Abstract
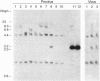
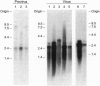
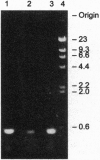
Oncogenic retroviruses carry coding sequences that are transduced from cellular protooncogenes. Natural transduction involves two nonhomologous recombinations and is thus extremely rare. Since transduction has never been reproduced experimentally, its mechanism has been studied in terms of two hypotheses: (i) the DNA model, which postulates two DNA recombinations, and (ii) the RNA model, which postulates a 5' DNA recombination and a 3' RNA recombination occurring during reverse transcription of viral and protooncogene RNA. Here we use two viral DNA constructs to test the prediction of the DNA model that the 3' DNA recombination is achieved by conventional integration of a retroviral DNA 3' of the chromosomal protooncogene coding region. For the DNA model to be viable, such recombinant viruses must be infectious without the purportedly essential polypurine tract (ppt) that precedes the 3' long terminal repeat (LTR) of all retroviruses. Our constructs consist of a ras coding region from Harvey sarcoma virus which is naturally linked at the 5' end to a retroviral LTR and artificially linked at the 3' end either directly (construct NdN) or by a cellular sequence (construct SU) to the 5' LTR of a retrovirus. Both constructs lack the ppt, and the LTR of NdN even lacks 30 nucleotides at the 5' end. Both constructs proved to be infectious, producing viruses at titers of 10(5) focus-forming units per ml. Sequence analysis proved that both viruses were colinear with input DNAs and that NdN virus lacked a ppt and the 5' 30 nucleotides of the LTR. The results indicate that DNA recombination is sufficient for retroviral transduction and that neither the ppt nor the complete LTR is essential for retrovirus replication. DNA recombination explains the following observations by others that cannot be reconciled with the RNA model: (i) experimental transduction is independent of the packaging efficiency of viral RNA, and (ii) experimental transduction may invert sequences with respect to others, as expected for DNA recombination during transfection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Cichutek K., Duesberg P. H. Transforming function of proto-ras genes depends on heterologous promoters and is enhanced by specific point mutations. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2217–2221. doi: 10.1073/pnas.88.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Harvey ras genes transform without mutant codons, apparently activated by truncation of a 5' exon (exon -1). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2340–2344. doi: 10.1073/pnas.83.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichutek K., Duesberg P. H. Recombinant BALB and Harvey sarcoma viruses with normal proto-ras-coding regions transform embryo cells in culture and cause tumors in mice. J Virol. 1989 Mar;63(3):1377–1383. doi: 10.1128/jvi.63.3.1377-1383.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Structure of the provirus within NIH 3T3 cells transfected with Harvey sarcoma virus DNA. J Virol. 1981 Apr;38(1):125–135. doi: 10.1128/jvi.38.1.125-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Evidence that retroviral transduction is mediated by DNA not by RNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3604–3608. doi: 10.1073/pnas.87.9.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral recombination during reverse transcription. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2052–2056. doi: 10.1073/pnas.87.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral transduction of oncogenic sequences involves viral DNA instead of RNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3733–3737. doi: 10.1073/pnas.85.11.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hay N., Bishop J. M. The role of RNA molecules in transduction of the proto-oncogene c-fps. Cell. 1986 Mar 28;44(6):935–940. doi: 10.1016/0092-8674(86)90016-4. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Repaske R., Garon C. F., Chan H. W., Rowe W. P., Martin M. A. Characterization of proviruses cloned from mink cell focus-forming virus-infected cellular DNA. J Virol. 1982 Feb;41(2):435–448. doi: 10.1128/jvi.41.2.435-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Maisel J., Dina D., Duesberg P. Murine sarcoma viruses: the helper-independence reported for a Moloney variant is unconfirmed; distinct strains differ in the size of their RNAs. Virology. 1977 Jan;76(1):295–312. doi: 10.1016/0042-6822(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Olson P., Temin H. M., Dornburg R. Unusually high frequency of reconstitution of long terminal repeats in U3-minus retrovirus vectors by DNA recombination or gene conversion. J Virol. 1992 Mar;66(3):1336–1343. doi: 10.1128/jvi.66.3.1336-1343.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Hughes S. H. Polypurine tract adjacent to the U3 region of the Rous sarcoma virus genome provides a cis-acting function. J Virol. 1982 Aug;43(2):482–488. doi: 10.1128/jvi.43.2.482-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Dieckmann M., Berg P. Transduction of cellular neo mRNA by retrovirus-mediated recombination. J Virol. 1990 Dec;64(12):5783–5796. doi: 10.1128/jvi.64.12.5783-5796.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A., Coffin J. M. Mechanism of transduction by retroviruses. Science. 1992 Feb 14;255(5046):841–845. doi: 10.1126/science.1371365. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Weiss R. A. How RNA makes DNA. Science. 1994 Jun 24;264(5167):1954–1955. doi: 10.1126/science.264.5167.1954-a. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Zhang J., Temin H. M. Rate and mechanism of nonhomologous recombination during a single cycle of retroviral replication. Science. 1993 Jan 8;259(5092):234–238. doi: 10.1126/science.8421784. [DOI] [PubMed] [Google Scholar]