Abstract
Background
Matrix metalloproteinase-1 (MMP-1) is a member of a family of enzymes that can degrade most extracellular matrix macromolecules. Extracellularly, MMPs are controlled by tissue inhibitors of metalloproteinases (TIMPs) and by mechanisms of pro-MMP activation. Levels of MMPs and TIMPs change during healing, inflammation, and normal tissue turnover. Herein we aimed to evaluate the levels of MMP-1 and TIMP-1 in gingival crevicular fluid (GCF) from periodontally healthy patients (control group) and chronic periodontitis patients before and after phase 1 therapy.
Methods
In this study we examined 30 patients who had chronic periodontitis with probing depth sites ⩾5 mm and a clinical attachment level (CAL) ⩾5 mm. We included 30 periodontally healthy patients as a control. Clinical measurements such as plaque (PI) and gingival (GI) indices, papillary bleeding index (PBI), probing depths (PD), and CAL were recorded both before treatment (BT) and after phase I periodontal treatment (AT). Assays for MMP-1 and TIMP-1 were performed with an enzyme-linked immunosorbent assay (ELISA) method.
Results
All clinical parameters were significantly reduced at the post-therapy visit. MMP-1 levels were significantly higher in patients BT than the controls; however, the patients AT were not statistically different than the controls. TIMP-1 levels in patients BT were significantly lower than in the controls and significantly lower than patients AT. We observed a significant positive correlation between GCF volume and MMP-1 levels. Furthermore, TIMP-1 levels were significantly negatively correlated with both GCF volume and all clinical parameters.
Conclusions
We observed that as the extent of periodontal destruction increases, MMP-1 concentration increases and TIMP-1 concentration decreases in GCF. When chronic periodontitis patients were treated by scaling and root planing (SRP), the average MMP-1 concentrations decreased and TIMP-1 concentrations increased in GCF.
Keywords: MMP-1, TIMP-1, Chronic periodontitis, Gingival crevicular fluid
1. Introduction
Periodontitis is an infectious disease characterized by periodontal attachment loss, bone destruction, and eventually tooth loss (Socransky and Haffajee, 1992). The tissue destruction appears to result from a complex interaction between bacteria and the host immune system and a complex network of cytokines, prostaglandins, reactive oxygen species, and proteolytic enzymes. Matrix metalloproteinases (MMPs) are a genetically distinct but structurally related family of host-derived proteolytic enzymes involved in the physiological and pathological degradation of extracellular matrix and basement membrane proteins (Birkedal-Hansen, 1993). To date, 24 different MMPs have been cloned and 23 of those are found in humans.
Fibrillar collagens are resistant to most proteinases and have the potential to initiate collagenolysis during periodontal inflammation. MMP-1, or interstitial collagenase, is one of the key proteolytic enzymes that degrade fibrillar collagens, especially types I and III, which are the predominant types of interstitial collagens in gingiva. MMP-1 is expressed by fibroblasts, endothelial cells, macrophages, hepatocytes, chondrocytes, osteoblasts, tumor cells, and migrating epidermal keratinocytes, but not neutrophils (Birkedal-Hansen, 1993). Tissue inhibitors of metalloproteinases (TIMPs) regulate the proteolytic activity of MMPs. For example, TIMP-1 effectively inhibits the activity of interstitial collagenase, such as MMP-1 (Shibata et al., 1991). TIMPs are expressed by many cells, including fibroblasts, keratinocytes, monocytes, macrophages, endothelial cells, and osteoblasts. There are four identified types of TIMPs, TIMP-1, -2, -3, and -4, which are widely distributed in oral and body fluids. The correct balance of MMPs and TIMPs is critical to degrade the connective tissue matrix under both physiological and pathological conditions. Thus, it is important to study the relationship between MMPs and TIMPs, as they reflect the status of periodontal diseases. The present study aimed to study MMP-1 and TIMP-1 levels in healthy patients and chronic periodontitis patients. Further, we examined the effect of phase 1 therapy on MMP-1 and TIMP-1 levels.
2. Materials and methods
2.1. Study population
A total of 60 subjects between 25 and 55 years of age were selected from patients visiting the Department of Periodontics of Vidya Shikshan Prasarak Mandal Dental College and Research Centre, Nagpur, India. These patients were assessed clinically and biochemically. All protocols were cleared by the Institutional Ethics Committee of our institute. We designed a survey to allow systematic recording of information and observations. The survey included a detailed case history, clinical examination, periodontal indices, and the patient’s written consent. The study comprised of two groups: group I, a control group of 30 periodontally healthy subjects, and group II, a test group of 30 patients with chronic periodontitis who were assessed before therapy (BT) and after phase 1 therapy (AT) – It included oral hygiene instructions and scaling and root planing. Patients were considered healthy if they exhibited a probing depth <3 mm and there was no clinical attachment loss (CAL). The average age of this group was 35.13 ± 7.14. Patients were diagnosed with chronic periodontitis if they exhibited a probing pocket depth ⩾5 mm and CAL ⩾5 mm at multiple sites. The average age of this group was 29.6 ± 4.84 years. All subjects were generally in good health and none had received periodontal treatment or medication during the past 6 months. No participants had a history of systemic conditions such as heart disease, diabetes, or other disorders that could influence the course of periodontal disease. Patients were not on any medication that could affect the manifestations of periodontal disease such as antibiotics, or their use in the 6 months prior, phenytoin, cyclosporine, anti-inflammatory drugs, or calcium channel blockers. Postmenopausal women and smokers were excluded from the study.
The clinical evaluation of patients was based on the following indices: plaque index (PI) (Silness & Loe, 1964), gingival index (GI) (Loe & Silness, 1963), probing depths (PD), CAL, and papillary bleeding index (Muhlemann, 1977). All parameters, which were recorded for the complete dentition, were measured using a Williams probe calibrated in mm. The most severely affected upper anterior sextant (maxillary incisors and the canine teeth) was chosen for collection of gingival crevicular fluid (GCF). GCF sampling and clinical index scores were recorded at baseline before treatment and 6 weeks after phase I periodontal therapy. All patients underwent therapy including oral hygiene instruction, scaling, root planning, and gingival curettage under local anesthesia.
2.2. GCF sampling and processing
All of the patients were informed about the procedure and were seated comfortably in the dental chair. Prior to GCF collection, the supragingival soft deposits were removed without causing trauma to the gingival crevice. If hemorrhage was evident after this procedure the GCF was not collected from that site. GCF samples were collected from 13 to 23 regions because the regions were easy to isolate and salivary contamination could be avoided. A microcapillary pipette was used to collect GCF samples. The area was thoroughly irrigated with distilled water, isolated by cotton rolls, and dried by stream of air. Suction was also used frequently to aspirate saliva to avoid GCF contamination. In subjects with clinically healthy gingiva, a ring of clear GCF was seen 15–20 min after the isolation of maxillary teeth at the gingival margin and interdental areas. In chronic gingivitis and chronic periodontitis patients, the clear ring of GCF could easily be seen after 5–10 min. The microcapillary pipette was then placed extracrevicularly in the accumulated fluid, and the GCF was collected in the microcapillary tube. Approximately 10 μl of GCF was collected from patients with chronic periodontitis and 4–5 μl of GCF was collected from patients with healthy gingiva. The samples were stored for a maximum time of 24 h at −20 °C before analysis.
2.3. Estimation of MMP-1 and TIMP-1 levels using ELISA
We used a Ray Bio® (Ray Biotech, Inc., USA) Human MMP-1 and TIMP-1 Enzyme Linked Immunosorbent Assay (ELISA). This kit is an in vitro ELISA that quantitatively measures human MMP-1 and TIMP-1 in serum, plasma, cell culture supernatants, and urine. This assay uses human specific MMP-1 and TIMP-1 antibodies coated on a 96-well plate. Standards and samples were pipetted into the wells, and the MMP-1 and TIMP-1 bound to the wells through the immobilized antibody. The wells were washed and biotinylated anti-human MMP-1 and TIMP-1 antibodies were added. After washing to remove unbound biotinylated antibody, we pipetted HRP-conjugated streptavidin into the wells. The wells were washed, and subsequently, a Tetra methylbenzidine (TMB) substrate solution was added to the wells. Color developed in proportion to the amount of MMP-1 and TIMP-1 bound to the well. The stop solution changed the color from blue to yellow, and the color intensity was measured at 450 nm. MMP-1 and TIMP-1 concentrations were determined using a standard curve.
2.4. Statistical analysis
Statistical analysis was done using STRATA software version 10.0. Clinical parameters such as PD, CAL, PI, GI, and papillary bleeding index, and MMP-1and TIMP-1 levels were calculated for non normalized data. Paired ‘t’ tests were performed to compare BT and AT for all parameters. BT and AT were compared with control using an unpaired ‘t’ test. A correlation coefficient (r) was calculated to assess the relationship between clinical parameters and MMP-1 or TIMP-1. A p value <.05 was considered statistically significant.
3. Results
For all clinical parameters we observed a statistically significant difference between BT patients and the control group and between AT patients and their control group. Additionally, all of the clinical parameters were significantly reduced from BT to AT. MMP-1 levels were significantly higher BT compared to the control subjects, but MMP-1 levels were not significantly different between patients AT compared to the control group. Finally, AT patients had significantly lower MMP-1 levels than BT patients. We noted a significant positive correlation between GCF volume and MMP-1 levels.
TIMP-1 levels were significantly higher in the control group than in patients BT, and TIMP-1 levels were also significantly higher AT than BT. We also observed a significant difference in TIMP-1 levels between AT patients and the control group. Finally, TIMP-1 was significantly negatively correlated with GCF volume and all clinical parameters.
4. Discussion
Periodontal diseases are characterized by a loss of extracellular matrix constituents, such as collagen fibers, in periodontal tissues. A possible mechanism for periodontal extracellular matrix degradation is the independent and/or cooperative action of human and bacterial proteinases (Sorsa et al., 1992). It is likely that a significant portion of periodontal tissue destruction is mediated by a host of cell-derived MMPs. There are four known mechanisms that regulate MMP functional activity: (1) positive and negative transcriptional controls of MMP genes; (2) the activation from a latent state; (3) differences in substrate specificity; and (4) modulation by serum inhibitors or TIMPs (Birkedal-Hansen, 1993).
A number of studies have suggested that osteoblasts express fibroblast collagenase when stimulated by bone-resorbing agents (Otsuka et al., 1984; Civitelli et al., 1989). MMP-1 is expressed by osteoblasts in response to resorptive signals such as parathyroid hormone. This leads to the initiation of osteoclastic bone resorption, which in turn leads to degradation of the unmineralized collagenous osteoid layer. Thus MMP-1 contributes to collagen degradation and bone resorption which leads to worsening of periodontal disease. Ingman et al. (1996) reported that MMP-1 is the predominant collagenase in the GCF of patients with localized juvenile periodontitis. Further, MMP-1 mRNA increases in inflammatory lesions of adult patients with periodontitis (Aiba et al., 1996; Nomura et al., 1993; Meikle et al., 1994). Thus, MMP-1 may also participate in collagen degradation in advanced periodontal disease.
We found that MMP-1 levels are increased in patients with chronic periodontitis compared to a healthy group, and MMP-1 levels decrease after phase I therapy. TIMP-1 levels were lower in patients with chronic periodontitis when compared to a healthy group, and TIMP-1 levels increased significantly after phase I therapy. These results are in accordance with results published by Tuter et al. (2002), who also investigated the levels of MMP-1 and TIMP-1 before and after phase I therapy.
Haerian et al. (1996) used a sandwich ELISA to evaluate the effect of scaling and root planing on the levels of fibroblast collagenase, stromelysin, and TIMP in the GCF of patients with advanced periodontal disease. They reported that TIMP levels in GCF were significantly increased after the phase I therapy, and TIMP levels were significantly reduced at the follow-up visit 3 months later. Utilizing ELISA we found that TIMP-1 levels were higher AT than BT; however, we measured total enzyme, which includes both latent and active enzymes. It is possible that post-treatment levels of active enzyme were low.
There are several possible explanations for the increased TIMP-1 levels we observed after periodontal therapy. We observed a reduction in MMP-1, which if present would bind to free TIMP; however, TIMP-1 regulation may not solely be dependent on MMP-1 (Howard et al., 1991). Decreased TIMP-1 levels in periodontally diseased patients may be due to selective degradation of TIMP-1 by neutrophil elastase or the inactivation of TIMP-1 by neutrophils following oxidant release (Okada et al., 1988). Phase I periodontal therapy may have reduced the number of neutrophils and the elastase released due to resolved gingival inflammation and tissue healing, thus resulting in increased TIMP-1 levels. Another possibility is that the increased TIMP-1 levels reflect its involvement in the healing process (Haerian et al., 1996). In addition, there might be clearance of MMP-TIMP-1 complexes by an as yet unidentified mechanism. Figueredo et al. (2004) demonstrated that non-surgical treatment is effective in both improving clinical parameters and reducing protease activity in GCF of chronic periodontitis patients. Similar to our study, both Kumar et al. (2013) and Tuter et al. (2005) reported an increase in TIMP-1 levels after phase I therapy.
Soell et al. (2002) evaluated MMP and TIMP levels in GCF and reported that MMP-1 levels were high in patients with chronic periodontitis when compared to controls; whereas TIMP-1 levels were lower in patients with chronic periodontitis compared to controls. Tuter et al. (2005, 2007) reported similar results. Haerian et al. (1996) pooled data from healthy, gingivitis, and periodontitis sites and observed a moderately positive, yet significant, correlation between the biochemical parameters, GCF, stromelysin, and TIMP levels with clinical parameters.
We found a significant, strong positive correlation between MMP-1 levels and both PI and GI; whereas we found a significant strong negative correlation between TIMP-1 levels and both PI and GI. These results indicate that the basis for dividing patients into the two groups in our study was appropriate. This result of our study is in accordance with Tuter et al. (2005), who also reported a positive correlation between MMP-1 levels and all the clinical parameters and a negative correlation between TIMP-1 levels and all the clinical parameters. Similarly, Pozo et al. (2005) reported a significant positive correlation between MMP and PD, CAL, and bleeding on probing, and they reported that metalloproteinase has a negative correlation with TIMP-1 levels. Notably, some studies have also found correlation between MMP-1 levels and clinical parameters (Pozo et al., 2005 and Tuter et al., 2005), while some studies have not (Tuter et al., 2002). These variations can likely be attributed to the clinical inclusion criteria, as clinical parameters do not necessarily reflect active periodontal disease.
Here we report a higher ratio of MMP-1/TIMP-1 in patients with chronic periodontitis BT, which is reduced AT. These results are in accordance with previous studies (Tuter et al., 2002; Mouzakiti et al., 2012). TIMPs have the widest range of actions among all metalloproteinase inhibitors, and they bind to most MMPs. Thus, the assessment of the MMP-1/TIMP-1 ratios is important because an imbalance between MMPs and TIMPs causes tissue degradation (Graphs A–C, Tables A and B).
Graph A.
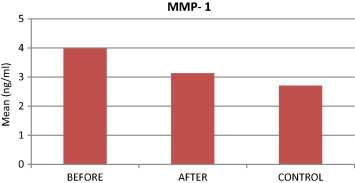
Comparison of MMP-1 levels among study groups.
Graph B.
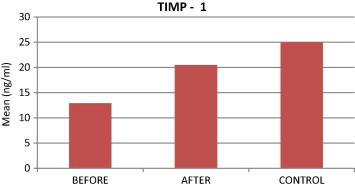
Comparison of TIMP-1 levels among study groups.
Graph C.
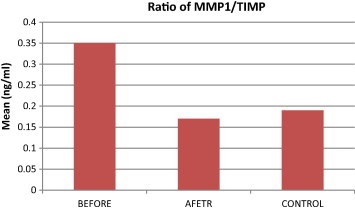
Comparison of MMP-1/TIMP-1 ratio among study groups.
Table A.
Comparison of clinical parameters, and GCF levels of Matrix metalloproteinase (MMP)-1 and Tissue inhibitors of metalloproteinase (TIMP)-1 before and after therapy.
| BT | AT | Controls | BT vs AT | BT vs control | AT vs control | |
|---|---|---|---|---|---|---|
| MMP-1 (ng/ml) | 3.98 | 3.13 | 2.71 | <0.001⁎ | <0.001⁎ | 0.1043⁎ |
| TIMP-1 | 12.88 | 20.46 | 25.01 | <0.001⁎ | <0.001⁎ | 0.0214⁎ |
| Pocket depth (mm) | 4.50 | 3.35 | 2.17 | <0.001⁎ | <0.001⁎ | <0.001⁎ |
| Clinical attachment level (in mm) | 4.71 | 3.57 | 2.17 | <0.001⁎ | <0.001⁎ | <0.001⁎ |
| Plaque index | 4.14 | 2.48 | 2.19 | <0.001⁎ | <0.001⁎ | 0.0014⁎ |
| Gingival index | 1.97 | 1.34 | 0 | <0.001⁎ | <0.001⁎ | 0.0014⁎ |
P < 0.05 - Significant.
Table B.
Correlation of levels of MMP-1 and TIMP-1 with clinical parameters.
| PD | CAL | PI | GI | PBI | ||
|---|---|---|---|---|---|---|
| MMP-1 | r-Value | 0.1139 | 0.0036 | 0.5321 | 0.4914 | 0.2399 |
| p-Value | 0.5489 | 0.9851 | 0.0025** | 0.0058** | 0.2016 | |
| TIMP-1 | r-Value | −0.2737 | −0.2512 | −0.2733 | −0.3614 | −0.0726 |
| p-Value | 0.1434 | 0.1802 | 0.0239⁎ | 0.0498⁎ | 0.7032 |
P < 0.05 , Significant.
Highly significant.
5. Conclusion
We conclude that MMP-1 concentrations increase and TIMP-1 concentrations decrease in GCF as periodontal destruction becomes greater. After chronic periodontitis subjects are treated by Scaling and root planing, the average concentration of MMP-1 reduces and the average concentration of TIMP-1 increases in GCF. However, further longitudinal studies are needed to evaluate MMP-1 and TIMP-1 levels in periodontal disease tissues.
Conflict of interest
The authors hereby declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Pallavi S. Ghodpage, Email: pghodpage@gmail.com.
Rajashri A. Kolte, Email: drrajashrikolte@gmail.com.
Abhay P. Kolte, Email: drabhaypkolte@gmail.com.
Madhur Gupta, Email: drmadhur20@rediffmail.com.
References
- Aiba T., Akeno N., Kawane T., Okamoto H., Horiuchi N. Matrix metalloproteinases-1 and -8 and TIMP-1 mRNA levels in normal and diseased human gingivae. Eur. J. Oral Sci. 1996;104:562–569. doi: 10.1111/j.1600-0722.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- Civitelli R., Hruska K., Jeffrey J., Kahn A., Avioli L., Partridge N. Second messenger signaling in the regulation of collagenase production by osteogenic sarcoma cells. Endocrinology. 1989;124:2928–2934. doi: 10.1210/endo-124-6-2928. [DOI] [PubMed] [Google Scholar]
- Figueredo C., Areas A., Mirandal L., Fischer R., Gustafsson A. The short-term effectiveness of non-surgical treatment in reducing protease activity in gingival crevicular fluid from chronic periodontitis patients. J. Clin. Periodontol. 2004;31:615–619. doi: 10.1111/j.1600-051X.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- Haerian A., Adonogianaki E., Mooney J., Manos A., Kinane D.F. Effects of treatment on gingival crevicular collagenase, stromelysin and tissue inhibitor of metalloproteinases and their ability to predict response to treatment. J. Clin. Periodontol. 1996;23:83–91. doi: 10.1111/j.1600-051x.1996.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Howard E., Bullen E., Banda M. Regulation of the autoactivation of human 72-kDa progelatinase by tissue inhibitor of metalloproteinases-2. Biol. Chem. 1991;266:13064–13069. [PubMed] [Google Scholar]
- Ingman T., Tervahartiala T., Ding Y. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J. Clin. Periodontol. 1996;23:1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Kumar M., Reddy R., Deepa A., Babu M., Kumar K., Chavan V. Comparison of matrix metalloproteinase-3 and tissue inhibitor of matrix metalloproteinase-1 levels in gingival crevicular fluid in periodontal health, disease and after treatment: A clinic biochemical study. J. Dent. Res. 2013;10:434–439. [PMC free article] [PubMed] [Google Scholar]
- Meikle M., Hembry R., Holley J., Horton C., McFarlane C., Reynolds J. Immunolocalization of matrix metalloproteinases and TIMP-1 in human gingival tissue from periodontitis patients. J. Periodontal Res. 1994;29:118–126. doi: 10.1111/j.1600-0765.1994.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Mouzakiti E., Pepelassi E., Fanourakis G., Markopoulou C., Tseleni-Balafouta S., Vrotsos I. Expression of MMPs and TIMP-1 in smoker and nonsmoker chronic periodontitis patients before and after periodontal treatment. J. Periodontal Res. 2012;47:532–542. doi: 10.1111/j.1600-0765.2011.01465.x. [DOI] [PubMed] [Google Scholar]
- Nomura T., Takahashi T., Hara K. Expression of TIMP-1, TIMP-2 and collagenase mRNA in periodontitis-affected human gingival tissue. J. Periodontal Res. 1993;28:354–362. doi: 10.1111/j.1600-0765.1993.tb01079.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Watanabe S., Nakanishi I. Inactivation of tissue inhibitor of metalloproteinases by neutrophil elastase and other serine proteinases. FEBS Lett. 1988;229:157–160. doi: 10.1016/0014-5793(88)80817-2. [DOI] [PubMed] [Google Scholar]
- Otsuka K., Sodek J., Limeback H. Synthesis of collagenase and collagenase inhibitors by osteoblast-like cells in culture. Eur. J. Biochem. 1984;145:123–129. doi: 10.1111/j.1432-1033.1984.tb08530.x. [DOI] [PubMed] [Google Scholar]
- Pozo P., Valenzuela M., Melej C. Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis-affected patients. J. Periodontal Res. 2005;40:199–207. doi: 10.1111/j.1600-0765.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- Shibata Y., Takiguchi H., Abiko Y. Antisense oligonucleotide of tissue inhibitor of metalloproteinase-1 induces the plasminogen activator activity in periodontal ligament cells. J. Periodontol. 1991;70:1158–1165. doi: 10.1902/jop.1999.70.10.1158. [DOI] [PubMed] [Google Scholar]
- Socransky S., Haffajee A. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- Soell M., Elkaim R., Tenenbaum H. Cathepsin C, matrix metalloproteinases, and their tissue inhibitors in gingival and gingival crevicular fluid from periodontitis affected patients. J. Dent. Res. 2002;81:174–178. [PubMed] [Google Scholar]
- Sorsa T., Ingman T., Suomalainen K., Haapasola M., Konttinen Y., Lindy O. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect. Immun. 1992;60:4491–4495. doi: 10.1128/iai.60.11.4491-4495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuter G., Kurtis B., Serdar M. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1. J. Periodontol. 2002;73:487–493. doi: 10.1902/jop.2002.73.5.487. [DOI] [PubMed] [Google Scholar]
- Tuter G., Kurtis B., Serdar M. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1. J. Clin. Periodontol. 2005;32:1011–1015. doi: 10.1111/j.1600-051X.2005.00816.x. [DOI] [PubMed] [Google Scholar]
- Tuter G., Kurtis B., Serdar M., Aykan T., Okyay K., Yucel A., Toyman U., Cemri M., Cengel A., Walker S., Golub L. Effects of scaling and root planing and subantimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J. Clin. Periodontol. 2007;34:673–681. doi: 10.1111/j.1600-051X.2007.01104.x. [DOI] [PubMed] [Google Scholar]


