Abstract
We present a case of rhino-orbitary mucormycosis which progressed despite liposomal amphotericin and early surgical debridement. Combined echinocandin and high dose liposomal amphotericin, repeated debridement, prolonged therapy with hyperbaric oxygen and continued therapy with posaconazole, along with strict diabetic control, allowed cure without disfigurement.
Keywords: Zygomycosis, Hyperbaric oxygen, Amphotericin B, Diabetes mellitus, Posazonale, Rhizopus arrhizu
1. Introduction
Mucormycosis (zygomycosis) is a low incidence by highly lethal disease of immunodepressed and diabetic patients, which needs a prompt diagnosis, aggressive surgical therapy and high dose antifungal therapy. We present a recently diagnosed diabetic patient who experienced rapid progression despite early debridement and liposomal amphotericin B therapy. The addition of echinocandin with increased dosage of amphotericin B, repeated debridement, transfer to a hyperbaric oxygen therapy unit, continuation therapy with posaconazole, along with strict glycemic control, allowed microbiological cure without disfigurement.
2. Case
A 65 year-old Peruvian male with a history of thalassemia minor, high blood pressure, dyslipidemia, obesity and ischemic and valvular heart disease had been submitted to surgical valve repair and coronary artery bypass surgery five years before admission. One month prior to admission (day −30) he suffered polyuria, thirst and weight loss. His primary care physician diagnosed “prediabetes” and prescribed metformin. The patient spent the following month in his country and on day −3 he complained right facial pain and rhinorrhea. Azithromycin was prescribed, to no avail. The patient suffered increasing right periocular pain, right hemicraneal headache, and hemifacial hypoesthesia, nausea, and vomiting.
In the emergency room (day 0), blood pressure was 135/69 mmHg, temperature 36.6 °C, heart rate 69 bpm, oxygen saturation of 97% with room air. Right orbitary edema and slight right palpebral ptosis were noticed on physical examination, as well as a linear ulceration on right hard palate with necrotic mucosa (Fig. 1). The remaining physical examination was unremarkable, a neurologist noticed right V1 and V2 hypoesthesia. Otherwise neurological examination did not reveal abnormal findings.
Fig. 1.
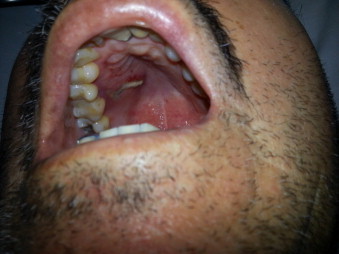
Hard palate befote treatment. Linear ulceration with necrotic mucosa.
The serum chemistries revealed glucose 773 mg/dL, creatinine 1.21 mg/dL, Na 118 mEq/L, K 4.4 mEq/L, plasma osmolality 330 m Osm/kg, C-reactive protein 143 mg/dL (reference range <10). Hemoglobin 11.2 g/dL, mean corpuscular volume 60.8 fl (thalassemia minor), 11,360 leukocytes/µL (polymorphonuclear leukocytes, 83.7%, lymphocytes, 8.8%, monocytes 5.6%, eosinophils 0.7%, basophils 0.2%).
CT scan revealed a full right maxillary sinus, right ethmoidal cells and inferior meatus, probably of inflammatory nature. Bone septa were thin. There was a slight increase in prebulbar fat on the right eye (Figs. 2–4).
Fig. 2.
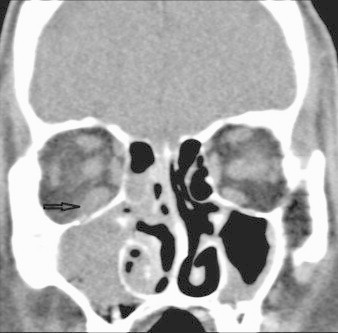
Pretreatment coronal CT: right maxillary sinus opacification with intraorbital extension without bone erosion. Arrow: thickening of inferior rectus muscle and infiltration of adjacent fat.
Fig. 3.
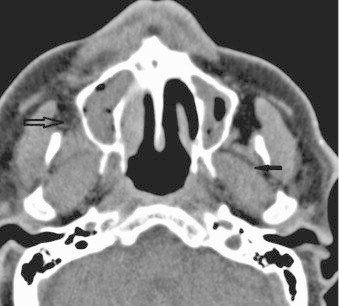
Axial CT: extension of infection in the buccal space and masticator space. Hollow arrow: hyperattenuation right retromaxillary fat pad. Small arrow: thickening masticator space musculature and obliteration of the fat planes.
Fig. 4.
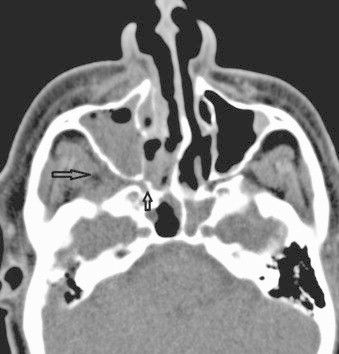
Pretreatment axial CT: note spread from the nasal cavity through the sphenopalatine foramen (small arrow) involving the right pterygopalatine fossa (big arrow). There is extension of infection into the right premaxillary soft tissues.
Rhino-orbital mucormycosis was suspected, and on day +1 an extensive unilateral endoscopic nasosinusal surgery was performed, with debridement of anterior and posterior ethmoidal cells, opening of the maxillar antrum and extensive removal of invaded mucosa in the right sphenoidal sinus. Furthermore resection of the right middle turbinate and body and tail of the lower turbinate was required. Extensive fungal masses were excised from maxillary and sphenoidal sinus (disease-free in previous CT scan). Mucosa appeared necrotic and its removal was unaccompanied by bleeding. Liposomal amphotericin B (5 mg/kg) was immediately started. Rhizopus arrhizus was recovered from all plating samples on Sabouraud-cloramphenicol agar (Fig. 5). This fungus was characterized by the production of thin-walled, broad coenocytic hyphae, producing typical sporangiophores and rhizoids, being classified as pertaining to the genus Rhizopus; and phenotypically identified as Rhizopus arrhizus, because of their capacity to grow at 37 °C (but not at 45 °C) on 1% malt extract agar, and due to the production of long (>1 mm) sporangiophores and relatively small (<250 μm diameter) sporangia [1].
Fig. 5.

Rhizopus arrhizus.
Initially the patient improved, but headache increased and on day +18 MRI showed postsurgical changes plus persistence of fungal invasion of right ethmoidal and frontal sinus, pre and retromaxillary spaces, right masticator space and right inferior rectus muscle (Fig. 6).
Fig. 6.
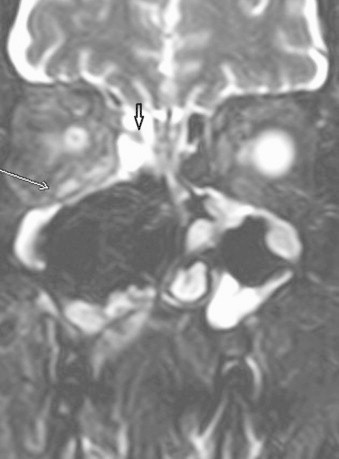
Coronal T2 FS MRI: black arrow shows a fungal concretion. White arrow reveals an enlargement and hyperintense signal of the right inferior rectus muscle and infiltration orbital fat.
On day +26 a new unilateral endoscopic debridement was performed, cleaning right frontal and sphenoidal sinus, rostral septum, sphenopalatinal ostium and the floor of the right nasal fossa. Liposomal amphotericin B dosage was increased (7.5 mg/kg), with addition of caspofungin. The patient suffered treatment-related hypokalemia (2.6 mEq/L) and transient worsening of renal function. Hemoglobin nadir was 8.3 g/dL and transfusion could be avoided.
On day +30 there was a new worsening of the headache, and the patient was transferred to another hospital to add hyperbaric oxygen therapy (40 sessions of 90 min). The patient needed three additional surgical debridements, continued intravenous liposomal amphotericin B (initially daily and later weekly infusions) and caspofungin, and topical amphotericin (10 mg daily). All surgical sample cultures had been negative. On day +104 he was discharged under treatment with posaconazole, 200 mg q.i.d. A last surgical revision was performed on day +240, without pathological evidence of fungus and a negative PCR on the sample, and posaconazole was discontinued on day +250. The patient is well, he suffered frequent epistaxis until the last surgical review but was asymptomatic thereafter. There is no residual disfigurement.
3. Discussion
Mucormycosis is an infrequent disease, with an annual incidence of 0.4–1.7 cases/1,000,000 population and worldwide distribution. In patients with acute myelogenous leukemia, its incidence can be as high as 8% [2]. It appears mainly in immunodepressed patients, mainly patients, with hematologic malignancies or with uncontrolled diabetes mellitus. Other risk factors are prolonged high dose of corticosteroid treatment, stem cell transplantation, solid organ transplantation, deferoxamine therapy, iron overload, severe neutropenia, intravenous drug use, trauma, burns, preterm infants and prolonged voriconazole therapy. It may occur in immunocompetent patients. It is usually acquired by inhalation, but yatrogenic transmission through transdermal patches, intravenous catheters and wooden tongue depressors has been described.
Rhizopus arrhizus is the most common cause of mucormycosis [3]. It is a fast growing organism. Microscopically, wide pauciseptate hiphae with right angle dichotomous branching are seen.
Mucormycosis has various clinical presentations, rhino-sino-orbital and rhinocerebral mucormycosis are the most common. Paranasal sinuses are the most frequent location. The fungus invades blood vessels, causing thrombosis and ischemic tissue necrosis. A black necrotic eschar is the most typical lesion, but its absence does not rule out the disease. Initial symptoms are usually those of a sinusitis preseptal or orbitary cellulitis. In predisposed patients, multiple cranial nerve palsies (mainly III, IV and VI), unilateral periorbitary and facial pain, eyelid edema, blepharoptosis, proptosis, diplopia, headache and acute visual loss are seen. The most frequent intracranial complications are epidural and subdural abscesses and cavernous and longitudinal sinus thrombosis [4].
The gold standard imaging method is contrast-enhanced computed tomography which shows opacification of sinuses, edematous mucosa and bone destruction, soft tissue swelling and swelling of extraocular muscles [5]. T2-weighted MR images demonstrate intracerebral infection and enhanced images may detect early vascular invasion [5]. The need for nephrotoxic drugs during treatment argues in favor of the use of MRI, since repeated imaging is usually needed for follow-up. Clinicians should not forget that bone destruction can be a late finding, that patients with early rhino-orbito-cerebral mucormycosis may have normal CT and MRI, and that surgical exploration with biopsy of the areas of suspected infection should always be performed in high-risk patients [6].
Diagnosis requires direct microscopy of samples, and culture, which allows differentiation from aspergillosis and species identification [7].
Therapy guidelines have been recently published [8]. The disease requires extensive and urgent surgical debridement and immediate first-line antifungal treatment with liposomal or lipid-complex amphotericin B with a minimum dose of 5 mg/kg/day. Reversal of predisposing conditions is strongly recommended, i.e. using granulocyte colony-stimulating factor in hematological patients with ongoing neutropenia, controlling hyperglycemia and ketoacidosis in diabetic patients, and limiting glucocorticosteroids to the minimum dose required.
In our patient the first aggressive debridement and standard dose of liposomal amphotericin B were unable to stop progression, thus we had to increase dosage and repeat surgical debridements. We chose the addition of caspofungin as rescue therapy. Despite the lack of in vitro susceptibility of mucorales to echinocandins, there are preclinical studies and a small retrospective study in which the success rate treating rhino-orbito-cerebral mucormycosis is higher with a combination of polyene–caspofungin [9]. For prolonged treatment we chose oral posaconazole. The abovementioned European guidelines strongly recommend posaconazole for salvage therapy, and support with moderate strength combination of lipid formulations of amphotericin B with posaconazole.
The value of hyperbaric oxygen therapy (HBOT) has been related to direct antifungal activity mediated through increased oxygen-based free radicals, reversal of fungal growth promoting lactic acidosis, restoration of phagocytosis and oxydative burst of polymorphonuclear leukocytes, and enhanced healing [10]. Survival of up to 94% of patients has been described in diabetic patients treated with HBOT [11].
Several adjunctive measures were important in the management of this patient. Glycemic control was emphasized throughout the course. No iron was administered and transfusions were withheld, despite anemia, to starve the fungus from iron. We considered the possibility of deferasirox therapy, but finally it was not used. Increases in serum creatinine have been described with this drug [12], and the risk of adding renal toxicity to liposomal amphotericin B seemed unacceptable.
Since we tried almost every possible measure to control this progressive infection (increasing dosage of amphotericin B and addition of topical amphotericin, combining caspofungin, and sequential treatment with posaconazole repeated debridement and HBOT) it is impossible to be certain about which therapies were more helpful. The close collaboration of otolaryngologists, infectious disease physicians, microbiologists, diabetologists, and prompt transfer to a military hospital with HBOT capabilities was crucial in the favorable outcome of this patient. Its importance cannot be overemphasized.
Duration of therapy is an important issue, given the expense of antifungal treatment. European guidelines state “continue treatment until complete response (on imaging) and permanent reversal of immunosuppression are achieved” but no prospective studies have been performed [8]. Discriminating residual changes on imaging studies from active lesions is difficult. The concretions of very hyperproteinaceous secretions may show a signal void simulating aeration on both T1 and T2 weighted images.
Our patient had increased volume of the right rectus muscle (the orbit was not entered at surgery) and other changes. We decided continuing treatment until the last surgical revision (day +240) and confirmation of negative PCR. Good glycemic control had been achieved much earlier. Every additional day of posaconazole treatment adds 115 € (156 USD)
A conservative estimate of the total management cost is shown in Table 1. It does not include non-antifungal therapy, and outpatient visits and several other items. It amounted roughly 174,000 € (237,000 USD). As a reference, the lifetime treatment cost of an HIV infection is estimated at $379,668 (in 2010 dollars) [13]. Table 1.
Table 1.
Estimation of direct health costs. Non-antifungal drugs and outpatient visits not included.
| Item | n | Daily or unitary cost (€) | Total (€) | Total (USD) |
|---|---|---|---|---|
| Inpatient day | 90 | 170 | 15,300 | 20,808 |
| Liposomal amphotericin B | 90 | 1295 | 116,550 | 158,508 |
| Caspofungin | 30 | 367 | 11,010 | 14,974 |
| Posaconazole | 154 | 115 | 17,710 | 24,086 |
| CT scan | 7 | 107 | 749 | 1019 |
| Magnetic resonance imaging | 3 | 207 | 621 | 845 |
| Surgical intervention | 6 | 2000 | 12,000 | 16,320 |
| Hyperbaric oxygen therapy (per hour) | 60 | 46 | 2760 | 3754 |
| Total | 173,940 | 236,558 |
Conflict of interest statement
The authors declare no conflict of interests relevant to this case.
Acknowledgments
The authors would like to thank Jesús Fortún, of the Hospital Ramón y Cajal, in Madrid, for his advice in the management of this difficult case, Miguel Ángel Brinquis of the Hyperbaric Oxygen Therapy Unit of the Hospital Central de la Defensa, Madrid, and most sincerely to the patient, for his role in the success of therapy, his cooperation in a long and difficult treatment, and his written permission to use a recognizable picture.
References
- 1.de Hoog GS, Guarro J, Gené J, Figueras MJ. Atlas of Clinical Fungo USB version. CBS-KNAW Fungal Biodiversity Centre and University Rovira i Virgili; 2014.
- 2.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl. 1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 3.Bonifaz A., Tirado-Sanchez A., Calderon L., Romero-Cabello R., Kassack J., Ponce R.M. Mucormycosis in children: a study of 22 cases in a Mexican hospital. Mycoses. 2014 doi: 10.1111/myc.12233. [DOI] [PubMed] [Google Scholar]
- 4.Kontoyiannis D.P., Lewis R.E. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am. 2006;20(3):581–607. doi: 10.1016/j.idc.2006.06.003. (vi) [DOI] [PubMed] [Google Scholar]
- 5.Prasad K., Lalitha R.M., Reddy E.K., Ranganath K., Srinivas D.R., Singh J. Role of early diagnosis and multimodal treatment in rhinocerebral mucormycosis: experience of 4 cases. J Oral Maxillofac Surg. 2012;70(2):354–362. doi: 10.1016/j.joms.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Rapidis A.D. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;15(Suppl. 5):98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh T.J., Gamaletsou M.N., McGinnis M.R., Hayden R.T., Kontoyiannis D.P. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis) Clin Infect Dis. 2012;54(Suppl. 1):S55–S60. doi: 10.1093/cid/cir868. [DOI] [PubMed] [Google Scholar]
- 8.Cornely O.A., Arikan-Akdagli S., Dannaoui E., Groll A.H., Lagrou K., Chakrabarti A. ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Mucormycosis 2013. Clin Microbiol Infect. 2014;20(Suppl. S3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 9.Reed C., Bryant R., Ibrahim A.S., Edwards J., Jr., Filler S.G., Goldberg R. Combination polyene–caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tragiannidis A., Groll A.H. Hyperbaric oxygen therapy and other adjunctive treatments for zygomycosis. Clin Microbiol Infect. 2009;15(Suppl. 5):82–86. doi: 10.1111/j.1469-0691.2009.02986.x. [DOI] [PubMed] [Google Scholar]
- 11.John B.V., Chamilos G., Kontoyiannis D.P. Hyperbaric oxygen as an adjunctive treatment for zygomycosis. Clin Microbiol Infect. 2005;11(7):515–517. doi: 10.1111/j.1469-0691.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 12.Cappellini M.D., Cohen A., Piga A., Bejaoui M., Perrotta S., Agaoglu L. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107(9):3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 13.Prevention CfDCa. HIV Cost-effectiveness 2013 [updated April 16, 2013 1 November 2013]. Available from: 〈http://www.cdc.gov/hiv/prevention/ongoing/costeffectiveness〉.


