SUMMARY
The Piwi/piRNA pathway protects the germ line from the activity of foreign sequences such as transposons. Remarkably, tens of thousands of piRNAs arise from a minimal number of discrete genomic regions. The extent to which clustering of these small RNA genes contributes to their coordinated expression remains unclear. We show that C. elegans SNPC-4, the Myb-like DNA-binding subunit of the small nuclear RNA activating protein complex (SNAPc), binds piRNA clusters in a germline-specific manner and is required for global piRNA expression. SNPC-4 localization is mutually dependent with that of piRNA biogenesis factor PRDE-1. SNPC-4 exhibits an atypical widely distributed binding pattern that “coats” piRNA domains. Discrete peaks within the domains occur frequently at RNA polymerase III-occupied tRNA genes, which have been implicated in chromatin organization. We suggest that SNPC-4 binding establishes a positive expression environment across piRNA domains, providing an explanation for the conserved clustering of individually transcribed piRNA genes.
INTRODUCTION
Piwi-interacting RNAs (piRNAs) are a class of small (21–30 bp) RNAs that associate with Piwi, a member of the highly conserved Argonaute/Piwi protein family involved in diverse small RNA-mediated regulatory pathways (Saxe and Lin, 2011). The Piwi/piRNA pathway is necessary for gametogenesis, and to protect genome integrity in the germ line by recognizing and silencing non-self elements such as transposons (Luteijn and Ketting, 2013). piRNAs are key effectors in this pathway because they have the ability to target many types of transcripts due to their abundance and sequence diversity. Although tens of thousands of piRNAs exist, they originate from a discrete number of clustered genomic loci, and their expression is generally limited to the germ line and associated cell types (Ishizu et al., 2012). The organization of other complex loci, such as the protocadherin gene cluster, the β-globin LCR, and Hox gene clusters, into specialized domains proves to be essential for their appropriate expression patterns (Andrey et al., 2013; Chen and Maniatis, 2013; Deng et al., 2012). However, the extent to which arrangement into clusters fully contributes to piRNA gene regulation remains unclear.
Based on association with the Piwi ortholog PRG-1, C. elegans has greater than 15,000 annotated piRNAs, known as 21U-RNAs. 21U-RNAs distribute roughly equally between two classes (Gu et al., 2012). Type I 21U-RNA genes are clustered into two discrete domains of 2.5 Mb (4.5–7 Mb) and 3.7 Mb (13.5–17.2 Mb) on Chromosome (Chr) IV, and primarily reside in introns and intergenic regions (Batista et al., 2008; Ruby et al., 2006). Type II 21U-RNAs arise from annotated coding gene promoters throughout the genome and are unclustered (Gu et al., 2012). Multiple lines of evidence indicate that each 21U-RNA is independently transcribed as a short RNA (approximately 26–70 bp in length) by RNA polymerase II (Pol II) and is processed into its mature, PRG-1-associated form through poorly characterized events (Batista et al., 2008; Billi et al., 2013; Cecere et al., 2012; Gu et al., 2012; Ruby et al., 2006). This process contrasts with mammals and Drosophila, in which a long precursor is processed into many piRNAs (Aravin et al., 2006; Brennecke et al., 2007; Lau et al., 2006). Factors implicated in the regulation of Type I 21U-RNAs include the novel factor PRDE-1 (Weick et al., 2014), and members of the forkhead (FKH)-related transcription factor family, which can bind to 21U-RNA consensus motif upstream of individual Type I 21U-RNA genes in order to promote their autonomous expression (Cecere et al., 2012). Type II 21U-RNAs are presumably under the regulation of the transcriptional machinery at the promoters from which they arise (Gu et al., 2012).
Although individual transcription should obviate the requirement for genomic clustering of Type I 21U-RNA loci, their clustering is highly conserved between C. elegans and its close nematode relative C. briggsae (Batista et al., 2008; Ruby et al., 2006; Shi et al., 2013). Intriguingly, the clustered Type I loci account for approximately 95% of the mature 21U-RNA population in C. elegans, whereas Type II 21U-RNAs have minimal contribution (Gu et al., 2012), suggesting that organization of 21U-RNAs into discrete domains is advantageous for effectively promoting robust and appropriate spatial expression. However, the mechanisms involved in creating an environment that instructs germline-specific expression of abundantly expressed Type I 21U-RNAs within these domains are unknown.
Here we use genome-wide analysis to demonstrate that the transcription factor SNPC-4, the ortholog of SNAPc subunit 4, binds the two major 21U-RNA domains and regulates the global expression of mature 21U-RNAs in C. elegans. SNPC-4 binding at 21U-RNA regions is developmentally regulated and germline-specific, and correlates with SNPC-4 subnuclear foci visible only in undifferentiated germ cells. Moreover, the formation of SNPC-4 foci, and the enriched binding at 21U-RNA regions, depends upon the activity of the 21U-RNA biogenesis factor PRDE-1. Conversely, SNPC-4 is required for PRDE-1 localization in germ nuclei. Given that SNPC-4 orthologs are sequence-specific transcription factors, the SNPC-4 binding profile within the 21U-RNA-rich genomic domains is unusual and striking: broad binding coats each domain, interspersed with stronger discrete peaks that do not necessarily correspond to individual 21U-RNA loci. At discrete sites, SNPC-4, in conjunction with RNA polymerase III (Pol III), binds tRNA genes with elevated frequency and strength within 21U-RNA domains. tRNA genes are known regulators of chromatin organization in yeast and humans (Donze, 2012), suggesting that these sites might contribute to defining 21U-RNA domains. Additionally, SNPC-4 contains Myb/SANT protein motifs, which are capable of influencing nucleosome positioning and histone modifications (Boyer et al., 2004). Together the data presented here define a highly specialized function for SNPC-4 in establishing a unified domain of regulation to coordinate the robust expression of tens of thousands of individually transcribed 21U-RNA genes.
RESULTS
Localization and expression of SNPC-4
Our interest in 21U-RNA regulation led us to further explore a striking germline localization pattern for a factor we investigated through the modENCODE project (Gerstein et al., 2010). This factor, initially called GEI-11, was re-named SNPC-4 to better reflect its orthology and function. SNPC-4 is the only C. elegans ortholog of mammalian SNAPC4, the DNA binding subunit of the small nuclear RNA activating protein complex SNAPc (Jawdekar and Henry, 2008; Wong et al., 1998) (Figure S1A and Supplemental Table 1). SNAPc binds to a consensus element called the proximal sequence element (PSE) and interacts with both Pol II and Pol III (Figure S1B) (Yoon et al., 1995). With Pol II, it promotes transcription of the U1, U2, U4 and U5 snRNAs involved in splicing. It also initiates transcription of Type 3 Pol III-regulated genes, such as U6 snRNAs, the scan RNA, RNase P RNA, RNase MRP RNA, vault RNA, 7SK RNA, and Y RNAs (Canella et al., 2010).
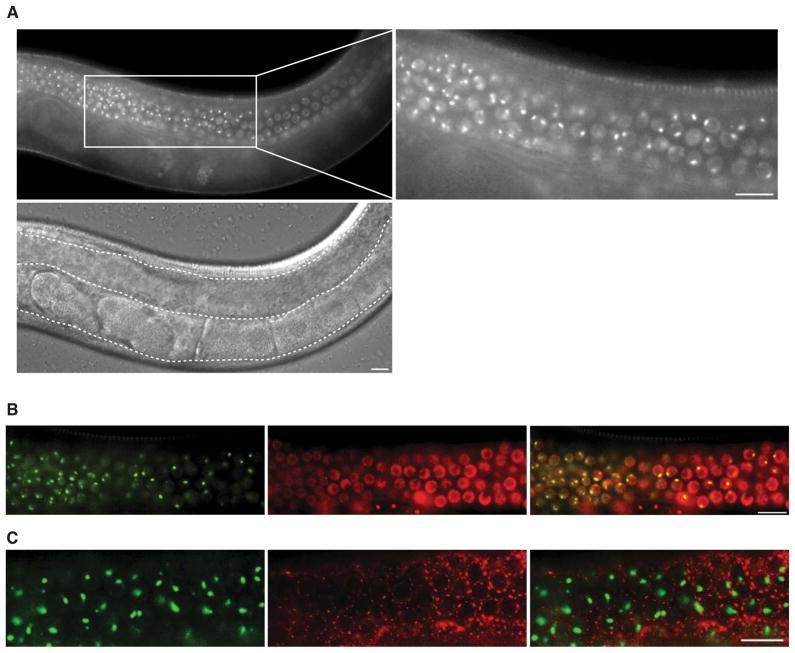
We investigated SNPC-4 localization and protein function using a transgenic line containing a fosmid that encompasses the endogenous snpc-4 genomic locus with an inserted GFP-3xFLAG tag at the carboxy terminus (Niu et al., 2011; Sarov et al., 2012; Zhong et al., 2010). This transgene rescued the lethal phenotype of snpc-4(tm4568) mutants, resulting in viable, fertile animals (Figure S1C). Consistent with its known function, SNPC-4:GFP was broadly expressed throughout all tissues, where it exhibited diffuse nuclear localization (Figure S1D–F). Notably, SNPC-4:GFP was also concentrated in prominent foci only within germline nuclei. These germline-specific SNPC-4 foci were present throughout development of both hermaphrodites and males (Figure 1A and S1G). SNPC-4:GFP foci co-localized with a marker for chromatin (mCherry:H2B), but not with a marker for perinuclear germ granules (PGL-1:RFP), consistent with the expectation that SNPC-4 associates with DNA (Figure 1B and 1C).
Figure 1. SNPC-4 localizes to germline-specific chromatin-associated foci.
(A) SNPC-4:GFP localization in one young adult gonad arm (outlined by a dotted line in the DIC image). Zoomed region shows SNPC-4:GFP localization to sub-nuclear foci in addition to diffuse nuclear expression in undifferentiated germ nuclei. (B) Co-localization of SNPC-4:GFP (green) with the chromatin marker mCherry:H2B (red) in young adult germ nuclei proceeding from the transition zone to the meiotic zone (left to right). (C) Overlay of SNPC-4:GFP (green) with the germ granule marker PGL-1::RFP (red) in young adult meiotic germ nuclei. Scale bars are 10 μm.
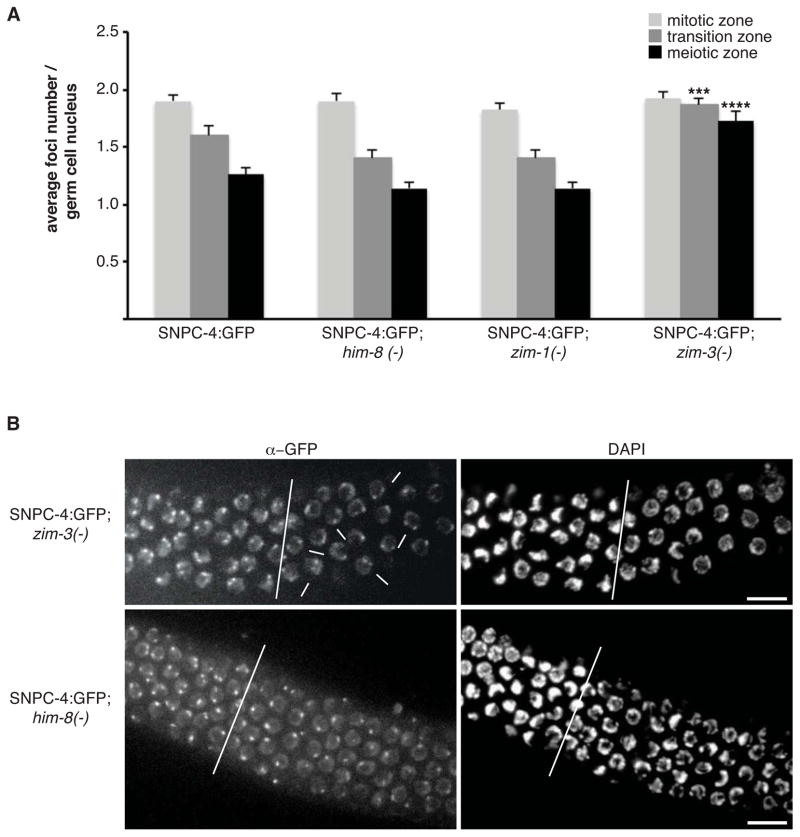
SNPC-4:GFP foci were most apparent in the distal population of proliferating and pachytene germ cells, and decreased substantially in intensity as proximal germ cells entered oogenesis. Typically, two foci were present in mitotic germ nuclei, whereas only a single, larger focus was detected in nuclei that had entered the pachytene stage of meiosis I (Figure 1A (zoom) and 2A). We hypothesized that the double foci in proliferating germ nuclei corresponded to a discrete genomic locus detectable on a pair of homologous chromosomes, and that these foci coalesce as chromosomes align and undergo synapsis in meiotic nuclei. We therefore utilized mutants that selectively disrupt homologous pairing and synapsis of specific chromosomes during meiosis: him-8(e1489) - Chr X, zim-1(tm1813) - Chr II & III, and zim-3(tm2303) - Chr I & IV (Phillips and Dernburg, 2006). After crossing these mutants to the SNPC-4:GFP transgenic line, we quantified the number of SNPC-4:GFP foci present in the mitotic, transition, and meiotic zones. A single focus was still detected in the pachytene region of him-8 and zim-1 mutants, as in wildtype, whereas in the absence of zim-3 activity, most meiotic nuclei contained two foci (p = 0.001; Figure 2A, 2B and S2). zim-3 is required for pairing of Chr I and IV, suggesting that SNPC-4:GFP foci might be associated with a region on one of these two chromosomes.
Figure 2. SNPC-4:GFP focal pattern is altered upon disruption of pairing and synapsis of the chromosome harboring 21U-RNA clusters.
(A) Bars represent average foci number per germ nucleus (n = 50 germ nuclei / zone) from at least 5 dissected SNPC-4:GFP gonads per genotype in wildtype and chromosome-specific pairing mutants him-8 (Chr X), zim-1 (Chr II & III), and zim-3 (Chr I & IV). Statistical analysis was performed by comparing wildtype versus mutant within each zone (***p ≤ 0.001 and ****p ≤ 0.0001, Fisher’s exact test). Error bars represent SEM. (B) Representative confocal z-stack projection of dissected young adult gonads stained with αGFP and DAPI from zim-3 and him-8 mutants, in which SNPC-4:GFP foci were quantified (see A and Figure S2). White line separates the transition (left) and meiotic (right) zones. White arrows indicate representative zim-3 mutant nuclei in the meiotic zone in which two SNPC-4:GFP foci persist. Scale bars are 10 μm.
SNPC-4 binds to the 21U-RNA domains in addition to canonical SNAPc targets
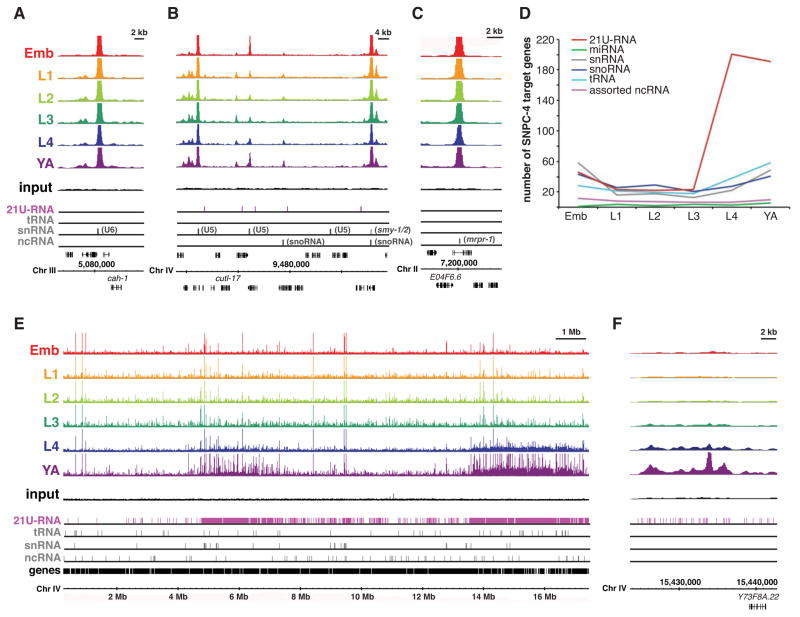
To more precisely determine the location of SNPC-4 binding in the genome, we performed chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) of SNPC-4:GFP at six stages of hermaphrodite development (embryos, L1, L2, L3, L4, and young adult) (Supplemental Table 2). As expected based on studies in mammals and Drosophila (James Faresse et al., 2012; Su et al., 1997), SNPC-4 was found at the promoters of many Pol II- and Pol III-transcribed snRNA genes (Figure 3A and 3B), as well as Type 3 Pol III-transcribed noncoding RNA (ncRNA) genes (Figure 3B and 3C). SNPC-4:GFP bound many snoRNAs and some tRNA genes as well, although tRNAs are not expected targets. Binding at the canonical ncRNA targets was generally consistent across developmental stages (Figure 3D). Additionally, SNPC-4:GFP was bound at specialized ncRNA genes whose products are involved in trans-splicing of multi-cistronic transcripts arising from operons (Blumenthal, 2005), including the smy- and SL2 splice leader genes, although not the SL1 genes.
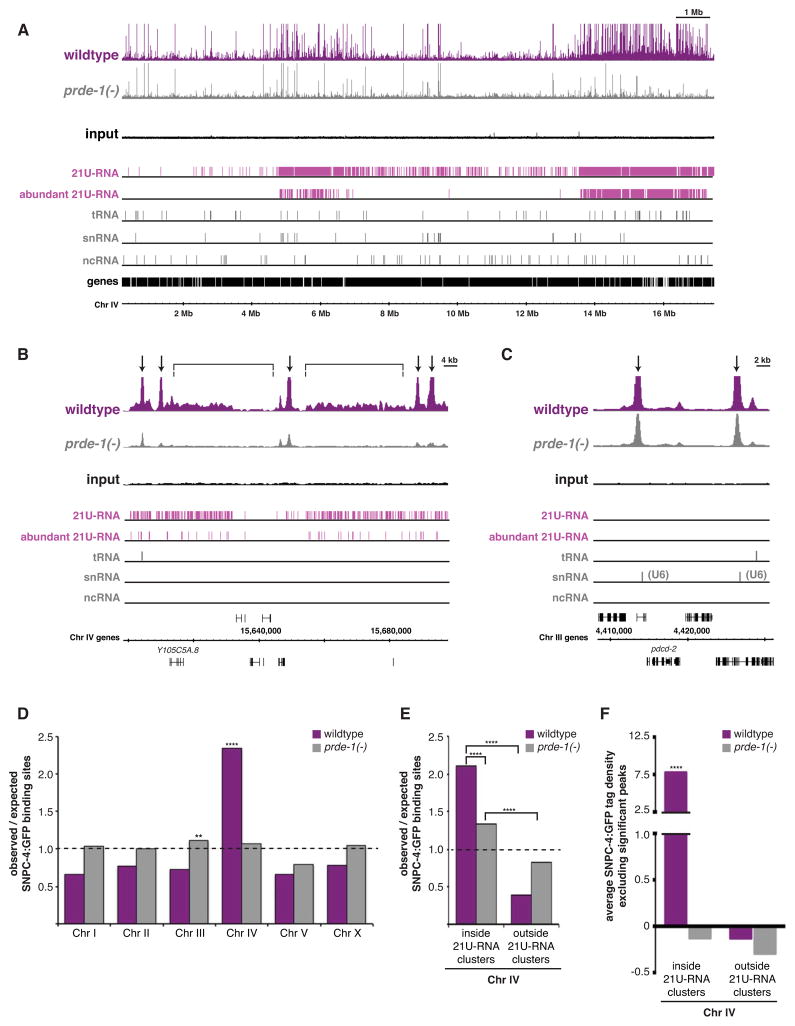
Figure 3. SNPC-4:GFP preferentially binds 21U-RNA clusters.
(A–C) SNPC-4:GFP ChIP-seq binding profiles for combined replicates at known ncRNA targets for all the major developmental stages. Read counts for each ChIP-seq dataset are normalized by the total number of reads. A representative input from the young adult dataset is shown. Annotated gene tracks include 21U-RNAs (pink) and various other ncRNA classes (gray). The snRNA track combines snRNA, sls-, and smy- genes. One gene per image is annotated for reference. (D) Quantification of SNPC-4:GFP significant peak binding for various ncRNA gene classes across development. The number of SNPC-4-bound targets was normalized to the total number of significant binding sites in each ChIP-seq dataset. Assorted ncRNA genes includes a variety of Type 3 Pol III targets as well as various single copy or small family ncRNAs. (E) SNPC-4:GFP ChIP-seq binding profiles of combined replicates across Chr IV for all the major developmental stages; tracks are as in (A). (F) An example of SNPC-4:GFP binding within the 3.7 Mb 21U-RNA cluster.
Most remarkably, SNPC-4:GFP exhibited developmentally regulated binding at two specific locations on Chr IV that corresponded precisely to the genomic regions containing clustered 21U-RNAs (Figure 3E). The highest level of binding occurred in the L4 and young adult stages and was much lower in other developmental stages (Figure 3D–3F). In the young adult stage, 50% (532/1073) of SNPC-4:GFP binding sites were on Chr IV, and of these, 79% (419/532) were within the two 21U-RNA domains. ncRNA genes were not enriched within the 21U-RNA domains relative to the rest of Chr IV (Figure S3A and S3B), and therefore cannot fully account for the preferential binding of SNPC-4 in these regions. Moreover, concentrated binding was not seen on any other chromosomes (e.g. Figure S3C). These data demonstrate that SNPC-4:GFP strongly associates with 21U-RNA domains.
Germline-specific binding of SNPC-4 at 21U-RNA regions
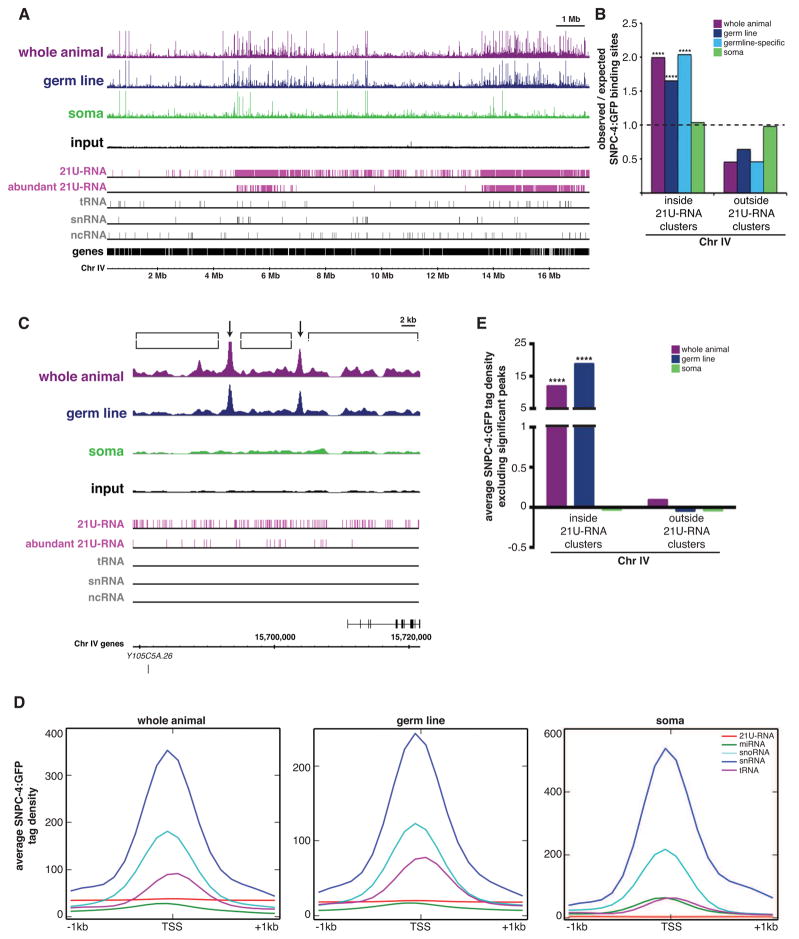
21U-RNAs are expressed primarily, if not exclusively, in the germ line, and enriched SNPC-4:GFP binding across the 21U-RNA-containing domains correlated with germline development. To determine directly whether SNPC-4 binding at the 21U-RNA regions occurs only in the germ line, we performed tissue-specific ChIP-seq (Supplemental Table 2). To examine somatic tissues, we crossed the SNPC-4:GFP transgenic strain to the glp-1(q224) mutant, which lacks virtually all germ cells at the restrictive temperature (Figure S4A). To query binding in the germ line, we generated a separate transgenic line using the pie-1 promoter to enrich SNPC-4:GFP expression in the germ line. Notably, pie-1-driven SNPC-4:GFP also concentrated in foci within germ nuclei (Figure S4B). SNPC-4:GFP binding sites in the whole animal and germ line datasets were preferentially located on Chr IV specifically in 21U-RNA regions (p ≤ 1.8 × 10−17), but not in the soma dataset (p = 0.7) (Figure 4A, 4B, and S4C). Importantly, in the soma, SNPC-4:GFP still occupied snRNA and other ncRNA target genes (e.g. Figure S4D). Consistent with the preferential expression and activity of 21U-RNAs in the germ line, SNPC-4 peaks in the germline dataset that were absent in the soma dataset (germline-specific SNPC-4 binding events) also were primarily located within the 21U-RNA regions (p = 3.8 × 10−27; Figure 4B). Based on these observations and the disrupted focal pattern in the zim-3 synapsis mutant (Figure 2A), we suggest that the SNPC-4:GFP foci seen specifically in germ nuclei likely correspond to 21U-RNA clusters. Several other germline-expressed transcription factors do not exhibit this binding pattern or concentrate in subnuclear foci (Kudron et al., 2013), indicating that this pattern is not simply a consequence of accessible chromatin in this tissue. We conclude that in addition to its role as a broadly expressed factor that regulates multiple classes of essential ncRNAs, SNPC-4 exhibits germline-specific binding at 21U-RNA regions.
Figure 4. Germline-specific binding of SNPC-4:GFP at 21U-RNA clusters consists of discrete and distributed binding.
(A) Young adult ChIP-seq binding profiles for combined replicates across Chr IV when SNPC-4:GFP is expressed broadly (whole animal, purple), specifically in the germ line (navy), and specifically in the soma (green). Annotated gene tracks as in Figure 3A. Abundantly expressed 21U-RNAs (pink) have >50 reads in the RNA-seq dataset in Figure 5A. (B) Distribution of significant discrete SNPC-4:GFP binding sites on Chr IV relative to genomic length for all tissue-specific ChIP-seq datasets. Germline-specific SNPC-4 binding sites (teal) consist of germline SNPC-4 sites not present in the soma ChIP-seq data. Statistically significant deviations greater than the expected value of 1 (marked with a black line) are indicated by asterisks (****p ≤ 0.0001, Pearson’s chi-square test). (C) Example of a SNPC-4:GFP ChIP-seq binding profile within the 3.7 Mb 21U-RNA cluster as described in (A). Arrows indicate significant, discrete peaks identified by the SPP algorithm present in the whole animal and germline datasets. Brackets denote regions where distributed binding occurs. (D) Distribution of mean tag density values (100 bp bins, input-subtracted) for SNPC-4:GFP across 21U-RNA genes and other ncRNA gene classes for the tissue-specific ChIP-seq datasets. Tag density bins were filtered for positive values, and were not normalized between datasets. (E) Average tag density excluding significant peaks was calculated across Chr IV in order to quantify the extent of distributed binding inside and outside 21U-RNA clusters by SNPC-4:GFP. Tag density values represent 100 bp bins with input subtracted and normalized to average tag density for the entire dataset. Statistically significant differences between inside and outside 21U-RNA clusters within each dataset are marked with asterisks (****p ≤ 0.0001, Welch’s t-test).
SNPC-4 exhibits two distinct forms of binding in the 21U-RNA domains that we have termed “discrete” and “distributed”. Discrete binding sites are statistically significant, clearly defined peaks typical of a sequence-specific transcription factor, whereas distributed binding is below significance, but clearly above background levels. Discrete peaks occurred at an interval of about 28 kb, whereas 21U-RNA genes are typically spaced 200–300 bp apart (Batista et al., 2008), such that the vast majority of 21U-RNAs are distant from discrete SNPC-4 peaks. Moreover, these peaks did not preferentially associate with the subset of loci that produce the most abundant 21U-RNAs (e.g. Figure 4C), suggesting that 21U-RNA expression is not directly linked to discrete SNPC-4 binding events. Strikingly however, the broad, distributed SNPC-4:GFP binding across both 21U-RNA clusters was much stronger in the whole animal and germline datasets than in the soma dataset (Figure 4C).
To better characterize the SNPC-4 binding pattern across individual 21U-RNA genes, we determined the mean signal intensity (tag density) of SNPC-4 occupancy spanning one kilobase upstream and downstream from the transcription start site of 21U-RNAs, as well as other classes of structural ncRNAs for comparison, within a given dataset. SNPC-4 was evenly bound across 21U-RNA loci, with no preferential accumulation at any specific feature, whereas SNPC-4 binding at the other classes of structural RNA genes was strongly centered at their transcription start sites (Figure 4D and S4E). Moreover, detectable binding across 21U-RNA genes was apparent in the whole animal and germline datasets, but at baseline levels in the soma. To confirm that the distributed SNPC-4 binding pattern at 21U-RNAs genes was specific to the 21U-RNA clusters, we measured the signal intensity for whole animal, germline, and soma SNPC-4 datasets across Chr IV, excluding intervals that correspond to discrete, significant peaks. The mean signal level of distributed binding within the 21U-RNA domains was >12-fold higher than baseline in the whole animal and germline datasets (p < 0.0001), whereas the soma dataset is equivalent to baseline (p ≥ 0.05) (Figure 4E). This distributed binding pattern was not detected on Chr IV outside the 21U-RNA regions in any sample (p ≥ 0.05). In sum, SNPC-4 exhibits specialized chromatin-binding properties that are specific to the 21U-RNA domains and to the germ line.
SNPC-4 is required for normal expression of mature 21U-RNAs
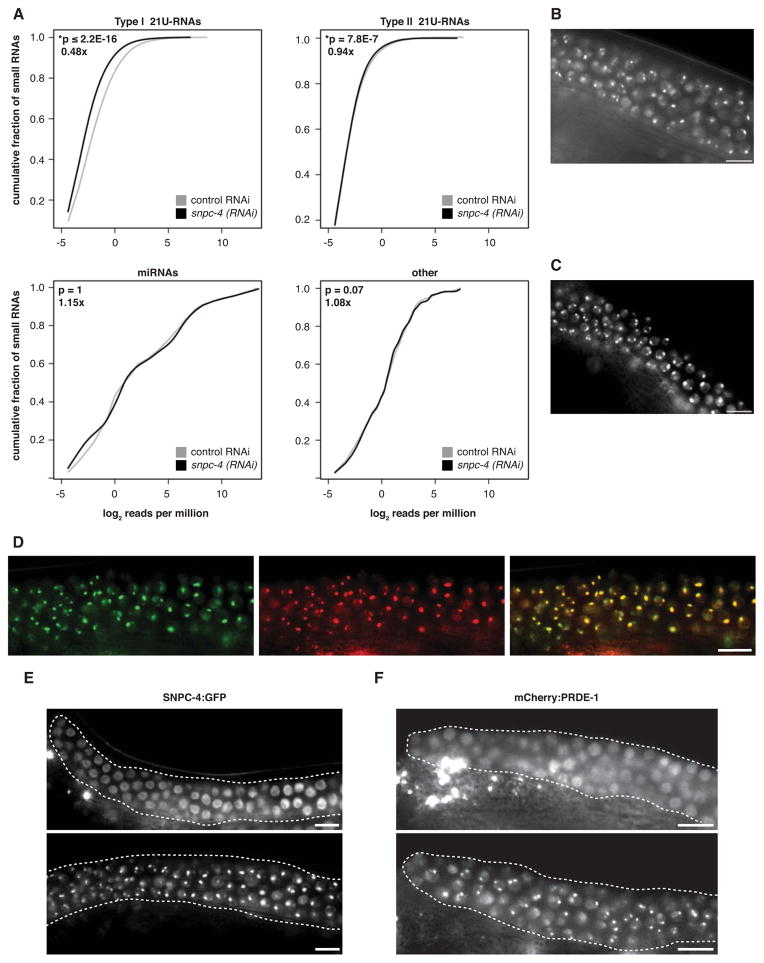
The germline-specific SNPC-4:GFP binding properties at 21U-RNA loci suggested that SNPC-4 might regulate 21U-RNA expression in the germ line. Investigating this possibility was complicated by the fact that SNPC-4 is required for germ cell viability (Figure S5A), precluding investigation of 21U-RNA levels in its absence. To bypass this limitation, we treated L1 larval animals with snpc-4(RNAi) to achieve a partial reduction of snpc-4 expression in adults (typically ~60–70% reduction of mRNA levels; Figure S5B–C), which did not result in obvious morphological germ cell defects or a significant decrease in progeny production (Figure S5D). Levels of an individual 21U-RNA (21ur-1) were reduced in snpc-4(RNAi) animals compared to controls by northern analysis (Figure S5E).
Using these conditions, we performed high throughput sequencing of small RNA populations from control and snpc-4(RNAi) young adults (Supplemental Table 3). Reads mapping to Type I 21U-RNA loci decreased 52% upon snpc-4(RNAi) (p ≤ 2.2 × 10−16), whereas reads corresponding to miRNAs (p = 1) or other types of small ncRNAs (p = 0.9) were unaffected, indicating that reduced 21U-RNA abundance is not simply a consequence of defects in germline development that broadly affect small RNA production (Figure 5A). The vast majority of Type I 21U-RNAs with detectable expression in the control sample decreased in the snpc-4(RNAi) sample, demonstrating that this reduction affects 21U-RNA levels globally. Intriguingly, this effect was limited to clustered Type I 21U-RNAs; unclustered Type II RNAs were minimally affected, decreasing only 6% (p = 7.8 × 10−7; Figure 5A). We subsequently validated the small RNA deep-sequencing results by quantitative reverse transcription polymerase chain reaction (qRT-PCR), and showed that expression was significantly reduced for four out of five Type I 21U-RNAs tested in animals treated with snpc-4(RNAi) compared to control (Figure S5F).
Figure 5. SNPC-4 functions early in biogenesis to promote normal levels of mature 21U-RNAs.
(A) Global levels of Type I and Type II 21U-RNA, miRNA, and other small RNA reads relative to the total number of reads (reads per million) in young adult animals treated with snpc-4(RNAi) (black) compared to vector control RNAi (gray). P values listed in the top left corner of each panel were determined by Wilcoxon-signed rank test. Fold expression differences between snpc-4(RNAi) relative to control RNAi are listed below P values. (B–C) SNPC-4:GFP focal pattern in prg-1(tm0872) (B) and unc-130(ev505) (C) young adult germ nuclei. (D) Co-localization of SNPC-4:GFP (green) with mCherry:PRDE-1 (red) in young adult germ nuclei. (E) SNPC-4:GFP expression in prde-1(mj207) and wildtype (prde-1(+)) young adult gonads. (F) Image depicts a young adult gonad expressing mCherry:PRDE-1 that was strongly affected by snpc-4(RNAi) (top) compared to a representative gonad treated with control RNAi (bottom). We increased RNAi strength to achieve more complete loss of snpc-4 activity (e.g. Figure S5I), disrupting germ cell proliferation slightly. RNAi of several other genes that decrease germ cell proliferation did not disrupt mCherry:PRDE-1 foci (Figure S5J). Scale bars are 10 μm.
Concentration of SNPC-4 at 21U-RNA loci depends upon PRDE-1
To determine whether SNPC-4 might act early, late, or indirectly in the generation of 21U-RNAs, we tested whether it interacted with other known biogenesis pathway components, specifically PRG-1, UNC-130 and PRDE-1. PRG-1, the C. elegans ortholog of Piwi, is required for the presence of mature 21U-RNAs, and presumably acts late in their biogenesis and/or function in germ granules (Batista et al., 2008; Das et al., 2008; Wang and Reinke, 2008). In prg-1(tm0872) mutants, SNPC-4:GFP foci were still present (Figure 5B) and SNPC-4:GFP binding still occurred at target sites residing in both 21U-RNA clusters, as well as at an snRNA gene (Figure S5G). These data suggest that SNPC-4 might act prior to PRG-1 in 21U-RNA production.
Next, we examined whether SNPC-4 genetically interacts with UNC-130, one of several members of the C. elegans FKH family of transcription factors implicated in the regulation of clustered 21U-RNA loci. UNC-130 binds the 21U-RNA upstream regulatory sequence in vivo, and unc-130(ev505) mutants have a partial reduction in the levels of select 21U-RNAs (Cecere et al., 2012). SNPC-4:GFP foci were still present in unc-130 mutant germ lines, indicating that UNC-130 is not essential for their formation (Figure 5C). Moreover, qRT-PCR analysis showed that disrupting snpc-4 activity still led to decreased 21U-RNA levels in unc-130 mutants (Figure S5H). These data suggest that SNPC-4 contributes to normal levels of 21U-RNA expression separately from UNC-130.
By contrast to PRG-1 and UNC-130, we did uncover a clear interaction with the early biogenesis factor, PRDE-1. PRDE-1 is a novel germline-specific factor required for the production of both precursor and mature Type I 21U-RNAs but not Type II 21U-RNAs (Weick et al., 2014). Like SNPC-4, PRDE-1 is concentrated in subnuclear foci on Chr IV that likely correspond to 21U-RNA domains (Weick et al., 2014). We found that SNPC-4:GFP and mCherry:PRDE-1 foci co-localize and exhibit essentially identical patterns during germ cell differentiation (Figure 5D). Strikingly, SNPC-4:GFP foci were dependent upon PRDE-1. In prde-1(mj207) mutants, SNPC-4:GFP expression became diffuse in the nucleus, no longer coalescing into prominent foci in any part of the germ line (Figure 5E). Conversely, mCherry:PRDE-1 foci were similarly disrupted in affected snpc-4(RNAi) animals (Figure 5F and S5I). Taken together, these data indicate that SNPC-4 and PRDE-1 co-localize to germline-specific foci in a mutually dependent fashion. Thus, SNPC-4 specifically and profoundly affects a factor that functions during the initial steps of 21U-RNA biogenesis, and vice versa, indicating that SNPC-4 is also an early-acting factor.
Both discrete and distributed SNPC-4 binding in the 21U-RNA region requires PRDE-1
The interaction between SNPC-4 and PRDE-1 foci led us to examine whether PRDE-1 was required for SNPC-4:GFP binding activity. ChIP-seq analysis of SNPC-4:GFP in the prde-1 mutant background (Supplemental Table 2) demonstrated that both discrete and distributed SNPC-4:GFP binding was greatly diminished in the 21U-RNA domains, while 93% (142/152) of SNAPc target loci such as snRNAs and other structural small RNAs, were retained in prde-1 mutants genome-wide (Figure 6A–C; Figure S6A). Quantification of these data demonstrates that discrete SNPC-4 binding events were no longer preferentially enriched on Chr IV in the absence of prde-1 (Figure 6D), and within 21U-RNA domains, there were 1.6-fold fewer peaks than expected in prde-1 mutants compared to wildtype (p = 6.5 × 10−52; Figure 6E). Additionally, distributed SNPC-4:GFP binding in the 21U-RNA domains was reduced to levels observed outside the domains when prde-1 activity was lost (Figure 6F).
Figure 6. SNPC-4 binding at 21U-RNA domains is diminished in prde-1 mutants.
(A–C) Young adult, whole animal SNPC-4:GFP ChIP-seq binding profiles for combined replicates across Chr IV (A), within in the 3.7 Mb 21U-RNA cluster (B), and at U6 snRNA genes (C) in wildtype (prde-1(+)) (purple) and prde-1 mutant (gray) backgrounds. Annotated gene tracks as in Figure 4A. Arrows indicate highly significant, discrete peaks (SPP enrichment signal value ≥ 1000) in wildtype, whereas brackets denote distributed binding. (D–E) Distribution of discrete SNPC-4:GFP binding sites across chromosomes (D) and on Chr IV (E) relative to genomic length for wildtype and prde-1 mutant ChIP-seq datasets. Statistically significant deviations greater than the expected value of 1 are indicated by asterisks (**p ≤ 0.01 and ****p ≤ 0.0001, Pearson’s chi-square test). (F) SNPC-4:GFP distributed binding as calculated in Figure 4E for wildtype and prde-1 mutants. To reduce possible SNPC-4 overexpression from the SNPC-4:GFP transgene (Figure S6D), ChIP-seq datasets associated with this figure (and Figure 7G) were generated using strains that lack the endogenous snpc-4 locus (YL487 and YL551).
Although depletion of SNPC-4 binding within 21U-RNA domains was profound in prde-1 mutants, it was not absolute; 19% of discrete SNPC-4:GFP peaks remained in the absence of prde-1 (e.g. Figure 6B). Discrete SNPC-4:GFP peaks in prde-1 mutants were still significantly enriched inside compared to outside 21U-RNA domains, but binding strength was often diminished (Figure 6E and S6B). Moreover, retained sites are enriched for the canonical PSE binding sequence (E = 5.9 × 10−348), despite the fact that most (53/59) of these PSE-enriched sites do not correspond to structural small RNA genes. These observations suggest that at least a subset of SNPC-4 binding events might occur independently of PRDE-1 in these regions (Figure S6C). In summary, both classes of SNPC-4 binding in the 21U-RNA domains, discrete and distributed, are largely dependent on prde-1 activity. These results further link enriched SNPC-4 binding at 21U-RNA regions with the regulation of 21U-RNA biogenesis.
Co-occupancy of SNPC-4 and Pol III within 21U-RNA clusters often occurs at tRNA genes
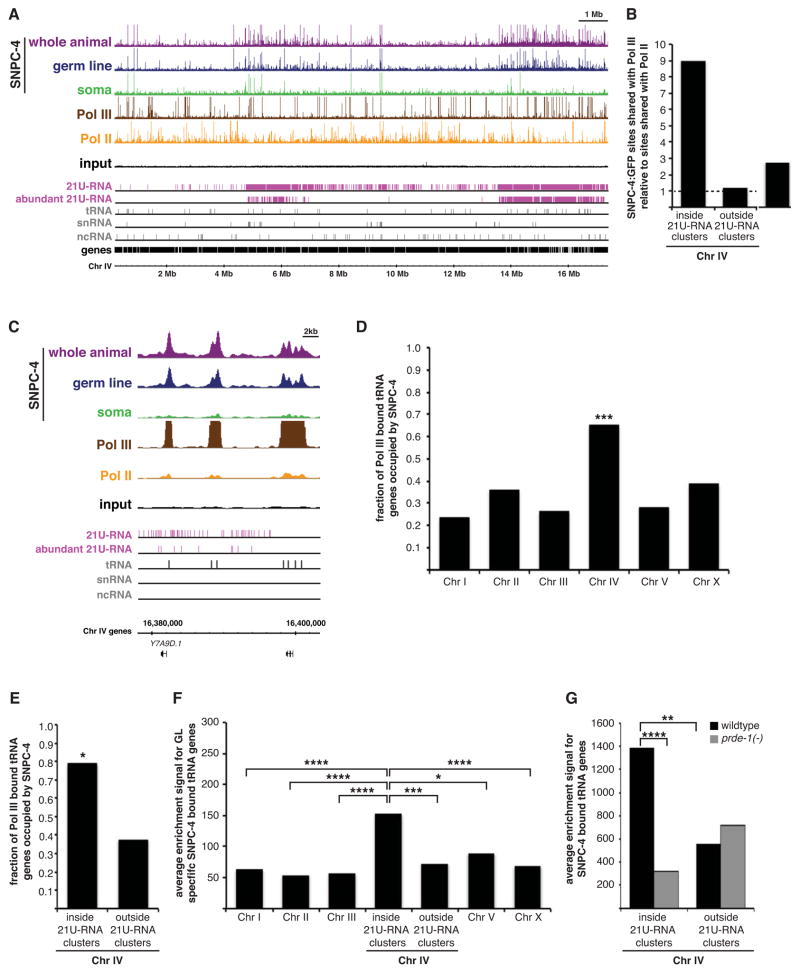
The co-dependency between SNPC-4 and PRDE-1, an early-acting factor in the 21U-RNA biogenesis pathway, strongly suggests that SNPC-4 also acts at a very early step in this process. SNAPc can interact with either Pol II or Pol III to activate its established ncRNA gene targets (Yoon et al., 1995). Therefore, we investigated the co-occupancy of Pol II and Pol III with SNPC-4 at 21U-RNA clusters to better understand how SNPC-4 may regulate 21U-RNA expression. For this analysis, we utilized published Pol II (AMA-1) ChIP-seq data (Gerstein et al., 2010), and generated ChIP-seq data for the large subunit of Pol III (RPC-1) in young adult whole animals (Figure 7A and Supplemental Table 2). When we quantified the frequency of discrete binding sites shared between the SNPC-4:GFP and polymerase datasets, we discovered that SNPC-4 occupied Pol III-bound sites nine-fold more frequently than Pol II-bound sites within 21U-RNA clusters (Figure 7B). By contrast, co-occupancy of SNPC-4 was roughly equivalent between polymerases in regions outside 21U-RNA domains on Chr IV and on other autosomes (Figure 7B and S7A). Because convincing evidence indicates that Pol II promotes transcription of individual 21U-RNAs (Gu et al., 2012; Weick et al., 2014), we examined whether the association between SNPC-4 and Pol III might influence aspects of 21U-RNA production other than transcription initiation.
Figure 7. SNPC-4 co-localizes with Pol III within 21U-RNA clusters, and often at tRNA genes.
(A) Young adult SNPC-4:GFP ChIP-seq binding profiles for combined replicates across Chr IV and annotated ncRNA gene tracks as described in Figure 4A, as well as young adult, whole animal ChIP-seq binding profiles for combined replicates of Pol III (brown) and Pol II (yellow). (B) Ratio of significant SNPC-4:GFP sites shared with Pol III compared to sites shared with Pol II inside and outside 21U-RNA clusters on Chr IV. (C) SNPC-4:GFP and RNA polymerase ChIP-seq binding profiles as described in (A) at tRNA loci located within the 3.7 Mb 21U-RNA cluster. One gene is annotated for reference. (D–E) Fraction of Pol III-bound tRNAs occupied by SNPC-4 across chromosomes (D) and inside and outside 21U-RNA clusters on Chr IV (E) in the whole animal. Statistical significance was determined by comparing observed versus expected number of occurrences (*p ≤ 0.05 and ***p ≤ 0.001, Pearson’s chi-square test). (F–G) Graphs depict average SNPC-4:GFP binding strength of significant peaks (enrichment signal as determined by the SPP algorithm) at SNPC-4:GFP-occupied tRNA genes for germline-specific (F) and for whole animal (G) targets in wildtype and prde-1 mutant backgrounds. Statistically significant differences between inside 21U-RNA clusters and other genomic regions are marked with asterisks (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001, Student’s t-test).
We noted that many (but not all) discrete SNPC-4 sites co-bound by Pol III are unexpectedly associated with tRNA genes (Figure 7C), which are not canonical targets of SNAPc (James Faresse et al., 2012). SNPC-4/Pol III co-occupancy at tRNA genes was greatest on Chr IV compared to other chromosomes (p = 0.0002; Figure 7D), and twice as frequent inside 21U-RNA clusters compared to outside those clusters (p = 0.03; Figure 7E), even though overall Pol III binding at tRNA genes was randomly distributed genome-wide (Figure S7B–C). The average strength of germline-specific SNPC-4 binding at occupied tRNA genes was significantly greater inside 21U-RNA domains compared to other regions on Chr IV and on other chromosomes (Figure 7F and S7D). Intriguingly, in prde-1 mutants, 28% (8/29) of tRNA genes were no longer bound by SNPC-4, and SNPC-4 binding strength at remaining tRNA genes was vastly reduced (Figure 6B and 7G), suggesting that PRDE-1 might also be important for this activity. Finally, sequences enriched in the strongest SNPC-4 peaks included known tRNA gene regulatory elements (the A and B box), which were primarily concentrated on Chr IV in the whole animal and germline datasets (E ≤ 7.1 × 10−108), but not the somatic dataset (Figure S7E–G). By contrast, the consensus SNAPc element, the PSE, was over-represented in all datasets (E ≤ 7.0 × 10−327).
Altogether, these results demonstrate a germline-specific association of SNPC-4 with Pol III at tRNA genes, a non-canonical target of SNAPc. tRNA genes, often in conjunction with partially or fully assembled Pol III complexes, have been previously implicated in chromatin organization in yeast and in humans (Donze, 2012), suggesting that the presence of SNPC-4 at these loci might contribute to the spatial organization of 21U-RNA domains in the germ line (see Discussion).
DISCUSSION
The piRNA clusters of C. elegans are remarkable because they invoke the coordinated, tissue-specific regulation of thousands of individual noncoding 21U-RNA genes over a large region that contains many intervening coding genes with diverse expression patterns. Here we used genome-scale analyses to provide evidence that SNPC-4 promotes the abundant expression of 21U-RNAs. Specifically in the germ line, SNPC-4 localizes to subnuclear foci and exhibits concentrated binding across the two 21U-RNA-rich domains on Chr IV. To our knowledge, extended occupancy of such large, contiguous chromosomal regions has not been described for other sequence-specific transcription factors, except for components involved in X chromosome dosage compensation (Ercan et al., 2007; Pferdehirt et al., 2011). Given the tissue-specific SNPC-4 binding profile, the mutual dependency with PRDE-1, and the reduction of mature 21U-RNA levels upon decreased SNPC-4 activity, the simplest interpretation of our data is that SNPC-4 acts at an early step in Type I 21U-RNA biogenesis, perhaps facilitating transcription and/or early processing events. Thus, a factor known to act at promoters to regulate transcription of diverse essential small ncRNA genes can adopt germline-specific properties to globally and uniformly regulate thousands of individually-transcribed 21U-RNA genes across large domains.
Strikingly, our data also provide a second instance in which a Myb-like factor functions in piRNA regulation, as the A-MYB protein binds and regulates mouse pachytene piRNA clusters (Li et al., 2013). Unlike SNPC-4, A-MYB does not display distributed binding and is likely acting primarily as a sequence-specific transcription factor. However, the repeated involvement of a Myb-related protein in piRNA regulation, despite the many differences in piRNA cluster organization and mechanism of transcription between worm and mouse, suggests an ancient regulatory relationship.
Trans-acting factors contributing to germline-specific SNPC-4 function
Constitutive binding of SNPC-4 at most target genes raises the question of how its developmentally-regulated germline-specific function at the two 21U-RNA clusters is achieved. Our discovery that SNPC-4 depends upon PRDE-1 for this binding pattern provides one likely mechanism for this specificity, as PRDE-1 is expressed only in the germ line, and has no obvious function beyond 21U-RNA regulation. Given the mutual dependency of each protein on the other for recruitment to the same germline-specific foci, it is possible that both proteins must be present for the spreading of SNPC-4 (and possibly PRDE-1) across the 21U-RNA domains. However, there are at least some SNPC-4 binding sites in the 21U-RNA domains that are PRDE-1-independent, and that contain the known SNAPc binding element, the PSE. Therefore, it is possible that SNPC-4 uses a subset of these sites to initiate binding and the germline-specific presence of PRDE-1 permits spreading throughout the domain. Experiments investigating the PRDE-1 binding pattern in comparison to that of SNPC-4, and whether it changes upon loss of SNPC-4 activity, will further enlighten our understanding of this important regulatory relationship.
Other mechanisms that could contribute to the germline-specific properties of SNPC-4 may involve other transcription factors. C. elegans encodes eight proteins (SNPC-1.1–1.4 and SNPC-3.1–3.4; see Supplemental Table 1) orthologous to the two mammalian SNAPc subunits that provide ancillary DNA contacts to support SNAPC4 binding (Li et al., 2004). One or more of these eight proteins might be expressed specifically in the germ line and modulate SNAPc binding in 21U-RNA-rich regions. However, whether SNPC-4 is acting as part of a canonical SNAPc complex within the 21U-RNA domains, or has been co-opted to function via a completely different mechanism, is unknown. Additionally, currently unidentified factor(s) that bind to the 21U-RNA upstream regulatory sequence only in the germ line might also facilitate SNPC-4 binding across the 21U-RNA domains in that tissue.
Differential binding patterns of SNPC-4 and potential chromatin interactions
SNPC-4 exhibits both discrete and distributed binding events within the 21U-RNA regions in the germ line. However, neither form of binding is enriched at any feature of the 21U-RNA locus, including the upstream 21U-RNA motif, transcription start site, or 5′ nucleotide. Moreover, ~99% of 21U-RNA loci are more than 500 bp from discrete binding sites, and proximity of these sites does not correlate with 21U-RNA abundance. These observations, along with pervasive, low-level SNPC-4 binding that coats the 21U-RNA domains, suggest that SNPC-4 might facilitate the coordinated, germline-specific expression of 21U-RNAs by affecting the genomic organization of the entire domain rather than separately regulating individual 21U-RNA loci.
One possible mechanism for promoting such an organization is suggested by the presence of multiple SANT domains within SNPC-4. SANT domains are commonly found in components of chromatin modifying complexes, such as Ada2, which promotes histone acetylation, and SmRT, a co-repressor that promotes deacetylation (Boyer et al., 2004). SANT domains can interact with histone tails and stabilize their conformation, potentially facilitating presentation of the tail to an associated enzymatic activity. Biochemical studies of SNAPc in other organisms indicate that it interacts with nucleosomes at snRNA promoters (Zhao et al., 2001). Thus, the SANT domains of SNPC-4 might influence chromatin state in the 21U-RNA regions through modulating nucleosome organization and/or histone modifications. Intriguingly, data collected from young adult animals suggest that the 21U-RNA regions have decreased nucleosome density (Cecere et al., 2012). The ability to analyze multiple properties of chromatin, including histone modifications, in the germ line separately from the soma will be necessary to directly explore the relationship between the chromatin state, SNPC-4, and 21U-RNA expression.
Another mechanism by which SNPC-4 could contribute to the genomic organization of 21U-RNA domains is through the interaction with Pol III and tRNAs in the 21U-RNA domains. This association was not expected a priori, because tRNA genes are regulated by Pol III through an internal A/B box motif and not via SNAPc (Canella et al., 2010). Intriguingly, Pol III and tRNA loci have been implicated in various large-scale gene regulatory processes, including enhancer blocking, insulator activity, and establishment of chromatin boundaries (Donze, 2012). A current hypothesis is that expression from tRNA loci disrupts the spread of heterochromatin through “transcriptional interference”, or by serving as entry sites for chromatin remodeling activities. Additionally, proteins that influence chromatin loop formation, such as cohesin, are often found in association with components of Pol III at tRNA genes and additional non-tRNA sites (Moqtaderi et al., 2010). Therefore, the tissue-specific association of SNPC-4 with RNA Pol III at tRNA genes and other genomic sites might promote the arrangement of the 21U-RNA-rich chromosomal domains into a conformation or location that is permissive for 21U-RNA expression, specifically in the germ line. For example, SNPC-4 may utilize Pol-III occupied tRNA genes as specific chromatin entry sites to facilitate its dispersion across the 21U-RNA domain, or as contact points to help establish particular chromatin loop structures in order to facilitate robust 21U-RNA expression. Alternatively, interaction with tRNA genes might help to position 21U-RNA clusters at the nuclear periphery, as has been recently shown for the association of the nuclear pore protein NPP-13 with tRNA and snoRNA genes (Ikegami and Lieb, 2013). Thus, SNPC-4 foci might be found near nuclear pores via interaction with Pol III and tRNA genes, aiding in either permissive chromatin conformation, post-transcriptional processing events of 21U-RNAs, and/or transport of 21U-RNAs to germ granules.
CONCLUSION
The mechanisms that translate information from chromatin-associated regulatory factors and sequence elements to the epigenetic states and three-dimensional genomic domains to govern gene expression are still mysterious. The unusual pattern and remarkable specificity of SNPC-4 binding at 21U-RNA domains suggest that the genomic clustering of these individually transcribed but coordinately regulated genes contributes to their robust and germline-specific expression. This system provides a unique and exciting opportunity to dissect how regulation of defined genomic domains is achieved.
EXPERIMENTAL PROCEDURES
Detailed methods are available in Supplemental Experimental Procedures.
C. elegans strains
Strains were maintained at 20°C using standard methods unless otherwise noted (Brenner, 1974). N2 Bristol was used as a wildtype strain. See Supplemental Experimental Procedures for transgenic strain construction and all strains used in this study.
SNPC-4:GFP foci analysis
Young adult SNPC-4:GFP gonads were antibody stained with a 1:100 dilution of rabbit α-GFP antibody (BD BioSciences, San Jose, CA), 1:100 dilution of Alexa Fluor 488 goat α-rabbit IgG, and 0.5 μg/mL DAPI as described in the Supplemental Experimental Procedures. Immunostained gonad images were subdivided based on nuclear morphology into mitotic, transition, and meiotic zones. SNPC-4 foci number was determined for ten nuclei in each zone for at least five animals per genotype. See Figure S2 for a representative example of foci quantification.
ChIP-sequencing
Worm samples were crosslinked in 2% formaldehyde for 30 minutes. Sonicated extract containing 2 mg of protein was immunoprecipitated as described (Zhong et al., 2010) with 7.5 μg of αGFP antibody (gift from Anthony Hyman) (Zhong et al., 2010) or αPol III antibody (gift from Jason Lieb) (Ikegami and Lieb, 2013) for the majority of samples. For ChIP-seq data comparing wildtype and prde-1 mutant backgrounds, 4 mg of sonicated lysate was immunoprecipitated and prepared for HiSeq sequencing using Ovation Ultralow DR Multiplex System (NuGEN Technologies Inc., San Carlos, CA). Reads were aligned to C. elegans WS220 genome build and significant peaks were identified with SPP and IDR algorithms (Landt et al., 2012). Target calling was performed separately for 21U-RNAs and other gene models (i.e. coding and ncRNAs). The gene in closest proximity to the peak maximum of a binding site was defined as a transcription factor candidate target. See Supplemental Experimental Procedures for detailed protocols for ChIP-seq, as well as downstream analyses such as metagene and motif analysis.
ChIP-qPCR
Chromatin was immunoprecipitated as described above, except control samples were immunoprecipitated with 7.5 μg of goat α-IgG antibody (R&D Systems, Minneapolis, MN). See Supplemental Experimental Procedures for quantitative PCR conditions.
RNA isolation and analysis
L1 animals were fed bacteria expressing RNAi clones from the Ahringer library (Fraser et al., 2000) until collection at the young adult stage. RNAi-treated young adults were harvested in TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was then treated with DNA-free DNAseI (Ambion, Austin, TX).
For small RNA-sequencing, a total of 5 μg of DNAse-treated RNA harvested from snpc-4(RNAi) and wildtype young adults was subjected to the Illumina small RNA v1.5 sample preparation kit and 22–30 bp small RNAs were sequenced (Illumina, San Diego, CA). Trimmed reads were counted within WS235 transcript sets mapped to WS220 coordinates, and normalized to reads per million. Read abundance for sequences present with at least one read in both conditions was analyzed in R using a paired-sample, one sided Wilcoxon signed rank test.
For quantitative RT-PCR, SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) was performed to generate cDNA. qPCR reactions were performed in triplicate for three biological replicates. Small RNA primers were designed as described (Chen et al., 2005; Das et al., 2008). Relative expression levels were quantified using the ΔΔCt method. See Supplemental Experimental Procedures for detailed protocols.
Supplementary Material
Supplemental Figure 1. SNPC-4:GFP rescues the lethality of snpc-4 (tm4568) mutants (related to Figure 1). (A) Annotated gene model for SNPC-4, the C. elegans ortholog to mammalian SNAPC4. The snpc-4 (tm4568) mutant allele contains a deletion that removes portions of the Myb-like/ SANT DNA- and chromatin-binding domains (PFAM ID: PF13921 (blue) and PFAM ID: PF00249 (orange)), resulting in a premature stop codon (*TGA). (B) SNAPC4, the major DNA-binding subunit of the mammalian SNAPc complex (blue), binds the external promoter of snRNA genes at the proximal sequence element (PSE). (top) SNAPc with the Pol II pre-initiation complex consisting of Pol II (purple) and the multi-subunit TFII complex (pink) promotes the transcription of U1–U5 snRNA genes. (middle) SNAPc with the Pol III pre-initiation complex consisting of Pol III (green) and the multi-subunit TFIIIB complex (light green) initiates the transcription of U6 snRNA genes. (bottom) Independent of SNAPc, Pol III machinery consisting of Pol III, TFIIIB complex, and TFIIIC complex (magenta) binds the internal promoter of tRNA genes at the A box and B box motif to promote their transcription. Images adapted from (Jawdekar and Henry, 2008; (White, 2011). (C) Embryonic lethality of the snpc-4(tm4568) mutant is rescued by SNPC-4:GFP expression. The snpc-4 mutant is balanced by hT2. Parental strains containing the hT2 balancer or SNPC-4:GFP transgene alone are shown for comparison. Average number of dead embryos was assessed for at least 4 adult hermaphrodites per genotype. Error bars represent SD. (D–F) Diffuse SNPC-4:GFP nuclear localization in hermaphrodite somatic tissues, such as the head (D), vulva (E), and tail (F). Brackets indicate representative regions in which somatic, nuclear SNPC-4:GFP is detected. (G) SNPC-4:GFP localizes to sub-nuclear foci in the male germ line (dotted outline). Depicted germ line is in a him-8 mutant background. Arrows point to representative examples of nuclei with SNPC-4:GFP expression. Scale bars are 10 μm.
Supplemental Figure 2. Method of SNPC-4:GFP germline-specific foci quantification (related to Figure 2). Collage of representative images used in the quantification of SNPC-4:GFP foci in meiotic germ nuclei from dissected gonads stained with α-GFP. Numbers mark representative individual nuclei as they progress through thirteen consecutive 0.5 μm confocal slices of a z-stack for each genotype. In wildtype and him-8 mutant (defective Chr X synapsis) germ nuclei, two foci coalesce to a single focus as chromosomes undergo synapsis. Foci fail to coalesce in the meiotic zone of zim-3 mutants, which have defective synapsis for Chr I and Chr IV, as pictured here. Scale bars are 10 μm.
Supplemental Figure 3. SNPC-4:GFP is distributed evenly across other chromosomes (related to Figure 3). (A–B) Graphs depict a comprehensive list of annotated Wormbase ncRNA genes (excluding 21U-RNAs) per kb across chromosomes (A) and inside and outside 21U-RNA clusters on Chr IV (B). (C) SNPC-4:GFP ChIP-seq binding profiles of combined replicates for all the major developmental stages. A representative input from the young adult dataset is shown. Read counts for each ChIP-seq data set are normalized by the total number of reads. Annotated gene tracks include 21U-RNAs (pink) and various other ncRNA classes (grey). The snRNA track includes snRNA as well as sls- and smy- genes. The concentrated binding found on Chr IV was not observed for Chr III (pictured here) or for the other chromosomes.
Supplemental Figure 4. SNPC-4:GFP binding is highly enriched on Chr IV in the germ line (related to Figure 4). (A) SNPC-4:GFP expression is limited to the soma through the use of the temperature sensitive germline deficient glp-1(q224) allele. A representative image of somatic SNPC-4:GFP expression in nuclei of the young adult head (left). The pharynx is outlined in the DIC image as a reference. Outlined young adult gonad lacks germ cells and SNPC-4:GFP expression (right). Scale bars are 10 μm. (B) Localization of germline-expressed SNPC-4:GFP in the young adult hermaphrodite germ nuclei. Scale bar is 10 μm. (C) Distribution of significant SNPC-4:GFP binding sites relative to chromosomal length for all tissue-specific ChIP-seq data sets. Germline-specific SNPC-4 binding sites (teal) consist of germline SNPC-4 sites not present in the soma ChIP-seq data. Statistically significant deviations greater than the expected value of 1 (marked with a black line) are indicated by asterisks (*p ≤ 0.05, **p ≤ 0.01, and ****p ≤ 0.0001, Pearson’s chi-square test). (D) Young adult binding profiles for combined replicates at a representative snRNA gene (U6) when SNPC-4:GFP is expressed broadly (whole animal, purple), specifically in the germ line (navy), and specifically in the soma (green). A representative input from the whole animal dataset is shown. One gene is annotated for reference. (E) Heat maps showing the distribution of input-subtracted tag density values for SNPC-4:GFP across individual 21U-RNA and other ncRNA genes for the tissue-specific ChIP-seq data sets. Genes are ordered by SNPC-4:GFP tag density.
Supplemental Figure 5. SNPC-4 function at 21U-RNA domains is unaffected in prg-1 and unc-130 mutants (related to Figure 5). (A) To determine if SNPC-4 is required for germ cell viability, untagged SNPC-4 was expressed in the soma and not in the germline of snpc-4(tm4568) mutants, likely due to germline-silencing of the transgene (Praitis et al., 2001). For two independent extra-chromosomal transgenic lines, greater than 90% of snpc-4 mutants grew to late larval or adult stages without obvious somatic defects, but were completely sterile and essentially lacked germ cells. Representative image of rescued snpc-4 homozygous mutants, which grew to adulthood and had a small, empty gonads (dotted outline). Scale bar is 10 μm. (B–C) Average fold expression of snpc-4, normalized to act-3 levels and relative to vector control RNAi. Statistically significant differences between vector control RNAi and each RNAi treatment are denoted with an asterisk (*p ≤ 0.05 and **p ≤ 0.01, Student’s t-test). Error bars represent SEM for three biological replicates. (D) Average brood size comparing vector control RNAi and snpc-4(RNAi) for at least ten animals (p ≥ 0.05, Student’s t-test). Error bars represent SD. (E) Northern blot for 21ur-1 in young adult animals using tRNA levels as a loading control. prg-1 mutants lack mature 21U-RNAs and were therefore used as a negative control. (F) Average fold expression of 21U-RNAs, normalized to mir-52 levels and relative to vector control RNAi. Statistically significant differences between vector and RNAi treatment are denoted with asterisks (*p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.01, Student’s t-test). Error bars represent SEM for three biological replicates. (G) Average fold enrichment of SNPC-4:GFP binding relative to IgG control at three binding sites and an unbound intergenic control region on Chr IV in young adult wildtype and prg-1 mutant backgrounds was calculated using ChIP-qPCR. Non-transgenic prg-1 mutants were used as a negative control to show specificity of binding. Statistical analysis was performed by comparing transgenic wildtype with transgenic prg-1 mutant animals for each locus (p ≥ 0.05, Student’s t-test). Error bars represent SEM from three biological replicates. (H) See (F) above. (I) SNPC-4:GFP expression of the same young adult gonad (dotted outline) treated with snpc-4 and control RNAi in Figure 5F. (J) To control for possible indirect effects of germline underproliferation due to increased strength of snpc-4(RNAi), several genes (ddb-1, toe-1, nath-10, and K12H4.3) that cause germline underproliferation for reasons unrelated to piRNA biogenesis (Waters et al., 2010) were examined. SNPC-4:GFP and mCherry:PRDE-1 foci persisted in all genes tested despite exhibiting germline underproliferation. Representative image of a young adult gonad treated with ddb-1(RNAi). Scale bars are 10 μm.
Supplemental Figure 6. SNPC-4:GFP binding sites retained in prde-1 mutants are diminished in strength and are enriched for the PSE motif (related to Figure 6). (A) Young adult, whole animal SNPC-4:GFP ChIP-seq binding profiles for combined replicates across Chr I in wildtype (purple) and prde-1 mutant (gray) backgrounds. A representative input from the wildtype dataset is shown. Annotated tracks as in Figure 4A. The loss of SNPC-4:GFP binding in prde-1 mutants was specific to 21U-RNA domains and was not observed for Chr I (pictured here) or for other chromosomes. (B) Average binding strength (enrichment signal determined by the SPP algorithm) for significant SNPC-4:GFP peaks retained on Chr IV in prde-1 mutants. Statistically significant differences between genotypes and regions inside compared to outside 21U-RNA clusters are marked with an asterisk (***p ≤ 0.001, and ****p ≤ 0.0001, Welch’s t-test). (C) The most significantly enriched sequence associated with significant SNPC-4:GFP binding sites retained in prde-1 mutants in 21U-RNA domains was the SNAPc PSE motif as determined by MEME analysis. (D) Average fold expression of snpc-4 transcript levels, normalized to act-3 levels and relative to N2 (wildtype strain). The mild overexpression of snpc-4 transcripts in the SNPC-4:GFP strain OP179 is reduced to near physiological levels when the endogenous snpc-4 locus is removed (strain YL487). Error bars represent SEM for two biological replicates.
Supplemental Figure 7. SNPC-4:GFP binding with Pol III binding at tRNA genes (related to Figure 7). (A) Ratio of significant SNPC-4:GFP sites shared with Pol III compared to sites shared with Pol II across chromosomes. The high co-occupancy of SNPC-4 with Pol III relative to Pol II on Chr X is likely due to nearly half of all tRNA genes (which are transcribed by Pol III) residing on Chr X, as well as due to the general lack of Pol II transcription from Chr X in germ cells (which contribute significantly to the young adult ChIP-seq sample) (Kelly et al., 2002). (B–C) Fraction of tRNA genes that are occupied by Pol III across chromosomes (B) and inside and outside 21U-RNA domains on Chr IV (C). Statistical significance was determined by comparing observed versus expected number of occurrences (Pearson’s chi-square test, p ≥ 0.05). (D) Average SNPC-4:GFP binding strength of significant peaks (enrichment signal determined by the SPP algorithm) at SNPC-4:GFP occupied tRNA genes in the whole animal. Statistically significant differences between regions inside 21U-RNA clusters and other genomic regions are marked with an asterisk (*p ≤ 0.05, ***p ≤ 0.001, and ****p ≤ 0.0001, Student’s t-test). (E–G) Distribution of the three most significant SNPC-4:GFP binding motifs as determined by MEME relative to chromosome length for all tissue-specific ChIP-seq data sets. Asterisks indicate statistically significant deviations greater than the expected value of 1(marked with a black line) (**p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001, Pearson’s chi-square test).
Table catalogues the mammalian SNAPc units and their orthologous counterparts in C. elegans. The functional description of each subunit is based on mammalian studies of SNAPc (Hung and Stumph, 2011).
This table can be found in a separate Excel file and contains chromosomal coordinates and signal values for peaks determined significant by the SPP algorithm for all ChIP-seq data sets.
Supplemental Table 3. Normalized RNA-seq data for young adult animals treated with snpc-4(RNAi) (related to Figure 5). This table can be found in a separate Excel file and contains raw and normalized read counts for small RNAs present in young adult animals treated with control and snpc-4 RNAi.
Supplemental Table 4. SNPC-4 associated motifs determined by MEME analysis (related to Supplemental Figure 7). This table can be found in a separate Excel file and includes sequences associated with SNPC-4 discrete peaks that were input and output from MEME analysis.
Acknowledgments
We thank the modENCODE consortium, especially Wei Niu, for help with ChIP-sequencing, members from the University of Chicago IGSB for ChIP-seq data processing, LaDeana Hillier for target calling analysis, Tyson Edwards for technical assistance, Jason Lieb and Anthony Hyman for antibodies, Lynn Cooley and Marc Hammarlund for equipment, and Joseph Culotti for the unc-130(ev505) strain. We especially thank Eric Miska and his lab for providing several prde-1 reagents. Some strains were provided by the Caenorhabditis Genetics Center (NIH P40 OD010440). This work was supported by grants from the March of Dimes (FY10–433) and NHGRI (U54HG007002 and U01HG004267). K.E.G. is supported by a grant from the NIGMS (F32GM101778).
Footnotes
ACCESSION NUMBERS
ChIP-seq and RNA-seq experiments are submitted to GEO (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE53412.
Supplemental Information includes seven figures, four tables, Supplemental Experimental Procedures, and Supplemental References.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrey G, Montavon T, Mascrez B, Gonzalez F, Noordermeer D, Leleu M, Trono D, Spitz F, Duboule D. A switch between topological domains underlies HoxD genes collinearity in mouse limbs. Science. 2013;340:1234167. doi: 10.1126/science.1234167. [DOI] [PubMed] [Google Scholar]
- Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi AC, Freeberg MA, Day AM, Chun SY, Khivansara V, Kim JK. A conserved upstream motif orchestrates autonomous, germline-enriched expression of Caenorhabditis elegans piRNAs. PLoS Genet. 2013;9:e1003392. doi: 10.1371/journal.pgen.1003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T. Trans-splicing and operons. WormBook; 2005. pp. 1–9. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Latek RR, Peterson CL. The SANT domain: a unique histone-tail-binding module? Nat Rev Mol Cell Biol. 2004;5:158–163. doi: 10.1038/nrm1314. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canella D, Praz V, Reina JH, Cousin P, Hernandez N. Defining the RNA polymerase III transcriptome: Genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res. 2010;20:710–721. doi: 10.1101/gr.101337.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecere G, Zheng GX, Mansisidor AR, Klymko KE, Grishok A. Promoters recognized by forkhead proteins exist for individual 21U-RNAs. Mol Cell. 2012;47:734–745. doi: 10.1016/j.molcel.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WV, Maniatis T. Clustered protocadherins. Development. 2013;140:3297–3302. doi: 10.1242/dev.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PP, Bagijn MP, Goldstein LD, Woolford JR, Lehrbach NJ, Sapetschnig A, Buhecha HR, Gilchrist MJ, Howe KL, Stark R, et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol. Cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Lee J, Wang H, Miller J, Reik A, Gregory PD, Dean A, Blobel GA. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–1244. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D. Extra-transcriptional functions of RNA Polymerase III complexes: TFIIIC as a potential global chromatin bookmark. Gene. 2012;493:169–175. doi: 10.1016/j.gene.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Ercan S, Giresi PG, Whittle CM, Zhang X, Green RD, Lieb JD. X chromosome repression by localization of the C. elegans dosage compensation machinery to sites of transcription initiation. Nat Genet. 2007;39:403–408. doi: 10.1038/ng1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Gerstein MB, Lu ZJ, Van Nostrand EL, Cheng C, Arshinoff BI, Liu T, Yip KY, Robilotto R, Rechtsteiner A, Ikegami K, et al. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science. 2010;330:1775–1787. doi: 10.1126/science.1196914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Lee HC, Chaves D, Youngman EM, Pazour GJ, Conte D, Jr, Mello CC. CapSeq and CIP-TAP identify Pol II start sites and reveal capped small RNAs as C. elegans piRNA precursors. Cell. 2012;151:1488–1500. doi: 10.1016/j.cell.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Lieb JD. Integral nuclear pore proteins bind to Pol III-transcribed genes and are required for Pol III transcript processing in C. elegans. Mol. Cell. 2013;51:840–849. doi: 10.1016/j.molcel.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu H, Siomi H, Siomi MC. Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012;26:2361–2373. doi: 10.1101/gad.203786.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James Faresse N, Canella D, Praz V, Michaud J, Romascano D, Hernandez N. Genomic study of RNA polymerase II and III SNAPc-bound promoters reveals a gene transcribed by both enzymes and a broad use of common activators. PLoS Genet. 2012;8:e1003028. doi: 10.1371/journal.pgen.1003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawdekar GW, Henry RW. Transcriptional regulation of human small nuclear RNA genes. Biochimica et biophysica acta. 2008;1779:295–305. doi: 10.1016/j.bbagrm.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudron M, Niu W, Lu Z, Wang G, Gerstein M, Snyder M, Reinke V. Tissue-specific direct targets of Caenorhabditis elegans Rb/E2F dictate distinct somatic and germline programs. Genome Biol. 2013;14:R5. doi: 10.1186/gb-2013-14-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S, Bernstein BE, Bickel P, Brown JB, Cayting P, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22:1813–1831. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- Li C, Harding GA, Parise J, McNamara-Schroeder KJ, Stumph WE. Architectural arrangement of cloned proximal sequence element-binding protein subunits on Drosophila U1 and U6 snRNA gene promoters. Mol Cell Biol. 2004;24:1897–1906. doi: 10.1128/MCB.24.5.1897-1906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, Xu J, Moore MJ, Schimenti JC, Weng Z, et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol Cell. 2013;50:67–81. doi: 10.1016/j.molcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luteijn MJ, Ketting RF. PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nat Rev Genet. 2013;14:523–534. doi: 10.1038/nrg3495. [DOI] [PubMed] [Google Scholar]
- Moqtaderi Z, Wang J, Raha D, White RJ, Snyder M, Weng Z, Struhl K. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nat Struct Mol Biol. 2010;17:635–640. doi: 10.1038/nsmb.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Lu ZJ, Zhong M, Sarov M, Murray JI, Brdlik CM, Janette J, Chen C, Alves P, Preston E, et al. Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C. elegans. Genome Res. 2011;21:245–254. doi: 10.1101/gr.114587.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pferdehirt RR, Kruesi WS, Meyer BJ. An MLL/COMPASS subunit functions in the C. elegans dosage compensation complex to target X chromosomes for transcriptional regulation of gene expression. Genes Dev. 2011;25:499–515. doi: 10.1101/gad.2016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CM, Dernburg AF. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev. Cell. 2006;11:817–829. doi: 10.1016/j.devcel.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Sarov M, Murray JI, Schanze K, Pozniakovski A, Niu W, Angermann K, Hasse S, Rupprecht M, Vinis E, Tinney M, et al. A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell. 2012;150:855–866. doi: 10.1016/j.cell.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe JP, Lin H. Small noncoding RNAs in the germline. Cold Spring Harb Perspect Biol. 2011;3:a002717. doi: 10.1101/cshperspect.a002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Montgomery TA, Qi Y, Ruvkun G. High-throughput sequencing reveals extraordinary fluidity of miRNA, piRNA, and siRNA pathways in nematodes. Genome Res. 2013;23:497–508. doi: 10.1101/gr.149112.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Song Y, Wang Y, Jessop L, Zhan L, Stumph WE. Characterization of a Drosophila proximal-sequence-element-binding protein involved in transcription of small nuclear RNA genes. European journal of biochemistry / FEBS. 1997;248:231–237. doi: 10.1111/j.1432-1033.1997.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr Biol. 2008;18:861–867. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick EM, Sarkies P, Silva N, Chen RA, Moss SM, Cording AC, Ahringer J, Martinez-Perez E, Miska EA. PRDE-1 is a nuclear factor essential for the biogenesis of Ruby motif-dependent piRNAs in C. elegans. Genes Dev. 2014;28:783–796. doi: 10.1101/gad.238105.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MW, Henry RW, Ma B, Kobayashi R, Klages N, Matthias P, Strubin M, Hernandez N. The large subunit of basal transcription factor SNAPc is a Myb domain protein that interacts with Oct-1. Mol Cell Biol. 1998;18:368–377. doi: 10.1128/mcb.18.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JB, Murphy S, Bai L, Wang Z, Roeder RG. Proximal sequence element-binding transcription factor (PTF) is a multisubunit complex required for transcription of both RNA polymerase II- and RNA polymerase III-dependent small nuclear RNA genes. Mol Cell Biol. 1995;15:2019–2027. doi: 10.1128/mcb.15.4.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Pendergrast PS, Hernandez N. A positioned nucleosome on the human U6 promoter allows recruitment of SNAPc by the Oct-1 POU domain. Mol Cell. 2001;7:539–549. doi: 10.1016/s1097-2765(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Zhong M, Niu W, Lu ZJ, Sarov M, Murray JI, Janette J, Raha D, Sheaffer KL, Lam HY, Preston E, et al. Genome-wide identification of binding sites defines distinct functions for Caenorhabditis elegans PHA-4/FOXA in development and environmental response. PLoS Genet. 2010;6:e1000848. doi: 10.1371/journal.pgen.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. SNPC-4:GFP rescues the lethality of snpc-4 (tm4568) mutants (related to Figure 1). (A) Annotated gene model for SNPC-4, the C. elegans ortholog to mammalian SNAPC4. The snpc-4 (tm4568) mutant allele contains a deletion that removes portions of the Myb-like/ SANT DNA- and chromatin-binding domains (PFAM ID: PF13921 (blue) and PFAM ID: PF00249 (orange)), resulting in a premature stop codon (*TGA). (B) SNAPC4, the major DNA-binding subunit of the mammalian SNAPc complex (blue), binds the external promoter of snRNA genes at the proximal sequence element (PSE). (top) SNAPc with the Pol II pre-initiation complex consisting of Pol II (purple) and the multi-subunit TFII complex (pink) promotes the transcription of U1–U5 snRNA genes. (middle) SNAPc with the Pol III pre-initiation complex consisting of Pol III (green) and the multi-subunit TFIIIB complex (light green) initiates the transcription of U6 snRNA genes. (bottom) Independent of SNAPc, Pol III machinery consisting of Pol III, TFIIIB complex, and TFIIIC complex (magenta) binds the internal promoter of tRNA genes at the A box and B box motif to promote their transcription. Images adapted from (Jawdekar and Henry, 2008; (White, 2011). (C) Embryonic lethality of the snpc-4(tm4568) mutant is rescued by SNPC-4:GFP expression. The snpc-4 mutant is balanced by hT2. Parental strains containing the hT2 balancer or SNPC-4:GFP transgene alone are shown for comparison. Average number of dead embryos was assessed for at least 4 adult hermaphrodites per genotype. Error bars represent SD. (D–F) Diffuse SNPC-4:GFP nuclear localization in hermaphrodite somatic tissues, such as the head (D), vulva (E), and tail (F). Brackets indicate representative regions in which somatic, nuclear SNPC-4:GFP is detected. (G) SNPC-4:GFP localizes to sub-nuclear foci in the male germ line (dotted outline). Depicted germ line is in a him-8 mutant background. Arrows point to representative examples of nuclei with SNPC-4:GFP expression. Scale bars are 10 μm.
Supplemental Figure 2. Method of SNPC-4:GFP germline-specific foci quantification (related to Figure 2). Collage of representative images used in the quantification of SNPC-4:GFP foci in meiotic germ nuclei from dissected gonads stained with α-GFP. Numbers mark representative individual nuclei as they progress through thirteen consecutive 0.5 μm confocal slices of a z-stack for each genotype. In wildtype and him-8 mutant (defective Chr X synapsis) germ nuclei, two foci coalesce to a single focus as chromosomes undergo synapsis. Foci fail to coalesce in the meiotic zone of zim-3 mutants, which have defective synapsis for Chr I and Chr IV, as pictured here. Scale bars are 10 μm.
Supplemental Figure 3. SNPC-4:GFP is distributed evenly across other chromosomes (related to Figure 3). (A–B) Graphs depict a comprehensive list of annotated Wormbase ncRNA genes (excluding 21U-RNAs) per kb across chromosomes (A) and inside and outside 21U-RNA clusters on Chr IV (B). (C) SNPC-4:GFP ChIP-seq binding profiles of combined replicates for all the major developmental stages. A representative input from the young adult dataset is shown. Read counts for each ChIP-seq data set are normalized by the total number of reads. Annotated gene tracks include 21U-RNAs (pink) and various other ncRNA classes (grey). The snRNA track includes snRNA as well as sls- and smy- genes. The concentrated binding found on Chr IV was not observed for Chr III (pictured here) or for the other chromosomes.
Supplemental Figure 4. SNPC-4:GFP binding is highly enriched on Chr IV in the germ line (related to Figure 4). (A) SNPC-4:GFP expression is limited to the soma through the use of the temperature sensitive germline deficient glp-1(q224) allele. A representative image of somatic SNPC-4:GFP expression in nuclei of the young adult head (left). The pharynx is outlined in the DIC image as a reference. Outlined young adult gonad lacks germ cells and SNPC-4:GFP expression (right). Scale bars are 10 μm. (B) Localization of germline-expressed SNPC-4:GFP in the young adult hermaphrodite germ nuclei. Scale bar is 10 μm. (C) Distribution of significant SNPC-4:GFP binding sites relative to chromosomal length for all tissue-specific ChIP-seq data sets. Germline-specific SNPC-4 binding sites (teal) consist of germline SNPC-4 sites not present in the soma ChIP-seq data. Statistically significant deviations greater than the expected value of 1 (marked with a black line) are indicated by asterisks (*p ≤ 0.05, **p ≤ 0.01, and ****p ≤ 0.0001, Pearson’s chi-square test). (D) Young adult binding profiles for combined replicates at a representative snRNA gene (U6) when SNPC-4:GFP is expressed broadly (whole animal, purple), specifically in the germ line (navy), and specifically in the soma (green). A representative input from the whole animal dataset is shown. One gene is annotated for reference. (E) Heat maps showing the distribution of input-subtracted tag density values for SNPC-4:GFP across individual 21U-RNA and other ncRNA genes for the tissue-specific ChIP-seq data sets. Genes are ordered by SNPC-4:GFP tag density.
Supplemental Figure 5. SNPC-4 function at 21U-RNA domains is unaffected in prg-1 and unc-130 mutants (related to Figure 5). (A) To determine if SNPC-4 is required for germ cell viability, untagged SNPC-4 was expressed in the soma and not in the germline of snpc-4(tm4568) mutants, likely due to germline-silencing of the transgene (Praitis et al., 2001). For two independent extra-chromosomal transgenic lines, greater than 90% of snpc-4 mutants grew to late larval or adult stages without obvious somatic defects, but were completely sterile and essentially lacked germ cells. Representative image of rescued snpc-4 homozygous mutants, which grew to adulthood and had a small, empty gonads (dotted outline). Scale bar is 10 μm. (B–C) Average fold expression of snpc-4, normalized to act-3 levels and relative to vector control RNAi. Statistically significant differences between vector control RNAi and each RNAi treatment are denoted with an asterisk (*p ≤ 0.05 and **p ≤ 0.01, Student’s t-test). Error bars represent SEM for three biological replicates. (D) Average brood size comparing vector control RNAi and snpc-4(RNAi) for at least ten animals (p ≥ 0.05, Student’s t-test). Error bars represent SD. (E) Northern blot for 21ur-1 in young adult animals using tRNA levels as a loading control. prg-1 mutants lack mature 21U-RNAs and were therefore used as a negative control. (F) Average fold expression of 21U-RNAs, normalized to mir-52 levels and relative to vector control RNAi. Statistically significant differences between vector and RNAi treatment are denoted with asterisks (*p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.01, Student’s t-test). Error bars represent SEM for three biological replicates. (G) Average fold enrichment of SNPC-4:GFP binding relative to IgG control at three binding sites and an unbound intergenic control region on Chr IV in young adult wildtype and prg-1 mutant backgrounds was calculated using ChIP-qPCR. Non-transgenic prg-1 mutants were used as a negative control to show specificity of binding. Statistical analysis was performed by comparing transgenic wildtype with transgenic prg-1 mutant animals for each locus (p ≥ 0.05, Student’s t-test). Error bars represent SEM from three biological replicates. (H) See (F) above. (I) SNPC-4:GFP expression of the same young adult gonad (dotted outline) treated with snpc-4 and control RNAi in Figure 5F. (J) To control for possible indirect effects of germline underproliferation due to increased strength of snpc-4(RNAi), several genes (ddb-1, toe-1, nath-10, and K12H4.3) that cause germline underproliferation for reasons unrelated to piRNA biogenesis (Waters et al., 2010) were examined. SNPC-4:GFP and mCherry:PRDE-1 foci persisted in all genes tested despite exhibiting germline underproliferation. Representative image of a young adult gonad treated with ddb-1(RNAi). Scale bars are 10 μm.
Supplemental Figure 6. SNPC-4:GFP binding sites retained in prde-1 mutants are diminished in strength and are enriched for the PSE motif (related to Figure 6). (A) Young adult, whole animal SNPC-4:GFP ChIP-seq binding profiles for combined replicates across Chr I in wildtype (purple) and prde-1 mutant (gray) backgrounds. A representative input from the wildtype dataset is shown. Annotated tracks as in Figure 4A. The loss of SNPC-4:GFP binding in prde-1 mutants was specific to 21U-RNA domains and was not observed for Chr I (pictured here) or for other chromosomes. (B) Average binding strength (enrichment signal determined by the SPP algorithm) for significant SNPC-4:GFP peaks retained on Chr IV in prde-1 mutants. Statistically significant differences between genotypes and regions inside compared to outside 21U-RNA clusters are marked with an asterisk (***p ≤ 0.001, and ****p ≤ 0.0001, Welch’s t-test). (C) The most significantly enriched sequence associated with significant SNPC-4:GFP binding sites retained in prde-1 mutants in 21U-RNA domains was the SNAPc PSE motif as determined by MEME analysis. (D) Average fold expression of snpc-4 transcript levels, normalized to act-3 levels and relative to N2 (wildtype strain). The mild overexpression of snpc-4 transcripts in the SNPC-4:GFP strain OP179 is reduced to near physiological levels when the endogenous snpc-4 locus is removed (strain YL487). Error bars represent SEM for two biological replicates.
Supplemental Figure 7. SNPC-4:GFP binding with Pol III binding at tRNA genes (related to Figure 7). (A) Ratio of significant SNPC-4:GFP sites shared with Pol III compared to sites shared with Pol II across chromosomes. The high co-occupancy of SNPC-4 with Pol III relative to Pol II on Chr X is likely due to nearly half of all tRNA genes (which are transcribed by Pol III) residing on Chr X, as well as due to the general lack of Pol II transcription from Chr X in germ cells (which contribute significantly to the young adult ChIP-seq sample) (Kelly et al., 2002). (B–C) Fraction of tRNA genes that are occupied by Pol III across chromosomes (B) and inside and outside 21U-RNA domains on Chr IV (C). Statistical significance was determined by comparing observed versus expected number of occurrences (Pearson’s chi-square test, p ≥ 0.05). (D) Average SNPC-4:GFP binding strength of significant peaks (enrichment signal determined by the SPP algorithm) at SNPC-4:GFP occupied tRNA genes in the whole animal. Statistically significant differences between regions inside 21U-RNA clusters and other genomic regions are marked with an asterisk (*p ≤ 0.05, ***p ≤ 0.001, and ****p ≤ 0.0001, Student’s t-test). (E–G) Distribution of the three most significant SNPC-4:GFP binding motifs as determined by MEME relative to chromosome length for all tissue-specific ChIP-seq data sets. Asterisks indicate statistically significant deviations greater than the expected value of 1(marked with a black line) (**p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001, Pearson’s chi-square test).
Table catalogues the mammalian SNAPc units and their orthologous counterparts in C. elegans. The functional description of each subunit is based on mammalian studies of SNAPc (Hung and Stumph, 2011).
This table can be found in a separate Excel file and contains chromosomal coordinates and signal values for peaks determined significant by the SPP algorithm for all ChIP-seq data sets.
Supplemental Table 3. Normalized RNA-seq data for young adult animals treated with snpc-4(RNAi) (related to Figure 5). This table can be found in a separate Excel file and contains raw and normalized read counts for small RNAs present in young adult animals treated with control and snpc-4 RNAi.
Supplemental Table 4. SNPC-4 associated motifs determined by MEME analysis (related to Supplemental Figure 7). This table can be found in a separate Excel file and includes sequences associated with SNPC-4 discrete peaks that were input and output from MEME analysis.