Summary
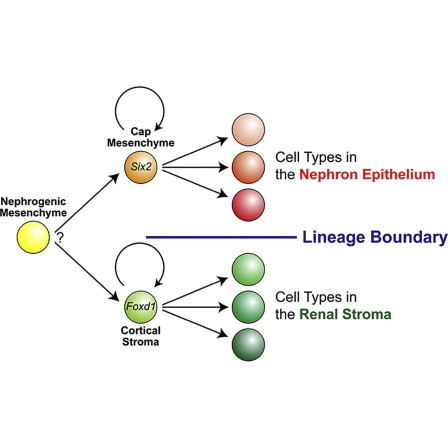
The mammalian kidney is a complex organ consisting of multiple cell types. We previously showed that the Six2-expressing cap mesenchyme is a multipotent self-renewing progenitor population for the main body of the nephron, the basic functional unit of the kidney. However, the cellular mechanisms establishing stromal tissues are less clear. We demonstrate that the Foxd1-expressing cortical stroma represents a distinct multipotent self-renewing progenitor population that gives rise to stromal tissues of the interstitium, mesangium, and pericytes throughout kidney organogenesis. Fate map analysis of Foxd1-expressing cells demonstrates that a small subset of these cells contributes to Six2-expressing cells at the early stage of kidney outgrowth. Thereafter, there appears to be a strict nephron and stromal lineage boundary derived from Six2-expressing and Foxd1-expressing cell types, respectively. Taken together, our observations suggest that distinct multipotent self-renewing progenitor populations coordinate cellular differentiation of the nephron epithelium and renal stroma during mammalian kidney organogenesis.
Graphical Abstract
Highlights
-
•
The Foxd1+ cortical stroma is a multipotent self-renewing stromal progenitor pool
-
•
A lineage boundary forms between the nephron and stromal progenitor compartments
-
•
A small fraction of Foxd1+ cells becomes Six2+ nephron progenitors at early stages
Although progenitor populations for functional units of organs (also known as “parenchyma”) have been relatively well studied, origins of surrounding stromal cells are less clear in many organs, including the kidney. In this article, Kobayashi and colleagues discovered a multipotent self-renewing stromal progenitor population during mammalian kidney development.
Introduction
The kidney contains multiple specialized cell types that have district physiological functions. During embryogenesis, the urogenital system, including the kidney, is derived from the intermediate mesoderm of the developing embryo (Herzlinger, 1995; Saxen, 1987). Formation of the definitive kidney or metanephros in mammals is initiated by reciprocal interactions between two tissues types, the ureteric bud and metanephric mesenchyme, starting around 10.5 days postcoitum (dpc) in the mouse (Costantini and Kopan, 2010; Dressler, 2009; Little and McMahon, 2012; Schedl, 2007). At this time, analysis of transcription factor expression distinguishes two morphologically distinct populations: a core of Six2-expressing (Six2+) mesenchyme, and an outer layer of Foxd1+ cells that likely emerge from a common Osr1+ mesenchymal progenitor (Kobayashi et al., 2008; Levinson and Mendelsohn, 2003; Mugford et al., 2008). As the outgrowing ureteric bud enters this mesenchyme and branches, Six2+ cells condense around the bud tip forming the cap mesenchyme, whereas Foxd1+ cortical stromal cells make up the renal capsule and nephrogenic interstitium between the Six2+ population and the outermost connective tissue (the presumptive renal fascia or Gerota’s fascia) that surrounds the kidney and adrenal gland (Costantini, 2006; Kobayashi et al., 2008; Levinson et al., 2005).
The Six2+ cap mesenchyme is a multipotent self-renewing nephron progenitor population (Kobayashi et al., 2008). In conjunction with ureteric branching, a ureteric epithelium-derived WNT9B signal induces a pathway of nephrogenesis within a subset of Six2+ progenitors (Carroll et al., 2005; Karner et al., 2011; Park et al., 2007, 2012). Induced cells undergo an initial aggregation to form the pretubular aggregate. Subsequently, through a mesenchymal-to-epithelial transition, the pretubular aggregate transitions to the renal vesicle that undergoes a series of morphological transformations and patterning processes generating the main body of the nephron from the proximal glomerulus to the distal connecting segment.
The mature nephron, and its accompanying vascular network, is embedded within the cortical and medullary interstitium (Little et al., 2007). This comprises pericytes and mesangial cell types that are intimately associated with the general kidney vasculature and the specialized vasculature of the glomerular capillary loops, respectively (Quaggin and Kreidberg, 2008; Wiggins, 2007), and interstitial fibroblast-like cells that are most prevalent within medullary regions of the mature kidney. Currently, the origins and interrelationships among these cell types are unclear, and the precise role of these stromal components in development, normal kidney function, and disease is poorly understood.
In this study, we have determined the fate map of the Foxd1+ cortical stromal cells during kidney development in vivo in the mouse. These studies demonstrate that the Foxd1+ cortical stroma is a multipotent self-renewing progenitor population for stromal cells in the kidney, giving rise to cortical and medullary interstitial cells, mesangial cells, and pericytes of the kidney. Interestingly, Foxd1+ stromal progenitors and Six2+ nephron progenitors form two mutually exclusive progenitor compartments shortly after the onset of ureteric branching. Prior to this stage, we observed a small but significant contribution of Foxd1+ cells to the Six2+ progenitor population. Our observations also suggest that the Foxd1+ stromal progenitor and Six2+ nephron progenitor populations temporally and spatially coordinate cellular differentiation. These data highlight the roles of distinct progenitor compartments in the assembly of the mammalian kidney.
Results
Generation of Foxd1-Cre Knockin Mouse Alleles
During early stages of kidney development, Foxd1 is specifically expressed in the cortical stroma of the nephrogenic zone (Das et al., 2013; Hatini et al., 1996; Levinson et al., 2005). To determine the fate map of this Foxd1-expressing (Foxd1+) population, we generated three Foxd1-Cre knockin alleles in the mouse, where eGFPCre (Foxd1GC/+), eGFPCreERT2 (Foxd1GCE/+), and CreERT2 (Foxd1CE/+) transgenes were introduced into the 5′ UTR of the endogenous Foxd1 locus (Figure S1 available online). These Foxd1-Cre knockin alleles ablate Foxd1 function; however, mice heterozygous for these and previously described null alleles are phenotypically normal and fertile (Hatini et al., 1996; Levinson et al., 2005) (data not shown). The GCE and CE alleles allow tamoxifen-dependent regulation of Cre recombinase activity (Indra et al., 1999; Kobayashi et al., 2008).
To validate transgene expression patterns of the Foxd1-Cre knockin alleles, we examined GFP expression in the developing kidney of Foxd1GC/+ and Foxd1GCE/+ embryos. In both lines, GFP expression was observed in the cortical stroma during kidney development (Figure S2; data not shown). The nuclear FOXD1 protein colocalized with nuclear GFP in Foxd1GC/+ kidneys (Figure S2I), whereas FOXD1 was surrounded by cytoplasmic GFP in Foxd1GCE/+ kidneys (Figure S2J). These observations confirmed GFP expression in FOXD1+ cortical stromal cells in the Foxd1-GC and Foxd1-GCE alleles.
Genome-wide gene expression projects (GenePaint and GUDMAP) have documented Foxd1 expression in the glomerulus at a low level at 14.5 dpc and at a higher level at 19.5 dpc (Figures S3A and S3B) (Harding et al., 2011; Visel et al., 2004), and microarray analysis suggests podocytes as the likely cell source (Brunskill et al., 2011). Although Foxd1 mRNA appears to be expressed in most podocytes of maturing-stage glomeruli (Figures S3A and S3B), a recent study showed that Cre recombination was observed only in a subset of podocytes in Foxd1-eGFPCre mice during kidney development (Boyle et al., 2014), indicating posttranscriptional regulation for Foxd1 expression or different sensitivity of detection methods. Consistent with these findings, we detected expression of GFP and FOXD1 in a subset of both podocytes and parietal epithelial cells of maturing-stage glomeruli, but not in less-differentiated capillary loop-stage glomeruli, in the Foxd1GC/+ kidney at 15.5 and 18.5 dpc (Figure S3B and S3C; data not shown). We observed Foxd1-GFP expression only in the cortical stroma, the visceral (podocytes), and the parietal epithelial cells of the glomerulus. No Foxd1-GFP expression was observed in any other tissues of the developing kidney. Thus, the Foxd1-Cre knockin alleles faithfully document endogenous FOXD1 expression.
Foxd1+ Cells within the Cortical Stroma Show a Distinct Fate Map to that of Six2+ Nephron Progenitors in the Cap Mesenchyme
The fate map of the Foxd1+ cortical stroma was compared to that of the Six2+ cap mesenchyme. Foxd1GC/+ and Six2-tetoff-eGFPCre (Six2TGC/+) (Kobayashi et al., 2008) mice were intercrossed with mice carrying a R26R-lacZ reporter allele (R26RlacZ/+) (Soriano, 1999) to permanently label descendant cells from the Foxd1+ cortical stromal and Six2+ cap mesenchymal cells by β-galactosidase (β-gal) expression.
As expected from our previous study (Kobayashi et al., 2008), analysis of Six2TGC/+; R26RlacZ/+ kidneys at 14.5 dpc showed β-gal activity confined to the cap mesenchyme and all nephron epithelia including the renal vesicle, S-shaped body, nephron tubule, and visceral and parietal epithelia of the glomerulus (Figures 1A, 1C, and 1E). In striking contrast, Foxd1GC/+; R26RlacZ/+ displayed a reciprocal pattern of β-gal activity restricted to the cortical stroma, cortical and medullary interstitium, and the glomerular mesangium region (Figures 1B, 1D, and 1F). At later stages, we also observed β-gal activity within podocytes of glomeruli at the maturing stage consistent with Foxd1 expression within this population (Figures S4A and S4C). A low-variable incomplete β-gal activity was observed in a subset of nephrons (Figures 1D and S4E). Taken together, our observations suggest that the fate maps of Six2+ cap mesenchyme and Foxd1+ cortical stroma are largely distinct: the former contributing to nephron epithelial cells, and the latter to renal stromal cell types.
Figure 1.
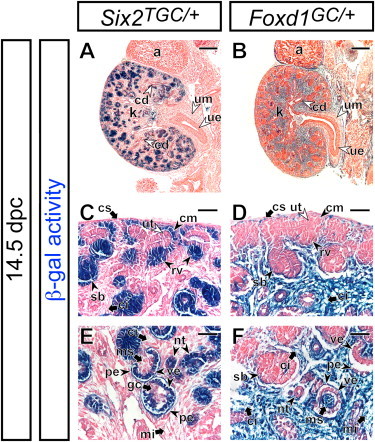
The Six2+ Cap Mesenchyme and Foxd1+ Cortical Stroma Give Rise to Distinct Cellular Lineages of the Kidney
β-gal-stained (blue) kidneys from Six2TGC/+; R26RlacZ/+ (A, C, and E), and Foxd1GC/+; R26RlacZ/+ (B, D, and F) embryos at 14.5 dpc counterstained with eosin (pink).
(A and B) Whole kidney.
(C–F) Higher magnification of the nephrogenic zone (C and D) and renal cortex with glomeruli (E and F).
a, adrenal gland; cd, collecting duct; ci, renal cortical interstitium; cm, cap mesenchyme; cs, cortical stroma; k, kidney; mi, renal medullary interstitium; ms, mesangium; nt, nephron tubule; pe, parietal epithelium; rv, renal vesicle; sb, S-shaped body; ue, ureter epithelium; um, ureter mesenchyme; ut, ureteric tip; ve, visceral epithelium (podocyte). Scale bars, 200 μm (A and B) and 50 μm (C–F).
The low contribution of Foxd1+ descendant cells to regions of the nephron epithelium, normally populated only by cells originating from the Six2+ cap mesenchyme, outside of the Foxd1+ visceral and parietal epithelia suggests a more complicated relationship among these stromal and nephron progenitor populations. There are three possible explanations for this result. First, Foxd1 may be activated transiently in a variable pattern within multiple regions of a subset of maturing nephrons. A molecular heterogeneity of this kind seems unlikely given our knowledge of nephron patterning and function. Furthermore, even if Foxd1 is transiently expressed in differentiating nephron epithelia, it is expected that such transient expression will result in a low level of Cre protein insufficient for DNA recombination. Second, there is a continual low-level contribution of Foxd1+ cortical stromal cells to repopulate the Six2+ nephron progenitors as kidney development proceeds. However, pulse labeling of Foxd1+ cortical stromal cells in Foxd1GCE/+; R26RlacZ/+ kidneys by tamoxifen injections at 11.5, 12.5, or 14.5 dpc did not result in β-gal+ cells in the nephron epithelium perinatally (Figures S4B, S4D, and S4F; data not shown), indicating that there is no contribution of Foxd1+ cells to the nephron epithelial cells at these stages after the onset of kidney development.
Finally, in the initial separation of Foxd1+ and Six2+ lineages at the onset of kidney development, some plasticity may remain within Foxd1+ cells such that they can secondarily convert to the Six2+ lineage. Although we did not detect Foxd1-GFPCre transgene expression in the SIX2+ cap mesenchyme at 11.5, 12.5, and 15.5 dpc, β-gal+ descendant cells from the Foxd1+ population were observed in the SIX2+ cap mesenchyme of Foxd1GC/+; R26RlacZ/+ kidneys by 11.5 dpc (Figure S5). β-gal+ SIX2+ cells were more frequently observed in the outer half of the SIX2+ population close to the cortical stroma (65.9% at 11.5 dpc [n = 41] and 57.1% at 12.5 dpc [n = 21]). Subsequently, although we never observed overlap of Foxd1-driven GFP expression and SIX2, the fraction of β-gal+ cells within the SIX2+ population increased significantly as nephrons differentiate between 12.5 and 15.5 dpc (1.53% ± 0.48% to 3.31% ± 0.16%, n = 3; p < 0.05, unpaired t test) (Figure S4I).
Together, our results indicate that there is a low transient reassignment within a Foxd1+ cell type to Six2+Foxd1− nephron progenitors but likely only at the onset of kidney development. By 11.5 dpc, all Six2+ and Foxd1+ mesenchymal cells are restricted to mutually exclusive nephron and stromal lineages, respectively. The marginal contribution of Foxd1+ descendants to the Six2+ population results in a sporadic contribution to the nephrons of the mature kidney: most nephrons are unlabeled by mesenchymal Foxd1+ descendants. Those that show a variable contribution likely reflect the proportion of these descendants in the nephron precursor, the renal vesicle. Our data cannot exclude the possibility of transient SIX2+ FOXD1+ cells at different time points that our methods cannot detect.
The Foxd1+ Cortical Stroma Is a Stromal Progenitor Population
We further determined cell types derived from Foxd1+ cortical stromal cells at a molecular level. We examined Foxd1+ cortical stroma-derived β-gal+ cells in Foxd1GC/+; R26RlacZ/+ kidneys at 15.5 dpc using the following cell-type-specific markers: cytokeratin (KRT) (ureteric tip and collecting duct); LIV2 (capsule and the outermost connective tissue); Wilms tumor 1 (WT1) (cap mesenchyme, visceral epithelium/podocytes); platelet-derived growth factor (PDGF) receptor β (PDGFRB; interstitium, glomerular mesangium, and pericytes); FLK1 (endothelium); CDKN1C (P57KIP2; a subset of the medullary interstitium); F4/80 (EMR1; macrophages); and smooth muscle actin α (aSMA/ACTA2; smooth muscle) (Figure 3).
Figure 3.
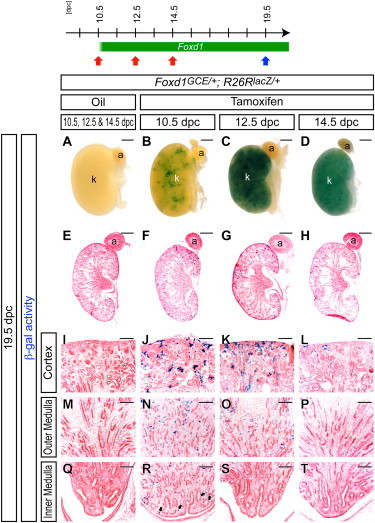
The Foxd1+ Cortical Stroma Contributes to Stromal Tissues throughout Kidney Organogenesis
β-gal-stained (blue) kidneys from Foxd1GCE/+; R26RlacZ/+ embryos at 19.5 dpc after three successive injections of oil vehicle only at 10.5, 12.5, and 14.5 dpc (A, E, I, M, and Q) or after a single injection of 2 mg tamoxifen at 10.5 dpc (B, F, J, N, and R), 12.5 dpc (C, G, K, O, and S), or 14.5 dpc (D, H, L, P, and T).
(A–D) Whole-mount view.
(E–T) Sections counterstained with eosin (pink).
(E–H) Whole kidney.
(I–L) Nephrogenic zone and renal cortex.
(M–P) Outer renal medulla.
(Q–T) Inner renal medulla (papilla). Arrows in (R) indicate β-gal+ interstitial cells in the inner medulla adjacent to the pelvic urothelial lining.
a, adrenal gland; k, kidney. Scale bars, 500 μm (A–H) and 100 μm (I–T).
In the nephrogenic zone, all FOXD1+ cortical stromal cells were β-gal+, no overlap of β-gal+ cells was observed in KRT+ ureteric tip/stalk cells, and only rare β-gal+ cells were noted among the SIX2+ cap mesenchyme, as described above (Figures 2A–2C). LIV2 staining was observed in the two outermost tissue layers of the kidney: the surface connective tissue, and an underlying layer of the capsule (Levinson et al., 2005) (data not shown). Although β-gal+ cells were detected in the inner LIV2+ renal capsule, a subpopulation of the FOXD1+ cortical stroma, no β-gal+ cells were observed in the outermost LIV2+ connective tissue (Figure 2D). In the renal cortex, PDGFRB+ cells, including cortical interstitial cells, mesangial cells of the glomerulus, and pericytes outside of the glomerulus, were β-gal+, but FLK1+ endothelial cells were β-gal− (Figures 2E and 2F; data not shown). As expected, some Foxd1+ maturing podocytes were labeled such that all podocytes were β-gal+ in the adult kidney (black arrowheads in Figures 2E, 2F, and S4C; data not shown). In the renal medulla, a subset of medullary interstitial cells expressing CDKN1C+ (P57KIP2+) (Yu et al., 2009; Zhang et al., 1997) was β-gal+ (Figure 2G), but F4/80+ macrophages were β-gal− (Figure 2H). Although SMA+ cells in the kidney were derived from Foxd1+ cells, β-gal+ cells were not observed in the SMA+ smooth muscle of the ureter (Figure 2I). Importantly, we observed no evidence of direct Foxd1 expression in cortical/medullary interstitial cells, mesangial cells, or pericytes. Taken together, these data suggest that Foxd1+ cortical stromal cells contribute to stromal cells of the kidney including cortical and medullary interstitial cells, mesangial cells, and pericytes but that smooth muscle cells in the ureter have an independent origin (Bohnenpoll et al., 2013; Boyle et al., 2014; Humphreys et al., 2008, 2010; Lin et al., 2014; Wang et al., 2009).
Figure 2.
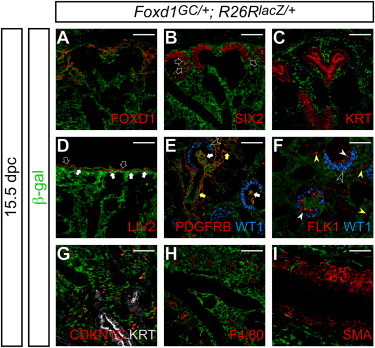
The Foxd1+ Cortical Stroma Is a Progenitor Population for Stromal Tissues in the Kidney
Confocal immunofluorescence images of Foxd1GC/+; R26RlacZ/+ kidneys at 15.5 dpc with anti-β-gal staining (green).
(A–D) Nephrogenic zone.
(A) FOXD1 (red).
(B) SIX2 (red). Black arrows indicate β-gal+ SIX2+ cap mesenchyme cells.
(C) KRT (red).
(D) LIV2 (red). White and black arrows indicate inner LIV2+ β-gal+ cells in the renal capsule and outer LIV2+ β-gal− cells in the connective tissue, respectively.
(E and F) Renal cortex with glomeruli.
(E) PDGFRB (red) and WT1 (blue). Yellow and white arrows indicate PDGFRB+ β-gal+ pericytes and mesangial cells, respectively.
(F) FLK1 (red) and WT1 (blue). Yellow and white arrowheads indicate FLK1+ β-gal− vascular cells in the renal cortex outside of the glomerulus and within the glomerular tuft, respectively. Black arrowheads in (E) and (F) indicate WT1+ β-gal+ podocytes.
(G and H) Renal medulla.
(G) CDKN1C (P57KIP2, red) and KRT (white).
(H) F4/80 (red).
(I) Ureter with SMA (red).
Scale bars, 50 μm.
Tempo-Spatial Patterns for Stroma Formation from the Foxd1+ Cortical Stroma during Kidney Development
To examine whether there is an ongoing commitment of Foxd1+ cortical stromal cells to stromal lineages, we used the tamoxifen-dependent Foxd1-GCE allele to label Foxd1+ cortical stromal cells at later stages of kidney development. We confirmed that the recombinase activity of the eGFPCreERT2 triple-fusion protein was completely tamoxifen dependent: no background recombination was detected without tamoxifen administration in the developing kidney (Figures 3A, 3E, 3I, 3M, and 3Q), as we previously observed with a Six2-eGFPCreERT2 allele (Kobayashi et al., 2008).
The Foxd1+ cortical stromal cells of Foxd1GCE/+; R26RlacZ/+ embryos were labeled by tamoxifen induction at 10.5, 12.5, or 14.5 dpc. β-gal activity was observed at 19.5 dpc in stromal cells of the kidney in embryos from dams injected with tamoxifen at all time points (Figures 3B–3D and 3F–3H). However, whereas tamoxifen injection at 10.5 dpc resulted in β-gal+ stromal cells along the entire radial axis of the kidney from the cortex to inner medulla (papilla) (Figures 3J, 3N, and 3R), 12.5 dpc injections did not label cells in the tip of the inner medulla (Figures 3K, 3O, and 3S), and 14.5 dpc injections showed β-gal+ stromal cells limited to the cortical interstitium (Figures 3L, 3P, and 3T). These data indicate that Foxd1+ cortical stromal cells continuously contributed to stromal lineages throughout kidney development and that there was a temporal restriction where later differentiating cells arising from the Foxd1+ progenitor compartment were cortically restricted. Interestingly, the radial restriction of β-gal+ stromal cells was still evident in the adult kidney, suggesting that radial domains once established were relatively stable within stromal tissues of the mature kidney (Figure S6).
The pulse labeling of Foxd1GCE/+; R26RlacZ/+ at 10.5, 12.5, and 14.5 dpc prior to FOXD1 expression in podocytes of maturing glomeruli did not label podocytes (Figures 3 and 4; data not shown), suggesting that podocytes are not derived from the Foxd1+ cortical stroma. The β-gal expression in a subset of podocytes in Foxd1GC/+; R26RlacZ/+ embryonic kidneys is due to Foxd1-eGFPCre activity directly in Foxd1+ podocytes.
Figure 4.
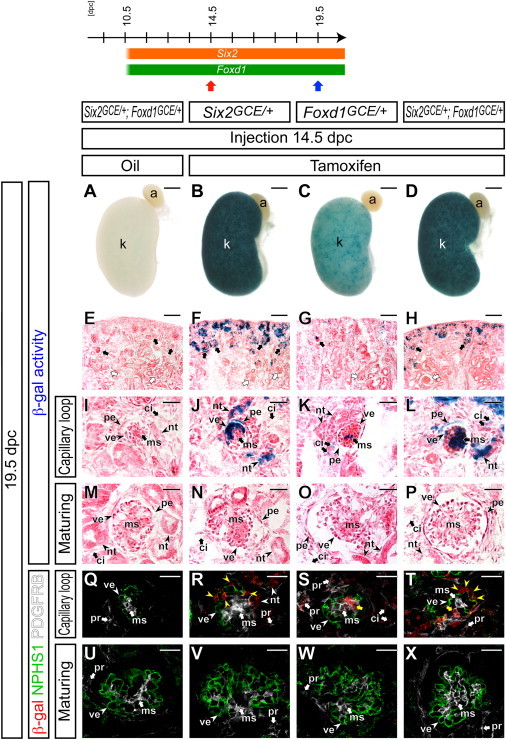
Six2+ Cap Mesenchymal Cells and Foxd1+ Cortical Stromal Cells Differentiate in a Coordinated Manner
Kidneys at 19.5 dpc from Six2GCE/+; Foxd1GCE/+; R26RlacZ/+ mice with oil-only injection at 14.5 dpc (A, E, I, M, Q, and U), Six2GCE/+; R26RlacZ/+ with 6 mg tamoxifen at 14.5 dpc (B, F, J, N, R, and V), Foxd1GCE/+; R26RlacZ/+ with 6 mg tamoxifen at 14.5 dpc (C, G, K, O, S, and W), and Six2GCE/+; Foxd1GCE/+; R26RlacZ/+ with 6 mg tamoxifen at 14.5 dpc (D, H, L, P, T, and X).
(A–D) β-gal-stained (blue) whole-mount kidney.
(E–P) β-gal-stained (blue) sections counterstained with eosin (pink).
(E–H) Nephrogenic zone and renal cortex. Black arrows and white arrows indicate capillary loop-stage and maturing-stage glomeruli, respectively.
(I–L) Capillary loop-stage glomerulus.
(M–P) Maturing-stage glomerulus.
(Q–X) Confocal immunofluorescence images with anti-β-gal (red), a podocyte marker anti-NPHS1 (nephrin, green), and a mesangium marker anti-PDGFRB (white).
(Q–T) Capillary loop-stage glomerulus. Yellow arrowheads and yellow arrows in (R)–(T) indicate the β-gal+ NPHS1+ podocytes and β-gal+ PDGFRB+ mesangial cells, respectively.
(U–X) Maturing-stage glomerulus.
a, adrenal gland; ci, renal cortical interstitium; k, kidney; ms, mesangium; nt, nephron tubule; pe, parietal epithelium; pr, pericyte; ve, visceral epithelium (podocyte). Scale bars, 500 μm (A–D), 100 μm (E–H), and 25 μm (I–X).
Coordinated Differentiation from Nephron and Stromal Progenitor Populations during Kidney Organogenesis
Nephron formation also demonstrates a temporal restriction along the radial axis of the kidney (Boyle et al., 2008), suggesting that nephron and stromal progenitor populations may differentiate in a coordinated manner during kidney organogenesis. To test this possibility, we examined the cellular contributions from the Six2+ cap mesenchyme and Foxd1+ cortical stroma at different stages of kidney organogenesis. Because the differentiating loop of Henle of newly formed nephrons extends into the previously generated medullary interstitium, we analyzed podocytes and mesangial cells derived from the cap mesenchyme and cortical stroma, respectively, within the glomerulus, where temporal differentiation of the nephron and stromal compartments is maintained.
Tamoxifen induction at 14.5 dpc in Six2GCE/+; R26RlacZ/+ embryos resulted in β-gal activity in the cap mesenchyme and main body of the nephron in the renal cortex at 19.5 dpc, including nephrin+ (NPHS1+) podocytes of the less-differentiated capillary loop-stage glomerulus localized to the outer cortex (Figures 4B, 4F, 4J, and 4R). However, β-gal+ cells were not observed in the more differentiated glomeruli that formed at earlier developmental stages and are positioned deeper within the cortical region (Figures 4F, 4N, and 4V). A similar labeling and analysis of the Foxd1+ cortical stroma in Foxd1GCE/+; R26RlacZ/+ kidneys resulted in β-gal activity in stromal tissues of the renal cortex, the PDGFRB+ mesangium of the capillary loop-stage glomerulus (Figures 4C, 4G, 4K, and 4S), but not maturing-stage glomeruli (Figures 4G, 4O, and 4W). To compare contributions from the Six2+ cap mesenchyme and Foxd1+ cortical stroma directly, both progenitor populations were labeled in Six2GCE/+; Foxd1GCE/+; R26RlacZ/+ kidneys at 14.5 dpc. β-gal activity was observed in both nephrin+ (NPHS1+) podocytes and PDGFRB+ mesangial cells of the capillary loop-stage glomerulus, but not the maturing-stage glomerulus (Figures 4D, 4H, 4L, 4P, 4T, and 4X). As expected, no β-gal activity was detected in oil-injected, carrier controls (Figures 4A, 4E, 4I, 4M, 4Q, and 4U). These data suggest that differentiation of stromal cells from the Foxd1+ progenitor population temporally and spatially coordinates with differentiation of the adjacent Six2+ progenitor pool into nephrons.
Foxd1+ Cortical Stromal Cells Are Maintained by Self-Renewal
To analyze the stromal progenitor population quantitatively, we performed fluorescence-activated cell sorting (FACS) analysis of Foxd1-GFP+ cells in Foxd1GCE/+ kidneys to determine the cell number of Foxd1+ cells during early-to-mid stages of nephrogenesis. We observed that Foxd1+ cells in a single kidney undergo a 22.5-fold increase from 4,485 ± 1,081 cells at 11.5 dpc (n = 12) to 101,068 ± 13,004 cells at 14.5 dpc (n = 6). Podocyte labeling is insignificant at this time (Figures 1B and 1F); thus, the majority of Foxd1+ cells at 14.5 dpc are within the cortical stroma that harbors Six2+ nephron progenitors and Ret+ ureteric epithelial progenitors (Shakya et al., 2005). Given a continual contribution of Foxd1+ progenitor cells to differentiated stromal derivatives, these data indicate an extensive expansion of the Foxd1+ stromal progenitor pool during kidney organogenesis.
To examine whether expansion of the Foxd1+ cell compartment occurs by self-renewal (Figure 5A) or through repopulation from external Foxd1− progenitor cells (Figure 5B), we performed a developmental pulse-chase experiment labeling Foxd1+ cells at the onset of kidney development (10.5 dpc) and examined retention of β-gal-labeled cells in the Foxd1+ stromal progenitors at birth (19.5 dpc). To determine the dynamics of Cre recombination activity, we injected 2 mg of tamoxifen into dams at 9.5 dpc, 1 day before the onset of Foxd1 expression in the metanephric region (Bohnenpoll et al., 2013; Mugford et al., 2008). No β-gal+ cells were observed in the 19.5 dpc kidney (Figures 5C, 5F, 5I, 5L, and 5O), consistent with a time window of tamoxifen activity of less than 24 hr for this dose as we reported previously (Kobayashi et al., 2008). When tamoxifen was injected at 10.5 dpc, we observed a significant contribution of β-gal+ cells within the Foxd1-GFP+ cortical stroma at 19.5 dpc (Figures 5E, 5H, 5K, 5N, and 5Q). Between 10% and 20% cells of Foxd1-GFP+ cortical stromal cells were β-gal+ at 19.5 dpc with some variation between dams and even between littermates (Figures 5N and 5Q; data not shown). No β-gal+ cells were detected in any oil-injected controls (Figures 5D, 5G, 5J, 5M, and 5P), indicating that these data are absolutely tamoxifen dependent. Considering our observations that even over a brief period from 11.5 to 14.5 dpc, the number of Foxd1+ cells increases 22.5-fold and that stromal cells are continuously differentiating from the Foxd1+ progenitor population, collectively, the results are most consistent with self-renewal within the Foxd1+ cortical stroma as the main driver for the maintenance and expansion of the stromal progenitor pool. Although we cannot exclude the minor contribution of Foxd1− cells to the cortical stroma, we did not observe a reduced contribution of labeled cells along the medullary and cortical interstitium (Figure 5H; data not shown), suggesting that any contribution from Foxd1− cells is not likely to constitute a substantive mechanism maintaining cortical stromal populations.
Figure 5.
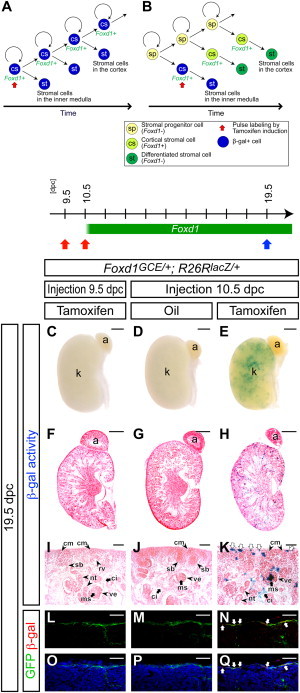
Foxd1+ Cortical Stromal Cells Self-Renew
(A and B) Models for maintenance of cortical stromal cells.
(A) A pulse-labeled β-gal+ (blue) Foxd1+ cortical stromal (cs) cell produces another β-gal+ Foxd1+ cortical stromal cell, which results in retention of β-gal+ cells in the Foxd1+ cortical stroma. Differentiated stromal cells (st) are β-gal+ cells along the cortico-medullary axis throughout kidney organogenesis.
(B) A Foxd1− stromal precursor population (sp) that generates Foxd1+ cortical stromal cells (cs) resides outside of the Foxd1+ population. Contribution of β-gal− cells from the Foxd1− stromal precursor population (sp) dilutes the pulse-labeled β-gal+ (blue) cells in the Foxd1+ cortical stroma (cs; light green). Only differentiated stromal cells (st) at early stages will be β-gal+ (blue) cells that will be seen in the inner medulla (papilla), but stromal cells in the renal cortex (st; dark green), which differentiate at later stages, will be β-gal−.
(C–Q) Foxd1GCE/+; R26RlacZ/+ kidneys at 19.5 dpc after injection of 2 mg tamoxifen at 9.5 dpc (C, F, I, L, and O) and 10.5 dpc (E, H, K, N, and Q), and oil only at 10.5 dpc (D, G, J, M, and P).
(C–E) Whole-mount β-gal-stained (blue) kidneys.
(F–H) Sections of β-gal-stained (blue) kidneys counterstained with eosin (pink).
(I–K) Higher magnification of the cortical region in (F)–(H), respectively. White arrows in (K) indicate multiple β-gal+ cells in the cortical stroma.
(L–Q) Confocal immunofluorescence images with anti-GFP (green), anti-β-gal (red), with (L–N) or without (O–Q) Hoechst (blue) staining. White arrows in (N) and (Q) indicate β-gal+ GFP+ cortical stromal cells.
a, adrenal gland; ci, renal cortical interstitium; cm, cap mesenchyme; k, kidney; ms, mesangium; nt, nephron tubule; pe, parietal epithelium; pr, pericyte; rv, renal vesicle; ve, visceral epithelium (podocyte). Scale bars, 500 μm (C–H) and 50 μm (I–Q).
A Foxd1+ Cell Can Give Rise to Multiple Stromal Cell Types
To determine whether multiple interstitial cell types can arise from clonal labeling of a Foxd1+ stromal progenitor cell (Figures 6A and 6B), we optimized dosages of tamoxifen for clonal analysis (Kobayashi et al., 2008). With injection of 0.1 mg tamoxifen at 12.5 dpc, we observed well-separated rare, tamoxifen-dependent β-gal+ clusters in the kidney at 19.5 dpc (1.95 ± 1.00 clusters per kidney; n = 20) (Figures 6C–6F), indicating that these rare clusters are likely derived from a single Foxd1+ cortical stromal cell. We analyzed 13 such clusters in kidneys with 3 clonal clusters or less at 19.5 dpc. Within a cluster, β-gal+ cells were observed within a limited area along the cortico-medullary axis (Figures 6D and 6F), indicating that renal stromal cells do not actively change relative positions not only along the radial axis (Figure 3) but also along the planes perpendicular to the cortico-medullary axis of the developing kidney. Multiple cells exhibiting β-gal activity were observed in the zone of Foxd1+ stromal progenitor cells, consistent with our earlier data on self-renewal of this population (white arrows in Figure 6G). In differentiated stromal tissues, β-gal activity was observed in the cortical interstitium, mesangium, and medullary interstitium within a single cluster (Figures 6F, 6G, and 6G′). Thus, Foxd1+ cortical stromal cells likely contain a multipotent cell type capable of generating regionally diverse stromal cell types.
Figure 6.
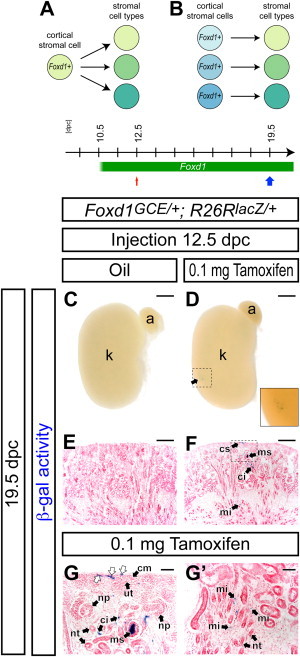
Foxd1+ Cortical Stromal Cells Can Be Multipotent Stromal Progenitors
(A and B) Models for developmental potential of Foxd1+ cells.
(A) A Foxd1+ cortical stromal cell retains multipotency to differentiate into different stromal tissues.
(B) Developmental potential of a Foxd1+ cell is restricted to limited stromal cell types within the Foxd1+ cortical stroma.
(C–G′) β-gal-stained (blue) kidneys from Foxd1GCE/+; R26RlacZ/+ embryos at 19.5 dpc after injection of oil only (C and E), and 0.1 mg tamoxifen (D, F, G, and G′) at 12.5 dpc.
(C and D) Whole-mount view. The inset in (D) shows a higher magnification of a clone in the dashed region. The arrow in (D) indicates a β-gal+ clone.
(E and F) Sections counterstained with eosin (pink).
(G and G′) Adjacent sections 96 μm apart showing the same clone. (G) is a higher magnification of the dashed region in (F). White arrows in (G) indicate multiple β-gal+ cells in the cortical stroma.
a, adrenal gland; ci, renal cortical interstitium; cm, cap mesenchyme; cs, cortical stroma; k, kidney; mi, renal medullary interstitium; ms, mesangium; np, nephron precursor; nt, nephron tubule; ut, ureteric tip. Scale bars, 500 μm (C and D), 200 μm (E and F), and 50 μm in (G and G′).
Foxd1 Function Is Dispensable for Regulation of the Stromal Lineage
The transcription factor Foxd1 plays important roles in kidney development. In the absence of Foxd1 function, the renal capsule formed abnormally, and the cap mesenchyme is thickened with retarded nephron differentiation (Fetting et al., 2014; Hatini et al., 1996; Levinson et al., 2005). Because Foxd1 is expressed in the stromal progenitor population, it is possible that Foxd1 regulates the stromal lineage during kidney development. To address this question, we examined the fate map of Foxd1+ cortical stromal cells in the absence of Foxd1 activity.
Foxd1GC/CE mice displayed a similar phenotype to that previously described for Foxd1 null mutant mice: the horseshoe kidneys containing the accumulated cap mesenchyme ectopically along the cortico-medullary axis, and the thickened capsule on the cortical surface (Hatini et al., 1996; Levinson et al., 2005). Thus, the creation of the targeted allele appears to phenocopy the existing Foxd1 null mutants. When Foxd1GC/CE; R26RlacZ/+ kidneys were examined, we observed β-gal activity in a similar range of cell types within the 15.5 dpc kidneys to Foxd1GC/+; R26RlacZ/+controls with a single active Foxd1 allele (Figures 7A and 7B). In the renal cortex, β-gal+ cells were detected in PDGFRB+ mesangial cells and pericytes (Figures 7C and 7D). No evidence was found for the additional contribution of β-gal+ cells to the Six2+ nephron progenitor pool or to cells of the nephron, except for the expected contribution to late-stage WT1+ podocytes (Figures 7C, 7D, 7I, and 7J). β-gal+ cells were observed in the CDKN1C+ (P57KIP2+) renal medullary interstitium, the LIV2+ renal capsule, but not in the KRT+ collecting duct (Figures 7E–7J). Thus, Foxd1 activity is dispensable for specification and differentiation of the stromal interstitial lineage within the mouse kidney.
Figure 7.
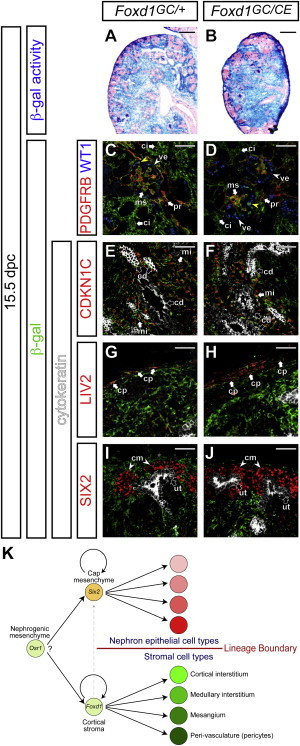
Foxd1 Activity Is Dispensable for Cell-type Specification of Stromal Tissues
Kidneys from Foxd1GC/+; R26RlacZ/+ (A, C, E, G, and I) and Foxd1GC/CE; R26RlacZ/+ (B, D, F, H, and J) embryos at 15.5 dpc.
(A and B) β-gal-stained (blue) sections counterstained with eosin (pink).
(C–J) Confocal immunofluorescence imaging with anti-β-gal (green) and anti-KRT (white) staining in (E)-(J). KRT is expressed in the ureteric tip and collecting duct.
(C and D) PDFGRB (red) and WT1 (blue). Yellow arrowheads indicate β-gal+ WT1+ podocytes.
(E and F) P57KIP2 (CDKN1C, red).
(G and H) LIV2 (red).
(I and J) SIX2 (red).
cd, collecting duct; ci, renal cortical interstitium; cm, cap mesenchyme; cp, capsule; mi, renal medullary interstitium; ms, mesangium; pr, pericyte; ut, ureteric tip; ve, visceral epithelium (podocyte).
(K) A model for cellular differentiation from mesenchymal progenitor populations during mammalian kidney development. The Six2+ cap mesenchyme and Foxd1+ cortical stroma are a multipotent self-renewing progenitor population for nephron epithelial and renal stromal cell types. Although Six2+ cells differentiate into all cell types of the main body of the nephron, Foxd1+ cortical stromal cells contribute to all stromal cells in the kidney, including cortical and medullary interstitial cells, mesangial cells, and pericytes. Only around the onset of kidney development, a small fraction of Foxd1+ cells becomes Six2+ cells (gray dashed arrow). Thereafter, the Six2+ cell-derived nephron lineage and Foxd1+ cell-derived stromal lineage form compartments with a lineage boundary throughout kidney development. Although the Osr1+ nephrogenic mesenchyme can contribute to both Six2+ cap mesenchyme and Foxd1+ cortical stroma, currently, it is unclear whether a single Osr1+ cell can differentiate into both Six2+ and Foxd1+ mesenchymal progenitor cells.
Scale bars, 200 μm (A and B) and 50 μm (C–J).
Discussion
The Foxd1+ Cortical Stroma Demarcates the Stromal Progenitor Population during Kidney Organogenesis
Our data indicate that Foxd1+ cortical stromal cells contribute to renal cortical and medullary interstitial cells, mesangial cells, and pericytes of the mammalian kidney (Figure 7K). The origin of the kidney stromal cells is somewhat controversial. In the mouse, analysis of Osr1+ intermediate and dorsal lateral plate mesoderm derivatives at 7.5–10.5 dpc showed that this population contributes to Foxd1+ stromal progenitors and Six2+ nephron progenitors. Thereafter, Osr1 expression becomes restricted to the Six2+ cells as the lineage boundary is established between Six2+ and Foxd1+ progenitor compartments (Mugford et al., 2008). In the chick, labeling studies have suggested a paraxial mesoderm origin for Foxd1+ stromal progenitors (Guillaume et al., 2009). If Foxd1 demarcates stromal progenitors in both systems, these data may suggest species difference or alternate mechanisms for distinct subsets of Foxd1+ cells.
Our analysis does not address whether the Foxd1+ cortical stroma is a homogeneous population where all Foxd1+ cells are stromal progenitor cells. Alternatively, the Foxd1+ cortical stroma may be a heterogeneous population where only a subset of Foxd1+ cells maintains a stromal-repopulating and broad differentiation capability. Indeed, the Foxd1+ cortical stroma consists of a cortical LIV2+ capsule and a medullary LIV2− nephrogenic interstitium. The current study does not distinguish whether stromal progenitor cells exist in the capsule, nephrogenic interstitium, or both. Moreover, it is possible that each domain contains heterogeneous cells with different properties. Additional fate mapping for subdomains within the Foxd1+ cortical stroma will be required to address these possibilities.
Distinct Multipotent Self-Renewing Progenitor Populations Direct Mammalian Kidney Organogenesis
Our data reinforce the critical cellular makeup and distinct developmental properties of the niche present at each branch tip of the developing ureteric tree in the mammalian kidney. Within the ureteric epithelium are the mitotically active Ret+ progenitors whose renewal establishes new branch tips in the arborizing collecting duct system and whose commitment generates the maturing medullary and cortical stalks of the differentiating ureteric epithelial network (Shakya et al., 2005). Immediately adjacent to the ureteric branch tips lie the Six2+ cap mesenchyme, a multipotent self-renewing progenitor population for the main body of the nephron. Our data demonstrate that the Foxd1+ population that surrounds the Six2+ domain is a distinct multipotent self-renewing progenitor population for renal stromal cells.
Six2 and Foxd1 expression correlates with distinct mesenchymal progenitor populations. Both genes are rapidly downregulated on commitment and movement of cells from the ureteric tip zone suggesting that expression of these markers, and maintenance of progenitor properties, is dependent on this cortical niche. Our observation of the lineage boundary between the Six2+ cap mesenchyme-derived nephron epithelial compartment and Foxd1+ cortical stroma-derived stromal compartment suggests that these cell types are largely independent progenitor pools from the onset of kidney organogenesis at 10.5 dpc, consistent with our previous observation from a clonal analysis of Osr1+ metanephric mesenchyme cells at 9.5 dpc (Mugford et al., 2008). However, our cell fate analysis indicates that a fraction of cells transiently activates Foxd1 in a cell that becomes a Six2+ nephron progenitor. Thus, Foxd1 activity does not appear to result in an irreversible commitment to a stromal progenitor fate, consistent with our analysis of Foxd1 null kidneys. The factor(s) responsible for establishing these lineage boundaries is unclear, but a number of transcriptional regulators have been mapped to each of these cellular compartments in the developing kidney (Brunskill et al., 2008; Mugford et al., 2009).
Throughout kidney organogenesis, the Six2+ and Foxd1+ cells are retained in the cortical niche. As the developing kidney grows outward, these progenitor populations leave behind differentiated derivatives, and the kidney grows along a radial medullary to cortical axis with less mature nephron and stromal cells in more cortical regions at all developmental stages. The cellular localization of progenitor compartments and their progressive commitment could facilitate the coordination of differentiation of the distinct nephron and stromal lineages during kidney organogenesis. Consistent with this view, pulse labeling shows corecruitment of cell types derived from Six2+ and Foxd1+ cells into distinct components of assembling glomeruli.
Recently, it was proposed that the outer and inner regions within the cap mesenchyme may have different properties (Das et al., 2013). Foxd1+ descendant Six2+ cells tend to localize in the outer half of the SIX2+ cap mesenchyme just after the onset of kidney development. Although this bias along the outer-inner axis in the cap mesenchyme decreases gradually, the fraction of Foxd1+ descendant cells among the entire Six2+ population increases during kidney development, though there is no evidence of additional Foxd1+ cell recruitment into the Six2+ pool during these stages. These data indicate that Foxd1+ descendant Six2+ cells in the outer region closer to the capsule may differentiate into the nephron less frequently than Six2+ cells lying closer to the ureteric epithelium, contrary to the recently proposed model where the outer cap mesenchymal cells differentiate, whereas the inner cap mesenchymal cells self-renew (Das et al., 2013). Alternatively, these Foxd1+ descendant Six2+ cells may have different properties from other Six2+ cells in the outer cap mesenchyme in cell proliferation/death and/or migration.
The relationship between developmental progenitor compartments within the kidney and the precise origins of some mature cell types that include the vasculature, macrophage, smooth muscle, and neural-associated components remain less clear, although our previous data suggest that vascular cells in the kidney and smooth muscle cells around the ureter are derived from parts of the Osr1+ intermediate mesoderm and lateral plate mesoderm at 8.5 dpc (Mugford et al., 2008). Our data also shed light on differences between the kidney and ureter. The ureter mesenchyme does not originate from the cap mesenchyme, cortical stroma, or ureteric bud. Consistent with this, the Foxd1− Tbx18+ mesenchyme between the kidney and nephric duct at the onset of kidney development contributes to the ureter mesenchyme (Bohnenpoll et al., 2013; Wang et al., 2009). Other evidence suggests a tail bud origin for these cells in the chick (Brenner-Anantharam et al., 2007), whereas the contribution of Osr1+ cell derivatives from 8.5 to 10.5 dpc to this population in the mouse points to a possible dorsal lateral plate origin (Mugford et al., 2008). Fate mapping analysis using additional tissue-specific transgenes will define progenitor populations for these cell types in the future.
Experimental Procedures
Mouse Strains
Mice were generated and maintained as described in the Supplemental Experimental Procedures. All animal work was performed under the oversight by an institutional review board at the Harvard Center for Comparative Medicine.
FACS Analysis
FACS analysis of kidney cells was performed as described in the Supplemental Experimental Procedures.
Histology
Histology on kidneys was performed as described in the Supplemental Experimental Procedures.
X-Gal Staining
X-gal staining was performed as described previously (Kobayashi et al., 2004, 2005). Details are described in the Supplemental Experimental Procedures.
Immunofluorescence
Confocal immunofluorescence was performed as described previously (Kobayashi et al., 2008, 2011). Details are described in the Supplemental Experimental Procedures.
Acknowledgments
We thank Drs. Benjamin Humphreys, Joo-Seop Park, Stuart Shankland, Ed Laufer, and Cathy Mendelsohn for critical comments on the manuscript. We thank Dr. Kevin Eggan for v6.5 129/Sv × C57BL/6 F1 hybrid embryonic stem cells, Dr. Brian Sauer for EGFPCre construct, and Drs. Daniel Metzger and Pierre Chambon for CreERT2 construct. This study used data from the GenePaint database (http://www.genepaint.org) and GUDMAP database (http://www.gudmap.org). Work in A.K.’s laboratory was supported by a grant from the NIH (DK094933), Basil O’Connor Starter Scholar Research Award from the March of Dimes, Carl W. Gottschalk Research Scholar Grant from the American Society of Nephrology, and grants from the Harvard Stem Cell Institute. Work in A.P.M.’s laboratory was supported by a grant from the NIH (DK054364).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Bohnenpoll T., Bettenhausen E., Weiss A.C., Foik A.B., Trowe M.O., Blank P., Airik R., Kispert A. Tbx18 expression demarcates multipotent precursor populations in the developing urogenital system but is exclusively required within the ureteric mesenchymal lineage to suppress a renal stromal fate. Dev. Biol. 2013;380:25–36. doi: 10.1016/j.ydbio.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Boyle S., Misfeldt A., Chandler K.J., Deal K.K., Southard-Smith E.M., Mortlock D.P., Baldwin H.S., de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S.C., Liu Z., Kopan R. Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development. 2014;141:346–354. doi: 10.1242/dev.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner-Anantharam A., Cebrian C., Guillaume R., Hurtado R., Sun T.T., Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–1975. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- Brunskill E.W., Aronow B.J., Georgas K., Rumballe B., Valerius M.T., Aronow J., Kaimal V., Jegga A.G., Yu J., Grimmond S. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev. Cell. 2008;15:781–791. doi: 10.1016/j.devcel.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill E.W., Georgas K., Rumballe B., Little M.H., Potter S.S. Defining the molecular character of the developing and adult kidney podocyte. PLoS ONE. 2011;6:e24640. doi: 10.1371/journal.pone.0024640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll T.J., Park J.S., Hayashi S., Majumdar A., McMahon A.P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–421. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Costantini F., Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Tanigawa S., Karner C.M., Xin M., Lum L., Chen C., Olson E.N., Perantoni A.O., Carroll T.J. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat. Cell Biol. 2013;15:1035–1044. doi: 10.1038/ncb2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G.R. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetting J.L., Guay J.A., Karolak M.J., Iozzo R.V., Adams D.C., Maridas D.E., Brown A.C., Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume R., Bressan M., Herzlinger D. Paraxial mesoderm contributes stromal cells to the developing kidney. Dev. Biol. 2009;329:169–175. doi: 10.1016/j.ydbio.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding S.D., Armit C., Armstrong J., Brennan J., Cheng Y., Haggarty B., Houghton D., Lloyd-MacGilp S., Pi X., Roochun Y. The GUDMAP database—an online resource for genitourinary research. Development. 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V., Huh S.O., Herzlinger D., Soares V.C., Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Herzlinger D. Inductive interactions during kidney development. Semin. Nephrol. 1995;15:255–262. [PubMed] [Google Scholar]
- Humphreys B.D., Valerius M.T., Kobayashi A., Mugford J.W., Soeung S., Duffield J.S., McMahon A.P., Bonventre J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Humphreys B.D., Lin S.L., Kobayashi A., Hudson T.E., Nowlin B.T., Bonventre J.V., Valerius M.T., McMahon A.P., Duffield J.S. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra A.K., Warot X., Brocard J., Bornert J.M., Xiao J.H., Chambon P., Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner C.M., Das A., Ma Z., Self M., Chen C., Lum L., Oliver G., Carroll T.J. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Shawlot W., Kania A., Behringer R.R. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kwan K.M., Carroll T.J., McMahon A.P., Mendelsohn C.L., Behringer R.R. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Valerius M.T., Mugford J.W., Carroll T.J., Self M., Oliver G., McMahon A.P. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Stewart C.A., Wang Y., Fujioka K., Thomas N.C., Jamin S.P., Behringer R.R. β-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development. 2011;138:1967–1975. doi: 10.1242/dev.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson R., Mendelsohn C. Stromal progenitors are important for patterning epithelial and mesenchymal cell types in the embryonic kidney. Semin. Cell Dev. Biol. 2003;14:225–231. doi: 10.1016/s1084-9521(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Levinson R.S., Batourina E., Choi C., Vorontchikhina M., Kitajewski J., Mendelsohn C.L. Foxd1-dependent signals control cellularity in the renal capsule, a structure required for normal renal development. Development. 2005;132:529–539. doi: 10.1242/dev.01604. [DOI] [PubMed] [Google Scholar]
- Lin E.E., Sequeira-Lopez M.L., Gomez R.A. RBP-J in FOXD1+ renal stromal progenitors is crucial for the proper development and assembly of the kidney vasculature and glomerular mesangial cells. Am. J. Physiol. Renal Physiol. 2014;306:F249–F258. doi: 10.1152/ajprenal.00313.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M.H., McMahon A.P. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 2012;4:a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M.H., Brennan J., Georgas K., Davies J.A., Davidson D.R., Baldock R.A., Beverdam A., Bertram J.F., Capel B., Chiu H.S. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr. Patterns. 2007;7:680–699. doi: 10.1016/j.modgep.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford J.W., Sipilä P., McMahon J.A., McMahon A.P. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev. Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugford J.W., Yu J., Kobayashi A., McMahon A.P. High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev. Biol. 2009;333:312–323. doi: 10.1016/j.ydbio.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Valerius M.T., McMahon A.P. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–2539. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- Park J.S., Ma W., O’Brien L.L., Chung E., Guo J.J., Cheng J.G., Valerius M.T., McMahon J.A., Wong W.H., McMahon A.P. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev. Cell. 2012;23:637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin S.E., Kreidberg J.A. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- Saxen L. Cambridge University Press; New York: 1987. Organogenesis of the Kidney. [Google Scholar]
- Schedl A. Renal abnormalities and their developmental origin. Nat. Rev. Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- Shakya R., Watanabe T., Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Visel A., Thaller C., Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32(Database issue):D552–D556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tripathi P., Guo Q., Coussens M., Ma L., Chen F. Cre/lox recombination in the lower urinary tract. Genesis. 2009;47:409–413. doi: 10.1002/dvg.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- Yu J., Carroll T.J., Rajagopal J., Kobayashi A., Ren Q., McMahon A.P. A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Liégeois N.J., Wong C., Finegold M., Hou H., Thompson J.C., Silverman A., Harper J.W., DePinho R.A., Elledge S.J. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


