Abstract
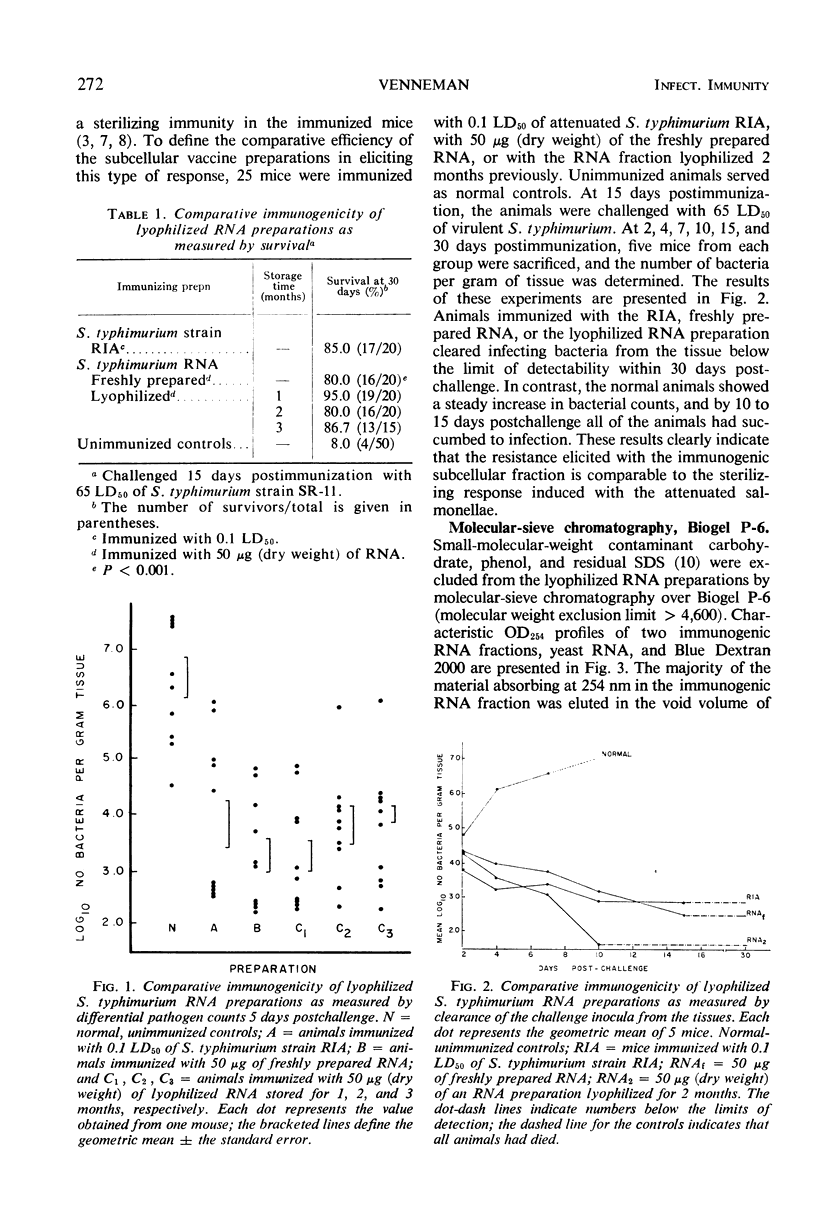
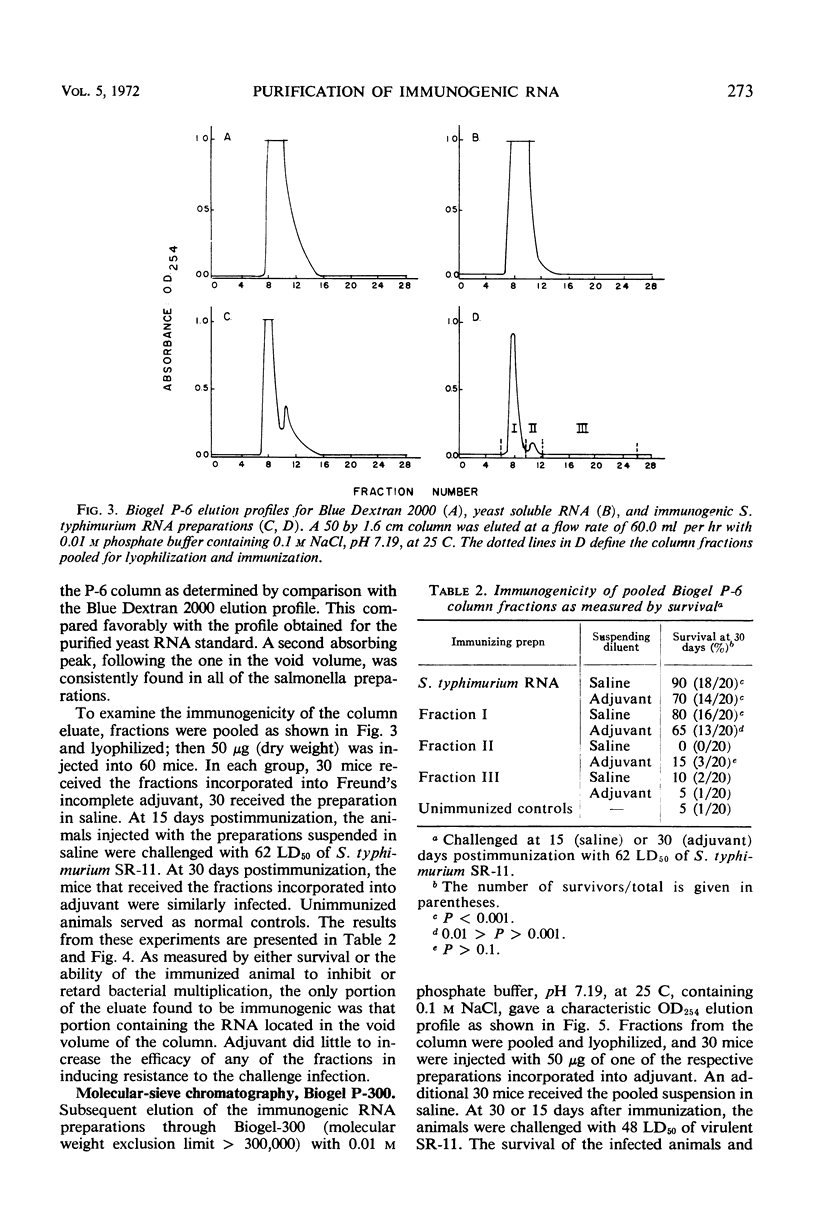
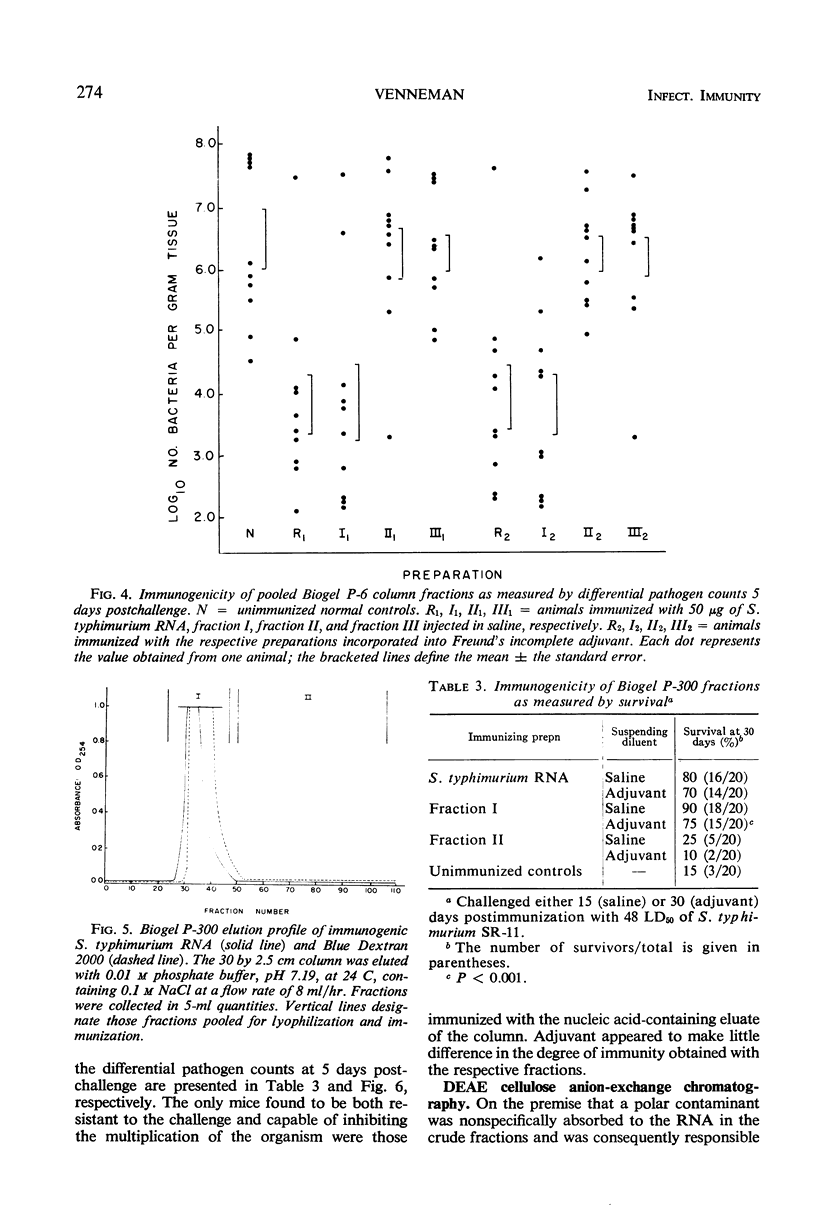
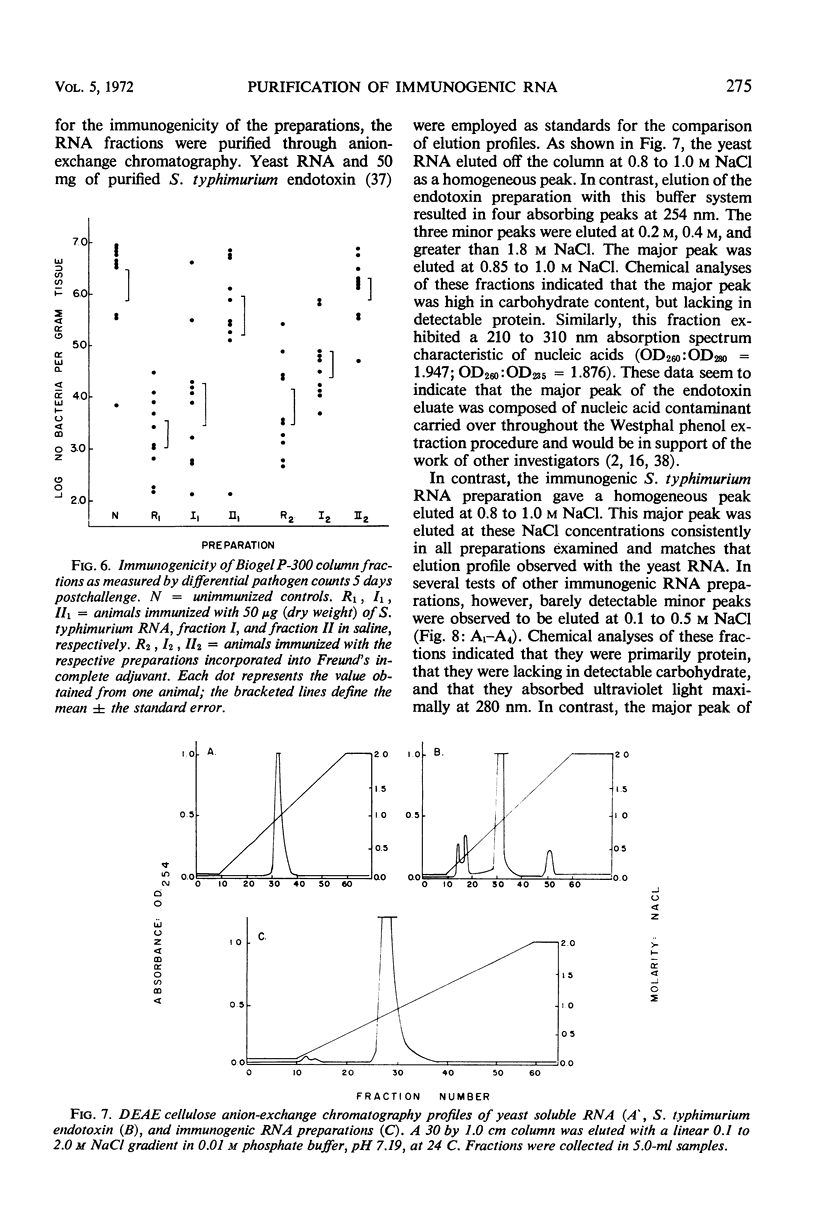
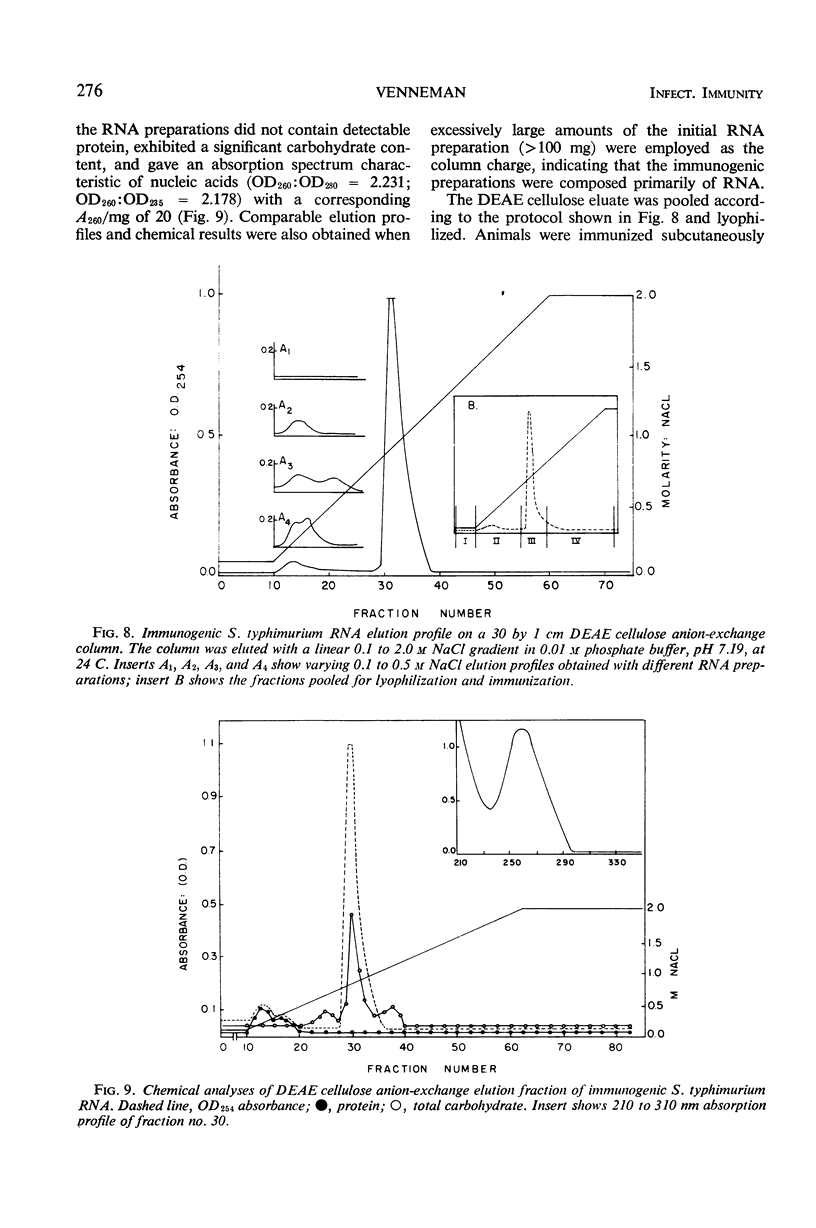
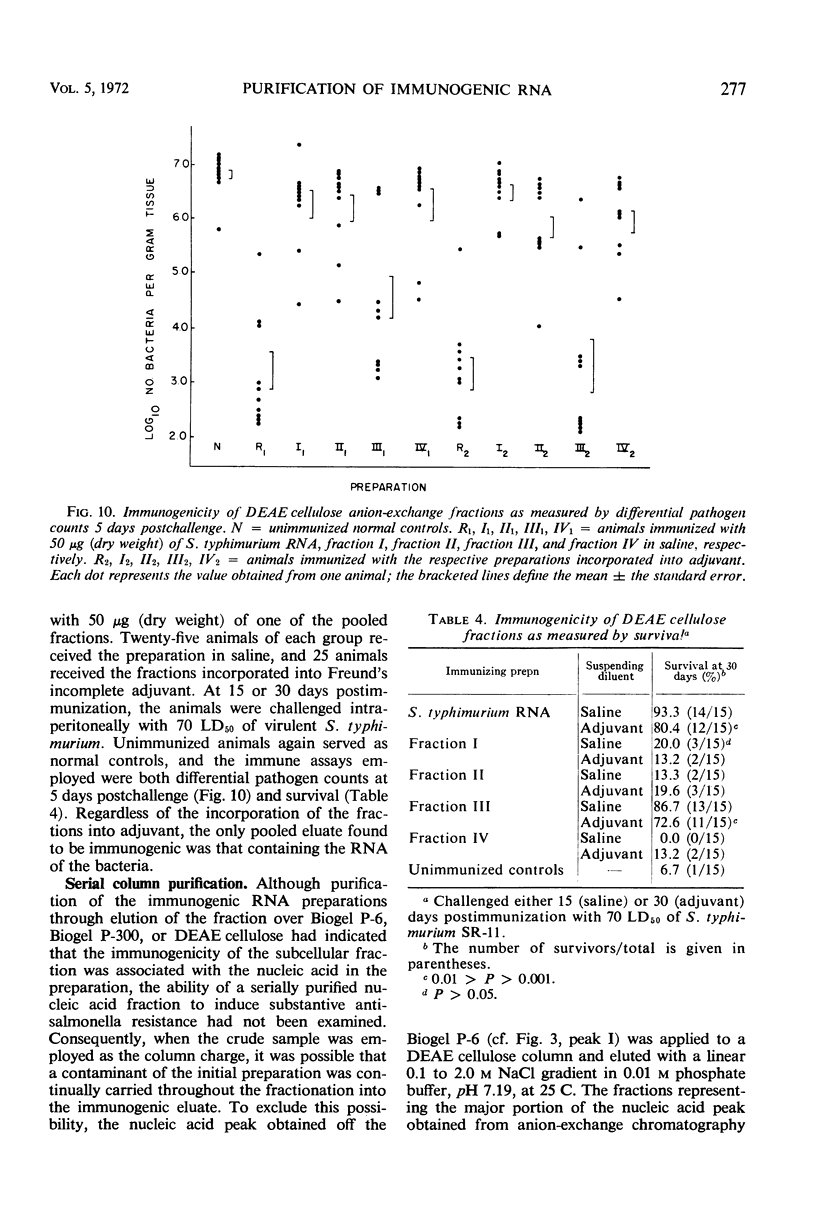
Immunogenic Salmonella typhimurium ribonucleic acid (RNA) preparations, prepared by differential centrifugation, phenol extraction at 65 C, and ethanol precipitation from 0.5% sodium dodecyl sulfate solution, maintained their immunogenicity through lyophilization. As measured by survival, differential pathogen counts 5 days postchallenge, or clearance of the infecting organism from the tissues, immunization with 50 μg (dry weight) of the lyophilized preparation proved as effective as immunization with 0.1 LD50 of attenuated S. typhimurium cells. Chromatography of the immunogenic fraction through Biogel P-6 (exclusion limit > 4,600) or through Biogel P-300 (exclusion limit > 300,000) resulted in only one immunogenically active protein of the eluate found in the void volume of the columns. Diethylaminoethyl (DEAE) cellulose anion-exchange chromatography of the RNA preparations showed that the immunogenic activity was eluted from the column at 0.8 to 1.0 m NaCl in a linear 0.1 to 2.0 m NaCl gradient. Nonimmunogenic, protein-containing minor peaks were eluted at 0.1 to 0.5 m NaCl. Serial fractionation of the crude RNA preparations over Biogel P-6 to DEAE cellulose to Biogel P-300 molecular-sieve or anion-exchange columns did not alter the immunogenicity of the RNA preparation. Incorporation of the column fractions into Freund's incomplete adjuvant did not increase their relative effectiveness in eliciting anti-salmonella resistance. Chemical analysis of the immunogenic preparations indicated that they were lacking in detectable protein, lipid, and deoxyribonucleic acid. These results suggest that the immunogenic moiety of the crude nucleic acid fraction is either RNA or an as yet undefined polysaccharide of greater than 300,000 molecular weight.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absher M., Stinebring W. R. Toxic properties of a synthetic double-stranded RNA. Endotoxin-like properties of poly I. poly C, an interferon stimulator. Nature. 1969 Aug 16;223(5207):715–717. doi: 10.1038/223715a0. [DOI] [PubMed] [Google Scholar]
- Albizo J. M., Surgalla M. J. Isolation and Biological Characterization of Pasteurella pestis Endotoxin. Infect Immun. 1970 Sep;2(3):229–236. doi: 10.1128/iai.2.3.229-236.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badakhsh F. F., Herzberg M. Deoxycholate-treated, nontoxic, whole-cell vaccine protective against experimental salmonellosis of mice. J Bacteriol. 1969 Nov;100(2):738–744. doi: 10.1128/jb.100.2.738-744.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. G., Offey D. S. Release of nuclear DNA template restrictions by specific polyribonucleotides. Science. 1971 Jan 15;171(3967):176–178. doi: 10.1126/science.171.3967.176. [DOI] [PubMed] [Google Scholar]
- Chaparas S. D., Thor D. E., Godfrey H. P., Baer H., Hedrick S. R. Tuberculin-active carbohydrate that induces inhibition of macrophage migration but not lymphocyte transformation. Science. 1970 Nov 6;170(3958):637–639. doi: 10.1126/science.170.3958.637. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Cross-protection against Salmonella enteritidis infection in mice. J Bacteriol. 1968 Apr;95(4):1343–1349. doi: 10.1128/jb.95.4.1343-1349.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in nonvaccinated mice challenged by various routes. J Bacteriol. 1969 Feb;97(2):667–675. doi: 10.1128/jb.97.2.667-675.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Milne M. Heat-labile antigens of Salmonella enteritidis. II. Mouse-protection studies. J Bacteriol. 1966 Sep;92(3):549–557. doi: 10.1128/jb.92.3.549-557.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUSHI K., ANACKER R. L., HASKINS W. T., LANDY M., MILNER K. C., RIBI E. EXTRACTION AND PURIFICATION OF ENDOTOXIN FROM ENTEROBACTERIACEAE: A COMPARISON OF SELECTED METHODS AND SOURCES. J Bacteriol. 1964 Feb;87:391–400. doi: 10.1128/jb.87.2.391-400.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. P., Stavitsky A. B., Solomon J. M. Induction in vitro of antibodies to phage T2: antigens in the RNA extract employed. Science. 1965 Sep 3;149(3688):1106–1107. doi: 10.1126/science.149.3688.1106. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P., Baer H., Chaparas S. D. Inhibition of macrophage migration by a skin-reactive polysaccharide from BCG culture filtrates. J Immunol. 1969 Jun;102(6):1466–1473. [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MERRITT K., JOHNSON A. G. STUDIES ON THE ADJUVANT ACTION OF BACTERIAL ENDOTOXINS ON ANTIBODY FORMATION. VI. ENHANCEMENT OF ANTIBODY FORMATION BY NUCLEIC ACIDS. J Immunol. 1965 Mar;94:416–422. [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Interferon: protection of cells infected with an intracellular protozoan (Toxoplasma gondii). Science. 1968 Aug 23;161(3843):804–806. doi: 10.1126/science.161.3843.804. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Synthetic polyanions protect mice against intracellular bacterial infection. Nature. 1970 Apr 25;226(5243):361–363. doi: 10.1038/226361a0. [DOI] [PubMed] [Google Scholar]
- STINEBRING W. R., YOUNGNER J. S. PATTERNS OF INTERFERON APPEARANCE IN MICE INFECTED WITH BACTERIA OR BACTERIAL ENDOTOXIN. Nature. 1964 Nov 14;204:712–712. doi: 10.1038/204712a0. [DOI] [PubMed] [Google Scholar]
- Thompson H. C., Snyder I. S. Protection against pneumococcal infection by a ribosomal preparation. Infect Immun. 1971 Jan;3(1):16–23. doi: 10.1128/iai.3.1.16-23.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiba D., Nakae T., Akiyama T., Kishimoto Y. Characterization of "clearance" factor and "cell-bound" antibody in experimental typhoid. J Bacteriol. 1966 May;91(5):1705–1712. doi: 10.1128/jb.91.5.1705-1712.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Cell-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):381–387. doi: 10.1128/iai.4.4.381-387.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Serum-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):374–380. doi: 10.1128/iai.4.4.374-380.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J., Berry L. J. Immunogenicity of Ribonucleic Acid Preparations Obtained from Salmonella typhimurium. Infect Immun. 1970 Jun;1(6):574–582. doi: 10.1128/iai.1.6.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Bigley N. J. Isolation and partial characterization of an immunogenic moiety obtained from Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):140–148. doi: 10.1128/jb.100.1.140-148.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat R. W., Berst M., Ruschmann E., Lüderitz O., Westphal O. Lipopolysaccharides of Salmonella T mutants. J Bacteriol. 1967 Nov;94(5):1366–1380. doi: 10.1128/jb.94.5.1366-1380.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S. H., Berry L. J. Antibacterial immunity induced by ribosomal vaccines. J Reticuloendothel Soc. 1970 Jul;8(1):13–24. [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Factors affecting immunogenic activity of mycobacterial ribosomal and ribonucleic acid preparations. J Bacteriol. 1969 Jul;99(1):42–50. doi: 10.1128/jb.99.1.42-50.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Failure of synthetic polynucleotides to affect the immunogenicity of mycobacterial ribonucleic Acid and ribosomal protein preparations. Infect Immun. 1971 Jan;3(1):149–153. doi: 10.1128/iai.3.1.149-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Immunogenic mycobacterial ribosomal and ribonucleic Acid preparations: chemical and physical characteristics. Infect Immun. 1970 Nov;2(5):659–668. doi: 10.1128/iai.2.5.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation and effect of different adjuvants on the immunogenic activity of mycobacterial ribosomal fraction. J Bacteriol. 1967 Oct;94(4):836–843. doi: 10.1128/jb.94.4.836-843.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J Bacteriol. 1966 Jun;91(6):2139–2145. doi: 10.1128/jb.91.6.2139-2145.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



