Abstract
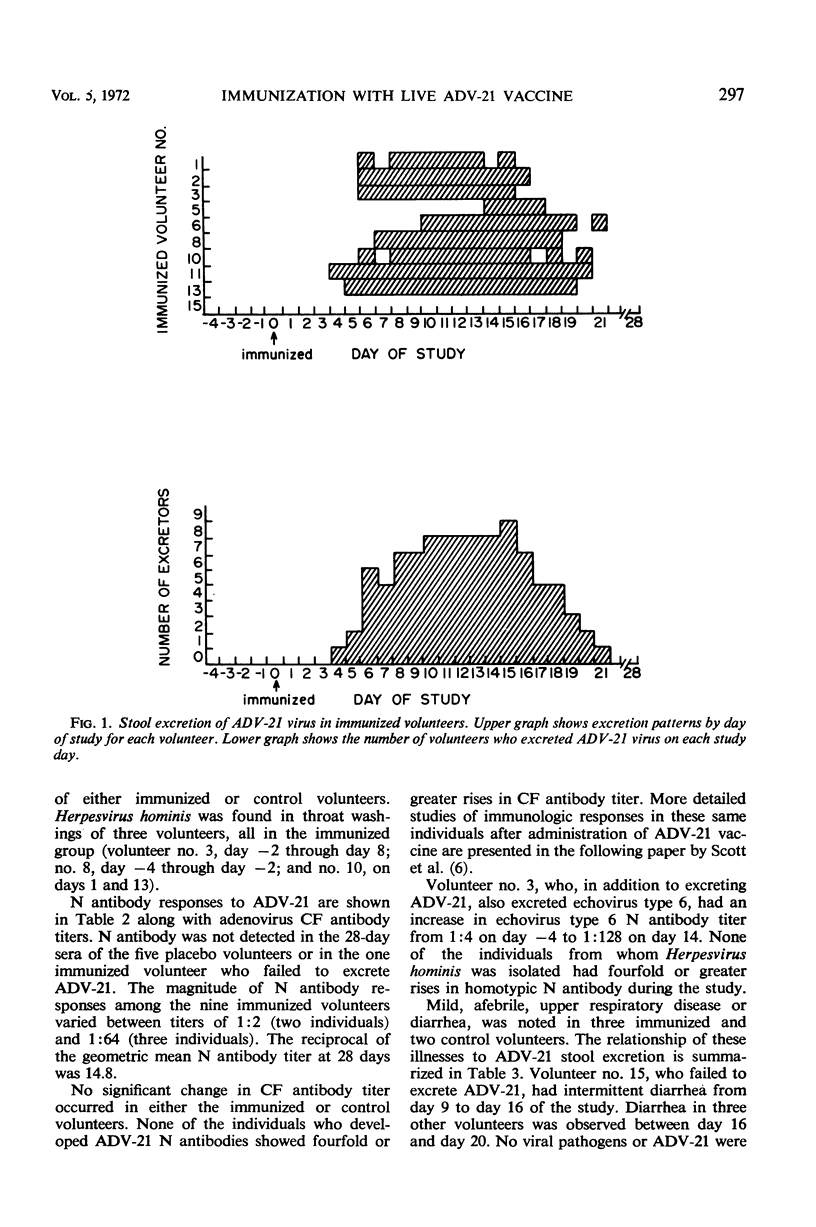
Studies were undertaken in volunteers to determine whether living adenovirus type 21 (ADV-21) vaccine could be safely administered orally to susceptible young adults. In the first study, ten volunteers were fed 106.4 tissue culture infectious dose50 (TCID50) of ADV-21 vaccine virus, and five received placebo tablets. Nine of ten infected volunteers shed ADV-21 in stools (mean duration, 10.1 days; range, 4 to 17 days). No pharyngeal excretion of ADV-21 was observed in any of these volunteers. Each of the nine developed type-specific neutralizing (N) antibodies to ADV-21. No evidence for person-to-person transmission of vaccine was observed. In a second study, volunteers were immunized with ADV-21 vaccines containing 106.8, 104.6, and 102.4 TCID50. ADV-21 N antibody responses were detected in nine of eleven who received the highest dose, six of twelve who received the middle dose, and two of twelve who were fed the lowest dose. None of twelve susceptible volunteers receiving the placebo capsule developed ADV-21 N antibodies postimmunization. This study established that the human infectious dose50 for these lots of ADV-21 vaccine was approximately 104.6 TCID50 and that the dose response to ADV-21 vaccine was lower than those previously reported for live ADV-4 and ADV-7 enteric vaccines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chanock R. M., Ludwig W., Heubner R. J., Cate T. R., Chu L. W. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA. 1966 Feb 7;195(6):445–452. [PubMed] [Google Scholar]
- Gutekunst R. R., White R. J., Edmondson W. P., Chanock R. M. Immunization with live type 4 adenovirus: determination of infectious virus dose and protective effect of enteric infection. Am J Epidemiol. 1967 Sep;86(2):341–349. doi: 10.1093/oxfordjournals.aje.a120744. [DOI] [PubMed] [Google Scholar]
- Rose H. M., Lamson T. H., Buescher E. L. Adenoviral infection in military recruits. Arch Environ Health. 1970 Sep;21(3):356–361. doi: 10.1080/00039896.1970.10667250. [DOI] [PubMed] [Google Scholar]
- Scott R. M., Dudding B. A., Romano S. V., Russell P. K. Enteric immunization with live adenovirus type 21 vaccine. II. Systemic and local immune responses following immunization. Infect Immun. 1972 Mar;5(3):300–304. doi: 10.1128/iai.5.3.300-304.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top F. H., Jr, Dudding B. A., Russell P. K., Buescher E. L. Control of respiratory disease in recruits with types 4 and 7 adenovirus vaccines. Am J Epidemiol. 1971 Aug;94(2):142–146. doi: 10.1093/oxfordjournals.aje.a121306. [DOI] [PubMed] [Google Scholar]
- Top F. H., Jr, Grossman R. A., Bartelloni P. J., Segal H. E., Dudding B. A., Russell P. K., Buescher E. L. Immunization with live types 7 and 4 adenovirus vaccines. I. Safety, infectivity, antigenicity, and potency of adenovirus type 7 vaccine in humans. J Infect Dis. 1971 Aug;124(2):148–154. doi: 10.1093/infdis/124.2.148. [DOI] [PubMed] [Google Scholar]



