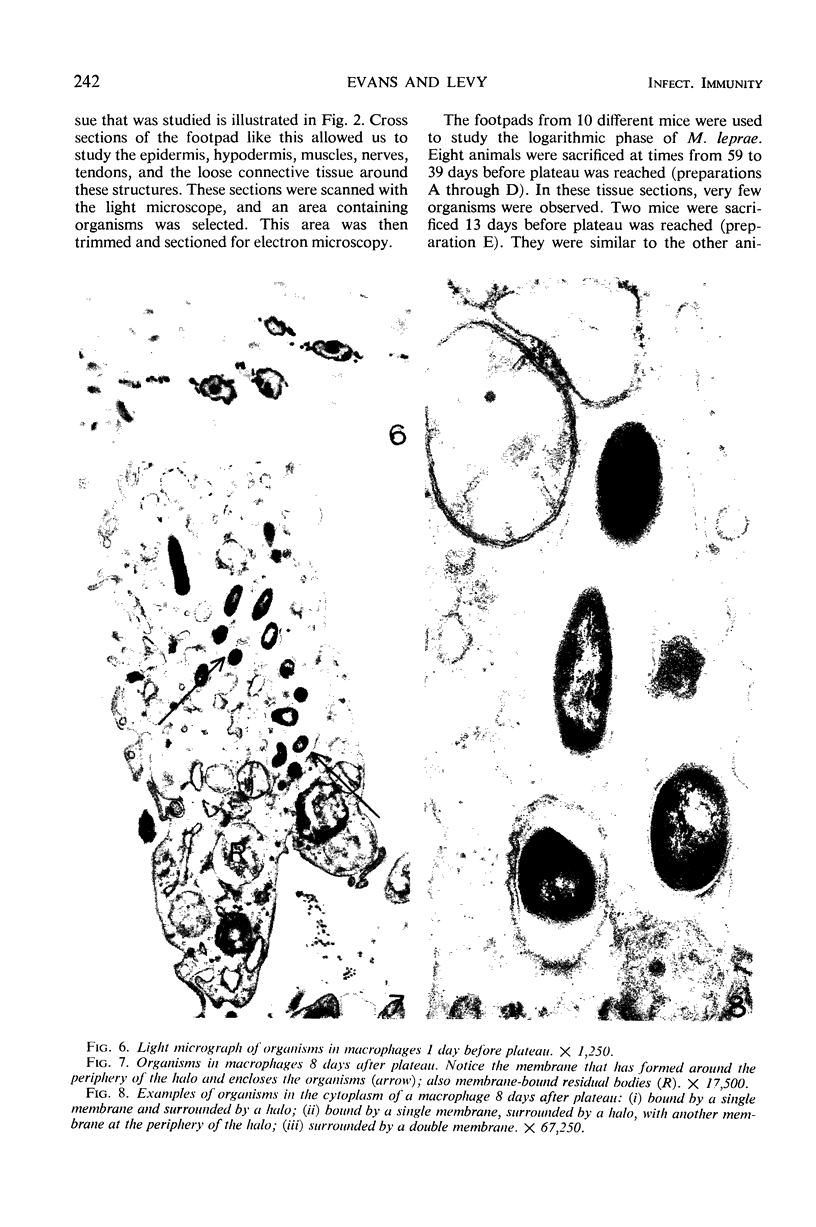
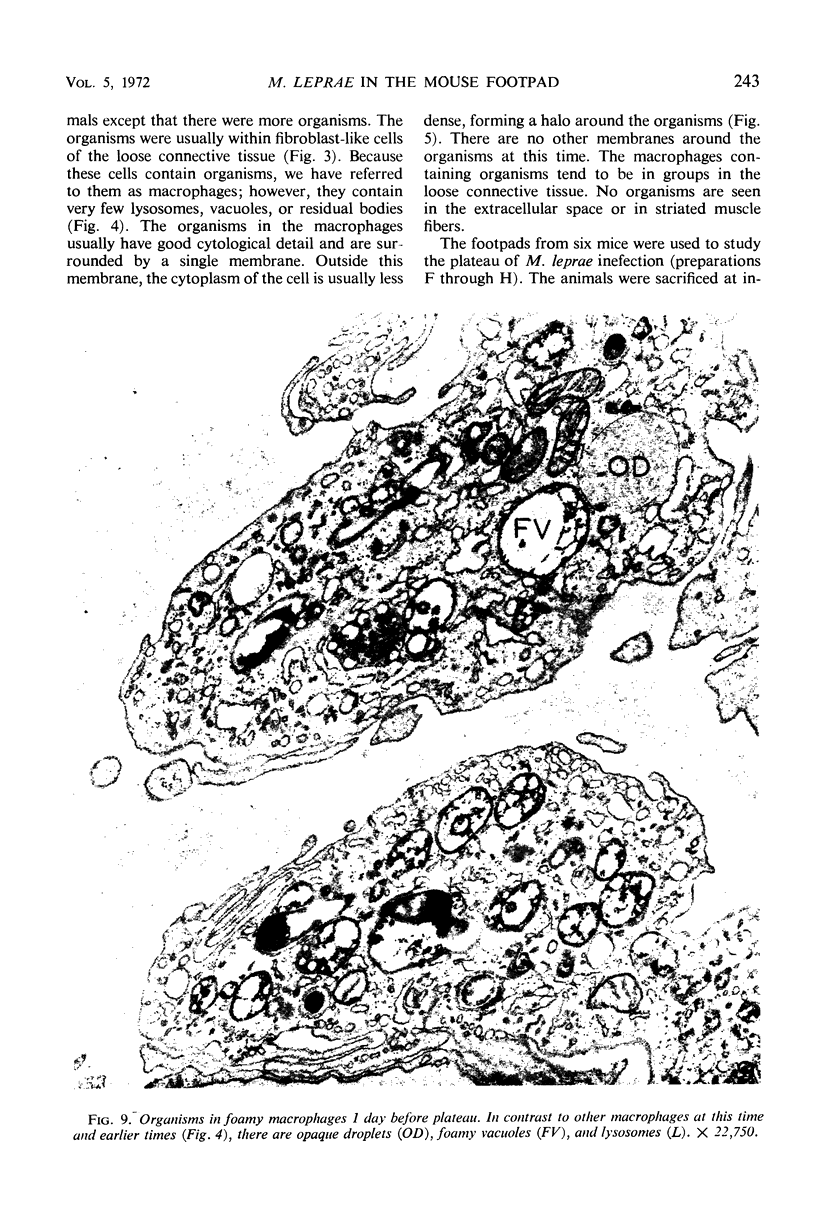
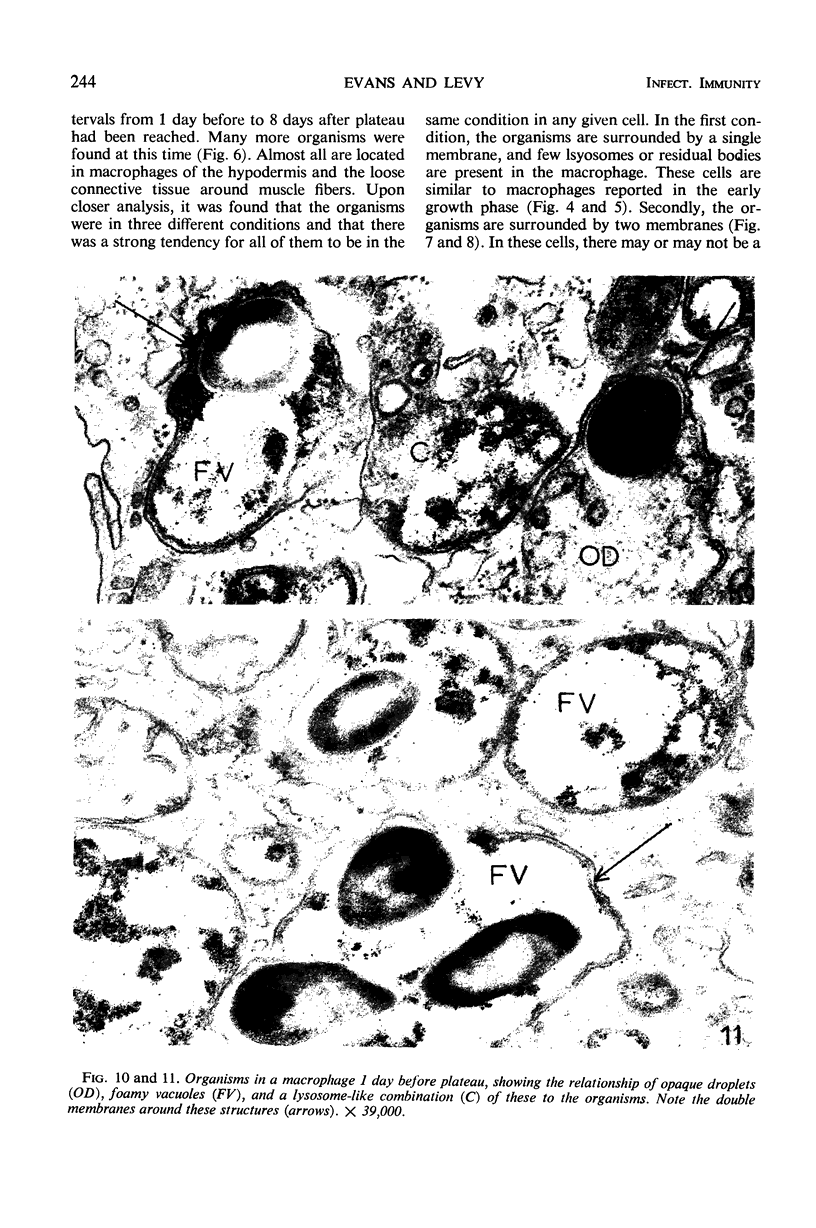
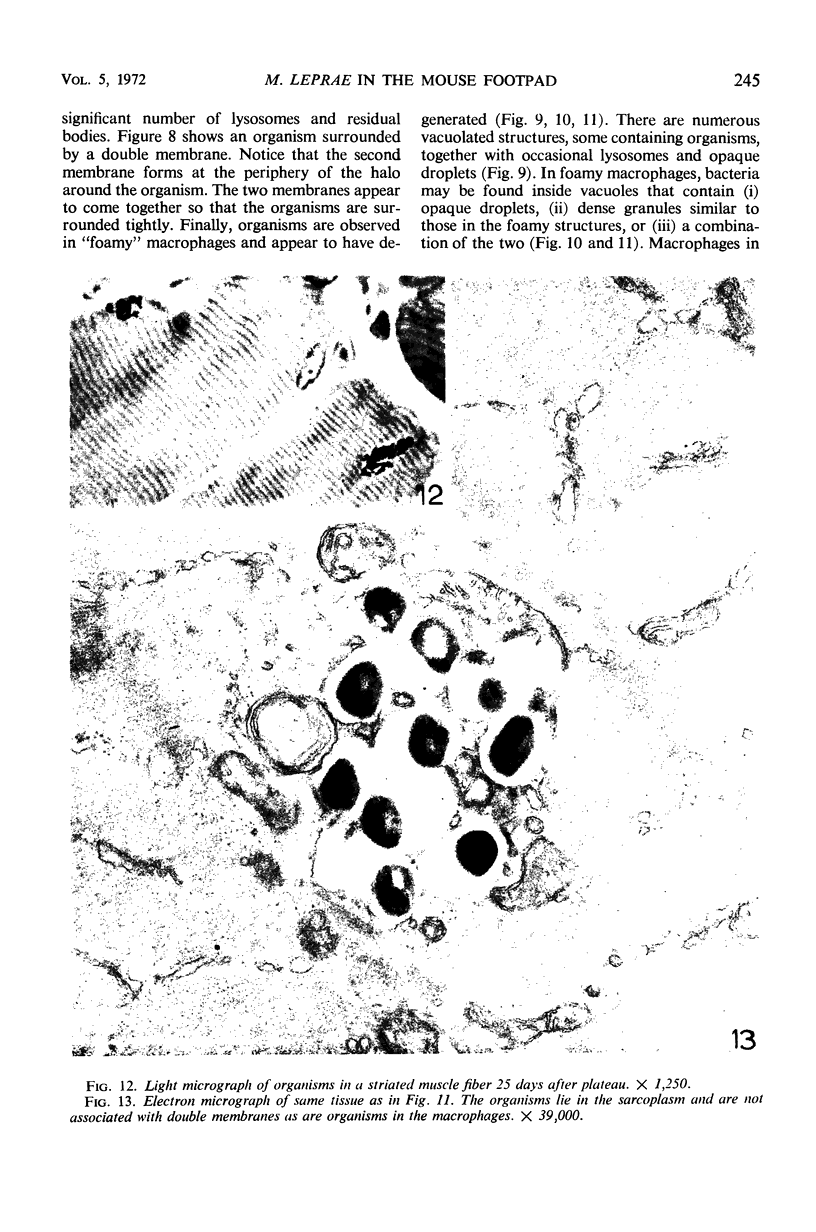


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aquino T. I., Skinsnes O. K. Pathobiologic significance of the subcellular organelles of lepra cells. Int J Lepr Other Mycobact Dis. 1970 Apr-Jun;38(2):134–148. [PubMed] [Google Scholar]
- Berthrong M. Biology of the mycobacterioses. The macrophage-tubercle bacillus relationship and resistance to tuberculosis. Ann N Y Acad Sci. 1968 Sep 5;154(1):157–166. doi: 10.1111/j.1749-6632.1968.tb16706.x. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L. Death of Mycobacterium leprae in mice, and the additional effect of dapsone administration. Proc Soc Exp Biol Med. 1970 Dec;135(3):745–749. doi: 10.3181/00379727-135-35134. [DOI] [PubMed] [Google Scholar]
- Palmer E., Rees R. J., Weddell A. G. Site of multiplication of human leprosy bacilli inoculated into the foot-pads of mice. Nature. 1965 May 1;206(983):521–522. doi: 10.1038/206521a0. [DOI] [PubMed] [Google Scholar]
- Pearson J. M., Rees R. J., Weddell A. G. Mycobacterium leprae in the striated muscle of patients with leprosy. Lepr Rev. 1970 Jul;41(3):155–166. doi: 10.5935/0305-7518.19700023. [DOI] [PubMed] [Google Scholar]
- Rees R. J., Weddell A. G. Biology of the mycobacterioses. Experimental models for studying leprosy. Ann N Y Acad Sci. 1968 Sep 5;154(1):214–236. doi: 10.1111/j.1749-6632.1968.tb16711.x. [DOI] [PubMed] [Google Scholar]
- Rees R. J., Weddell A. G. Transmission of human leprosy to the mouse and its clinical implications. Trans R Soc Trop Med Hyg. 1970;64(1):31–47. doi: 10.1016/0035-9203(70)90194-x. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Chang Y. T. Effect of DDS on established infections with Mycobacterium leprae in mice. Int J Lepr Other Mycobact Dis. 1967 Jan-Mar;35(1):52–57. [PubMed] [Google Scholar]
- Shepard C. C., Congdon C. C. Increased growth of Mycobacterium leprae in thymectomized-irradiated mice after foot pad inoculation. Int J Lepr Other Mycobact Dis. 1968 Apr-Jun;36(2):224–227. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Skinsnes O. K. Biology of the mycobacterioses. Comparative pathogenesis of mycobacterioses. Ann N Y Acad Sci. 1968 Sep 5;154(1):19–31. doi: 10.1111/j.1749-6632.1968.tb16692.x. [DOI] [PubMed] [Google Scholar]
- Spector W. G. The granulomatous inflammatory exudate. Int Rev Exp Pathol. 1969;8:1–55. [PubMed] [Google Scholar]
- Weissmann G., Dukor P. The role of lysosomes in immune responses. Adv Immunol. 1970;12:283–331. doi: 10.1016/s0065-2776(08)60172-8. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Skinsnes O. K. Intracellular modulation in cellular immunity. I. Morphologic studies of macrophages in murine leprosy under conditions of immunity enhancement and suppression. Int J Lepr Other Mycobact Dis. 1969 Apr-Jun;37(2):111–129. [PubMed] [Google Scholar]