Summary
This study investigated human-induced pluripotent stem cell (hiPSC) -derived neurons for their ability to secrete neurotransmitters in an activity-dependent manner, the fundamental property required for chemical neurotransmission. Cultured hiPSC neurons showed KCl stimulation of activity-dependent secretion of catecholamines—dopamine (DA), norepinephrine (NE), and epinephrine (Epi)—and the peptide neurotransmitters dynorphin and enkephlain. hiPSC neurons express the biosynthetic enzymes for catecholamines and neuropeptides. Because altered neurotransmission contributes to schizophrenia (SZ), we compared SZ to control cultures of hiPSC neurons and found that SZ cases showed elevated levels of secreted DA, NE, and Epi. Consistent with increased catecholamines, the SZ neuronal cultures showed a higher percentage of tyrosine hydroxylase (TH)-positive neurons, the first enzymatic step for catecholamine biosynthesis. These findings show that hiPSC neurons possess the fundamental property of activity-dependent neurotransmitter secretion and can be advantageously utilized to examine regulation of neurotransmitter release related to brain disorders.
Graphical Abstract
Highlights
-
•
hiPSC neurons show activity-dependent secretion of catecholamines and neuropeptides
-
•
hiPSC neurons express enzymes for production of catecholamines and neuropeptides
-
•
SZ hiPSC neurons show changes in catecholamines secreted
-
•
SZ hiPSC neuronal cultures display increased percentage of TH-positive neurons
In this article, Hook and colleagues show that human induced pluripotent stem cell (hiPSC) neurons possess the fundamental property of activity-dependent neurotransmitter secretion, and examination of several case studies of schizophrenia (SZ) patient-derived schizophrenia hiPSC neurons illustrate aberrant catecholamine neurotransmitters with increased percentage of tyrosine hydroxylase neurons.
Introduction
Neurotransmitters are critical for nervous system control of behaviors and physiological functions (Kandel et al., 2000; Holz and Fisher, 2012). The challenge in gaining insight into regulation of neurotransmitter secretion in human brain disorders has been the lack of human neuronal models from individual clinical cases. The human-induced pluripotent stem cell (hiPSC) neuronal approach has the capability to assess human brain cellular properties and may potentially be able to address the fundamental property of activity-dependent secretion of neurotransmitters required for chemical neurotransmission.
We therefore asked whether neurons differentiated from hiPSCs (Brennand et al., 2011; Peitz et al., 2013) possess the ability to secrete neurotransmitters in an activity-dependent manner, and whether biosynthetic enzymes for neurotransmitters are expressed. Further, to determine whether hiPSC neurons can be utilized to study neurotransmitter secretion related to a psychiatric brain disorder, we tested schizophrenia (SZ) hiPSC-derived neurons to answer the question of whether alterations in secreted neurotransmitters are recapitulated, including dopamine, which plays a role in SZ (Seeman, 1987; Coyle and Konopask, 2012; Eyles et al., 2012).
hiPSC neurons displayed activity-dependent, regulated secretion of catecholamine and peptide neurotransmitters (neuropeptides), and expressed the biosynthetic enzymes for these neurotransmitters. Relative to healthy controls, hiPSC neuronal cultures derived from SZ patients showed increases of the catecholamines dopamine (DA), norepinephrine (NE), and epinephrine (Epi). Consistent with this, SZ-derived hiPSC neuronal populations had a higher percentage of tyrosine hydroxylase (TH)-positive neurons, the first enzymatic step for catecholamine biosynthesis, suggesting a change in the number of catecholaminergic neurons in SZ hiPSC neuronal cultures. Overall, these findings show that hiPSC neurons possess the fundamental property of activity-dependent neurotransmitter release, demonstrating their value as a platform with which to investigate normal and abnormal neurotransmitter secretion related to brain function.
Results
Activity-Dependent Secretion of Catecholamine and Peptide Neurotransmitters in hiPSC Neurons
Neurotransmission requires activity-dependent secretion of neurotransmitters (Kandel et al., 2000), and KCl-stimulated secretion is widely used as a model of activity-dependent, regulated secretion of neurotransmitters (Cavallero et al., 2009; Toneff et al., 2013). We incubated neurons with high KCl (50 mM) in the culture media to stimulate depolarization-mediated secretion and measured the release of catecholamines and several peptide neurotransmitters in control hiPSC neurons during stimulation with KCl. KCl induced the secretion of DA, NE, and Epi at levels that were two to three times above those of basal secretion (Figures 1A–1C). The secretion of the neuropeptides dynorphin A and (Met)enkephalin was also stimulated by KCl (Figures 1D and 1E). These results demonstrate that hiPSC neurons possess the property of activity-dependent, regulated secretion of neurotransmitters.
Figure 1.
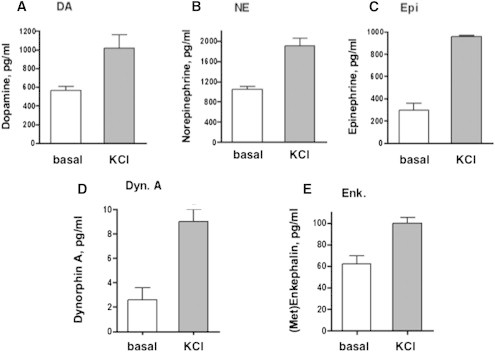
Activity-Dependent Secretion of Catecholamines and Neuropeptides from hiPSC Neurons
(A–C) Activity-dependent, regulated secretion was stimulated by high KCl in the medium (50 mM KCl, 30 min). The hiPSC neurons were derived from biopsies from healthy control individuals and were subjected to secretion in the absence or presence of KCl for basal and stimulated secretion, respectively. The collected media were assayed for levels of catecholamines dopamine (DA), norepinephrine (NE), and epinephrine (Epi), illustrated in (A), (B), and (C), respectively (from control cell lines 94293A, 4506CB, and BJ3E, respectively).
(D and E) In addition, the peptide neurotransmitters (neuropeptides) dynorphin A (Dyn. A) and (Met)enkephalin (Enk.) in the secretion media were measured by radioimmunoassay, illustrated in (D) and (E), respectively (from control cell line 4506CB).
Results are shown as the mean ± SEM, for neurotransmitters secreted under conditions of KCl-stimulated and basal secretion (n = 3 per experiment. Experiments utilized triplicate wells of cells for each condition; neurotransmitter measurements for each well were conducted in triplicate assays, and experiments were conducted twice. This experiment was also conducted with independent cell lines from three controls and 3 SZ patients, shown in Figure S1.
Neurotransmitter Biosynthetic Enzymes in hiPSC Neurons
The presence of the biosynthetic enzymes for catecholamines and neuropeptides in hiPSC neurons was assessed by immunofluorescence confocal microscopy. The catecholamine biosynthetic enzymes DOPA decarboxylase (DDC), dopamine β-hydroxylase (DBH), and phenylethanolamine N-methyltransferase (PNMT) generate DA, NE, and Epi, respectively. All of these enzymes were observed in hiPSC neurons. DDC was distributed throughout the neurons, consistent with the cytoplasmic location of DDC (Figure 2Ai). The discrete, punctate pattern of DBH subcellular distribution is consistent with its known presence in secretory vesicles for conversion of DA to NE (after transport of cytoplasmic DA into secretory vesicles, Figure 2Aii). PNMT was observed throughout hiPSC neurons, consistent with its known presence in the cytoplasm for biosynthesis of Epi, which then is transported to secretory vesicles for secretion (Figure 2Aiii).
Figure 2.
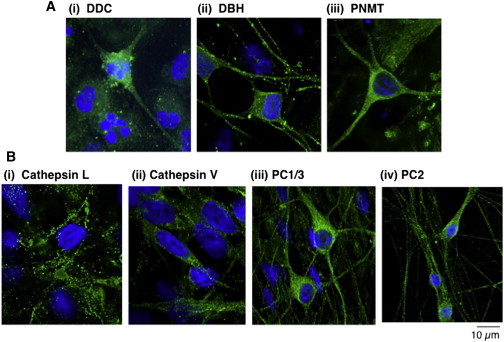
Biosynthetic Enzymes of Catecholamines and Neuropeptides Expressed in hiPSC Neurons
(A) Catecholamine biosynthetic enzymes in hiPSC neurons. Immunofluorescence confocal microscopy demonstrated the presence of the catecholamine biosynthetic enzymes DOPA decarboxylase (DDC), dopamine β-hydroxylase (DBH), and phenylethanolamine N-methyltransferase (PNMT) as shown in i, ii, and iii, respectively. These enzymes participate in the synthesis of DA, NE, and Epi, respectively. Enzyme immunofluorescence (green) was visualized with secondary anti-rabbit Alexa Fluor 488 (from Invitrogen). Nuclei were stained with DAPI blue. Controls conducted without primary antisera (directed to the enzymes) resulted in lack of immunofluorescence, demonstrating specific detection of the enzymes.
(B) Neuropeptide biosynthetic enzymes in hiPSC neurons. The presence of the proneuropeptide processing enzymes was assessed by immunofluorescence confocal microscopy for the cysteine proteases cathepsin L and cathepsin V (human) (Hook et al., 2008; Funkelstein et al., 2012) (i and ii, respectively) and the subtilisin-like proteases of prohormone convertases 1 and 2 (PC1/3 and PC2) (iii and iv, respectively) (Seidah and Prat, 2012). Enzyme immunofluorescence was visualized with secondary anti-rabbit Alexa Fluor 488 (green fluorescence, from Invitrogen). Nuclei were stained with DAPI blue. The discrete localization of these neuropeptide biosynthetic enzymes is consistent with their localization in secretory vesicles, the primary intracellular site of neuropeptide production (Hook et al., 2008).
Experiments in (A) and (B) were conducted twice with two or three different control neuronal hiPSC cell lines, with each experiment conducted with duplicate or triplicate cell samples.
Neuropeptide transmitters are synthesized as proneuropeptide precursor proteins that undergo proteolytic processing by two main protease pathways composed of the cysteine proteases cathepsin L and cathepsin V, and the subtilisin-like proteases prohormone convertases 1/3 and 2 (PC1/3 and PC2) (Hook et al., 2008; Seidah and Prat, 2012; Funkelstein et al., 2012). Cathepsins L and V were both observed in hiPSC neurons by immunofluorescence microscopy (Figure 2Bi and ii) and were present in a punctate pattern of subcellular localization consistent with their known localization to secretory vesicles for neuropeptide production. PC1/3 and PC2 immunofluorescence was also observed in a discrete pattern of subcellular distribution (Figure 2Biii and iv). These data indicate that hiPSC neurons possess neurotransmitter-synthesizing enzymes for catecholamines and neuropeptides.
SZ hiPSC Neurons Display Increased Secretion of DA, NE, and Epi Released in an Activity-Dependent Manner
We compared catecholamines secreted by SZ hiPSC neurons to those derived from healthy controls. Secretion of DA, NE, and Epi under basal conditions was greater in SZ hiPSC neurons relative to controls (Figures 3A–3C). Importantly, SZ hiPSC neurons showed increased amounts of secreted DA, NE, and Epi levels during KCl treatment, relative to controls (Figures 3A–3C). The ratios of these catecholamines secreted in an activity-dependent manner, stimulated by KCl, above baseline untreated levels was similar in SZ relative to control hiPSC neurons (Table S1 available online). These neurotransmitter secretion data were obtained as averages from hiPSC neurons derived from three SZ patients and three healthy controls (average values shown in Table S1). Values for individual SZ and control cell lines are shown in Table S2 and graphed in Figure S1.
Figure 3.
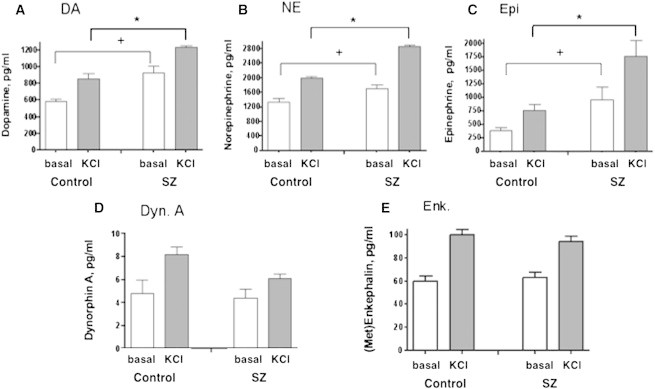
SZ hiPSC Neurons Display Increases in Dopamine, Norepinephrine, and Epinephrine Secreted in an Activity-Dependent Manner, Compared to Control hiPSC Neurons
Schizophrenia (SZ) and control hiPSC neurons were compared with respect to levels of neurotransmitters secreted in an activity-dependent manner, stimulated by KCl. The hiPSC neurons were derived from three SZ patients and from three healthy controls (as described in Experimental Procedures). Experimental conditions in each hiPSC neuronal cell line were conducted in triplicate. Neurotransmitter levels in basal and KCl-stimulated secretion media were measured for the catecholamines DA, NE, and Epi, and for the neuropeptides dynorphin A (Dyn. A) and (Met)enkephalin (Enk.). Results are shown as the mean ± SEM of three different control and three different SZ hiPSC neuronal cell lines; each cell line was studied in triplicate samples of neuronal cells. Average values of the three different cell lines for control, and SZ groups are shown in Table S1. Neurotransmitter secretion data for each individual control and SZ hiPSC neuronal cell line are shown in Figure S1 and Table S2, and experiments were conducted twice.
Data showed significant differences in levels of catecholamines secreted from SZ hiPSC neurons compared to control hiPSC neurons upon KCl stimulation (∗p < 0.05, Student’s t test). In addition, basal secretion of catecholamines was significantly increased in SZ compared to control neurons (+p < 0.05, Student’s t test).
With respect to secretion of neuropeptides, the SZ hiPSC neurons showed a 25% decrease in KCl-stimulated dynorphin A secretion (Figure 3D) compared to controls, but the decrease was not significant. There was no change in the amounts of (Met)enkephalin secreted from SZ and control hiPSC neurons (Figure 3E). Table S1 shows the average secretion values of cell lines from the three SZ cases and three control cases. Values for individual hiPSC cell lines are shown in Table S2 with graphs shown in Figure S1.
SZ hiPSC Cultures Show Increased Percentage of TH-Positive Neurons
Elevated secretion of all three catecholamines tested (DA, NE, and Epi) in SZ hiPSC neurons suggested that tyrosine hydroxylase (TH), the first and common enzymatic step of the catecholamine biosynthetic pathway, might be affected. Cell counts of TH-positive cells revealed that the percentage of TH-positive βIII-TUBULIN-positive hiPSC neurons was significantly increased in SZ relative to healthy controls (Figure 4). Notably, this difference was only observed when calculating the percentage of TH-positive cells relative to βIII-TUBULIN-positive neurons, and not when relative to total DAPI-positive nuclei (data not shown). However, analyses of DDC, DBH, and PNMT by gene expression microarray (Brennand et al., 2011) and western blots showed no change in levels of these biosynthetic enzymes for DA, DBH, and Epi, respectively (data not shown). This result is consistent with elevations in secreted amounts of all three catecholamines (Figure 3).
Figure 4.
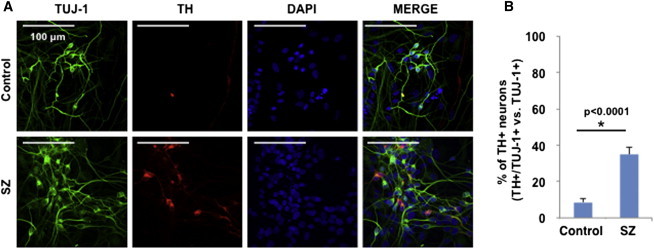
Tyrosine Hydroxylase Neurons in SZ hiPSC Neuronal Cultures
SZ hiPSC neurons displayed an increased percentage of TH-positive neurons, illustrated by anti-TH immunofluorescence microscopy (A) with quantitation (B).
(A) TH immunofluorescence (red) was compared to that of the neuronal marker TUJ-1 (green), providing an indication of the percentage of TH-positive neurons (colocalized yellow immunofluorescence).
(B) The percentage of TH-positive neurons in SZ hiPSC neuronal cultures was significantly increased compared to controls (mean ± SEM, with ∗statistical significance of p < 0.05, Student’s t test, n = 3).
See also Figure S2. Results were compiled from three different control and three different SZ neuronal cell lines (each derived from an individual patient), conducted in three experiments, with each experiment having three cellular replicates.
Discussion
Activity-dependent regulated neurotransmitter secretion is a fundamental requirement for neuronal communication. This study shows that hiPSC neurons display activity-dependent, regulated secretion of the catecholamines DA, NE, and Epi, as well as the peptide neurotransmitters dynorphin A and (Met)enkephalin, when stimulated by KCl depolarization. Secretion and biosynthesis of these neurotransmitters is supported by the presence of the catecholamine biosynthetic enzymes DDC, DBH, and PNMT, as well as TH, the first enzymatic step for biosynthesis of catecholamines. hiPSC neurons also express the neuropeptide biosynthetic enzymes composed of cysteine proteases, cathepsin L and cathepsin V, and the subtilisin-like prohormone convertases 1 and 2 (PC1/3 and PC2). Thus, hiPSC neurons produce and secrete neurotransmitter molecules, indicating that they are a relevant platform for the study of aberrant neurotransmitter systems in human disease.
hiPSC neurons derived from three patients with SZ, compared to controls, showed elevated secretion of DA, NE, and Epi. The increase in secretion of all three catecholamines suggests an increase in TH, the first enzymatic step common to all catecholamine biosynthesis. Consistent with the increase in catecholamine secretion, we detected an increased percentage of TH-positive neurons in hiPSC neurons derived from these cases of SZ patients. SZ hiPSC neurons (compared to controls), thus, appear to have differentiated into, and/or preferentially survived as, neuronal populations with a larger portion of TH-positive neurons. These case studies of several SZ patients provide insight into the hypothesis that catecholamine neurotransmitters may be regulated in SZ.
It is important to clarify that increased catecholamine release does not necessarily indicate increased production in SZ hiPSC neurons but may instead imply a difference in the overall activity of one or more neuronal subtypes (Yu et al., 2014) and/or a change in the composition of neuronal populations (Robicsek et al., 2013) throughout the differentiation of these heterogeneous SZ hiPSC neuronal cultures. Indeed, an increased percentage of TH-positive neurons was observed in the SZ hiPSC neurons relative to controls, which likely accounts for the elevated catecholamines detected. A number of possible explanations might account for the increased number of TH-positive neurons observed, including, but not limited to, different rates of TH specification, maturation, or survival relative to other neurons in the culture. Given that our previously reported microarray comparisons of SZ and control hiPSC neural progenitor cells (NPCs) (Brennand et al., 2014) and neurons (Brennand et al., 2011) failed to detect increased patterning of DA and catecholamine fate, we posit that the differences observed more likely reflect differential survival of TH neurons rather than increased specification. Because changes in TH staining were not reflected in gene expression differences, this increase might instead indicate graded levels of protein, changing protein stability, and/or changes in protein translation. It is also possible that aberrant regulation of reuptake and metabolism of catecholamines in SZ hiPSC neurons may occur. We further speculate that the increased oxidative stress that we (Brennand et al., 2014) and others (Paulsen et al., 2012; Robicsek et al., 2013) have reported in SZ hiPSC neurons may be contributing to our observed results, and note that increased TH staining is generally reflected in neurons derived from higher passage NPCs, which might have been exposed to increasing amounts of stress with increased time in culture. Finally, because the observed increase in TH-positive neurons was heavily skewed by results in two of three SZ patients, we predict that in future studies of larger cohorts, hiPSC neurons derived from some but not all SZ patients may show this effect. Thus, the variability between patient-derived neurons in vitro may reflect real variance between SZ patients and could underlie, in part, the differences in drug responsiveness among patients. The molecular and cellular mechanisms contributing to these differences remain to be elucidated.
The increase in the portion of TH-positive (relative to βIII-TUBULIN-positive) neurons in SZ hiPSC neurons is consistent with the upregulation of neuroregulin in SZ (Kato et al., 2011), which is thought to upregulate the expression of TH, resulting in increased DA and a hyperdopaminergic state (Kato et al., 2011). Alternately, it may be consistent with increased oxidative stress in SZ brain tissue causing preferential loss of non-catecholamine-producing neurons (Behrens et al., 2007). Though a recent postmortem study of SZ patients assessed TH protein levels in the substantia nigra/ventral tegmental brain area, great variability was observed among SZ brain samples compared to controls (Perez-Costas et al., 2012); another study found decreased TH-immunoreactive axons, but it is not known whether the data indicated fewer axons from individual neurons or fewer TH neurons (Akil et al., 2000). Nonetheless, these findings, when combined with our results, point to the importance of TH levels in SZ.
Increased DA secretion from SZ hiPSC neurons is consistent with the ability of current antipsychotics, functioning as D2 DA receptor antagonists, to ameliorate the positive symptoms of SZ (Seeman, 1987; Coyle and Konopask, 2012; Eyles et al., 2012). Interestingly, because current antipsychotics lack efficacy for improving the cognitive dysfunction of SZ (Heinrichs and Zakzanis, 1998; Young et al., 2012; Coyle and Konopask, 2012), it is possible that neurotransmitters other than DA contribute to SZ such as those identified in hiPSC neuronal models. Other neurotransmitter systems involved in SZ may include glutamate (Snyder and Gao, 2013; Menniti et al., 2013), serotonin (Meltzer et al., 2012), and GABA (Volk and Lewis, 2013). It will be advantageous in future studies to characterize the activity-dependent release of these other neurotransmitter systems in SZ hiPSC neurons.
Great progress has been achieved in recent years in development of hiPSC neuronal systems to model human brain diseases (Kiskinis and Eggan, 2010; Brennand et al., 2011; Young and Goldstein, 2012; Peitz et al., 2013). The ability to investigate the critical property of activity-dependent neurotransmitter release from hiPSC neurons derived from patients afflicted with mental disorders and neurological diseases can provide knowledge of chemical neurotransmitter mechanisms in brain disorders.
Experimental Procedures
hiPSC Neurons Derived from Control and SZ Patients
Control and SZ hiPSC neurons were derived from patient samples as we have described (Brennand et al., 2011). Patient samples were obtained from the Coriell collection. SZ patient GM01792 was a male, 26 years of age, Jewish Caucasian, who displayed agitation, delusions, and fear of assassination. Patient GM02038 was a male, 22 years old, who was diagnosed at 6 years of age and committed suicide at 22. Patient GM2497 was a male, 27 years old, Jewish Caucasian, who was diagnosed at age 15 years and showed symptoms including paralogical thinking, affective shielding, and suspiciousness. Healthy control hiPSC neurons were derived from AG09429 (female, 25 years old), GM04506 (female, 22 years of age), and BJ (male, newborn, from fibroblasts obtained from ATCC).
Preparation of hiPSC neurons was conducted as we have described (Brennand et al., 2011). Briefly, SZ and control fibroblasts were reprogrammed with tetracycline-inducible lentiviruses expressing the transcription factors OCT4, SOX2, KLF4, mCYC, and LIN28. The hiPSCs were differentiated to replicating neural progenitor cells and then to mature neurons via BDNF, GDNF, dibutyryl-cAMP, and ascorbic acid. Differentiation into neurons has been characterized with neuron-specific markers of βIII-tubulin, MAP2AB, PSD95, vGLUT, and GAD (Brennand et al., 2011).
KCl-Stimulated and Basal Neurotransmitter Secretion from hiPSC Neurons in Culture
Cultured hiPSC neuronal cell lines (3 months age) from the SZ and control patients were subjected to treatment with high KCl in the medium (50 mM, 30 min), which depolarizes neurons to result in activity-dependent stimulation of secretion (Toneff et al., 2013). KCl depolarization represents a model of activity-dependent neurotransmitter secretion from the regulated secretory pathway of cultured neurons. Basal secretion without KCl was conducted in parallel, which represents constitutive secretion. Secretion media were collected for measurements of the catecholamines DA, NE, and Epi, as well as the peptide neurotransmitters (Met)enkephalin and dynorphin A.
Measurement of Secreted Catecholamines and Peptide Neurotransmitters
The catecholamines DA, NE, and Epi were assayed in the media as described previously (Kennedy and Ziegler, 1990). The peptide neurotransmitters (Met)enkephalin and NPY were measured by radioimmunoassays (Phoenix Pharmaceuticals). Assays were conducted from triplicate cell-culture wells for basal and KCl-stimulated conditions.
Immunofluorescence Microscopy of Neurotransmitter Biosynthetic Enzymes
Immunofluorescence histochemistry of neurotransmitter biosynthetic enzymes was assessed by deconvolution microscopy, as we have described (Funkelstein et al., 2012). Briefly, hiPSC neurons were fixed in 4% paraformaldehyde (PFA), permeabilized with 0.1% Triton X-100 (5–10 min), blocked in 3% BSA in PBS (30 min), and incubated with primary antibodies directed to neurotransmitter biosynthetic enzymes. Antisera to DDC, DBH, and PNMT (rabbit antisera from Millipore, AB 1569, AB1585, and AB110, respectively, at 1:75, 1:100, and 1:100 dilution) were incubated with fixed cells for 2 hr at room temperature in 3% BSA in PBS, followed by incubation with secondary antibody consisting of goat anti-rabbit Alexa Fluor 488 for green immunofluorescence (1:300, Molecular Probes/Life Technologies) for 45 min at room temperature in 3% BSA in PBS. DAPI staining of nuclei was also conducted. Immunofluorescence was examined with the Delta Vision Spectris Image Deconvolution System on an Olympus IX70 microscope using Softworx Explorer software (Applied Precision). As control, incubation with only secondary antibodies was performed, which resulted in a lack of immunofluorescence, indicating specific immunofluorescence observed with the primary antisera.
For TH immunfluorescence staining, fixed neurons were blocked in 3% normal goat serum (in TBS), incubated with anti-TH (1:200, rabbit, from Pel-Freeze Biologicals) or anti-TUJ-1 (1:500, mouse, from Covance) at 4°C overnight, washed, incubated with secondary antibody (1:200, anti-rabbit-cy3 or anti-mouse-FITC, respectively) 45 min at room temperature followed by washing, and observed by LSM780 confocal microscopy (Zeiss).
Acknowledgments
This work was supported by an Academic Senate grant (RL040H) from UC San Diego, a 2013 NARSAD distinguished investigator grant, and NIH grant R01 MH077305 to V.H., as well as NIH grant U01GM092655 (to Dr. W. Sadee, Ohio State University, and V.H., UCSD). Grant support for F.H.G. was provided by the The Helmsely Foundation, The Engman Family, the JPB Foundation, the Lookout Foundation, The Streim Family, and a 2013 NARSAD distinguished investigator grant. K.J.B. is a New York Stem Cell Foundation–Robertson Investigator; K.J.B.’s laboratory is supported by a Brain and Behavior Young Investigator Grant, NIH grant R01 MH101454, and the New York Stem Cell Foundation. Grant support to M.Z. was provided by the NIH (UL1TR000100 and P01HL58120).
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Supplemental Information
References
- Akil M., Edgar C.L., Pierri J.N., Casali S., Lewis D.A. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol. Psychiatry. 2000;47:361–370. doi: 10.1016/s0006-3223(99)00282-6. [DOI] [PubMed] [Google Scholar]
- Behrens M.M., Ali S.S., Dao D.N., Lucero J., Shekhtman G., Quick K.L., Dugan L.L. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Brennand K.J., Simone A., Jou J., Gelboin-Burkhart C., Tran N., Sangar S., Li Y., Mu Y., Chen G., Yu D. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K., Savas J.N., Kim Y., Tran N., Simone A., Hashimoto-Torii K., Beaumont K.G., Kim H.-J., Topol A., Ladran I. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol. Psychiatry. 2014;2014:1. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallero A., Marte A., Fedele E. L-aspartate as an amino acid neurotransmitter: mechanisms of the depolarization-induced release from cerebrocortical synaptosomes. J. Neurochem. 2009;110:924–934. doi: 10.1111/j.1471-4159.2009.06187.x. [DOI] [PubMed] [Google Scholar]
- Coyle J.T., Konopask G.T. The neurochemistry of schizophrenia. In: Brady S.T., Siegel G.J., Albers R. Wayne, Price D.L., editors. Basic Neurochemistry. 8th edition. Elsevier; Amsterdam: 2012. pp. 1000–1011. [Google Scholar]
- Eyles D., Feldon J., Meyer U. Schizophrenia: do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl. Psychiatr. 2012;2:e81. doi: 10.1038/tp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L., Lu W.D., Koch B., Mosier C., Toneff T., Taupenot L., O’Connor D.T., Reinheckel T., Peters C., Hook V. Human cathepsin V protease participates in production of enkephalin and NPY neuropeptide neurotransmitters. J. Biol. Chem. 2012;287:15232–15241. doi: 10.1074/jbc.M111.310607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs R.W., Zakzanis K.K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Holz R.W., Fisher S.K. Synaptic transmission and cellular signaling: an overview. In: Braday S.T., Siegel G.J., Alberts R.W., Price D.L., editors. Basic Neurochemistry. Academic Press; Amsterdam: 2012. pp. 236–257. [Google Scholar]
- Hook V., Funkelstein L., Lu D., Bark S., Wegrzyn J., Hwang S.R. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R., Schwartz J.H., Jessell T.M. McGraw-Hill; New York: 2000. Principles of Neural Science; pp. 251–277. [Google Scholar]
- Kato T., Abe Y., Sotoyama H., Kakita A., Kominami R., Hirokawa S., Ozaki M., Takahashi H., Nawa H. Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopmental hypothesis for schizophrenia. Mol. Psychiatry. 2011;16:307–320. doi: 10.1038/mp.2010.10. [DOI] [PubMed] [Google Scholar]
- Kennedy B., Ziegler M.G. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- Kiskinis E., Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J. Clin. Invest. 2010;120:51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H.Y., Massey B.W., Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr. Pharm. Biotechnol. 2012;13:1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- Menniti F.S., Lindsley C.W., Conn P.J., Pandit J., Zagouras P., Volkmann R.A. Allosteric modulators for the treatment of schizophrenia: targeting glutamatergic networks. Curr. Top. Med. Chem. 2013;13:26–54. doi: 10.2174/1568026611313010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen Bda.S., de Moraes Maciel R., Galina A., Souza da Silveira M., dos Santos Souza C., Drummond H., Nascimento Pozzatto E., Silva H., Jr., Chicaybam L., Massuda R. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant. 2012;21:1547–1559. doi: 10.3727/096368911X600957. [DOI] [PubMed] [Google Scholar]
- Peitz M., Jungverdorben J., Brüstle O. Disease-specific iPS cell models in neuroscience. Curr. Mol. Med. 2013;13:832–841. doi: 10.2174/1566524011313050014. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E., Melendez-Ferro M., Rice M.W., Conley R.R., Roberts R.C. Dopamine pathology in hydroxylase in the substantia nigra. Front Psych. 2012;3:31. doi: 10.3389/fpsyt.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robicsek O., Karry R., Petit I., Salman-Kesner N., Müller F.J., Klein E., Aberdam D., Ben-Shachar D. Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol. Psychiatry. 2013;18:1067–1076. doi: 10.1038/mp.2013.67. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- Seidah N.G., Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat. Rev. Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- Snyder M.A., Gao W.J. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front. Cell. Neurosci. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneff T., Funkelstein L., Mosier C., Abagyan A., Ziegler M., Hook V. Beta-amyloid peptides undergo regulated co-secretion with neuropeptide and catecholamine neurotransmitters. Peptides. 2013;46:126–135. doi: 10.1016/j.peptides.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk D.W., Lewis D.A. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248C:154–164. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.E., Goldstein L.S. Alzheimer’s disease in a dish: promises and challenges of human stem cell models. Hum. Mol. Genet. 2012;21(R1):R82–R89. doi: 10.1093/hmg/dds319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.W., Powell S.B., Geyer M.A. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2012;62:1381–1390. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.X., Di Giorgio F.P., Yao J., Marchetto M.C., Brennand K., Wright R., Mei A., McHenry L., Lisuk D., Grasmick J.M. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2014;2:295–310. doi: 10.1016/j.stemcr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


