Summary
Canonical Wnt signaling in endothelial cells (ECs) is required for vascularization of the central nervous system (CNS) and for formation and maintenance of barrier properties unique to CNS vasculature. Gpr124 is an orphan member of the adhesion G-protein-coupled receptor family that is expressed in ECs and is essential for CNS angiogenesis and barrier formation via an unknown mechanism. Using canonical Wnt signaling assays in cell culture and genetic loss- and gain-of-function experiments in mice, we show that Gpr124 functions as a co-activator of Wnt7a-and Wnt7b-stimulated canonical Wnt signaling via a Frizzled receptor and Lrp co-receptor, and that Gpr124-stimulated signaling functions in concert with Norrin/Frizzled4 signaling to control CNS vascular development. These experiments identify Gpr124 as a ligand-specific co-activator of canonical Wnt signaling.
Keywords: endothelial cell, Frizzled, brain, vascular biology, TEM5
Introduction
Central nervous system (CNS) angiogenesis begins with endothelial cell (EC) invasion from the perineural vascular plexus, followed by EC migration and vascular branching within the CNS. Concomitant with development of the CNS vasculature, brain ECs acquire the characteristics of a blood-brain barrier (BBB) [or in the eye, a blood-retina barrier (BRB)]. Canonical Wnt signaling plays a central role in these processes. In the embryo, Wnt7a and Wnt7b produced by the neuro-epithelium of the developing brain and spinal cord promote angiogenesis and BBB formation by activating canonical Wnt signaling in ECs (Stenman et al., 2008; Liebner et al., 2008; Daneman et al. 2009). In the postnatal retina and cerebellum, an analogous response is mediated by glial-derived Norrin (a TGF-beta family member), which activates canonical Wnt signaling in ECs via the receptor Frizzled4 (Fz4) and co-receptors Lrp5 and Lrp6 (Xu et al., 2004; Ye et al., 2009; Wang et al., 2012). In retinal ECs, the cell-surface Norrin/Fz4/Lrp5 signaling complex also includes the tetra-spanin protein Tspan12 (Junge et al., 2009). In humans and mice, mutations in the genes coding for each of these polypeptides cause retinal hypovascularization syndromes that impair or eliminate vision (Nikopoulos et al., 2010; Ye et al, 2010).
Gpr124, a member of the “adhesion G-protein-coupled receptor (GPCR)” family, was first linked to vascular biology based on the enrichment of its transcripts in human colorectal tumor vasculature (St. Croix et al., 2000; Carson-Walter et al., 2001; Gpr124 is also referred to as tumor endothelial marker 5, TEM5). Gpr124 is expressed in developing ECs, and targeted mutation of Gpr124 in mice, either throughout the body or specifically in ECs, leads to defects in embryonic CNS angiogenesis and BBB formation that closely resemble the defects caused by loss of canonical Wnt signaling (Kuhnert et al., 2010; Anderson et al., 2011; Cullen et al., 2011). Like many “adhesion GPCRs”, Gpr124 is currently classified as an orphan receptor since the ligand(s) that activate it and the signal transduction pathway(s) to which it couples are unknown. Starting with the clue that Gpr124 is essential for CNS angiogenesis, we demonstrate here that Gpr124 functions as a ligand-specific co-activator of canonical Wnt signaling in the CNS vasculature during both embryonic and postnatal life.
Results
Gpr124 selectively activates Wnt7a/Wnt7b signaling via Fz4 and Lrp5 in a reporter cell line
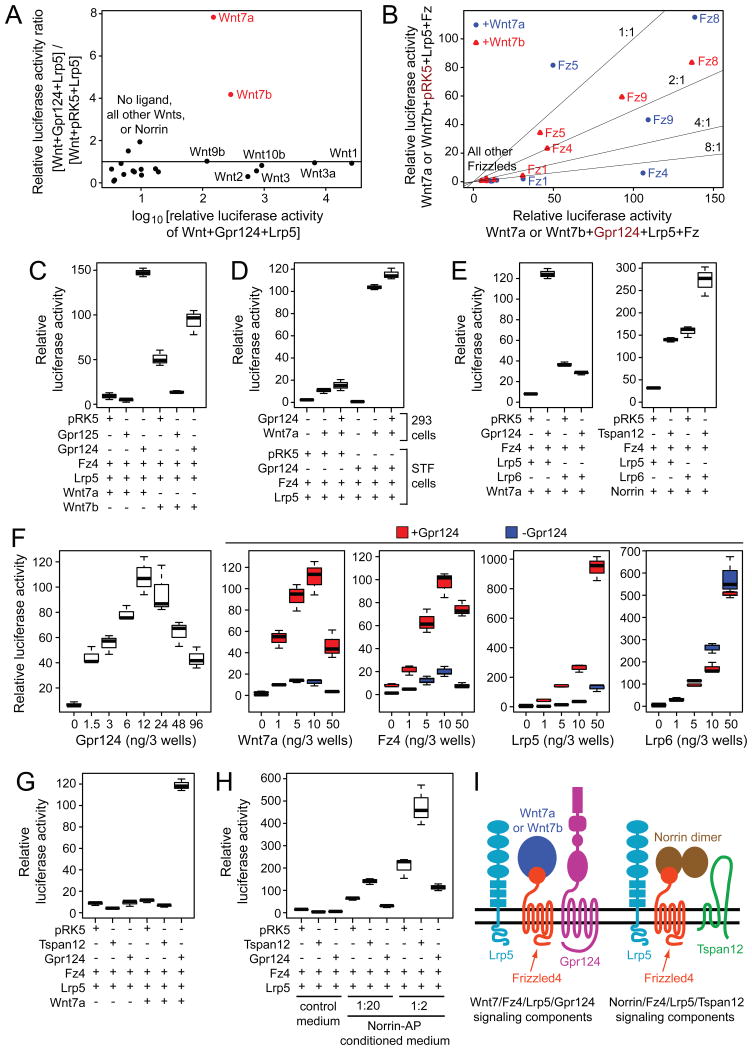
As an initial screen, we asked whether transfection of a canonical Wnt signaling reporter cell line (Super Top Flash; STF) with Lrp5 and each of the 19 mammalian Wnts or Norrin could reveal an effect of co-expressed Gpr124 (Figure 1A). STF cells express multiple Wnt signal transduction components at low level (Table S1), which likely accounts for their responses to some Wnts in the absence of co-transfected Frizzleds, the high-affinity Wnt receptors. Gpr124 was observed to increase Wnt7a- and Wnt7b-dependent signaling ∼8-fold and ∼4-fold, respectively (Figures 1A and S1A). Gpr124 had little or no effect on signaling by other Wnts or by Norrin. To determine which of the ten mammalian Frizzleds mediates the Gpr124 effect, STF cells were transfected with each Frizzled together with Lrp5 and Wnt7a or Wnt7b, with or without Gpr124 (Figure 1B). Among the five Frizzleds that exhibited a signal substantially above background, Fz1 and Fz4 showed the greatest enhancement by Gpr124 (up to ∼10-fold).
Figure 1. Specificity of Gpr124 for Wnt7a and Wnt7b activation of canonical Wnt signaling via Lrp5 and Fz4 in transfected STF cells.
(A) Ratio of luciferase activity induced in STF cells by Wnt+Gpr124+Lrp5 compared to Wnt+vector (pRK5)+Lrp5 plotted against luciferase activity induced by Wnt+Gpr124+Lrp5. The means are shown from triplicate determinations. See Figure S1A for an enlargement of the lower left part of the plot. Super Top Flash (STF) cells are a stable HEK/293 derivative that expresses firefly luciferase in response to canonical Wnt signaling (Xu et al., 2004).
(B) Relative luciferase activity in STF cells of Wnt7a+Lrp5+Fz or Wnt7b+Lrp5+Fz with vector (pRK5) vs. Gpr124.
(C) Relative luciferase activity in STF cells of Gpr124 vs. Gpr125 with Wnt7a or Wnt7b in the presence of Fz4 and Lrp5.
(D) Co-culture of 293 cells transfected with Wnt7a, with or without Gpr124, together with STF cells transfected with Fz4 and Lrp5, with or without Gpr124.
(E) Comparison of Wnt7a/Fz4/Gpr124 vs. Norrin/Fz4/Tspan12 signaling and their dependence on Lrp5 vs. Lrp6 determined by relative luciferase activity in STF cells.
(F) Titrations of Gpr124, Wnt7a, Fz4, and Lrp5 or Lrp6 plasmids in transfected STF cells. For each titration, the three other plasmids were held at a standard concentration [per three wells of a 96 well tray, these are: Gpr124, 50 ng; Fz4, 50 ng; Wnt7a, 10 ng; Lrp5, 5 ng].
(G) Comparison of Gpr124 vs. Tspan12 effects on Wnt7a/Fz4/Lrp5 signaling in STF cells.
(H) Comparison of Gpr124 vs. Tspan12 effects on Norrin/Fz4/Lrp5 signaling in STF cells. For this experiment, Norrin was delivered in conditioned medium as a Norrin-alkaline phosphatase (AP) fusion protein.
(I) Illustration of plasma membrane proteins that mediate Wnt7a or Wnt7b/Fz4/Lrp5/Gpr124 andNorrin/Fz4/Lrp5/Tspan12 signaling. See also Figure S1 and table S1.
We next used STF cells to compare Gpr124 activity to that of the closely related protein Gpr125 (Figure S1B). When Wnt7a or Wnt7b, Fz4, and Lrp5 were co-transfected with Gpr125, we observed a ∼2-3 fold depression in the STF signal relative to the vector control, whereas Gpr124 produced ∼15-fold and ∼2-fold increases in Wnt7a and Wnt7b signaling, respectively (Figure 1C). In a screen analogous to the one shown in Figure 1A (Lrp5 and each of the 19 mammalian Wnts or Norrin), Gpr125 showed little effect on signaling (Figure S1C).
The data presented thus far are consistent with a model in which Gpr124 promotes the formation or enhances the activity of the Wnt/Fz/Lrp transmembrane signaling complex. Alternately, Gpr124 could increase the bioactivities of Wnt7a and Wnt7b by promoting their folding, intra-cellular trafficking, or secretion. To distinguish these possibilities, we co-cultured STF cells transfected with Fz4 and Lrp5, with or without Gpr124, together with 293 cells that had been transfected with Wnt7a and/or Gpr124 (Figure 1D). This experiment showed that Gpr124 had no effect on Wnt7a production/secretion by co-cultured 293 cells, but instead exerted its effects only when expressed in STF cells, consistent with a role for Gpr124 as part of the Fz/Lrp signaling complex.
Lrp5 and Lrp6, the closely related co-receptors for canonical Wnt signaling (Figure S1B), are largely interchangeable in a variety of biological contexts (Joiner et al., 2013). In particular, prenatal CNS angiogenesis is unaffected by loss of either Lrp5 or Lrp6, but is severely compromised by the combined loss of both receptors (Zhou et al., 2014). Surprisingly, Gpr124 does not enhance Wnt7a/Fz4/Lrp6 signaling in STF cells (Figure 1E, left panel). A control experiment shows that both Lrp5 and Lrp6 mediate Tspan12-enhancement of signaling in response to Norrin, albeit with different levels of basal activity (Figure 1E, right panel). In a titration experiment (Figure 1F), Lrp6 showed no Gpr124 stimulation at any dose tested, whereas each component in the putative Wnt7a/Fz4/Lrp5/Gpr124 signaling complex showed well-behaved dose response curves. We note that in the Wnt7a, Fz4, and Lrp5 titrations, Gpr124 stimulation was observed at all concentrations tested. Earlier work demonstrated that Lrp5 and Lrp6 differ quantitatively in signal transduction efficiency (MacDonald et al., 2011), but the difference between Lrp5 and Lrp6 in Gpr124 responsiveness in the STF assay is striking as it represents an all-or-none functional difference between the two Lrp co-receptors.
The severe defects in CNS angiogenesis in Gpr124-/- embryos imply that loss of Gpr124 in vivo impairs both Lrp5 and Lrp6 signaling. We tested the idea that Gpr124 might enhance Wnt7a or Wnt7b signaling via Lrp6 in the context of Frizzleds other than Fz4 by surveying all ten Frizzleds in STF cells. This survey revealed only a modest (∼2-fold) enhancement for Fz1 (Figure S1D). We hypothesize that other still-unknown components facilitate Gpr124 stimulation of Wnt/Fz signaling via Lrp6 in CNS ECs and that these components are missing from STF cells.
Current evidence indicates that Tspan12 is an integral part of the Norrin/Fz4/Lrp5 signaling complex, and that Tspan12 enhances Norrin-induced but not Wnt-induced canonical signaling through Fz4 (Junge et al., 2012). To compare the ligand specificities of Tspan12 and Gpr124 in the context of Fz4/Lrp5 signaling, we measured canonical Wnt signaling in response to Wnt7a or Norrin (Figure 1G and H). These experiments show that Gpr124 enhances Wnt7a but not Norrin signaling, and that Tspan12 enhances Norrin but not Wnt7a signaling. Thus, Tspan12 and Gpr124 function as ligand-specific co-activators of canonical Wnt signaling in the context of the Fz4/Lrp5 complex. The components of these two signaling complexes are diagrammed in Figure 1I.
Gpr124 and Norrin/Fz4 play partially redundant roles in CNS angiogenesis and BBB development
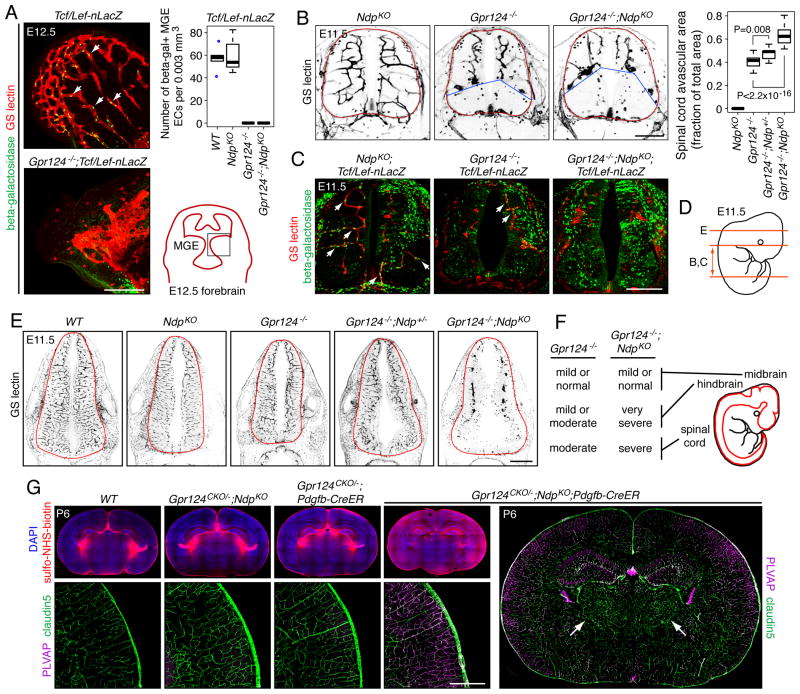
To test whether Gpr124 participates in canonical Wnt signaling in vivo, we examined the expression of a Tcf/Lef-nLacZ canonical Wnt signaling reporter transgene (BAT-gal; Maretto et al., 2003) in wild type (WT) vs. Gpr124-/- embryos. In the Gpr124-/- CNS between E11.5 and E13.5, the number of beta-galactosidase+ EC nuclei was dramatically reduced in multiple regions, including the medial ganglionic eminence (MGE), hindbrain, and spinal cord compared to WT controls (Figures 2A, 2C, S2A, and S2B), indicating a reduction in canonical Wnt signaling.
Figure 2. Gpr124 and Ndp are partially redundant in CNS angiogenesis.
(A) Canonical Wnt signaling in ECs in E12.5 MGE vasculature in WT, Gpr124-/-, NdpKO, and Gpr124-/-;NdpKO mice as measured by accumulation of a nuclear-localized beta-galactosidase reporter expressed from the Tcf/Lef-nLacZ transgene (left panels). Z-stacked images from WT and Gpr124-/- MGE are shown; white arrows, beta-galactosidase positive nuclei. Conveniently, there is little or no Tcf/Lef-nLacZ expression in neural progenitors in the MGE. Upper right: quantification. Lower right: drawing showing the MGE in a coronal brain section at E12.5, with the black square corresponding to the images at left. Figure S2B-D display the individual data points for this and other scatter plots.
(B) Avascular zone in the ventral spinal cord in Gpr124-/-, andGpr124-/-;NdpKO embryos at E11.5. The avascular zone is absent in NdpKO embryos and is expanded in Gpr124-/-;NdpKO embryos compared to Gpr124-/- embryos (left panels). The neuroepithelium is outlined in red, and the dorsal boundary of the ventral avascular zone is marked by blue lines. For quantification (right), the area of the avascular zone was compared to the area of the spinal cord.
(C) Tcf/Lef-nLacZ expression in NdpKO, Gpr124-/-, and Gpr124-/-;NdpKO spinal cord vasculature. Single Z-plane images are shown; white arrows, beta-galactosidase positive nuclei. Figure S2A shows the corresponding Z-stacked images from spinal cord and hindbrain.
(D) Locations and planes of section of E11.5 spinal cord (panels B and C) and hindbrain (panel E) images.
(E) Massive reduction in Gpr124-/-;NdpKO hindbrain vascularization at E11.5. NdpKO, Gpr124-/-, and Gpr124-/-;Ndp+/- hindbrains show normal or nearly normal vascularization at E11.5, although Tcf/Lef-nLacZ expression is reduced in the Gpr124-/- hindbrain vasculature (Figure S2A).
(F) Summary of hypovascularization phenotypes in the Gpr124-/- and Gpr124-/-;NdpKO CNS at E11.5.
(G) Effect of EC-specific postnatal ablation of Gpr124 in an NdpKO background (Gpr124CKO/-;NdpKO;Pdgfb-CreER): many CNS ECs convert from PLVAP-/claudin5+ to PLVAP+/claudin5- with extensive sulfo-NHS-biotion leakage into the brain parenchyma (shown here for cerebral cortex). Brains from three control genotypes are shown (WT, Gpr124CKO/-;NdpKO, and Gpr124CKO/-;Pdgfb-CreER): CNS ECs remain PLVAP-/claudin5+ and sulfo-NHS-biotin leakage is confined to the choroid plexus. Far right, vasculature within the Gpr124CKO/-;NdpKO;Pdgfb-CreER thalamus is largely PLVAP-/claudin5+ (white arrows). 50-100 ug 4HT was injected IP at P3-P4, and brains were examined at P6. Scale bars: A,B,C,G, 200 μm; E, 500 μm. See also Figure S2 and Table S2.
Embryos with an EC-specific knockout of beta-catenin exhibit defective CNS angiogenesis throughout the entire neuraxis (Stenman et al., 2008; Liebner et al., 2008; Daneman et al. 2009; Zhou et al., 2014), whereas Gpr124-/- embryos exhibit CNS angiogenesis defects in the forebrain and ventral spinal cord, but not in the midbrain, hindbrain, or dorsal spinal cord (Kuhnert et al., 2010; Anderson et al., 2011; Cullen et al., 2011). In embryos lacking Wnt7a and Wnt7b, the pattern of CNS angiogenesis defects resembles the pattern seen in Gpr124-/- embryos (Stenman et al., 2008; Daneman et al., 2009). Intriguingly, at midgestation, Ndp (Norrie disease protein; the gene coding for Norrin) is expressed in the dorsal spinal cord and in the hindbrain, but is not detectable in the forebrain (Ye et al., 2011), suggesting that Norrin/Fz4 signaling may play a complementary and/or partially redundant role with Wnt7a/Wnt7b/Gpr124 signaling. To test this hypothesis, we compared CNS angiogenesis in NdpKO, Gpr124-/-, and Gpr124-/-;NdpKO embryos at E11.5 (Figures 2B-F, S2A-C, and S2E; Ndp is an X-linked gene, and we refer to both Ndp-/- females and Ndp-/Y males as NdpKO). The CNS vasculature in NdpKO embryos is indistinguishable from WT and canonical Wnt signaling in ECs appears to be normal in NdpKO embryos as judged by Tcf/Lef-nLacZ expression (Figure 2A, 2C, and S2A). Unlike NdpKO or Gpr124-/-embryos,Gpr124-/-;NdpKO embryos show a severe defect in hindbrain vascularization with numerous glomeruloid-like vascular bodies (Figures 2E, S2A; multiple examples are shown in Figure S2E). Gpr124-/-;NdpKO embryos also show an enlarged avascular zone in the ventral spinal cord relative to Gpr124-/- embryos (Figures 2B, 2C, S2A, and S2C). These data are summarized in Figure 2F and Table S2. Consistent with these architectural defects, the fraction of EC nuclei in these regions that are beta-galactosidase+ is smaller in Gpr124-/-;NdpKO embryos than in Gpr124-/- embryos (Figures 2C and S2A).
Despite widespread postnatal expression of Gpr124 in CNS and non-CNS vasculature, early postnatal and EC-specific elimination of Gpr124 in Gpr124CKO/-;Pdgfb-CreER mice produces no detectable vascular phenotype in the brain or retina (Kuhnert et al., 2010; Figures 2G, S2G, S2H, and data not shown). Constitutive loss of Ndp leads to CNS vascular phenotypes that are confined to the retina (severe hypovascularization) and cerebellum (a mild BBB defect), despite the expansion of the Ndp expression domain around the time of birth to include astrocytes throughout the CNS in addition to Bergman glia in the cerebellum and Muller glia in the retina (Ye et al., 2010). The broad spatiotemporal expression domains observed for Ndp and Gpr124 suggest that these genes may play wider roles in postnatal CNS vascular development/homeostasis than previously appreciated, but these roles may have been masked by redundancy. Consistent with this idea, eliminating both Gpr124 and Ndp postnatally in Gpr124CKO/-;NdpKO;Pdgfb-CreER mice produced widespread loss of BBB integrity, as assessed by leakage of sulfo-NHS-biotin from the intravascular space to the parenchyma (Figures 2G and S2F). Vascular leakage was associated with suppression of the tight junction protein claudin5 and induction of plasmalemma vesicle-associated membrane protein (PLVAP), a structural component of EC fenestrations. This phenotype was observed in the cerebral cortex, hippocampus, colliculus, brainstem, and cerebellum, but it was largely absent from the thalamus and the roof of the anterior midbrain (Figures 2G, S2F, S2G, and data not shown). Presumably, CNS regions that were less affected express other activators of canonical Wnt signaling.
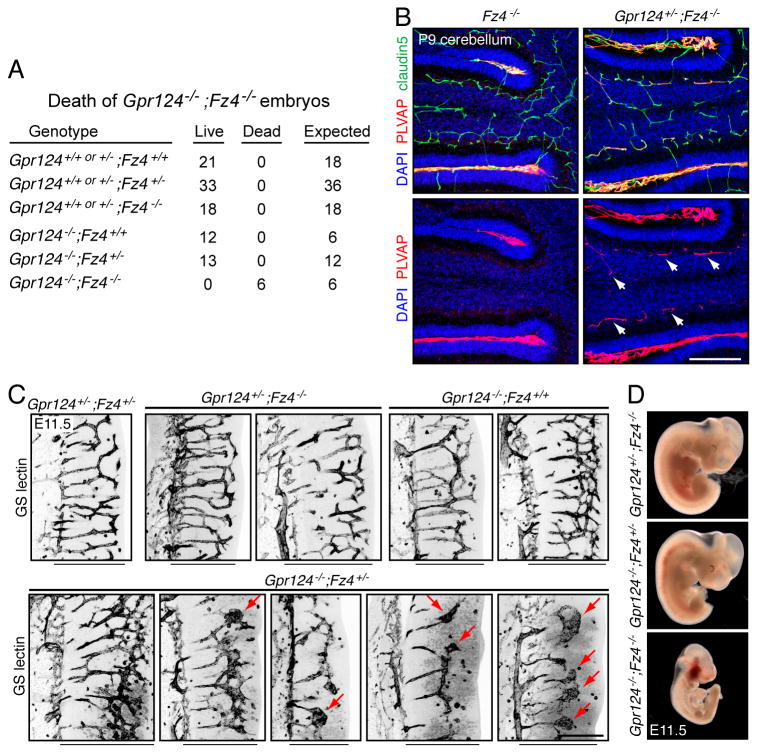
As a further test of the hypothesis that Gpr124 participates in canonical Wnt signaling in vivo, we asked whether the combined loss of Fz4 and Gpr124 produced a more severe phenotype than loss of either gene alone (Figure 3). In midgestation embryos, Fz4 and Gpr124 are expressed throughout the CNS vasculature (Ye et al., 2009; Kuhnert et al., 2010; Anderson et al., 2011; Cullen et al., 2011). Fz4-/-embryos have no vascular defects, and Gpr124-/- embryos survive through late gestation despite their CNS vascular defects. When we generated various combinations of Fz4 and Gpr124 alleles, the following phenotypes were observed (listed in order of increasing severity): (a) Fz4+/-;Gpr124+/-embryos, fetuses, and postnatal mice showed no vascular defects; (b) Fz4-/-;Gpr124+/- embryos showed no vascular defects, but postnatal Fz4-/-;Gpr124+/- mice showed an enhanced conversion of cerebellar ECs from a PLVAP- to a PLVAP+ state relative to Fz4-/- controls (Figure 3B); (c) Fz4+/-;Gpr124-/-embryos showed variable defects in hindbrain angiogenesis at E11.5 (Figure 3C); and (d), Fz4-/-;Gpr124-/- embryos exhibited a severe and lethal growth retardation by E10.5 (Figure 3D).
Figure 3. Gpr124 and Fz4 interact genetically in CNS angiogenesis and BBB maintenance.
(A) Genotypes and phenotypes of 97 embryos harvested at E11.5 from 15 timed pregnant females from a Gpr124+/-;Fz4+/- x Gpr124+/-;Fz4+/- intercross.
(B) Fz4-/- and Gpr124+/-;Fz-/- cerebella at P9. Gpr124+/-;Fz4-/- cerebella show accelerated conversion of granule cell layer ECs to a PLVAP+ state (arrows).
(C) Angiogenesis in E11.5 hindbrains. Within each image, the perineural vascular plexus is to the left and the ventricular surface is to the right. The width of the neuroepithelium is indicated by the black line beneath each image. Angiogenesis in Gpr124+/-;Fz4+/-, Gpr124+/-;Fz4-/-, and Gpr124-/-;Fz4+/+ hindbrains is indistinguishable from WT (upper panels). Angiogenesis in Gpr124-/-;Fz4+/- hindbrains is variably attenuated (lower panels). Hindbrain sections are shown from five Gpr124-/-;Fz4+/- embryos, and three show a severely impoverished vascular network with glomeruloid bodies (red arrows).
(D) Severe growth retardation of Gpr124-/-;Fz4-/- embryos at mid-gestation; all embryos were harvested at E11.5. Scale bars: 200 μm. See also table S2.
As summarized in Figure 3A, among 97 embryos harvested at E11.5 from a Gpr124+/-;Fz4+/- x Gpr124+/-;Fz4+/- intercross, 6 were Gpr124-/-;Fz4-/-. In this cohort, 6/97 embryos were growth retarded and/or dead, and this phenotype coincided precisely with the Gpr124-/-;Fz4-/- genotype. If genotype and lethality were uncorrelated, the probability of this coincidence would be 1.36×10-6.
This dosage experiment shows that with a progressive reduction in the number of WT copies of both Gpr124 and Fz4, there is a progressive increase in phenotypic severity. These experiments also imply that Gpr124 does not act exclusively through Fz4. If it did, then Fz4-/- embryos would have a vascular phenotype at least as severe as Gpr124-/- embryos. Presumably Gpr124 also enhances canonical Wnt signaling in ECs via one or more other Frizzleds. We note that multiple Frizzleds in addition to Fz4 are expressed in ECs (Goodwin et al., 2006) and are therefore candidates for interacting with Gpr124 in vivo, despite their lower synergy with Gpr124 in the STF assay.
Artificially activating canonical Wnt signaling rescues vascular defects in Gpr124-/- embryos
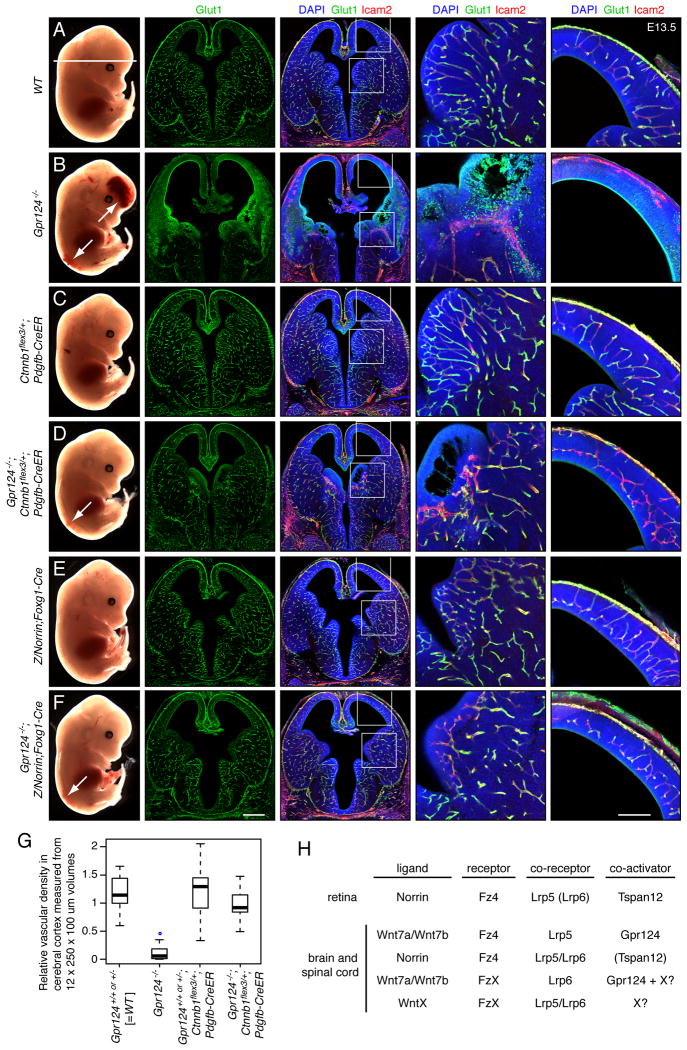
All of the data presented up to this point support the hypothesis that Gpr124 acts in the CNS vasculature by enhancing canonical Wnt signaling. A critical test of this hypothesis would be to determine whether the vascular phenotype of Gpr124-/- embryos can be rescued by artificially increasing canonical Wnt signaling. We have performed this test in two ways: (1) by stabilizing beta-catenin in ECs, and (2) by over-expressing Norrin in the forebrain. Beta-catenin communicates the canonical Wnt signal from cytoplasm to nucleus, and its artificial stabilization - by Cre-mediated excision of glycogen synthase kinase-3 (GSK-3) phosphorylation sites within exon 3 of the beta-catenin gene (Ctnnb1) in Ctnnb1flex3/+;Pdgfb-CreER mice (Harada et al., 1999) - should over-ride canonical Wnt signaling defects at the plasma membrane. The Norrin over-expression experiment - which utilizes a Cre-activated Norrin knock-in at the Ubiquitin-B (Ubb) locus (Z/Norrin; Ye et al., 2009; Wang et al., 2012) and an early embryonic forebrain-specific Cre driver (Foxg1-Cre; Hébert and McConnell, 2000) - tests whether expanding the domain of Norrin/Fz4 signaling in the early embryo can compensate for the loss of Wnt/Gpr124 signaling.
Figure 4B shows that E13.5 Gpr124-/- embryos exhibit forebrain and lower spinal cord hemorrhage (column 1; white arrows), severe defects in vascularization of the MGE (columns 2-4) and cerebral cortex (columns 2, 3, and 5), and loss of CNS vascular expression of Glucose transporter-1 (Glut-1; an EC marker for the BBB state; columns 2-5). The beta-catenin rescue experiment (Figures 4D, 4G, and S2D) shows that E13.5 Gpr124-/-;Ctnnb1flex3/+;Pdgfb-CreER embryos - treated at E10.5 with a maternal IP injection of 1.5 mg tamoxifen - exhibit almost complete suppression of the forebrain hemorrhage but not the spinal cord hemorrhage (column 1; white arrow), partial rescue of the MGE (columns 2-4) and cerebral cortical (columns 2, 3, and 5) vascularization defects, and partial rescue of the Glut-1 expression defect (columns 2-5). In E13.5 Gpr124-/-;Ctnnb1 flex3/+;Pdgfb-CreER ventral spinal cords, vascularization is largely rescued and Glut-1 expression is partially restored (Figure S3A). The incomplete rescue of the spinal cord hemorrhage most likely reflects the temporal overlap between spinal cord angiogenesis, which begins at ∼E10, and the initiation of Pdgfb-CreER–dependent recombination following tamoxifen administration at E10.5. Rescue of Gpr124-/-;Ctnnb1flex3/+;Pdgfb-CreER embryos did not occur without tamoxifen treatment (data not shown).
Figure 4. Gpr124-/- CNS angiogenesis defects can be rescued by artificially activating canonical Wnt signaling in ECs.
(A-F) E13.5 embryos. The horizontal white line in A (column 1) shows the plane of section. Regions within the rectangles in the third column are enlarged in the fourth and fifth columns.
(G) Quantification of vascular density in the cerebral cortex at E13.5.
(H) Summary of interacting ligand, receptor, co-receptor, and co-activator combinations for canonical Wnt signaling in CNS vascular development and barrier integrity. In the retina, Lrp6 plays a minor role, and it is therefore shown in parentheses. In the brain, it is not known whether Tspan12 participates in Norrin/Fz4 signaling, and it is therefore shown in parentheses. Unknown Wnts and Frizzleds are indicated by ‘WntX’ and ‘FzX’, respectively. Hypothesized co-activators are represented by ‘X?’. Scale bars: columns 2 and 3, 500 μm; columns 4 and 5, 200 μm. See also Figure S3 and Table S2.
The Norrin rescue experiment (Figure 4F and S3D) shows that Gpr124-/-;Z/Norrin;Foxg1-Cre embryos exhibit a nearly complete rescue of all Gpr124-/- defects except for the spinal cord hemorrhage, a result that is expected based on the forebrain-specific expression of Foxg1-Cre. When examined at different time points, the Gpr124-/-;Z/Norrin;Foxg1-Cre cerebral cortex shows retarded vascularization at E11.5, but nearly normal vascular architecture at E12.5 and E13, and the Gpr124−/−;Z/Norrin;Foxg1-Cre MGE showed normal pericyte coverage of the rescued vessels at E12.5, as judged by the distribution of NG2 (Figure S3B-D). We note that increasing canonical Wnt signaling on a WT background in control Ctnnb1flex3/+;Pdgfb-CreER and Z/Norrin;Foxg1-Cre embryos had little or no effect on embryonic vascular development at or prior to E13.5 (Figures 4C, 4E, 4G, S3A, S3B and S3D).
Discussion
The experiments reported here establish Gpr124 as a ligand-specific co-activator of canonical Wnt signaling in developing and mature ECs, yet they also suggest that additional components of this system remain to be discovered (Figure 4H). We speculate that Gpr124 evolved to ensure a high signal-to-noise ratio for Wnt7a- and Wnt7b-induced signaling in brain vascular development and homeostasis, analogous to the role of Tspan12 in the context of Norrin/Fz4 signaling. Co-activators of canonical Wnt signaling might be especially important in the context of CNS vascular biology because signal strength in this system appears to be close to the minimal threshold required for normal development and function: in both mice and humans, modest decrements in canonical Wnt signaling in ECs have been shown to subtly retard vascular development and/or BBB/BRB maintenance (Nikopoulos et al., 2010; Zhou et al., 2014).
The ligand and receptor specificities of Gpr124 might provide a partial resolution to two apparent paradoxes: (a) that Wnt knockout phenotypes are generally specific to particular tissues and developmental process, whereas the distribution of individual Wnt ligands is often more widespread (Wnt homepage), and (b) that the three-dimensional structure of a Wnt bound to a Frizzled ligand-binding domain shows that most ligand-receptor contacts involved residues (and a lipid) that are conserved across Wnt and Frizzled family members (Janda et al., 2012). We suggest that binding and signaling specificity between the 19 mammalian Wnts and the ten mammalian Frizzleds is sharpened by co-activators such as Gpr124, allowing cells such as ECs to exhibit greater discrimination in an environment in which multiple Wnts are competing for receptor binding. It would be of interest to test whether other members of the adhesion GPCR family function as co-activators in the context of other Wnt/Frizzled combinations or of other ligand-receptor systems.
The finding that Wnt/Gpr124 and Norrin/Fz4 function in a largely redundant manner to maintain the BBB in postnatal life lends support to an emerging picture of the BBB as a meta-stable state of EC differentiation that is maintained by canonical Wnt signaling (Liebner et al., 2008; Wang et al., 2012). It would be of great interest to determine whether alterations in canonical Wnt signaling play a role in pathologic BBB breakdown, as observed in the contexts of neuroinflammation, stroke, and trauma (Obermeier et al., 2013). Defining the molecules and pathways that control BBB integrity also suggests potential strategies for transient and/or localized modulation of BBB integrity to enhance CNS drug delivery (Paes et al., 2011).
The experiments reported here, together with the observation of high Gpr124 transcript levels in tumor vasculature (St Croix et al., 2000; Carson-Walter et al., 2001), implicate canonical Wnt signaling in tumor angiogenesis. This inference is consistent with earlier observations that Wnt antagonists can inhibit both vascularization of tumors and differentiation of endothelial cell progenitors (Hu et al., 2009). It seems likely that canonical Wnt signaling in ECs activates similar pro-angiogenic programs during CNS and tumor angiogenesis. Taken together, these data support the idea that inhibition of canonical Wnt signaling could synergize with inhibition of VEGF signaling as an anti-angiogenic therapy.
Experimental Procedures
Mice
The following transgenic mouse alleles were used: Gpr124CKO (JAX 016881; Cullen et al., 2011), Tcf/Lef-nLacZ (JAX 005317; Maretto et al., 2003), NdpKO (JAX 012287; Ye et al., 2009), Fz4- (JAX 012823; Wang et al., 2001), Z-Norrin (JAX 011077; Ye et al., 2009), Foxg1-Cre (JAX 004337; Hébert and McConnell, 2000), Ctnnb1flex3 (Harada et al., 1999), and Pdgfb-CreER (Claxton et al., 2008). Mice were handled and housed according to the approved Institutional Animal Care and Use Committee (IACUC) protocol MO13M469 of the Johns Hopkins Medical Institutions.
Antibodies
Antibodies used in this study were as follows: chicken anti-beta-galactosidase (Abcam 9361); rabbit anti-GLUT-1 (Thermo Scientific RB-9052-P0); rabbit anti-NG2 (Millipore AB5320); rat anti-mouse CD102/ICAM2 (BD Biosciences 553326); rat anti-PLVAP/ MECA-32 (BD Biosciences 553849); mouse anti-Claudin-5, Alexa 488 conjugate (Invitrogen 352588), and Texas Red streptavidin (Vector Laboratories SA-5006). AlexFluor-labeled secondary antibodies and GS lectin (Isolectin GS-IB4) were from Invitrogen. Primary antibodies were used at 1:200 to 1:500 dilution.
Luciferase assays
Luciferase assays were performed as described in Xu et al. (2004),
Tissue processing and immunohistochemistry
Tissue processing was performed as described in Wang et al. (2012), and Zhou et al. (2014).
RNAseq on STF cells
RNA was extracted from STF cells using Trizol (Life Technologies) and purified with the RNeasy kit (Qiagen). The cDNA library was synthesized using the TrueSeq kit (Illumina) and sequenced from one end on an Illumina HiSeq, with read lengths of 50 bases. 103,453,057 reads were obtained, of which 103,441,162 could be aligned to the human genome (hg19) using TopHat (v2.0.8). Transcript abundance (FPKM) was quantified using Cufflinks (v2.1.1).
A detailed description of the Experimental Procedures is presented in the Supplemental Information.
Supplementary Material
Figure S1, Related to Figure 1. Luciferase activity in STF cells in response to Gpr, Fz, Lrp, and Wnt transfection.
(A) Ratio of luciferase activity induced by Wnt+Gpr124+Lrp5 compared to Wnt+ vector (pRK5)+Lrp5 plotted against luciferase activity induced by Wnt+Gpr124+Lrp5. This panel is an enlargement of the lower left quadrant of Figure 1A, showing the subset of ligands that produced relatively low luciferase activities in STF cells.
(B) Amino acid identities of murine Gpr124 vs. Gpr125 and Lrp5 vs. Lrp6, shown for each domain.
(C) Gpr125 and Lrp5 tested with 19 Wnts and Norrin. Ratio of luciferase activity induced by Wnt+Gpr125+Lrp5 compared to Wnt+vector (pRK5)+Lrp5 plotted against luciferase activity induced by Wnt+Gpr125+Lrp5. Wnt8a and Wnt8b show a small enhancement of activity.
(D) Lrp6 shows little or no synergy with Gpr124. The plot shows the relative luciferase activity of Wnt7a+Lrp6+Fz or Wnt7b+Lrp6+Fz with vector (pRK5) vs. Gpr124. Among the ten mouse Frizzleds, six have activity substantially above background, but only Fz1 shows an enhancement of signaling with Gpr124+Lrp6 (∼2-fold).
Figure S2, Related to Figure 2. Genetic interaction between Gpr124 and Ndp in CNS angiogenesis and BBB postnatal BBB maintenance.
(A) Canonical Wnt signaling in the hindbrain and spinal cord vasculature visualized with the Tcf/Lef-nLacZ reporter. Z-stacked images of E11.5 hindbrain and spinal cord show numerous beta-galactosidase+ nuclei, most of which are in the CNS parenchyma. Among CNS vascular ECs, there is a greater density of beta-galactosidase+ nuclei in the NdpKO; Tcf/Lef-nLacZ samples and a lower density of beta-galactosidase+ nuclei in the Gpr124-/-;Tcf/Lef-nLacZ samples. In Z-stacked images, nuclei of ECs that are part of well-formed blood vessels can generally be distinguished from closely apposed nuclei in neuroepithelial cells because the EC nuclei are typically elongated in the direction of the long axis of the blood vessel. We note that Gpr124-/-;NdpKO;Tcf/Lef-nLacZ ECs are not well organized and it is therefore difficult to determine in a Z-stacked image whether beta-galactosidase+ nuclei superimposed on the GS lectin signal are vascular or neural. Analysis of single Z-plane images (Figure 2C) suggests that few if any are vascular. Scale bar: 200 μm.
(B-D) Scatter plots from Figures 2A, 2B, and 4G are reproduced here as panels B, C, and D, respectively, with the individual data points shown. In panel B, the Gpr124-/- and Gpr124-/-;NdpKO genotypes were represented by 8 and 4 sections, respectively, and have a mean of zero beta-gal+ ECs per section in the MGE. In panel B, the NdpKO genotype is represented by 14 sections and has a mean avascular area of zero um2 (as described in the Figure 2 legend).
(E) Massive reduction in Gpr124-/-;NdpKO hindbrain vascularization at E11.5. GS lectin-stained hindbrain sections from five Gpr124-/- embryos and five Gpr124-/-;NdpKO embryos. The Gpr124-/- embryos show normal or nearly normal vascularization, whereas the Gpr124-/-;NdpKO embryos show severely retarded vascularization with glomeruloid body formation throughout the ventral 90% of the hindbrain. These data confirm the phenotypes shown in Figure 2E. Red lines demarcate the borders of the neuroepithelium. Scale bar: 500 μm.
(F,G) Postnatal redundancy of Gpr124 and Ndp in maintaining the BBB state. In (F) Sulfo-NHS-biotin perfusion to monitor BBB integrity at P10 in Gpr124CKO/-;NdpKO;Pdgfb-CreER mice is shown in coronal sections at the level of the anterior hippocampus. In this experiment, residual CreER activity in the absence of tamoxifen or 4HT exposure provided sufficient recombination of the Gpr124CKO allele. Constitutive deletion of Ndp in the presence of one functional copy of Gpr124 (upper panels) is associated with an intact BBB, sulfo-NHS-biotin leakage that is confined to the choroid plexus, and an absence of PLVAP in ECs. Constitutive deletion of Ndp combined with EC-specific deletion of Gpr124 (lower panels) is associated with widely scattered BBB defects as shown by multiple zones of sulfo-NHS-biotin leakage in the cerebral cortex and hippocampus and by induction of PLVAP in a subset of ECs. The BBB is largely intact in the thalamus (white arrows). In (G) Coronal sections at the level of the colliculus/cerebellum junction show conversion of ECs from PLVAP-/claudin5+ to PLVAP+/claudin5- in the superior colliculus (top), cerebellum (center), and brainstem (bottom) with constitutive deletion of Ndp and postnatal EC-specific deletion of Gpr124 (Gpr124CKO/-;NdpKO;Pdgfb-CreER). Deletion of either gene alone shows little conversion. Mice were treated with 50-100 ug 4HT at P3-P4, and brains were examined at P6. Scale bars: F, 500 μm; G, 1 mm.
(H) Eliminating Gpr124 in postnatal ECs has little or no effect on retinal vascular architecture or EC differentiation. Comparison of P6 retina flat mounts with the following number of functional Gpr124 alleles in ECs following 50-100 ug 4HT treatment at P3-P4: two (Gpr124CKO/+), one (Gpr124CKO/+;Pdgfb-CreER), or zero (Gpr124CKO/-;Pdgfb-CreER). By contrast, a P6 NdpKO retina exhibits reduced vascular coverage, complete conversion of veins and capillaries from a PLVAP-/claudin5+ to a PLVAP+/claudin5- state, and partial conversion of arteries from a PLVAP-/claudin5+ to a PLVAP+/claudin5- state. Scale bar: 200 μm.
Figure S3, Related to Figure 4. Spinal cord vascularization defects in E13.5 Gpr124-/- embryos are largely rescued by EC-specific stabilization of beta-catenin, and ectopic Norrin expression corrects the Gpr124-/- angiogenesis phenotype in the cerebral cortex and MGE.
(A) For each genotype, sections at the forelimb level are shown from three E13.5 embryos treated at E10.5 with a maternal IP injection of 1.5 mg tamoxifen. The sections were stained for Glut1, Icam2, and GS lectin. Two of the three markers are shown in each image, with false coloring (red) of the channels for GS lectin (left) and Icam2 (right). The first and fourth columns derive from one section from the first embryo; the second and fifth columns derive from one section from the second embryo; and the third and sixth columns derive from one section from the third embryo. All Gpr124-/-embryos exhibit a marked reduction in vascularization and reduced Glut1 expression in the ventral spinal cord, and both of these phenotypes are partially rescued in the Gpr124-/-;Ctnnb1flex3/+;Pdgfb-CreER embryo three days after tamoxifen exposure. Scale bar: 500 μm.
(B) Rescue of the Gpr124-/- cerebral cortex angiogenesis defect at E11.5 and E12.5 by Z/Norrin;Foxg1-Cre (upper panels) and rescue of the MGE angiogenesis defect with restoration of normal vascular architecture and pericyte coverage (visualized with anti-NG2; lower panels).
(C) Rescue of the Gpr124-/- cerebral cortex angiogenesis defect at E13 by Z/Norrin;Foxg1-Cre. Vascular architecture and Glut1 expression are restored. An age-matched WT control is shown together with Gpr124-/- samples that are 0.5 days younger and 0.5 days older. The Gpr124-/- phenotype remains constant during this time window. Scale bars: 200 μm.
(D) Quantification of vascular density in the E13.5 cerebral cortex (based on GS lectin staining) shows a complete rescue of the Gpr124-/- angiogenesis defect by Z/Norrin;Foxg1-Cre. We note that GS lectin stains both ECs and invading macrophages, and the automated procedure used to quantify vessel density (skeletonizing GS lectin+ objects in ImageJ) does not discriminate between the two. The result is an over-estimate of vascular density in the Gpr124-/- cerebral cortex, i.e. an underestimate of the severity of the angiogenesis defect.
Table S1, Related to Figure 1. Abundance of transcripts coding for canonical Wnt signaling components in STF cells, determined by RNAseq.
Table S2, Related to Figures 2-4. Numbers of embryos analyzed for different genotypes shown in Figures 2-4.
Combinations of Gpr124 and Ndp alleles at E11.5
Combinations of Gpr124 and Fz4 alleles at E11.5
Combinations of Gpr124, Ctnnb1flex3, and Pdgfb-CreER alleles at E12.5-E13.5
Combinations of Gpr124, Z/Norrin, and Foxg1-Cre at E11.5-E13.5
Acknowledgments
The authors thank to Dr. Maketo Taketo (Kyoto University) for the Ctnnb1flex3 mice, Dr. Calvin Kuo (Stanford University) for the Gpr124 expression plasmid, Dr. Amir Rattner for providing the STF RNAseq data, Phil Smallwood with assistance in plasmid construction and characterization, and Drs. Amir Rattner and Hao Wu for comments on the manuscript. Supported by the National Eye Inistitute (NIH; EY018637 to J.N.), the Ellison Medical Foundation, and the Howard Hughes Medical Institute.
Footnotes
Author Contributions: YZ and JN designed experiments, analyzed data, and wrote the paper; YZ conducted the experiments.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue YZ, Wei Y, et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci USA. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, Croix BS. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li XJ, Chaudhary A, Xu LH, Hilton MB, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci USA. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:6422–6422. doi: 10.1073/pnas.0805165106. vol 106, pg 641, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin AM, Sullivan KM, D'Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dynam. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Hu J, Dong AW, Fernandez-Ruiz V, Shan JJ, Kawa M, Martinez-Anso E, Prieto J, Qian C. Blockade of Wnt Signaling Inhibits Angiogenesis and Tumor Growth in Hepatocellular Carcinoma. Cancer Res. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, Garcia KC. Structural Basis of Wnt Recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner DM, Ke JY, Zhong ZD, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrin Met. 2013;24:31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye WL. TSPAN12 Regulates Retinal Vascular Development by Promoting Norrin- but Not Wnt-Induced FZD4/beta-Catenin Signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, et al. Essential Regulation of CNS Angiogenesis by the Orphan G Protein-Coupled Receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Semenov MV, Huang H, He X. Dissecting Molecular Differences between Wnt Coreceptors LRP5 and LRP6. Plos One. 2011;6:e23537. doi: 10.1371/journal.pone.0023537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikopoulos K, Venselaar H, Collin RWJ, Riveiro-Alvarez R, Boonstra FN, Hooymans JMM, Mukhopadhyay A, Shears D, van Bers M, de Wijs IJ, et al. Overview of the Mutation Spectrum in Familial Exudative Vitreoretinopathy and Norrie Disease with Identification of 21 Novel Variants in FZD4, LRP5, and NDP. Hum Mutat. 2010;31:656–666. doi: 10.1002/humu.21250. [DOI] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes KT, Wang E, Henze K, Vogel P, Read R, Suwanichkul A, Kirkpatrick LL, Potter D, Newhouse MM, Rice DS. Frizzled 4 Is Required for Retinal Angiogenesis and Maintenance of the Blood-Retina Barrier. Invest Ophth Vis Sci. 2011;52:6452–6461. doi: 10.1167/iovs.10-7146. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt Signaling Regulates Organ-Specific Assembly and Differentiation of CNS Vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Wang YS, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YS, Rattner A, Zhou YL, Williams J, Smallwood PM, Nathans J. Norrin/Frizzled4 Signaling in Retinal Vascular Development and Blood Brain Barrier Plasticity. Cell. 2012;151:1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnt homepage: http://www.stanford.edu/group/nusselab/cgi-bin/wnt/
- Xu Q, Wang YS, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Smallwood P, Nathans J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns. 2011;11:151–155. doi: 10.1016/j.gep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang YS, Cahill H, Yu MZ, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, Frizzled-4, and Lrp5 Signaling in Endothelial Cells Controls a Genetic Program for Retinal Vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang YS, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Jeremy Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clinical Invest. 2014 doi: 10.1172/JCI76431. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Related to Figure 1. Luciferase activity in STF cells in response to Gpr, Fz, Lrp, and Wnt transfection.
(A) Ratio of luciferase activity induced by Wnt+Gpr124+Lrp5 compared to Wnt+ vector (pRK5)+Lrp5 plotted against luciferase activity induced by Wnt+Gpr124+Lrp5. This panel is an enlargement of the lower left quadrant of Figure 1A, showing the subset of ligands that produced relatively low luciferase activities in STF cells.
(B) Amino acid identities of murine Gpr124 vs. Gpr125 and Lrp5 vs. Lrp6, shown for each domain.
(C) Gpr125 and Lrp5 tested with 19 Wnts and Norrin. Ratio of luciferase activity induced by Wnt+Gpr125+Lrp5 compared to Wnt+vector (pRK5)+Lrp5 plotted against luciferase activity induced by Wnt+Gpr125+Lrp5. Wnt8a and Wnt8b show a small enhancement of activity.
(D) Lrp6 shows little or no synergy with Gpr124. The plot shows the relative luciferase activity of Wnt7a+Lrp6+Fz or Wnt7b+Lrp6+Fz with vector (pRK5) vs. Gpr124. Among the ten mouse Frizzleds, six have activity substantially above background, but only Fz1 shows an enhancement of signaling with Gpr124+Lrp6 (∼2-fold).
Figure S2, Related to Figure 2. Genetic interaction between Gpr124 and Ndp in CNS angiogenesis and BBB postnatal BBB maintenance.
(A) Canonical Wnt signaling in the hindbrain and spinal cord vasculature visualized with the Tcf/Lef-nLacZ reporter. Z-stacked images of E11.5 hindbrain and spinal cord show numerous beta-galactosidase+ nuclei, most of which are in the CNS parenchyma. Among CNS vascular ECs, there is a greater density of beta-galactosidase+ nuclei in the NdpKO; Tcf/Lef-nLacZ samples and a lower density of beta-galactosidase+ nuclei in the Gpr124-/-;Tcf/Lef-nLacZ samples. In Z-stacked images, nuclei of ECs that are part of well-formed blood vessels can generally be distinguished from closely apposed nuclei in neuroepithelial cells because the EC nuclei are typically elongated in the direction of the long axis of the blood vessel. We note that Gpr124-/-;NdpKO;Tcf/Lef-nLacZ ECs are not well organized and it is therefore difficult to determine in a Z-stacked image whether beta-galactosidase+ nuclei superimposed on the GS lectin signal are vascular or neural. Analysis of single Z-plane images (Figure 2C) suggests that few if any are vascular. Scale bar: 200 μm.
(B-D) Scatter plots from Figures 2A, 2B, and 4G are reproduced here as panels B, C, and D, respectively, with the individual data points shown. In panel B, the Gpr124-/- and Gpr124-/-;NdpKO genotypes were represented by 8 and 4 sections, respectively, and have a mean of zero beta-gal+ ECs per section in the MGE. In panel B, the NdpKO genotype is represented by 14 sections and has a mean avascular area of zero um2 (as described in the Figure 2 legend).
(E) Massive reduction in Gpr124-/-;NdpKO hindbrain vascularization at E11.5. GS lectin-stained hindbrain sections from five Gpr124-/- embryos and five Gpr124-/-;NdpKO embryos. The Gpr124-/- embryos show normal or nearly normal vascularization, whereas the Gpr124-/-;NdpKO embryos show severely retarded vascularization with glomeruloid body formation throughout the ventral 90% of the hindbrain. These data confirm the phenotypes shown in Figure 2E. Red lines demarcate the borders of the neuroepithelium. Scale bar: 500 μm.
(F,G) Postnatal redundancy of Gpr124 and Ndp in maintaining the BBB state. In (F) Sulfo-NHS-biotin perfusion to monitor BBB integrity at P10 in Gpr124CKO/-;NdpKO;Pdgfb-CreER mice is shown in coronal sections at the level of the anterior hippocampus. In this experiment, residual CreER activity in the absence of tamoxifen or 4HT exposure provided sufficient recombination of the Gpr124CKO allele. Constitutive deletion of Ndp in the presence of one functional copy of Gpr124 (upper panels) is associated with an intact BBB, sulfo-NHS-biotin leakage that is confined to the choroid plexus, and an absence of PLVAP in ECs. Constitutive deletion of Ndp combined with EC-specific deletion of Gpr124 (lower panels) is associated with widely scattered BBB defects as shown by multiple zones of sulfo-NHS-biotin leakage in the cerebral cortex and hippocampus and by induction of PLVAP in a subset of ECs. The BBB is largely intact in the thalamus (white arrows). In (G) Coronal sections at the level of the colliculus/cerebellum junction show conversion of ECs from PLVAP-/claudin5+ to PLVAP+/claudin5- in the superior colliculus (top), cerebellum (center), and brainstem (bottom) with constitutive deletion of Ndp and postnatal EC-specific deletion of Gpr124 (Gpr124CKO/-;NdpKO;Pdgfb-CreER). Deletion of either gene alone shows little conversion. Mice were treated with 50-100 ug 4HT at P3-P4, and brains were examined at P6. Scale bars: F, 500 μm; G, 1 mm.
(H) Eliminating Gpr124 in postnatal ECs has little or no effect on retinal vascular architecture or EC differentiation. Comparison of P6 retina flat mounts with the following number of functional Gpr124 alleles in ECs following 50-100 ug 4HT treatment at P3-P4: two (Gpr124CKO/+), one (Gpr124CKO/+;Pdgfb-CreER), or zero (Gpr124CKO/-;Pdgfb-CreER). By contrast, a P6 NdpKO retina exhibits reduced vascular coverage, complete conversion of veins and capillaries from a PLVAP-/claudin5+ to a PLVAP+/claudin5- state, and partial conversion of arteries from a PLVAP-/claudin5+ to a PLVAP+/claudin5- state. Scale bar: 200 μm.
Figure S3, Related to Figure 4. Spinal cord vascularization defects in E13.5 Gpr124-/- embryos are largely rescued by EC-specific stabilization of beta-catenin, and ectopic Norrin expression corrects the Gpr124-/- angiogenesis phenotype in the cerebral cortex and MGE.
(A) For each genotype, sections at the forelimb level are shown from three E13.5 embryos treated at E10.5 with a maternal IP injection of 1.5 mg tamoxifen. The sections were stained for Glut1, Icam2, and GS lectin. Two of the three markers are shown in each image, with false coloring (red) of the channels for GS lectin (left) and Icam2 (right). The first and fourth columns derive from one section from the first embryo; the second and fifth columns derive from one section from the second embryo; and the third and sixth columns derive from one section from the third embryo. All Gpr124-/-embryos exhibit a marked reduction in vascularization and reduced Glut1 expression in the ventral spinal cord, and both of these phenotypes are partially rescued in the Gpr124-/-;Ctnnb1flex3/+;Pdgfb-CreER embryo three days after tamoxifen exposure. Scale bar: 500 μm.
(B) Rescue of the Gpr124-/- cerebral cortex angiogenesis defect at E11.5 and E12.5 by Z/Norrin;Foxg1-Cre (upper panels) and rescue of the MGE angiogenesis defect with restoration of normal vascular architecture and pericyte coverage (visualized with anti-NG2; lower panels).
(C) Rescue of the Gpr124-/- cerebral cortex angiogenesis defect at E13 by Z/Norrin;Foxg1-Cre. Vascular architecture and Glut1 expression are restored. An age-matched WT control is shown together with Gpr124-/- samples that are 0.5 days younger and 0.5 days older. The Gpr124-/- phenotype remains constant during this time window. Scale bars: 200 μm.
(D) Quantification of vascular density in the E13.5 cerebral cortex (based on GS lectin staining) shows a complete rescue of the Gpr124-/- angiogenesis defect by Z/Norrin;Foxg1-Cre. We note that GS lectin stains both ECs and invading macrophages, and the automated procedure used to quantify vessel density (skeletonizing GS lectin+ objects in ImageJ) does not discriminate between the two. The result is an over-estimate of vascular density in the Gpr124-/- cerebral cortex, i.e. an underestimate of the severity of the angiogenesis defect.
Table S1, Related to Figure 1. Abundance of transcripts coding for canonical Wnt signaling components in STF cells, determined by RNAseq.
Table S2, Related to Figures 2-4. Numbers of embryos analyzed for different genotypes shown in Figures 2-4.
Combinations of Gpr124 and Ndp alleles at E11.5
Combinations of Gpr124 and Fz4 alleles at E11.5
Combinations of Gpr124, Ctnnb1flex3, and Pdgfb-CreER alleles at E12.5-E13.5
Combinations of Gpr124, Z/Norrin, and Foxg1-Cre at E11.5-E13.5