Abstract
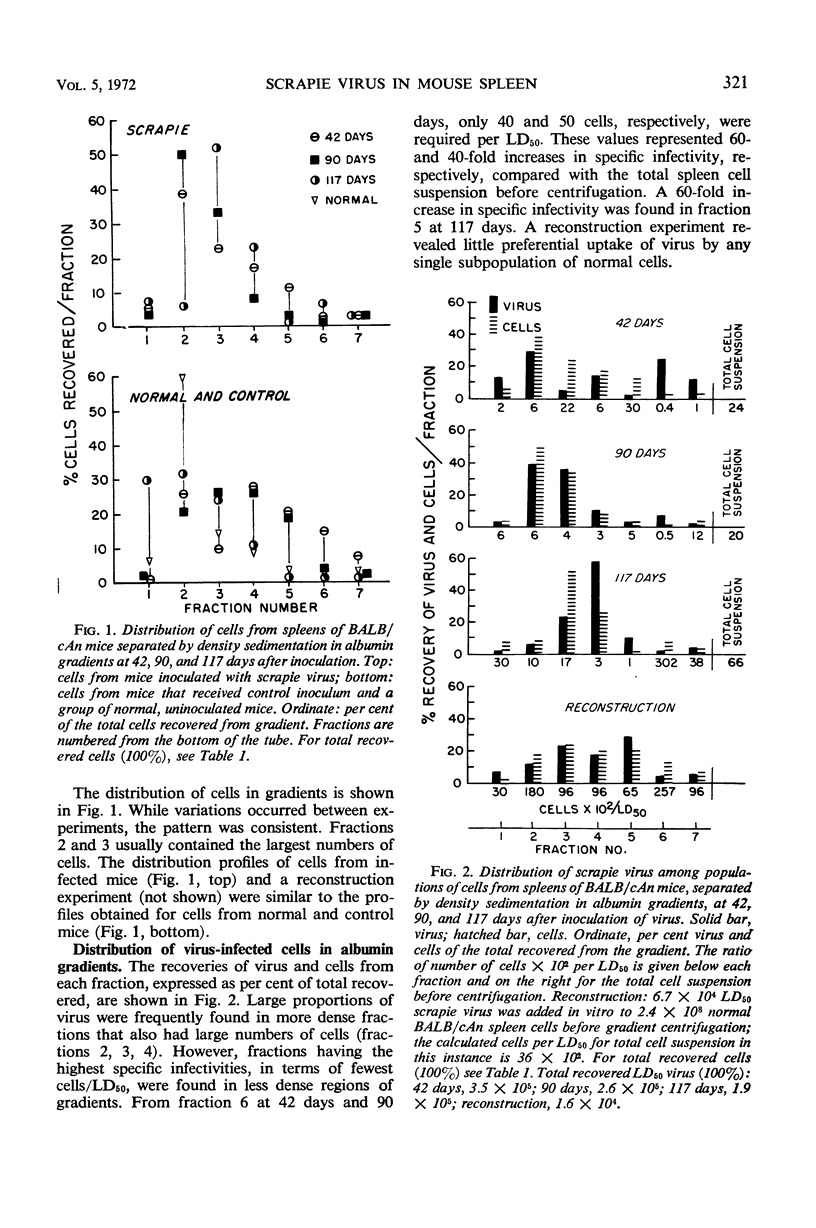
Spleen cells from mice infected with scrapie virus were separated into subpopulations on the basis of buoyant density in discontinuous gradients of isotonic albumin or differential adherence of cells to plastic. At three different intervals after infection, a population of “less dense” cells was found in albumin gradients that had 40-to 60-fold higher specific infectivity (cells per median lethal dose) than the total cell suspension before gradient sedimentation. The class of cells associated with high relative specific infectivity has a density characteristic of lymphoblasts, myeloblasts, and macrophages. Separation of “macrophage rich” cells on the basis of adherence to plastic did not result in significant enrichment of scrapie virus-infected cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspary E. A., Bell T. M. Growth potential of scrapie mouse brain in vitro. Nature. 1971 Jan 22;229(5282):269–270. doi: 10.1038/229269a0. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Fraser H. Genetical control of the concentration of ME7 scrapie agent in mouse spleen. J Comp Pathol. 1969 Jul;79(3):363–366. doi: 10.1016/0021-9975(69)90051-6. [DOI] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Specific role of each human leukocyte type in viral infections. II. Phytohemagglutinin-treated lymphocytes as host cells for vesicular stomatitis virus replication in vitro. J Virol. 1968 May;2(5):440–448. doi: 10.1128/jvi.2.5.440-448.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund C. M., Kennedy R. C., Hadlow W. J. Pathogenesis of scrapie virus infection in the mouse. J Infect Dis. 1967 Feb;117(1):15–22. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- JAHMIAS A. J., KIBRICK S., ROSAN R. C. VIRAL LEUKOCYTE INTERRELATIONSHIPS. I. MULTIPLICATION OF A DNA VIRUS--HERPES SIMPLEX--IN HUMAN LEUKOCYTE CULTURES. J Immunol. 1964 Jul;93:69–74. [PubMed] [Google Scholar]
- LEIF R. C., VINOGRAD J. THE DISTRIBUTION OF BUOYANT DENSITY OF HUMAN ERYTHROCYTES IN BOVINE ALBUMIN SOLUTIONS. Proc Natl Acad Sci U S A. 1964 Mar;51:520–528. doi: 10.1073/pnas.51.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Enders J. F. Vaccinia virus replication and cytopathic effect in cultures in phytohemagglutinin-treated human peripheral blood leukocytes. J Virol. 1968 Aug;2(8):787–792. doi: 10.1128/jvi.2.8.787-792.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Mould D. L., Dawson A. M., Rennie J. C. Very early replication of scrapie in lymphocytic tissue. Nature. 1970 Nov 21;228(5273):779–780. doi: 10.1038/228779a0. [DOI] [PubMed] [Google Scholar]
- Möller G., Hiesche K. Fractionation of immunocompetent spleen cells by albumin density gradient centrifugation. Immunology. 1970 Apr;18(4):585–594. [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K. The separation of different cell classes from lymphoid organs. II. The purification and analysis of lymphocyte populations by equilibrium density gradient centrifugation. Aust J Exp Biol Med Sci. 1968 Aug;46(4):375–396. doi: 10.1038/icb.1968.32. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Edelman R. Specific role of each human leukocyte type in viral infections. 3. 17D yellow fever virus replication and interferon production in homogeneous leukocyte cultures treated with phytohemagglutinin. J Immunol. 1969 Sep;103(3):429–436. [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dicke KA Hooft J. I., van Bekkum D. W. The selective elimination of immunologically competent cells from bone marrow and lymphatic cell mixtures. II. Mouse spleen cell fractionation on a discontinuous albumin gradient. Transplantation. 1968 Jul;6(4):562–570. doi: 10.1097/00007890-196807000-00009. [DOI] [PubMed] [Google Scholar]


