Abstract
Objective
To examine the relation of endothelial microparticles (EMPs) with cardiometabolic risk in the community.
Background
Circulating EMPs are small membrane vesicles released after endothelial cell injury. Endothelial microparticles are reportedly increased among individuals with a high burden of cardiovascular risk factors. However, prior investigations have been limited to small, highly selected samples.
Methods
We studied 844 individuals without a history of cardiovascular disease in the Framingham Offspring cohort (mean age 66 ± 9 years, 57% women). We used standardized flow cytometry methods to identify and quantify circulating CD144+ and CD31+/CD41− EMPs. We then used multivariable regression analyses to investigate the relations of EMP phenotypes with cardiovascular and metabolic risk factors.
Results
In multivariable analyses, the following cardiovascular risk factors were associated with one or more of the circulating EMP populations: hypertension (P = 0.025 for CD144+,), elevated triglycerides (P = 0.002 for CD144+, P < 0.0001 for CD31+/CD41−), and metabolic syndrome (P < 0.0001 for CD144+,). Overall, each tertile increase in the Framingham risk score corresponded to a 9% increase in log-CD31+/CD41− EMPs (P = 0.022). Furthermore, the presence of hypertriglyceridaemic waist status was associated with 38% higher levels of CD144+ EMPs (P < 0.0001) and 46% higher levels of CD31+/CD41− EMPs (P < 0.0001).
Conclusion
In a large community-based sample, circulating EMP levels were associated with the presence of cardiometabolic risk factors, particularly dyslipidaemia. These data underscore the potential influence of high-risk metabolic profiles on endothelial integrity.
Keywords: Microparticles, Cardiovascular risk factors, Endothelium
Introduction
Microparticles (MPs) are plasma membrane vesicle fragments (ranging in size between 0.1 and 1 μm) that are released from various cell types during activation by physical or chemical stress, including apoptosis.1 Recently, several reports have shed light on the relationships between alterations in the endothelial homeostasis and specific subpopulations of circulating MPs originating from the vascular endothelium [i.e. endothelial MPs (EMPs)].1,2 Experimental studies have demonstrated the ability of EMPs to promote vascular inflammation and interfere with coagulation pathways and regulation of vascular tone, thus pointing to a number of biologic mechanisms by which EMPs may contribute to cardiovascular disease progression.1,2 Clinical pilot studies in small numbers and highly selected patients have indicated that circulating EMPs levels are increased in the presence of cardiovascular risk factors such as diabetes, dyslipidaemia, and uncontrolled systemic hypertension.3–6
Research to date indicates that MPs derived from the endothelium represent potentially important surrogate markers of endothelial injury and cardiovascular risk.7 However, because data on EMPs in humans are limited to referral samples, the extent to which circulating EMPs may reflect chronic endothelial injury and cardiovascular risk in the general population is unknown. Therefore, we conducted a comprehensive study of circulating EMPs in a large community-based sample and investigated whether high EMP concentrations are associated with the presence of cardiovascular and metabolic risk factors.
Methods
Study sample
The longitudinal cohort study design of the original Framingham Heart Study (FHS) has been previously described.8 A total of 5124 offspring (and spouses of the offspring) of the original FHS study participants were enrolled in the FHS Offspring Study in 1971.9 All FHS Offspring Study participants receive routine examinations approximately every 4 years, and a total of 3021 participants attended the eighth examination cycle in 2005 through 2008. A total of 915 participants had fasting blood samples available for characterization of circulation EMP quantity (see details below). Of these individuals, 844 participants were free of cardiovascular disease (history of myocardial infarction, coronary insufficiency, stroke, and heart failure) and were included in the current analysis. All participants provided written informed consent, and the institutional review board of the Boston University School of Medicine approved all study protocols.
Clinical and risk factor assessment
All study participants underwent a standardized assessment of cardiovascular risk factors. Systolic and diastolic blood pressures were the average of two physician-measured readings. Fasting blood samples were drawn for glucose, total and high-density lipoprotein (HDL) cholesterol, and triglycerides. Use of medications and cigarette smoking status were self-reported. Diabetes mellitus was defined as having a fasting glucose ≥126 mg/dL or taking medications to treat diabetes mellitus. A physical activity index was calculated using the number of hours spent each day at various activity levels, weighted according to the estimated oxygen consumption required for each activity.10 Metabolic syndrome was defined as the presence of ≥3 of the following component traits:11 waist circumference ≥40 in. in men or ≥35 in. in women; serum triglycerides ≥150 mg/dL or taking lipid-lowering medication; HDL cholesterol <40 mg/dL in men or <50 mg/dL in women or taking medication for low HDL; systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or taking anti-hypertensive medication; and fasting glucose ≥100 mg/dL. The presence of hypertriglyceridaemic waist, considered a marker of atherogenic metabolic status, was defined as the presence of triglycerides >177 mg/dL and waist circumference ≥90 cm in men or ≥85 cm in women.12,13 C-reactive protein was assayed with the immunoturbidimetric latex-enhanced high-sensitivity assay (Roche Diagnostics, Indianapolis, IN, USA).
Circulating endothelial microparticle analyses
Participant blood samples were collected in citrated tubes following an overnight fast and initially processed to separate platelet rich from platelet-poor plasma, as previously described.14 All blood specimens underwent a two-step centrifugation protocol (860 × g for 15 min at 4°C, followed by 1700 × g for 5 min at 20°C), prior to being stored at −80°C. Analyses of EMP phenotypes were subsequently performed on thawed specimens using a Guava EasyCyte 8HT flow cytometer (Millipore, Billerica, MA, USA) that automatically processed batches of up to 10 plasma specimens per multi-well microplate. Over the course of the analysis period, a total of 10 batches (allowing the analysis of 100 samples total) were processed weekly. The following fluorochrome-coupled antibodies and their corresponding isotypes were used for EMP phenotyping: anti CD31+ PE (Beckman Coulter, Villepinte, France), anti CD41+ PC7 (Beckman Coulter, Villepinte, France) and anti-CD144+ PE (eBioscience, Paris, France) Fluorescent Megamix beads calibrated from 0.5 to 3.0 µm (Biocytex, Marseille, France) were used to define an analysis window (gate) consistent with the size of EMPs. Events <1.0 µm diameter were identified in forward scatter and side scatter intensity dot representation, gated as a microparticle, and then plotted on 1- or 2-color fluorescence histograms. Endothelial microparticles were defined as elements that were >0.1 and <1.0 µm in size and that were positively labelled by specific antibodies (Supplementary material online, Figure S1). Microparticle concentration was assessed by comparison with calibrator Flowcount beads (Beckman Coulter, Villepinte, France) with a predetermined concentration. PECAM+ (CD31+/CD41−) and VE-cadherin (CD144+) EMP populations were defined according to previous reports.2 Two independent examiners, unaware of the study participant clinical status, analysed the data off-line using the FlowJo 8.8 software (Tree Star, Ashland, OR, USA).
Statistical analyses
Due to right-skewed distribution of values, natural logarithmic transformation was applied to triglycerides and all EMP variables for analyses. We used linear mixed effects regression analyses adjusting for ‘batch’ as a random effects term in all models (and all other covariates as fixed effects).15 In regression analyses adjusting for age and sex, we examined the association of circulating EMP phenotypes (CD144+ and CD31+/CD41−) with traditional cardiovascular risk factors. We also examined the associations of EMP phenotypes with risk factors in multivariable regression models that adjusted simultaneously for several variables selected a priori, specifically: age, sex, waist circumference, hypertension, diabetes, total/HDL cholesterol, log triglycerides, and smoking status. Among participants without prevalent diabetes, we examined the association of each EMP phenotype with the presence vs. absence of metabolic syndrome, number of metabolic syndrome component traits, and the presence vs. absence of hypertriglyceridaemic waist. In the total sample, we also investigated the relation of each EMP phenotype with the Framingham risk score.13
In secondary analyses, we repeated the main multivariable analyses with additional adjustment for natural log-transformed C-reactive protein and statin medication use. We also tested for effect modification by age, sex, and diabetes status using multiplicative interaction terms. All analyses were performed with SAS statistical software (PROC MIXED), version 9.3 (SAS Institute Inc, Cary, NC, USA), with a two-tailed value of P < 0.05 considered statistically significant.
Results
Characteristics of the study sample are shown in Table 1. The median (and inter-quartile range) of circulating concentrations of EMP phenotypes in the sample were as follows: 131 (82, 178) events/µL for CD144+ EMPs and 75 (42, 125) events/µL for CD31+/CD41− EMPs.
Table 1.
Sample characteristics
| Characteristic | Total sample N = 844 |
|---|---|
| Age (years) | 66 ± 9 |
| Female (%) | 57 |
| Systolic blood pressure (mmHg) | 127 ± 17 |
| Diastolic blood pressure (mmHg) | 74 ± 10 |
| Body mass index (kg/m2) | 28.1 ± 5.1 |
| Waist circumference (cm) | 101 ± 14 |
| Cigarette smoking (%) | 8 |
| Physical activity index | 35.5 ± 5.7 |
| Total cholesterol (mg/dL) | 186 ± 36 |
| HDL cholesterol (mg/dL) | 59 ± 18 |
| Total/HDL cholesterol ratio | 3.4 ± 1.0 |
| LDL cholesterol | 106 ± 30 |
| Triglycerides (mg/dL) | 109 ± 55 |
| Hypertension (%) | 59 |
| Diabetes mellitus (%) | 12 |
| Anti-hypertensive therapy (%) | 48 |
| Cholesterol-lowering therapy (%) | 46 |
| Metabolic syndrome (%) | 48 |
| Framingham risk score | 8.46 ± 3.93 |
Values shown are means ± SD or percentages.
In age- and sex-adjusted analyses (Table 2), we observed significant associations of higher CD144+ and CD31+/CD41− EMPs with greater BMI, larger waist circumference, lower HDL cholesterol, and higher triglycerides. CD144+ was significantly higher in men compared with women and in the setting of hypertension. In multivariable-adjusted analyses (Table 3), the associations of CD144+ and CD31+/CD41− EMPs with triglyceride plasma levels remained statistically significant. Each 1-SD increment (55 mg/dL) in log triglycerides was associated with a 7% increase in log-CD144+ (P = 0.002) and a 16% increase in log-CD31+/CD41− EMPs (P < 0.0001). CD144+ EMPs also remained significantly associated with blood pressure; individuals with hypertension had 8% higher log-CD144+ (P = 0.025) when compared with individuals without hypertension. In spline analyses, we did not detect any non-linear relationships between covariates and EMP levels. We observed no significant collinearity between covariates included in the main multivariable model (data not shown).
Table 2.
Age- and sex-adjusted relations of clinical characteristics with endothelial microparticle phenotypes
| CD144+ EMPs |
CD31+/CD41− EMPs |
|||
|---|---|---|---|---|
| Regression coefficient (SE)a | P-value | Regression coefficient (SE)a | P-value | |
| Age (year) | −0.03 (0.03) | 0.2703 | 0.02 (0.03) | 0.52 |
| Female sex | −0.11 (0.06) | 0.045 | −0.06 (0.06) | 0.37 |
| Body mass index (kg/m2) | 0.07 (0.03) | 0.020 | 0.06 (0.03) | 0.043 |
| Waist circumference (cm) | 0.09 (0.03) | 0.002 | 0.09 (0.03) | 0.003 |
| Systolic blood pressure (mmHg) | 0.04 (0.03) | 0.15 | 0.05 (0.03) | 0.13 |
| Diastolic blood pressure (mmHg) | 0.07 (0.03) | 0.024 | 0.06 (0.03) | 0.050 |
| Hypertension | 0.20 (0.06) | 0.0007 | 0.06 (0.07) | 0.33 |
| Total/HDL cholesterol ratio | 0.11 (0.03) | <0.0001 | 0.18 (0.031) | <0.0001 |
| Total cholesterol (mg/dL) | <0.01 (<0.01) | 0.16 | <0.01 (<0.01) | 0.31 |
| LDL cholesterol (mg/dL) | 0.01 (0.03) | 0.76 | 0.04 (0.03) | 0.24 |
| HDL cholesterol (mg/dL) | −0.0046 (0.001628) | 0.005 | −0.00892 (0.001739) | <0.0001 |
| Log triglycerides | 0.15 (0.04) | <0.0001 | 0.23 (0.03) | <0.0001 |
| Diabetes | 0.05 (0.09) | 0.59 | 0.01 (0.10) | 0.95 |
| Current smoker | −0.04 (0.11) | 0.67 | 0.04 (0.12) | 0.70 |
| Physical activity index | −0.03 (0.03) | 0.33 | −0.02 (0.03) | 0.54 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
aRegression coefficients represent standardized change in the natural log-transformed dependent variable (endothelial microparticle phenotype) per 1 SD change in the independent variable (clinical characteristic) for continuous covariates and presence (vs. absence) for categorical covariates. For reference, 1 SD of log-CD144+ is 0.587 and log-CD31+/CD41− is 0.878.
Table 3.
Multivariable-adjusted relations of clinical characteristics with endothelial microparticle phenotypes
| CD144+ EMPs |
CD31+/CD41− EMPs |
|||
|---|---|---|---|---|
| Regression coefficient (SE)a | P-value | Regression coefficient (SE)a | P-value | |
| Age (year) | −0.04 (0.03) | 0.16 | 0.02 (0.03) | 0.59 |
| Female sex | −0.07 (0.06) | 0.22 | −0.02 (0.07) | 0.74 |
| Waist circumference (cm) | 0.03 (0.03) | 0.26 | 0.05 (0.03) | 0.17 |
| Hypertension | 0.14 (0.06) | 0.025 | −0.02 (0.07) | 0.81 |
| Diabetes | −0.07 (0.09) | 0.41 | −0.13 (0.10) | 0.20 |
| Total/HDL cholesterol ratio | 0.03 (0.04) | 0.40 | 0.04 (0.04) | 0.27 |
| Log triglycerides | 0.12 (0.04) | 0.002 | 0.19 (0.04) | <0.0001 |
| Current smoker | −0.03 (0.10) | 0.74 | 0.02 (0.11) | 0.83 |
HDL, high-density lipoprotein.
aRegression coefficients represent standardized change in the natural log-transformed dependent variable (endothelial microparticle phenotype) per 1 SD change in the independent variable (clinical characteristic) for continuous covariates and presence ( vs. absence) for categorical covariates. For reference, 1 SD of log-CD144+ is 0.587 and log-CD31+/CD41− is 0.878. All analyses adjusted for age, sex, waist circumference, hypertension, diabetes, total/HDL cholesterol, log triglycerides, and smoking status.
Notably, there was no association of diabetes status with either EMP trait (P ≥ 0.20); results were unchanged when analyses were repeated to account for diabetes status based on concurrent glucose-lowering medication or no medication use (P > 0.30). Even though diabetes appeared associated with marginally higher EMP levels in age- and sex-adjusted analyses (Table 2), these associations were not significant and remained non-significant in fully-adjusted multivariable models (Table 3). However, we did observe a significant effect modification of diabetes on the association of triglycerides with both CD144+ EMPs (β = 0.22, P = 0.01 for interaction) and CD31+/CD41− EMPs (β = 0.31, P = 0.0005 for interaction). Although analyses stratified by diabetes status were limited by sample size, we observed that each 1 SD increment in log triglycerides was associated with a larger magnitude of change in each EMP trait in diabetics (N = 102) than in non-diabetics (N = 741) (Supplementary material online, Table S1).
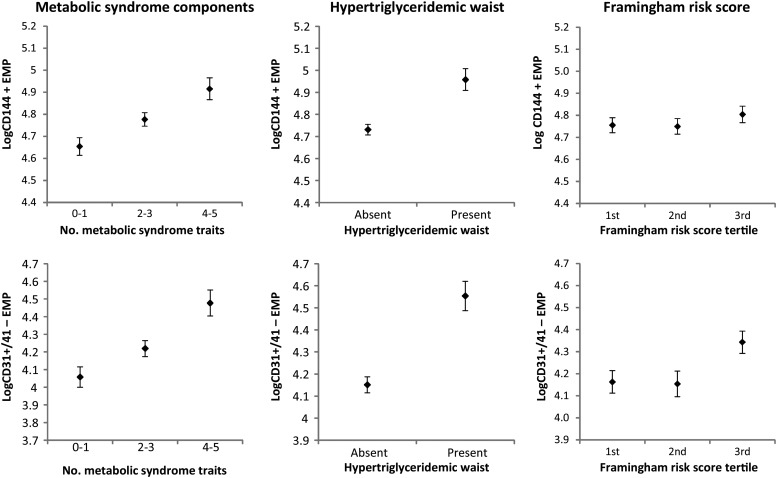
In participants without prevalent diabetes (N = 741), the presence of the metabolic syndrome was associated with higher levels of CD144+ (Table 4). The regression coefficient for this association was 0.249 ± 0.061 (P < 0.0001). Thus, individuals with metabolic syndrome had 15% higher log-CD144+ EMP levels when compared with individuals without metabolic syndrome. In fact, increasing number of metabolic syndrome component traits was correlated with both CD144+ EMPs (P < 0.0001) and CD31+/CD41− EMPs (P = 0.0002) in age- and sex-adjusted analyses (Table 4). Furthermore, the presence of hypertriglyceridaemic waist status was also associated with significantly higher levels of CD144+ EMPs and CD31+/CD41− EMPs (Figure 1). Individuals with hypertriglyceridaemic waist had 24% higher log-CD144+ EMP levels when compared with individuals without hypertriglyceridaemic waist, corresponding to a 27% increase in plasma levels of CD144+ EMPs. Similarly, individuals with hypertriglyceridaemic waist had 38% higher log-CD31+/CD41− EMP levels when compared with individuals without hypertriglyceridaemic waist, corresponding to a 46% increase in plasma levels of CD31+/CD41− EMPs (Figure 1).
Table 4.
Age- and sex-adjusted relations of metabolic traits with endothelial microparticle phenotypes in individuals without diabetes
| CD144+ EMPs |
CD31+/CD41− EMPs |
|||
|---|---|---|---|---|
| Regression coefficient (SE)a | P-value | Regression coefficient (SE)a | P-value | |
| Metabolic syndrome | 0.249 (0.061) | <0.0001 | 0.106 (0.065) | 0.10 |
| No. of metabolic syndrome components | 0.106 (0.022) | <0.0001 | 0.089 (0.024) | 0.0002 |
| Hypertriglyceridaemic waistb | 0.404 (0.080) | <0.0001 | 0.427 (0.087) | <0.0001 |
aRegression coefficients represent standardized change in the natural log-transformed dependent variable (endothelial microparticle phenotype) per 1 unit change in the independent variable (clinical characteristic) for continuous covariates and presence (vs. absence) for categorical covariates. For reference, 1 SD of log-CD144+ is 0.587 and log-CD31+/CD41− is 0.878. All analyses adjusted for age and sex.
bPresence of triglycerides >177 mg/dL and waist circumference ≥90 cm in men or ≥85 cm in women.
Figure 1.
Log-CD144+ and log-CD31+/41− endothelial microparticle values are shown (mean ± standard error) across increasing number of metabolic syndrome component traits, by hypertriglyceridaemic status, and across increasing tertiles of the Framingham risk score.
We examined the association of Framingham risk score with EMPs (Figure 1). Higher levels of circulating CD31+/CD41− EMPs were significantly associated with increased Framingham risk score. Each tertile increase in the Framingham risk score corresponded to a 9% increase in log-CD31+/CD41− EMPs (P = 0.022; corresponding to a 47% increase in plasma levels of CD31+/CD41− EMPs). There was no significant association of CD144+ EMPs with overall risk as represented by the score.
In secondary analyses, we also adjusted for log C-reactive protein and statin use. There was no significant association between any of the EMP phenotypes and C-reactive protein (P > 0.36). There was also no significant association between EMP phenotypes and statin use (P > 0.12). Accordingly, associations of each EMP phenotype with the clinical covariates reported above remained unchanged. There was also no significant effect modification by age or sex on any of the observed associations between individual clinical correlates and EMP phenotypes. In addition, we repeated the main analyses in the total sample with EMP data available (N = 915), including individuals with prevalent CVD. In these analyses, the main results reported above were not substantially changed; furthermore, the presence of CVD was not significantly associated with variation in either CD144+ EMPs (P = 0.77) or CD31+/CD41− EMPs (P = 0.81).
Discussion
In this first study investigating EMPs levels in a community cohort, we found that EMP plasma levels are associated with several cardiovascular risk factors, including higher triglyceride levels, hypertension, and metabolic syndrome. These findings raise the possibility that one consequence of a high burden of metabolic risk factors is increased endothelial activation or injury, in particular in individuals with hypertriglyceridaemic waist.
Microparticles are plasma membrane vesicle fragments that are released from various cell types during activation or apoptosis. In recent years, a growing body of literature has highlighted the role of MPs as mediators of intercellular communication, given that the biological properties of MPs are influenced by both their cellular origin and their composition. Routine MP measures in clinical samples require ongoing efforts to establish standardized protocols for plasma preparation, anticoagulation, and storage conditions in addition to techniques for quantification; these determinants are still under investigation. Nonetheless, in clinical studies performed to date, circulating MPs derived from the endothelium (EMPs) have been shown to demonstrate a special relationship with alterations in endothelial homeostasis. Increased levels of circulating EMPs have been observed in patients with stable or unstable coronary artery disease, stroke, and peripheral artery disease.2 These changes in plasma EMP levels usually averaged a 2- to 3-fold increase in patients with cardiovascular diseases, when compared with healthy controls. Elevated EMPs levels have also been measured in the presence of cardiovascular risk factors such as uncontrolled systemic hypertension, diabetes, and dyslipidemia.3–6 However, these results were observed in highly selected groups of patients and were limited by modest sample sizes. Thus, the influence of different CV risk factors and medications (i.e. cholesterol-lowering therapy, anti-hypertensive medications) on EMP levels has yet to be examined in a larger community-based population.
Our data indicate that elevated triglycerides are strongly associated with higher EMP levels. These results are consistent with observations from previous pilot studies in humans. Ferreira et al. showed that CD31+ EMP levels increase along with triglyceride levels following a high-fat meal in a sample of 18 healthy individuals without known cardiovascular risk factors.3 Similar findings were also reported for CD144+ EMPs in 27 patients with diabetes.16 High levels of triglycerides have been shown to impair endothelial function in vivo,17 which may be related to the pro-inflammatory and pro-apoptotic effects of remnant lipoprotein particles on the endothelium18 that are potentially mediated by changes in local oxidative stress.19
High triglyceride levels are associated with an increased risk for development of cardiovascular disease in certain subgroups such as women and young healthy men.18,20–22 Potential explanations for this association include increased atherogenic cholesterol-enriched remnant lipoprotein particle production, interference with HDL or LDL metabolism, and enhanced recruitment and attachment of monocytes to the vascular wall.18 Interestingly, the association of EMPs with triglycerides observed in our community-based sample was independent of total cholesterol levels, other cardiovascular risk factors, and the presence of any medication therapies for cardiovascular risk. Thus, our findings offer potentially new insights regarding the deleterious role of triglycerides on vascular homeostasis.18 As EMPs interfere with coagulation, inflammation, regulation of vascular tone, and angiogenesis,2 these vesicles might further support the initiation and promotion of vascular disease. We did not observe any independent relationship between HDL or total cholesterol values and EMPs levels. Although oxidized LDL cholesterol has been shown to increase EMP release in vitro,23 the current data suggest that the previously observed links in vivo between these parameters could have been influenced by the presence of confounding factors (such as associated cardiovascular risk factors, including hypertension and diabetes)6 or that the presence of lipid-lowering therapy might have affected this relationship.24–26
We also observed independent associations between circulating levels of EMPs and the presence of metabolic profiles associated with high cardiovascular risk. In particular, the present paper demonstrates robust correlations between circulating EMP levels and hypertryglyceridaemic waist, suggesting that the vascular endothelium of these participants at high risk of coronary artery disease12,13,27 may be prematurely injured or inflamed.28–32 Metabolic abnormalities are associated with impaired endothelial function and altered vascular structure.27,33 The pathophysiology of the deleterious effects on the endothelium has not been fully elucidated but may involve macrophage infiltration within white adipose tissue, release of adipokines by adipocytes and pro-inflammatory cytokines by leukocytes, insulin resistance, and changes in lipid profiles.34 All of these factors can negatively impact endothelial homeostasis, leading to local inflammation, increased oxidative stress, and cellular vesiculation.34 Inflammatory cytokines are elevated in the presence of metabolic syndrome34 and have been shown to stimulate EMP release in vitro.1,35 Thus, in turn, EMPs may interfere with coagulation, inflammation, or enhance endothelial damage, leading to increased endothelial vesiculation and MP release.
Our data also show that hypertension is associated with an increase in CD144 EMPs. Prior studies have reported increased circulating EMPs levels in patients with uncontrolled severe hypertension5 and an effect of anti-hypertensive drugs on platelet or monocyte-derived, but not endothelial, MPs.36,37 Our study highlights differences between the two EMP sub-populations (CD144+ or CD31+CD41− MPs) with regards to sex and hypertension, which could result from the preparation or the conservation of the plasma samples.38 Alternately, these differences might reflect a different condition or site of endothelial injury39 or a different affinity of CD144 and CD31 antibodies for their endothelial epitopes.
Notably, we observed no significant association between frank diabetes and EMP traits. Prior small studies of patients referred for coronary angiography have suggested that circulating EMP levels may be elevated in diabetics compared with non-diabetics.40,41 However, Sabatier et al. reported higher levels of EMPs compared with healthy controls in Type 1 but not Type 2 diabetics.4 Notably, 99% of individuals with diabetes in the Framingham Offspring cohort are known to have Type 2 diabetes.42 Thus, previously observed relations of EMPs with diabetes may be related to concomitant burden of cardiometabolic risk factors, active coronary artery disease, or both. Moreover, the absence of any apparent association between diabetes and EMP traits may also be related to the presence of confounding factors. Notably, we observed that the association between triglycerides and EMP levels was more pronounced in diabetics compared with non-diabetics. This observation suggests the possibility that diabetes enhances potentially deleterious effects of hypertriglyceridemia on endothelium. Alternately, the presence of diabetes in association with hypertriglyceridemia may reflect a more severe grade of metabolic disorder that leads to greater endothelial injury. Further research is needed to specifically investigate these observed relations.
Interestingly, there was no significant association between smoking status and EMP traits in our study sample. In previous work, both active and passive smoking exposure has been associated with a small but significant elevation in circulating EMPs in small samples of healthy adults, with mean ages ranging from 30 to 47 years.43,44 By contrast, our larger study sample was older (mean age 66 years) with a presumably larger burden of cardiometabolic risk factors. Thus, it is possible that whereas smoking leads to elevated EMP levels in otherwise healthy younger adults, this relation is attenuated with advancing age and the cumulative burden of cardiometabolic risk that is frequently seen in older adults.
Several limitations of this study merit consideration. The present study was not designed to precisely assess quantity of small-sized MPs, with diameters between 0.1 and 0.5 μm; thus, our conclusions are only valid for large EMPs with diameters ranging from 0.5 to 1.0 μm.45 The platelet-poor plasma preparation used in the present study might have included residual platelets which could generate artifactual MPs; thus, findings related to platelet-derived MPs or the overall pool of annexinV+ MPs expressing phosphatidylserine would need to be interpreted with extreme caution in this cohort. Because our analyses were cross sectional, we cannot determine whether high-EMPs preceded or followed the development of metabolic syndrome. Additionally, HOMA-IR and HbA1c data were not available as concurrent assessments of insulin resistance or glycaemic exposure. The lack of outcomes data for this ambulatory cohort with low event rates precluded analyses of associations with incident cardiovascular disease, which warrants future investigation. Concurrent data on surrogate measures of subclinical atherosclerosis, such as carotid intima media thickness, were also not available at the time of the EMP assessment. Thus, further research is needed to examine the relations of EMPs and such imaging based markers of subclinical vascular disease in the community. Anti-hypertensive medications including angiotensin receptors blockers, β-blockers or calcium channel blockers have an impact on various subgroups of circulating EMPs. Endothelial microparticle traits have been assessed in very few large samples because they are difficult to measure and require specialized specimen handling. In future prospective cohorts, the ability to obtain samples suitable for EMP and other cell-based phenotyping may help to provide confirmatory data for our findings. Additionally, our sample was predominantly white of European ancestry; therefore, the extent to which our findings are generalizable to other racial/ethnic populations is unknown.
In conclusion, in this large community-based study, higher levels of circulating plasma EMPs were associated with elevated triglycerides, hypertension, and the metabolic syndrome. Our results support the need for further studies exploring the potential mechanisms linking metabolic and lipid abnormalities to the production of EMPs. Further studies may also be warranted to determine whether EMP levels might represent an appropriate biomarker for identification of asymptomatic individuals at risk of developing cardiovascular events.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported in part by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195), grant R01-HL083197 (T.J.W.), Institut National de la Santé et de la Recherche Médicale (INSERM), and ANR-2010-EMMA-041 (C.M.B.) and R01-HL93328 (R.S.V.). Additional support was provided by National Institutes of Health grant R01-HL-08675. S.C. is supported in part by a Clinical Research Program grant from the American Heart Association and the Ellison Foundation.
Conflict of interest: None declared.
References
- 1.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 2.Amabile N, Rautou PE, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin Thromb Hemost. 2010;36:907–916. doi: 10.1055/s-0030-1267044. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira AC, Peter AA, Mendez AJ, Jimenez JJ, Mauro LM, Chirinos JA, Ghany R, Virani S, Garcia S, Horstman LL, Purow J, Jy W, Ahn YS, de Marchena E. Postprandial hypertriglyceridemia increases circulating levels of endothelial cell microparticles. Circulation. 2004;110:3599–3603. doi: 10.1161/01.CIR.0000148820.55611.6B. [DOI] [PubMed] [Google Scholar]
- 4.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, Sampol J, Dignat-George F. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51:2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 5.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 6.Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:2530–2535. doi: 10.1161/01.ATV.0000243941.72375.15. [DOI] [PubMed] [Google Scholar]
- 7.Amabile N, Boulanger CM. Circulating microparticle levels in patients with coronary artery disease: a new indicator of vulnerability? Eur Heart J. 2011;32:1958–1960. doi: 10.1093/eurheartj/ehr055. [DOI] [PubMed] [Google Scholar]
- 8.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nation's Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study . Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, Paffenbarger RS, Jr, Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986;111:1177–1192. doi: 10.1016/0002-8703(86)90022-0. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 12.Arsenault BJ, Lemieux I, Despres JP, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182:1427–1432. doi: 10.1503/cmaj.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 15.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 16.Tushuizen ME, Nieuwland R, Rustemeijer C, Hensgens BE, Sturk A, Heine RJ, Diamant M. Elevated endothelial microparticles following consecutive meals are associated with vascular endothelial dysfunction in type 2 diabetes. Diabetes Care. 2007;30:728–730. doi: 10.2337/dc06-1473. [DOI] [PubMed] [Google Scholar]
- 17.Lundman P, Eriksson MJ, Silveira A, Hansson L-O, Pernow J, Ericsson C-Gr, Hamsten A, Tornvall P. Relation of hypertriglyceridemia to plasma concentrations of biochemical markers of inflammation and endothelial activation (C-reactive protein, interleukin-6, soluble adhesion molecules, von Willebrand factor, and endothelin-1) Am J Cardiol. 2003;91:1128–1131. doi: 10.1016/s0002-9149(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 19.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 20.Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–437. doi: 10.1016/0002-8703(86)90296-6. [DOI] [PubMed] [Google Scholar]
- 21.Mazza A, Tikhonoff V, Schiavon L, Casiglia E. Triglycerides + high-density-lipoprotein-cholesterol dyslipidaemia, a coronary risk factor in elderly women: the CArdiovascular STudy in the ELderly. Intern Med J. 2005;35:604–610. doi: 10.1111/j.1445-5994.2005.00940.x. [DOI] [PubMed] [Google Scholar]
- 22.Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Israeli E, Henkin Y, Kochba I, Shai I. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147:377–385. doi: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 23.Nomura S, Shouzu A, Omoto S, Nishikawa M, Iwasaka T, Fukuhara S. Activated platelet and oxidized LDL induce endothelial membrane vesiculation: clinical significance of endothelial cell-derived microparticles in patients with type 2 diabetes. Clin Appl Thromb Hemost. 2004;10:205–215. doi: 10.1177/107602960401000302. [DOI] [PubMed] [Google Scholar]
- 24.Tramontano AF, O'Leary J, Black AD, Muniyappa R, Cutaia MV, El-Sherif N. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the Rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34–38. doi: 10.1016/j.bbrc.2004.05.127. [DOI] [PubMed] [Google Scholar]
- 25.Mobarrez F, Egberg N, Antovic J, Br√∂ijersen A, Jörneskog G, Wallén H. Release of endothelial microparticles in vivo during atorvastatin treatment; a randomized double-blind placebo-controlled study. Thromb Res. 2012;129:95–97. doi: 10.1016/j.thromres.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Huang B, Cheng Y, Xie Q, Lin G, Wu Y, Feng Y, Gao J, Xu D. Effect of 40 mg versus 10 mg of atorvastatin on oxidized low-density lipoprotein, high-sensitivity C-reactive protein, circulating endothelial-derived microparticles, and endothelial progenitor cells in patients with ischemic cardiomyopathy. Clin Cardiol. 2012;35:125–130. doi: 10.1002/clc.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–74. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 29.Esposito K, Ciotola M, Giugliano D. Pioglitazone reduces endothelial microparticles in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1926. doi: 10.1161/01.ATV.0000231512.15115.25. [DOI] [PubMed] [Google Scholar]
- 30.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Circulating endothelial progenitor cells and endothelial microparticles in patients with arterial erectile dysfunction and metabolic syndrome. J Androl. 2012;33:202–209. doi: 10.2164/jandrol.111.013136. [DOI] [PubMed] [Google Scholar]
- 32.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106:2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 33.Mattsson N, Rönnemaa T, Juonala M, Viikari JSA, Jokinen E, Hutri-Kähönen N, Kähönen M, Laitinen T, Raitakari OT. Arterial structure and function in young adults with the metabolic syndrome: the Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008;29:784–791. doi: 10.1093/eurheartj/ehm576. [DOI] [PubMed] [Google Scholar]
- 34.Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg. 2011;54:819–831. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodsky SV, Malinowski K, Golightly M, Jesty J, Goligorsky MS. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation. 2002;106:2372–2378. doi: 10.1161/01.cir.0000033972.90653.af. [DOI] [PubMed] [Google Scholar]
- 36.Nomura S, Shouzu A, Omoto S, Nishikawa M, Iwasaka T. Effects of losartan and simvastatin on monocyte-derived microparticles in hypertensive patients with and without type 2 diabetes mellitus. Clin Appl Thromb Hemost. 2004;10:133–141. doi: 10.1177/107602960401000203. [DOI] [PubMed] [Google Scholar]
- 37.Nomura S, Shouzu A, Omoto S, Nishikawa M, Iwasaka T. Long-term treatment with nifedipine modulates procoagulant marker and C-C chemokine in hypertensive patients with type 2 diabetes mellitus. Thromb Res. 2005;115:277–285. doi: 10.1016/j.thromres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 38.van Ierssel SH, Van Craenenbroeck EM, Conraads VM, Van Tendeloo VF, Vrints CJ, Jorens PG, Hoymans VY. Flow cytometric detection of endothelial microparticles (EMP): Effects of centrifugation and storage alter with the phenotype studied. Thromb Res. 2010;125:332–339. doi: 10.1016/j.thromres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, Tando Y, He M, Yamada M, Kurosawa S, Yamaya M, Kubo H. Differences in the released endothelial microparticle subtypes between human pulmonary microvascular endothelial cells and aortic endothelial cells in vitro. Exp Lung Res. 2013;39:155–161. doi: 10.3109/01902148.2013.784932. [DOI] [PubMed] [Google Scholar]
- 40.Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 41.Tramontano AF, Lyubarova R, Tsiakos J, Palaia T, Deleon JR, Ragolia L. Circulating endothelial microparticles in diabetes mellitus. Mediators Inflamm. 2010;2010:250476. doi: 10.1155/2010/250476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 43.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, Mezey JG, Crystal RG. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert S, Lacroix R, Poncelet P, Harhouri K, Bouriche T, Judicone C, Wischhusen J, Arnaud L, Dignat-George F. High-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles--brief report. Arterioscler Thromb Vasc Biol. 2012;32:1054–1058. doi: 10.1161/ATVBAHA.111.244616. [DOI] [PubMed] [Google Scholar]