Abstract
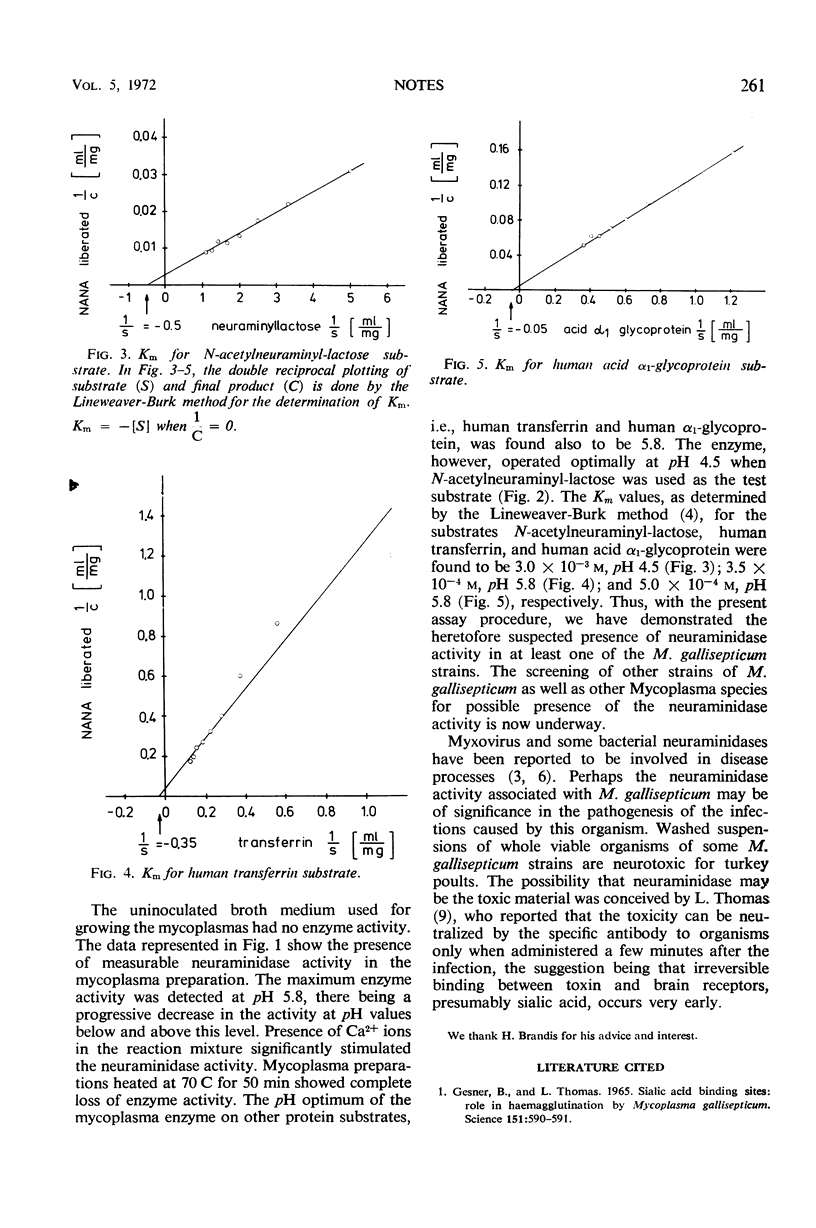
The whole viable Mycoplasma gallisepticum (strain TT) organisms were found to possess neuraminidase activity with a pH optimum of 5.8 on substrates such as human transferrin, human α1-glycoprotein, and rabbit serum. The enzyme operated optimally at pH 4.5 when N-acetylneuraminyl-lactose was used as the test substrate.
Full text
PDF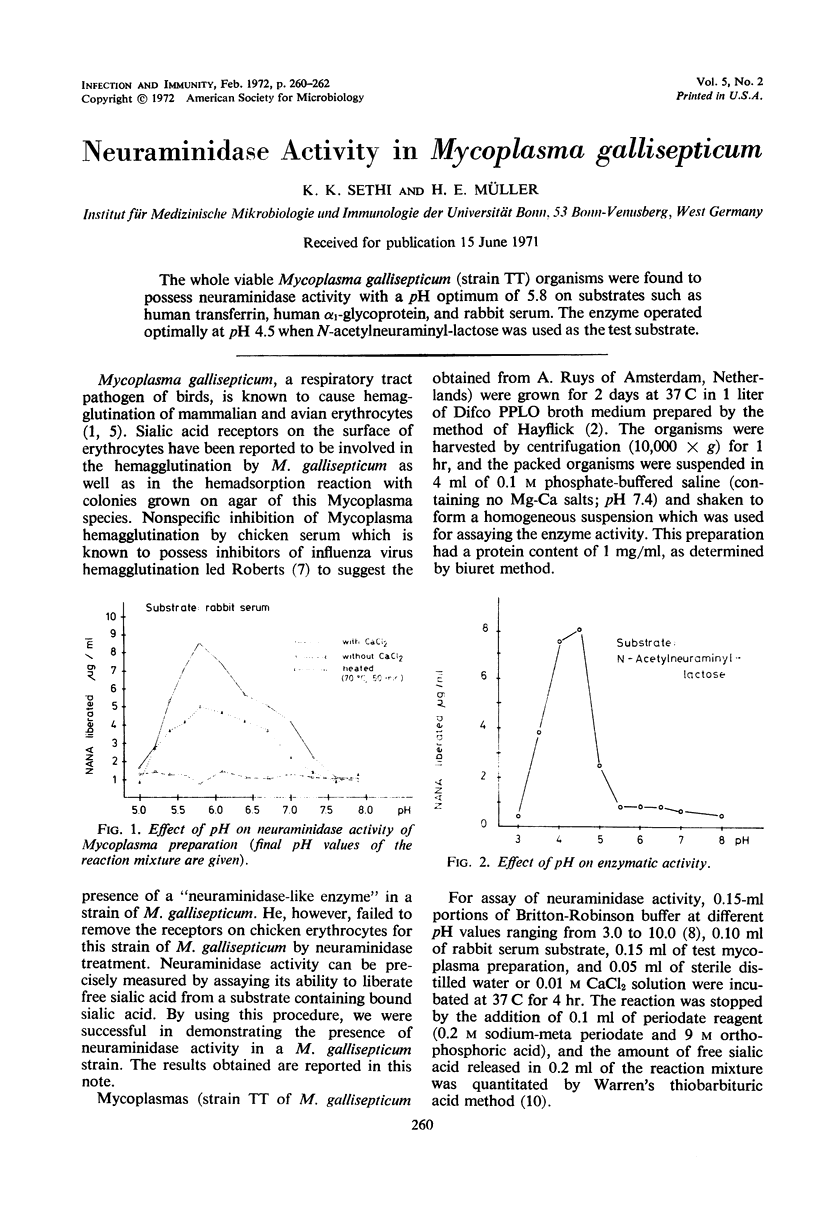

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gesner B., Thomas L. Sialic acid binding sites: role in hemagglutination by Mycoplasma gallisepticum. Science. 1966 Feb 4;151(3710):590–591. doi: 10.1126/science.151.3710.590. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- Müller H. E. Die Neuraminidase als pathogenetischer Faktor bei Pneumokokken-Infektionen. Dtsch Med Wochenschr. 1969 Oct 17;94(42):2149–passim. doi: 10.1055/s-0028-1110406. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]



