Abstract
Sphingosine-1-phosphate (S1P) is an intracellularly generated bioactive lipid essential for development, vascular integrity, and immunity. These functions are mediated by S1P-selective cell surface G-protein coupled receptors. S1P signaling therefore requires extracellular release of this lipid. Several cell types release S1P and evidence for both plasma membrane transporter-mediated and vesicle-dependent secretion has been presented. Platelets are an important source of S1P and can release it in response to agonists generated at sites of vascular injury. S1P release from agonist-stimulated platelets was measured in the presence of a carrier molecule (albumin) using HPLCMS/MS. The kinetics and agonist-dependence of S1P release were similar to that of other granule cargo e.g. platelet factor IV (PF4). Agonist-stimulated S1P release was defective in platelets from Unc13dJinx (Munc13-4 null) mice demonstrating a critical role for regulated membrane fusion in this process. Consistent with this observation, platelets efficiently converted fluorescent NBD-sphingosine to its phosphorylated derivative which accumulated in granules. Fractionation of platelet organelles revealed the presence of S1P in both the plasma membrane and in α-granules. Resting platelets contained a second pool of constitutively releasable S1P that was more rapidly labeled by exogenously added sphingosine. Our studies indicate that platelets contain two pools of S1P that are released extracellularly: a readily-exchangeable, metabolically active pool of S1P, perhaps in the plasma membrane, and a granular pool that requires platelet activation and regulated exocytosis for release.
Keywords: Platelets, sphingosine, secretion, Munc13-4, platelet activation
1. INTRODUCTION
Bioactive lipids contribute to many cellular signaling processes [1, 2]. In most cases, the biological actions of these lipids are mediated by cell surface receptors. These lipids therefore must be generated and/or released extracellularly. The focus of the present study is to examine the route by which a bioactive lipid, sphingosine-1-phosphate (S1P), is released from activated platelets. S1P regulates many physiological processes, e.g. cell migration, maturation, differentiation, vascular integrity, and angiogenesis [3]. Its effects are facilitated by a family of receptors called S1PR1-5 (previously called Endothelial Differentiation Gene receptors) [4]. A reciprocal axis of VEGF/S1P signaling is key to normal angiogenesis and absence of S1P results in hypersprouting of blood vessels leading to vascular leakage [5]. Localized availability of S1P is necessary to maintain the junctional integrity of vascular endothelial cells. S1P also regulates lymphocyte trafficking between the lymphatic system and peripheral circulation [6]. Therefore, regulating the availability of extracellular S1P is critical for several aspects of vascular function.
Blood contains both cellular and plasma associated pools of S1P. Plasma S1P is almost exclusively associated with carriers: the apolipoprotein M component of HDL or albumin [7, 8], and is predominantly produced by erythrocytes [9], and vascular endothelial cells [11]. Indeed, erythrocyte transfusion reduces the vascular leakiness seen in genetically-modified mice, deficient in plasma S1P [12] and human plasma S1P levels correlate with hematocrit [25]. Release of S1P from erythrocytes is passive, requiring only the presence of a carrier molecule to adsorb S1P [13]. Although not a primary determinant of circulating S1P levels, platelets contain a regulated pool of S1P that is mobilized in response to extracellular signals resulting in localized increases in extracellular S1P. The relevance of this pool is demonstrated by the role that platelet-released S1P plays to maintain the integrity of high endothelial venules (HEVs) [14]. Platelet production of S1P is robust given their high levels of sphingosine kinase (SphK) and lack of S1P phosphatase and S1P lyase [10, 15, 16]. How platelets release S1P has been the subject of several studies, yet the mechanisms are unclear [17]. Platelets passively release S1P and also exhibit an activation-dependent release, which requires stimulation with agonists such as thrombin [18]. Initial theories held that S1P, made on the cytoplasmic leaflet of platelet plasma membranes, is translocated to the extracellular space by a transporter, such as Spinster 2 (SPNS2), and/or an ABC transporter. Though SPNS2 is important for S1P efflux from endothelial cells, it is not required in platelets [19]. Studies with the ABC transporter inhibitor, glyburide (also known as glibenclamide), suggest that an ABCA-like transporter could be involved; but, the inhibitor's effects on other platelet processes were not tested [17]. Other ABC transporter inhibitors (i.e. verapamil, cyclosporine and MK571) were without effect [17, 20].
In this study, we sought to clarify the mechanisms by which platelets release S1P to determine if this bioactive lipid is stored in cargo-containing granules and is secreted upon stimulation. We used mass spectrometry to monitor both platelet-associated and released S1P. We examined S1P release from platelets obtained from a mouse strain with a genetic defect in the SNARE-based machinery that is required for platelet exocytosis [21]. Our results suggest a scheme to explain how platelet-derived S1P could be used to acutely and locally influence S1PR signaling in endothelial cells at vascular lesions.
2 MATERIALS AND METHODS
2.1 Platelet Preparation and Treatments
Human and mouse platelets were prepared as described [21-23]. The platelet preparations had no overt erythrocyte or nucleated cell contamination as assessed microscopically. Platelet concentrations were adjusted with Hepes Tyrode's (HT) buffer (10 mM HEPES/KOH, pH 7.4, 137 mM NaCl, 12 mM NaHCO3, 5.56 mM glucose, 2.7 mM KCl, 1 mM MgCl2·6H2O, 0.36 mM NaH2PO4·H2O) containing 1% (w/v) Fraction V, fatty acid-free, bovine serum albumin (BSA; Sigma Aldrich, St. Louis, MO), unless otherwise indicated. When stimulated with thrombin (Chrono–Log, Haverton, PA), the secretion reactions were stopped with a two-fold excess of hirudin (Sigma Aldrich). Other agonists: convulxin (Centerchem, Norwalk, CT) and ADP (Chrono–Log), were used at the indicated concentrations. Agonist concentrations were adjusted for optimal S1P release from the platelets obtained from different donors. All solvents were of reagent grade.
2.2 Lipid Extraction
Lipids were extracted in acidified organic solvents as described [24]. Platelets (2.5 × 107 in 50 μL) were added to a mixture of 2 mL CH3OH and 1 mL CHCl3 in 8 mL borosilicate glass tubes. Fifty microliters of 1 µM C17-S1P (Avanti Polar Lipids, Alabaster, AL) was added as an internal standard. The mixture was acidified with 0.45 mL of 0.1 M HCl, mixed for 5 min, and placed at 4°C for 1 h. The extraction volume was increased with 1 mL CHCl3 and 1.3 mL 0.1 M HCl. After mixing for 5 min, the samples were centrifuged for 10 min at 3,500×g. The lower phase was transferred to a glass vial avoiding the protein interface. Samples were dried under N2 stream and resuspended in 100 μL CH3OH for analysis.
2.3 Quantitation of S1P by High Performance Liquid Chromatography-Electron Spray Ionization-Mass Spectrometry/Mass Spectrometry (HPLC-ESI-MS/MS)
Extracted lipids were used for the detection of S1P as described in [25]. Analysis of S1P was carried out using a Shimadzu Ultra-Fast Liquid Chromatograph coupled with an ABSciex 4000-Qtrap hybrid linear ion trap triple quadrapole mass spectrometer operated in multiple reaction monitoring (MRM) mode. C17-S1P or hepta-deuterated S1P were used as internal standards. Lipids were separated using an Agilent Zorbax Eclipse XDB C8 column (5 μm, 4.6×150 mm). The mobile phase consisted of 75/25 of CH3OH/H2O with formic acid (0.5%) and 5 mM HCOONH4 (0.1%) as solvent A and 99/1 of CH3OH/H2O with formic acid (0.5%) and 5 mM HCOONH4 (0.1%) as solvent B. For the analysis of S1P, the separation was achieved using a gradient of 0% B for 1 min, 0% B to 100% B in the next minute, maintained at 100% B for the next 10 min and equilibrated back to the initial conditions in 3 min. The flow rate was 0.5 mL/min with a column temperature of 30°C. The sample injection volume was 10 μL. The mass spectrometer was operated in positive electro-spray ionization (ESI) mode with optimal ion source settings determined by synthetic standards of S1P and C17-S1P with a declustering potential of 61 V, entrance potential of 10 V, collision energy of 23 V, collision cell exit potential of 16 V, curtain gas of 20 psi, ion spray voltage of 5,500 V, ion source gas1/gas2 of 40 psi and temperature of 550°C. MRM transitions monitored were as follows: 366.141/250 for C17-S1P; and 380.124/264.1 for S1P.
2.4 Mouse Models
C57BL/6 (wild type), and Unc13dJinx mice were as described [21]. All experiments with animals were approved by the University of Kentucky Institutional Animal Care and Use Committee.
2.5 Fluorescence Microscopy
Washed platelets (5×108/mL) were incubated with 1 μM mepacrine dihydrochloride (Sigma Aldrich) or 1 μM omega (7-nitro-2-1, 3-benzoxadiazol-4-yl)(2S,3R,4E)-2-amino octadec-4-ene-1,3-diol (NBD–sphingosine; Avanti Polar Lipids) for 30 min at 37°C. The platelets were pelleted at 550×g for 8 min and resuspended in fresh HT buffer. Approximately 10 μL of the samples were placed onto a microscope slide and overlaid with a cover glass. The platelets were then allowed to settle at RT for 30 min and were viewed using a Nikon Eclipse 600 epifluorescence microscope equipped with a 100x oil objective (Melville, NY). Digital images were captured using Axiocam MRm (Carl Zeiss Microscopy, Thornwood, NY) and were processed using Axiovision software.
2.6 Thin Layer Chromatography
After liquid-phase lipid extraction, samples were separated by thin layer chromatography using Silica Gel 60 TLC plates (Merck KGaA, Darmstadt, Germany) with a mobile phase of CHCl3/CH3OH/H2O (60/35/8). After development and drying, the separated, NBD–sphingolipids were visualized using a Typhoon 9500 scanner (GE Healthcare Life Sciences, Piscataway, NJ) at excitation wavelength 457 nm and emission wavelength 532 nm. A NBD-S1P standard was a generous gift from Dr. Brian W. Wattenberg (University of Louisville).
2.7 Sucrose Gradient Sub-Cellular Fractionation
The procedure was modified from [26]. A unit of platelet rich plasma (PRP) was subjected to centrifugation at 137×g for 15 min. Platelets, in the supernatant, were pelleted at 695×g for 15 min at RT and washed twice with 50 mL Tris-citrate buffer (63 mM Tris/HCl, pH 6.5, 95 mM NaCl, 12 mM citric acid, 5 mM KCl). The platelet pellet was finally resuspended in 12 mL Tris-citrate buffer and was transferred to the cell-disruption bomb (Parr 4639, Parr Instrument Co., Moline, IL). The Parr bomb was pressurized with N2 to 1,200 psi on ice for 15 min and the pressure was rapidly released. This cycle was repeated thrice and the platelet homogenates were cleared by centrifugation at 610×g for 10 min, prior to loading the supernatant onto sucrose gradients. Linear sucrose gradients were generated by layering 1.5 mL of the sucrose solutions (containing 5mM EDTA) of decreasing concentrations (from 60 to 30%, w/v) into an ultracentrifuge tube (Beckman Instruments, Inc., Fullerton, CA). The tubes were incubated overnight at 4°C. Homogenate (1.5 mL) was layered onto the top of the gradient and subjected to centrifugation at 284,061×g at 4°C using a SW-40 rotor (Beckman Instruments). After 90 min, the tubes were recovered and fractions were collected. Aliquots of the fractions were utilized for lipid extraction and measurement of S1P or subjected to trichloroacetic acid (TCA) precipitation to recover proteins. Proteins from each fraction were analyzed by SDS-PAGE and western blotting.
2.8 Metrizamide Gradient Sub–Cellular Fractionation
The procedure for fractionation of dense granules was modified from [27]. PRP was labeled with 50 μM mepacrine dihydrochloride for 30 min at RT and platelets were isolated. Pelleted platelets were washed twice in buffer (10 mM HEPES/KOH, pH 7.4, 130 mM NaCl, 24 mM NaHCO3, 12.5 mM sucrose, 10 mM glucose, 5 mM KCl, 2 mM Na2EDTA, 1 mM NaH2PO4·H2O, 0.35% (w/v) BSA) and finally resuspended in homogenization medium (25 mM HEPES/KOH, pH 7.0, 100 mM KCl, 25 mM NaCl, 12 mM Na3citrate, 10 mM glucose, 5 mM ATP, 2 mM MgSO4, 0.35% (w/v) BSA). Platelets were sonicated twice at 5 W for 10 sec using a Sonic Dismembrator model 100 (Fisher Scientific, Pittsburg, PA). Unbroken platelets were removed by centrifugation at 610×g for 10 min. Disrupted platelets (0.7 mL) were over-laid onto a metrizamide (Sigma Aldrich) step gradient (1 mL, 35%; 0.5 mL, 38%) in 11×34 mm (2.2 mL) ultracentrifuge tubes and subjected to centrifugation at 76,974×g at 4°C for 1 hr in a TLS-55 rotor (Beckman Instruments). Fractions were harvested and used for epifluorescence microscopy, lipid extraction, and western blotting.
3 RESULTS
3.1 Detection of released S1P from platelets is enhanced by the presence of a carrier molecule in the media
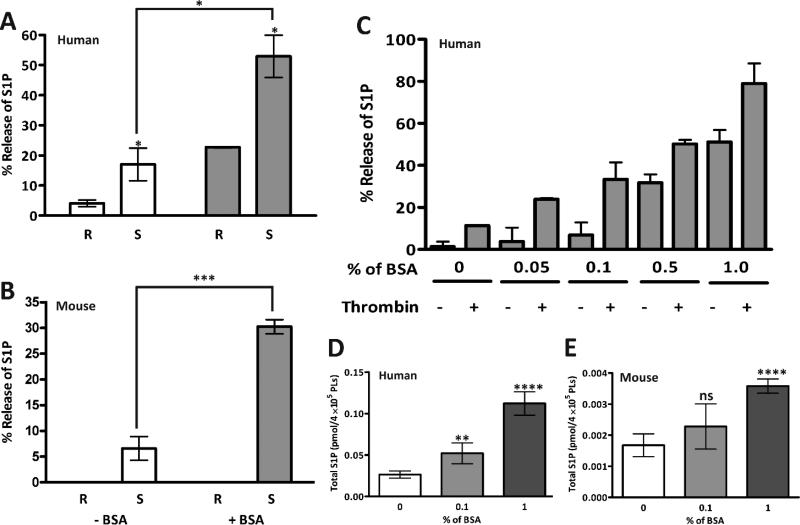
S1P in plasma is predominantly associated with albumin or HDL [7, 8], thus we initially tested whether such a carrier was needed to measure S1P release. No S1P was detected in the preparations of fatty acid-free BSA used throughout our studies (data not shown). Both human (Fig. 1A) and mouse (Fig. 1B) platelets were either kept resting or stimulated with thrombin (0.05 U/mL) in the absence or presence of BSA. S1P release could be detected in thrombin-stimulated, washed human or mouse platelets in the absence of BSA. This implies the presence of an endogenous carrier protein in the washed platelet preparations, which is plausible since albumin is known to be present in platelet granules. However, for both species, BSA (1% w/v) increased the recovery of S1P in the supernatant of stimulated platelets confirming that BSA is an effective acceptor of released S1P. When human platelets were incubated with increasing concentrations of BSA (Fig. 1C), more S1P was detected in the supernatants of resting and stimulated platelets. Despite the net increase, thrombin stimulation always resulted in more S1P release, consistent with the previous reports [17, 28]. Interestingly, increasing the concentration of BSA in the incubation mixture increased the total S1P detected in both human (Fig. 1D) and mouse (Fig. 1E) platelets. This suggests that albumin, by extracting S1P from resting platelets, may affect a homeostatic mechanism that maintains platelet S1P levels. For all subsequent experiments, 1% (w/v) fatty acid-free BSA was included.
Figure 1. Albumin facilitates S1P release from platelets.
Both human (A) and mouse (B) platelets (2.5×108/mL) were prepared as described in Materials and Methods. They were left unstimulated (R) or stimulated with thrombin (0.05 U/mL) for 45 sec (S) and the reactions were stopped with hirudin. Reactions were carried out in the absence (open bars) or presence (shaded bars) of fatty acid-free BSA (1% (w/v)) as indicated. The samples were separated by centrifugation (13,000×g for 2 min) to generate a supernatant and pellet fraction. Lipids from the two fractions were extracted as in Materials and Methods and S1P was quantified by HPLCMS/MS. A percent release value was calculated ([supernatant/{supernatant + pellet}] x 100) and the data are presented as the average ± SD (n = 3). (C) Human platelets were stimulated (+) or held resting (−) in the presence of increasing concentrations of fatty acid-free BSA. Release of S1P was then measured as in A and B. Human (D) and mouse (E) platelets (2.5×108/mL) were incubated in increasing concentrations of BSA and total S1P was measured. Statistical analysis was done using the unpaired two-tailed student's t-test (*, p≤ 0.05; **, p≤0.0005; ***, p≤0.0005; ****, p≤0.0001).
3.2 Release of S1P from platelets requires exocytosis
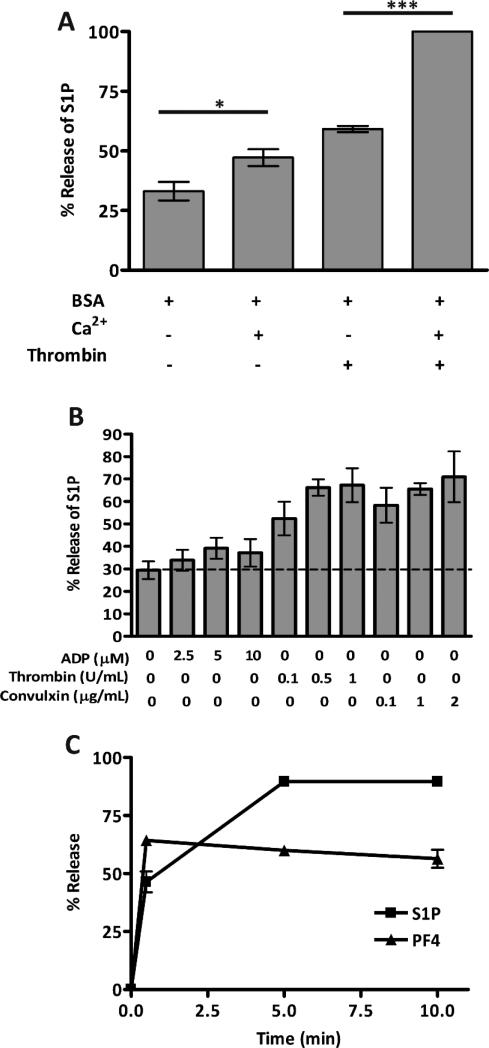
As with most platelet secretion processes, the presence of Ca2+ increased the release of S1P from platelets upon thrombin stimulation (Fig. 2A). Release of S1P depended on the agonist-potency. A weaker agonist, ADP, which stimulates aggregation but only limited secretion of granule cargo [29], induced lower level of S1P release. Stronger agonists, such as thrombin or convulxin, stimulated greater release (Fig. 2B). The kinetics of S1P release closely mirrored that of the α-granule marker, PF4 (Fig. 2C). Taken together, these data indicate that S1P release exhibits many of the same characteristics as the release of well-characterized platelet granule cargo.
Figure 2. Kinetics of S1P release from resting and stimulated platelets.
(A) Human platelets (2.5×108/mL) were resuspended in buffer with fatty acid-free BSA (1% (w/v)), with (+) or without (−) Ca2+ (1 mM). The platelets were incubated at RT for 30 min and 50 μL of the suspension was stimulated with thrombin (0.05 U/mL) for 5 min and the reactions were stopped with hirudin. Statistical analysis was done using the unpaired two-tailed student's t-test (*, p≤0.005; **, p≤0.005; ***, p≤0.0005; ****, p≤0.0001). (B) Human platelets (5×108/mL) were stimulated with increasing concentrations of ADP, thrombin, or convulxin, as indicated, for 5 min at RT and percent release of S1P was measured. (C) Human platelets (2.5×108/mL) in buffer with fatty acid-free BSA (1% (w/v)) were stimulated with thrombin (0.2 U/mL) at 37°C for the indicated times and the reactions were stopped with hirudin. All cargo release measurements were in triplicate. Lipids were extracted and S1P (■) was measured by HPLC-MS/MS analysis; PF4 (▲) was measured by ELISA (C). Percent release of S1P was calculated and the data are represented as the average ± SD, (n = 3).
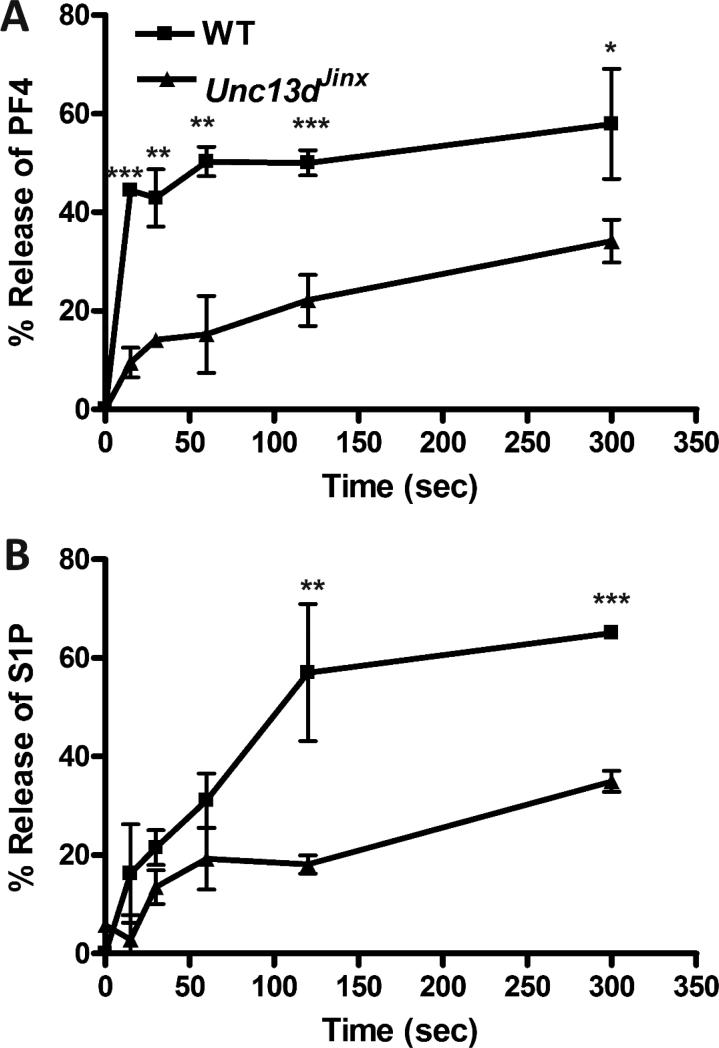
Because these observations suggested that granule fusion might mediate S1P release, we assayed platelets from a secretion-deficient mouse strain. Platelets from Munc 13-4-deficient, Unc13dJinx mice were stimulated with thrombin and release of PF4 (Fig. 3A) and S1P (Fig. 3B) was measured and compared to release from wild type (WT) platelets. Secretion of S1P from Unc13dJinx mouse platelets was reduced as was also seen for PF4 release. This effect was likely not due to differences in platelet-associated S1P, since platelet S1P levels (in the presence of BSA) were 23 ± 8, and 15 ± 7 fmol/4×105 platelets were comparable for wild type and Unc13dJinx mice, respectively. Similarly, plasma S1P levels in wild type and Unc13dJinx mice were 0.15 ± 0.02 pmol/μL and 0.11 ± 0.02 pmol/μL, respectively.
Figure 3. Release of S1P from secretion-defective mouse platelets.
Platelets (2.5×108/mL) were isolated from C57BL/6 (WT, ■), or Unc13dJinx (▲) mice and resuspended in buffer with fatty acid-free BSA (1% (w/v)). They were stimulated with thrombin (0.05 U/mL) for increasing times. The reactions were stopped with hirudin and the platelets were separated from releasate by centrifugation at 13,000×g for 2 min. All the assays for granule cargo were in triplicate. The percentage release of PF4 was determined by ELISA (A) and of S1P by HPLC-MS/MS (B). The data are plotted as percent release vs. time. In both plots, the background of unstimulated release has been subtracted (1.5-5% in A and 5-12% in B). Statistical analysis, at the indicated time points comparing WT to Unc13dJinx platelets, was done using the unpaired two-tailed student's t-test (*, p≤0.05; **, p≤0.01; ***, p≤0.005).
3.3 Granular localization of S1P in platelets
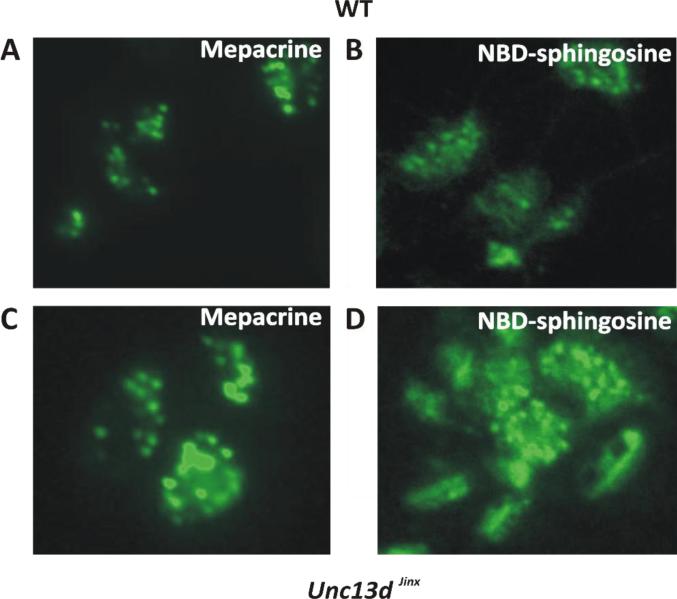
Our observations indicate that a portion of platelet S1P is in a granule compartment. To address this issue, platelets were incubated for at least one hour, with fluorescently labeled sphingosine (NBD-sphingosine). As SphK is highly active in platelets, this derivative was efficiently converted into NBD-S1P (data not shown and Figure 6B). To examine the localization of S1P, wild type and Unc13dJinx platelets were labeled with the fluorescent dye, mepacrine (Fig. 4A and C), to identify dense granules [30], and with NBD-sphingosine (NBD-Sph, Fig. 4B and D). The punctate appearance of the NBD staining and the relative sizes of the punctae, compared to the dense granules stained with mepacrine, suggests that at least some of the NBD-S1P was concentrated in the granule lumen or partitioned into granule membranes. There were apparently more NBD-stained granules present than mepacrine-positive granules, especially in the Unc13dJinx platelets, where granule exocytosis is blocked. The additional, non-granule-associated NBD-staining is consistent with either a cytosolic or plasma membrane pool of the labeled lipid. Similar observations were made when human platelets were incubated with NBD-sphingosine (data not shown).
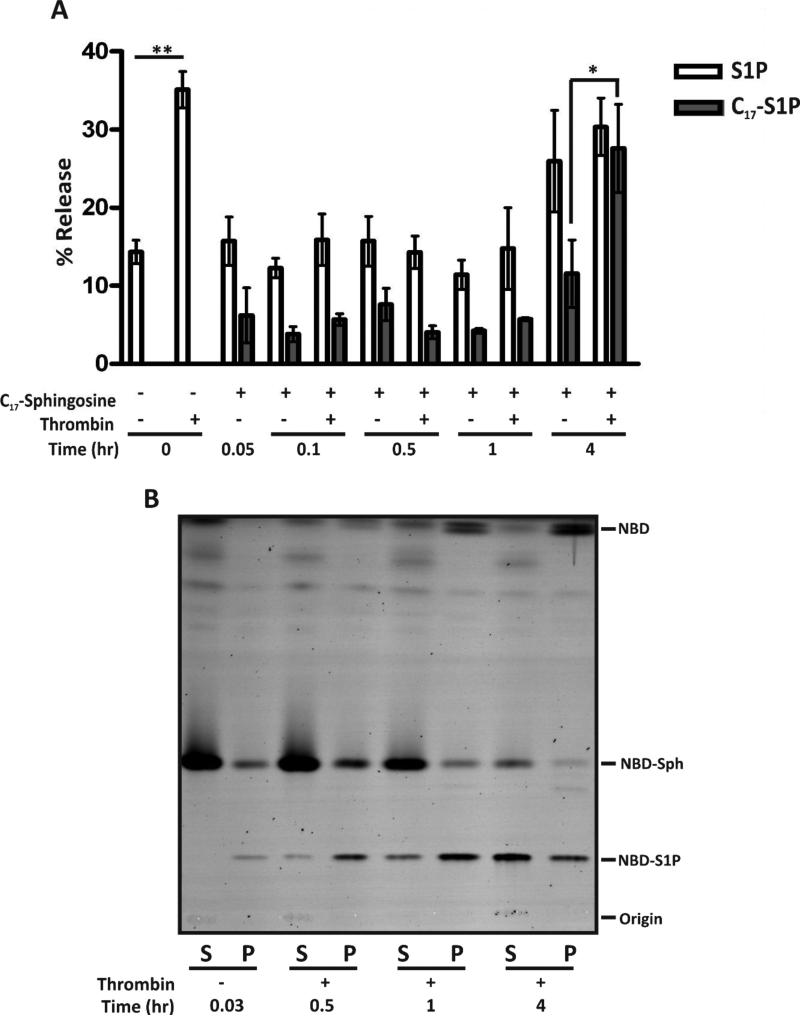
Figure 6. Release of newly synthesized S1P from platelets. (A).
Human platelets (5×108/mL) were incubated in buffer with or without C17-sphingosine (20 μM) for the indicated times with Ca2+ (1 mM) and fatty acid-free BSA (1% (w/v)). The platelets were kept resting or stimulated with thrombin (0.1 U/mL), as indicated, for 5 min and the reactions were stopped with hirudin. Platelets were separated from the releasate, lipids were isolated, and S1P was quantified by HPLC-MS/MS. The data are plotted as percent total platelet-associated S1P released. Statistical analysis, at the indicated time points, was done using the unpaired two-tailed student's t-test (*, p≤0.05; **, p≤0.001. (B) Human platelets (4×109/mL) were incubated with NBD-sphingosine (2 μM) for the indicated times in the presence of Ca2+ and fatty acid-free BSA. The platelets were kept resting (−) or stimulated (+) with thrombin for 2 min and the reactions were stopped with hirudin. After centrifugation, reactions were separated into supernatant (S) and pellet (P) fractions. The lipids were extracted, analyzed by TLC and imaged using a Typhoon 9500 imager.
Figure 4. NBD-Sphingosine-1-Phosphate is localized to platelet granules.
Platelets from wild type (A, B) and Unc13d Jinx (C, D) mice (5×108/mL) were resuspended in buffer containing fatty acid-free BSA (1% (w/v)) and incubated with mepacrine (1 μM) or NBD-sphingosine (1 μM) for at least 60 min at 37°C. The platelets were imaged by epifluorescence using a 100X oil objective on a Nikon Eclipse 600 microscope.
3.4 Granular pools of platelet S1P
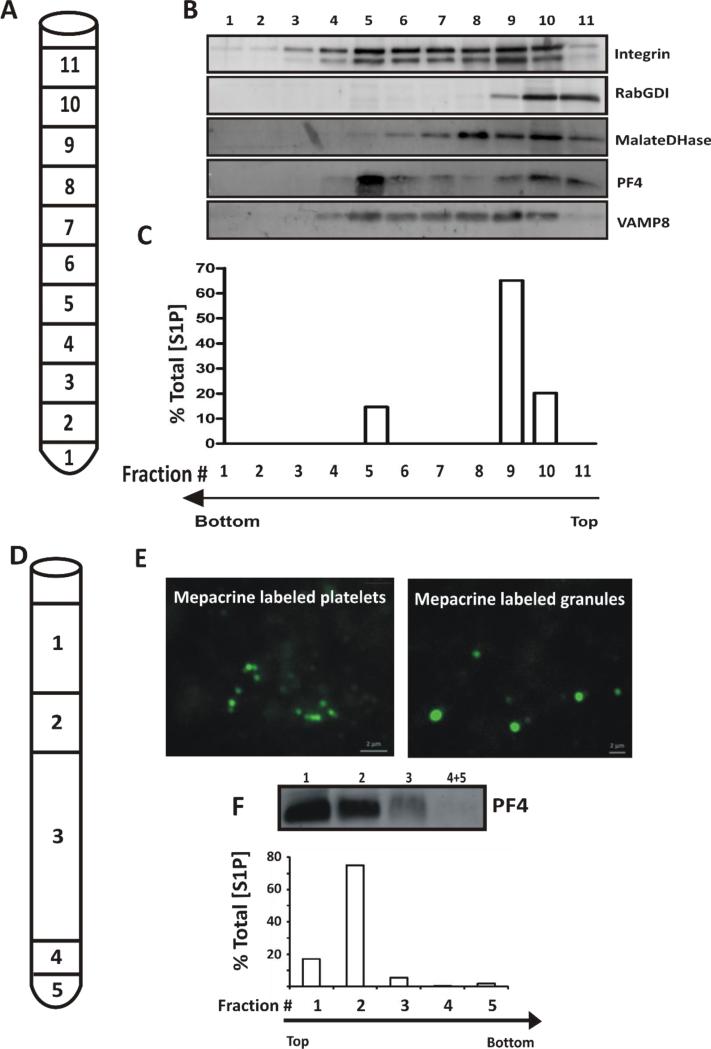
Given the staining patterns seen with NBD-S1P and the limitations of the available fluorophores for further imaging of platelet-associated S1P, we assessed the distribution of platelet S1P by sub-cellular fractionation. For the analysis of α-granules, platelets were disrupted by nitrogen cavitation and sub-cellular fractions were separated by sucrose-gradient ultracentrifugation (Fig. 5A). Marker proteins were examined by western blotting of each fraction, (Fig. 5B) and S1P was measured by HPLC-MS/MS (Fig. 5C). A plasma membrane marker, αIIbβ3 integrin peaked in fraction 9, but was spread across the gradient in fractions 3-10, perhaps reflecting pools of the integrin that may also be in granules and endosomal compartments. The soluble cytosolic marker RabGDI (Rab GDP Dissociation Inhibitor) was found mainly at the top of the gradient (fractions 10-11). The α-granule marker PF4 peaked in fraction 5 where the v-SNARE, VAMP-8, was also found. S1P was found in fractions containing the plasma membrane marker, αIIbβ3 and in fraction 5, which contained PF4. These data are consistent with at least a fraction of the total platelet S1P being present in α-granules.
Figure 5. Localization of S1P to platelet granules.
(A) Human platelets were disrupted by nitrogen cavitation and the material was layered onto a continuous, sucrose gradient (30-60% (w/v)) and organelles were separated by ultracentrifugation (n=4). 1 mL fractions were collected as indicated. (B) TCA precipitated proteins from each fraction were probed by western blotting for the indicated markers. (C) Lipids were extracted from the corresponding fractions and S1P was measured by HPLC-MS/MS. S1P in each fraction is expressed as percent of Total S1P in the starting platelets. The data are representative of three independent experiments. (D) Platelets were incubated with mepacrine to label dense granules before disruption by sonication and fractionation of the material obtained by layering onto a 35%, 38% metrizamide step gradient and ultracentrifugation. The fractions were harvested as indicated: Fraction 1, 500 μL; 2, 200 μL; 3, 1.2 mL; 4, 100 μL; 5 (pellet), 100 μL (n=4). (E) Mepacrine-labeled, whole platelets and Fraction 5, containing platelet dense granules were imaged by epifluorescence microscopy. (F) Lipids were extracted from the gradient fractions and their S1P content was measured by HPLC-MS. The values are represented as percent of Total [S1P] in each fraction. The corresponding fractions were TCA precipitated to detect PF4 by western blotting. The data are representative of three different experiments.
Due to their increased density, analysis of dense granules requires a different fractionation scheme using a metrizamide gradient (Fig. 5D). To monitor dense granules, platelets were labeled with mepacrine and then disrupted by sonication prior to ultracentrifugation (Fig. 5D). Fractions were observed by fluorescence microscopy to identify mepacrine-stained dense granules (Fig. 5E). The positive staining assures that the granules are intact since mepacrine is a vital stain that requires sealed granular membranes [31]. The dense granule population, in the bottom fraction, contained only minimal quantities of S1P (<2% of total) while the bulk of the bioactive lipid was found in the lighter fractions containing plasma membranes and α-granules (Fig. 5F). These data indicate that S1P was not concentrated in dense granules.
3.5 Time course for loading the releasable pool of S1P in platelets
Our data in Figure 1 suggest that platelets could contain at least two pools of S1P, a passively extractable pool and a pool that is acutely mobilized by platelet activation. We sought to determine the time required for newly synthesized S1P to become incorporated into either pool. Platelets were incubated with either C17-sphingosine (Fig. 6A) or NBD-sphingosine (Fig. 6B) over a 4 hr time course and were stimulated at the indicated time points with thrombin. In both cases the sphingosine analogs were phosphorylated. While newly phosphorylated C17- or NBD-sphingosine (Fig. 6A or B, respectively) were detected in the thrombin-stimulated releasate following less than 30 min of incubation, robust incorporation of the S1P analogues into the stimulation-dependent, releasable pool did not occur until 4 hr, under our incubation conditions. Interestingly, the release of endogenous S1P (containing C18-sphingosine) decreased upon addition of exogenous C17-sphingosine (20 μM). The cause of this effect is not clear but again suggests that homeostatic mechanisms control the total level of platelet-associated S1P. This phenomenon was reversible because normal S1P secretion occurred after 4 hr.
4 DISCUSSION
The major source of plasma S1P, at least in mice, is reported to be erythrocytes [13] with potential contributions by platelets [32], and endothelial cells [11]. Based on our studies and those of others [17, 28], platelets can constitutively release S1P to extracellular carriers such as albumin. In addition, platelets release S1P upon stimulation and thus are distinct from erythrocytes [13, 33]. In this manuscript, we show that a significant amount of released S1P from platelets requires regulated exocytosis. Consistently, we observed that S1P is stored in platelet granules and newly synthesized S1P required about an hour to enter the stimulation-dependent, releasable pool. Activated platelets are known to release their granule contents at the site of vascular damage. Our studies identify S1P as an α-granule cargo. Such a granule store would enable platelets to mediate localized release of S1P, which may contribute to the maintenance of vascular integrity upon injury and during the post-thrombus sequelae associated with wound healing.
Inclusion of BSA was important for efficient detection of released S1P (Fig. 1). Interestingly, as the concentrations of BSA were increased there was a concomitant increase in total S1P in both human and mouse platelets. This implies that platelets can synthesize more S1P if there is an extracellular “trap” for the lipid. The S1P released from resting platelets showed the greatest change when BSA concentrations were increased, implying that resting platelets contain a pool of S1P that readily partitions into soluble carriers in the plasma. This pool can be replenished rapidly via de novo synthesis of S1P and may be similar to the pool of S1P in erythrocytes, which is constitutively released. Given the short half-life of S1P (<15 min) in plasma [11], the constitutively released pool from erythrocytes, and possibly from platelets, may serve a buffering function to control basal plasma S1P levels. Our labeling studies with C17- and NBD-sphingosine (Fig. 6), as well as studies by other groups [34], suggest that S1P in platelets can be generated by phosphorylation of extra-platelet sources of sphingosine. We did not measure the flux of sphingosine into ceramide or other higher order sphingolipids.
A second pool of S1P is released upon stimulation. This pool appears more insulated from extraction by BSA and is loaded less efficiently by de novo synthesis, as indicated by our metabolic labeling experiments (Fig. 6). Secretion of this pool requires the platelet secretory machinery (i.e. Munc13-4) and occurs with kinetics similar to the release of other platelet granule cargo molecules (Fig. 2, 3). The NBD-sphingosine labeling experiments (Fig. 4) are qualitatively consistent with this conclusion as were the sub-cellular fractionation experiments (Fig. 5). The sub-cellular distribution of S1P indicated that a fraction of the total platelet S1P was present in α-granules, but not detected in dense granules. It should be noted that imaging of NBD-staining yields information about localization but not quantitative distribution of platelet-associated S1P. In particular, a greater total mass of NBD-S1P could be present in the plasma membrane (as suggested by Fig. 6) but it would be less evident because it is more diffuse than the material that is concentrated in granules.
Although S1P levels and S1P synthesis from extracellular sphingosine were comparable to platelets from wild type animals, S1P release was attenuated in platelets from transgenic mice with defective platelet secretory machinery. Unc13dJinx mice are deficient in Munc13-4, a key regulatory molecule for the formation of the transbilayer SNARE complexes needed for granule-plasma membrane fusion [21]. Munc13-4 is required for efficient, regulated release of cargo in dense granule, α-granule, and lysosomes. Previously, we showed that release of the various soluble components of the platelet releasate requires SNARE-mediated membrane fusion [35]. The data presented here imply that activation-dependent release of S1P also requires regulated membrane fusion. These data clarify previous reports that S1P is released from activated platelets and perhaps explain the inhibitory effects of staurosporine and the ATP requirement, previously noted [17]. Exocytosis of platelet granule cargo is dependent on both PKC [36, 37] and on ATP hydrolysis [38]. Our data offer some of the first evidence that the release of a bioactive lipid requires the same exocytosis machinery needed for release of other small molecules and polypeptides.
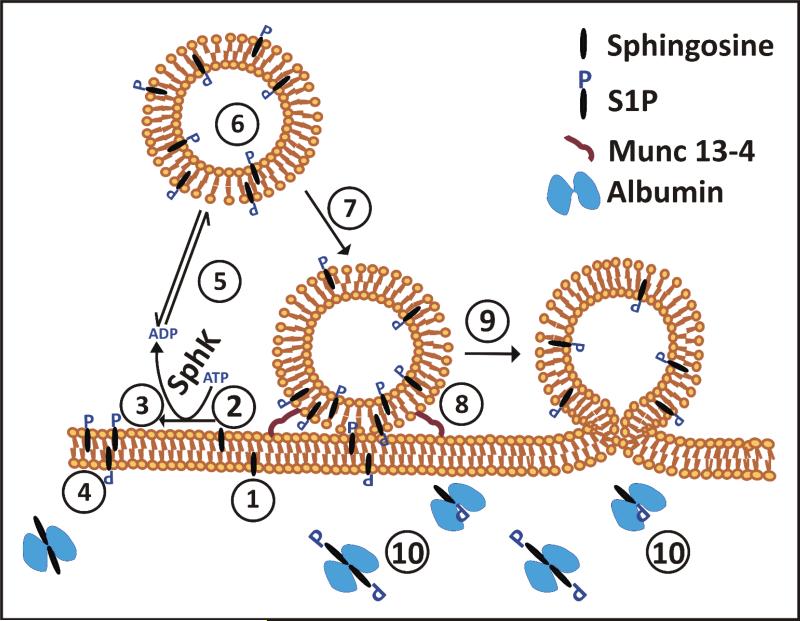
Our data suggest a framework for understanding the intra-platelet trafficking of sphingosine and S1P (Fig. 7). Initially, sphingosine could partition into the outer leaflet of the platelets’ plasma membrane (step 1) and translocate to the cytoplasmic leaflet where it could be converted to other sphingolipids or phosphorylated to make S1P (steps 2, 3). The resulting S1P could be translocated back to the outer leaflet and contribute to the passive, carrier-extractable pool of platelet S1P (step 4). Alternatively, the S1P could be transported to the granule membrane and then translocated to the luminal leaflet (steps 5-6). Once on the luminal side, the S1P could be “trapped” by a binding protein. Platelet activation (step 7) initiates SNARE-dependent exocytosis (fusion of the granule and plasma membranes, Step 8-9) exteriorizing the luminal S1P for extraction by plasma carrier proteins (Step 10) or for binding to proximal S1P receptors. S1P has numerous effects but it is clearly important for maintaining vascular integrity. Many of the effects of S1P on endothelial cells (migration, proliferation, and morphogenesis) can promote or mediate the wound-healing responses initiated by platelets. The fact that platelets also release VEGF [39] suggests a mechanism by which platelets can contribute to the S1P/VEGF, reciprocal axis that is important for proper angiogenesis. Our data show that platelets contain a granule pool of S1P that is responsive to stimulation and that can be released, like other platelet cargo, at the site of vascular injury. This adds S1P to the myriad molecules that platelets employ to modulate vascular microenvironments.
Figure 7. Trafficking and release of S1P by platelets.
Depicted is a trafficking scheme for sphingosine and S1P in platelets, based on the data presented in this paper. The steps are: 1) Sphingosine insertion into the outer leaflet of the plasma membrane (PM); 2) Translocation to the inner leaflet; 3) Phosphorylation by SphK to make S1P; 4) Translocation of S1P to the outer leaflet (This pool can be readily extracted by carrier proteins e.g. albumin and HDL); 5) Transport to the cytoplasmic leaflet of a platelet granule; 6) Translocation to the granule's luminal leaflet; 7) Platelet activation; 8) Granule docking; 9) Granule exocytosis; 10) Extraction of S1P by a plasma carrier protein, e.g. albumin or the apolipoprotein M component of HDL.
HIGHLIGHTS.
– Sphingosine-1-phosphate (S1P) promotes endothelial integrity.
– Platelets release S1P both constitutively and in an activation-dependent manner.
– Platelets have non-granular and granular S1P pools.
– Release of the granular pool of S1P involves regulated exocytosis.
ACKNOWLEDGEMENTS
We acknowledge Dr. Brian W. Wattenberg (University of Louisville) for providing the NBD-S1P standard for chromatography. We thank the staff of the Kentucky Blood Center for their help. We also thank members of the Whiteheart Laboratory for their critical reading of this manuscript.
FUNDING
*This work was supported by Grant HL56652 and HL082193 from the National Heart, Lung, and Blood Institute (National Institutes of Health) to S.W.W. and by Grant GM50388 and HL112788 from the National Heart, Lung, and Blood Institute (National Institutes of Health) to A.J.M. The microscopy was performed using facilities of the Molecular Basis of Human Disease COBRE Imaging Core, which is supported by grant P20RR020171 from the National Institutes of Health.
ABBREVIATIONS
- S1P
Sphingosine-1-Phosphate
- S1PR
S1P Receptor
- ABC
ATP Binding Cassette
- SPNS2
Spinster 2
- NBDSphingosine
7-nitro-2-1,3-benzoxadiazol-4-yl)(2S,3R,4E)-2-amino octadec-4-ene-1,3-diol
- MS
Mass Spectrometry
- RT
Room Temperature
- PF4
Platelet Factor IV
- SphK
Sphingosine Kinase
- SNARE
Soluble N-ethylmaleimide Sensitive Factor Attachment Protein Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts to disclose.
REFERENCES
- 1.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 3.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Seminars in Cell & Developmental Biology. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- 5.Gaengel K, Niaudet C, Hagikura K, Lavina B, Muhl L, Hofmann JJ, Ebarasi L, Nystrom S, Rymo S, Chen LL, Pang MF, Jin Y, Raschperger E, Roswall P, Schulte D, Benedito R, Larsson J, Hellstrom M, Fuxe J, Uhlen P, Adams R, Jakobsson L, Majumdar A, Vestweber D, Uv A, Betsholtz C. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Hisano Y, Nishi T, Kawahara A. The functional roles of S1P in immunity. J Biochem. 2012;152:305–311. doi: 10.1093/jb/mvs090. [DOI] [PubMed] [Google Scholar]
- 7.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Seminars in Immunopathology. 2012;34:73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends in Immunology. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 10.Yatomi Y, Ozaki Y, Ohmori T, Igarashi Y. Sphingosine 1-phosphate: synthesis and release. Prostaglandins & Other Lipid Mediators. 2001;64:107–122. doi: 10.1016/s0090-6980(01)00103-4. [DOI] [PubMed] [Google Scholar]
- 11.Venkataraman K, Lee Y-M, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular Endothelium As a Contributor of Plasma Sphingosine 1-Phosphate. Circulation Research. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. The Journal of Clinical Investigation. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hänel P, Andréani P, Gräler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. The FASEB Journal. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 14.Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan Y, Sheng M, Yago T, Silasi-Mansat R, McGee S, May F, Nieswandt B, Morris AJ, Lupu F, Coughlin SR, McEver RP, Chen H, Kahn ML, Xia L. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–109. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 16.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-Phosphate Induces Platelet Activation through an Extracellular Action and Shares a Platelet Surface Receptor with Lysophosphatidic Acid. Journal of Biological Chemistry. 1997;272:5291–5297. doi: 10.1074/jbc.272.8.5291. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. Journal of Lipid Research. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- 19.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 Functions as a Sphingosine-1-Phosphate Transporter in Vascular Endothelial Cells. PLoS ONE. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anada Y, Igarashi Y, Kihara A. The immunomodulator FTY720 is phosphorylated and released from platelets. European Journal of Pharmacology. 2007;568:106–111. doi: 10.1016/j.ejphar.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 21.Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120:5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Q, Barber HK, Crawford GL, Karim ZA, Zhao C, Choi W, Wang C-C, Hong W, Whiteheart SW. Endobrevin/VAMP-8 Is the Primary v-SNARE for the Platelet Release Reaction. Molecular Biology of the Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathews TP, Kennedy AJ, Kharel Y, Kennedy PC, Nicoara O, Sunkara M, Morris AJ, Wamhoff BR, Lynch KR, Macdonald TL. Discovery, Biological Evaluation, and Structure−Activity Relationship of Amidine Based Sphingosine Kinase Inhibitors. Journal of Medicinal Chemistry. 2010;53:2766–2778. doi: 10.1021/jm901860h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selim S, Sunkara M, Salous AK, Leung SW, Berdyshev EV, Bailey A, Campbell CL, Charnigo R, Morris AJ, Smyth SS. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci (Lond) 2011;121:565–572. doi: 10.1042/CS20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broekman MJ. Homogenization by nitrogen cavitation technique applied to platelet subcellular fractionation. Methods Enzymol. 1992;215:21–32. doi: 10.1016/0076-6879(92)15049-i. [DOI] [PubMed] [Google Scholar]
- 27.Rendu F, Lebret M, Nurden AT, Caen JP. Initial characterization of human platelet mepacrine-labelled granules isolated using a short metrizamide gradient. British Journal of Haematology. 1982;52:241–251. doi: 10.1111/j.1365-2141.1982.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 28.Aoki S, Yatomi Y, Ohta M, Osada M, Kazama F, Satoh K, Nakahara K, Ozaki Y. Sphingosine 1-Phosphate–Related Metabolism in the Blood Vessel. Journal of Biochemistry. 2005;138:47–55. doi: 10.1093/jb/mvi100. [DOI] [PubMed] [Google Scholar]
- 29.Gachet C. ADP Receptors of Platelets and their Inhibition. Thrombosis and Haemostasis. 2001;86:222–232. [PubMed] [Google Scholar]
- 30.Wall J, Buijs-Wilts M, Arnold JT, Wang W, White MM, Jennings LK, Jackson CW. A flow cytometric assay using mepacrine for study of uptake and release of platelet dense granule contents. Br J Haematol. 1995;89:380–385. doi: 10.1111/j.1365-2141.1995.tb03315.x. [DOI] [PubMed] [Google Scholar]
- 31.Reddington M, Novak E, Hurley E, Medda C, McGarry M, Swank R. Immature dense granules in platelets from mice with platelet storage pool disease. Blood. 1987;69:1300–1306. [PubMed] [Google Scholar]
- 32.Yang L, Yatomi Y, Satoh K, Igarashi Y, Ozaki Y. Sphingosine 1-Phosphate Formation and Intracellular Ca2+ Mobilization in Human Platelets: Evaluation with Sphingosine Kinase Inhibitors. Journal of Biochemistry. 1999;126:84–89. doi: 10.1093/oxfordjournals.jbchem.a022440. [DOI] [PubMed] [Google Scholar]
- 33.Morris AJ, Panchatcharam M, Cheng HY, Federico L, Fulkerson Z, Selim S, Miriyala S, Escalante-Alcalde D, Smyth SS. Regulation of blood and vascular cell function by bioactive lysophospholipids. Journal of Thrombosis and Haemostasis. 2009;7:38–43. doi: 10.1111/j.1538-7836.2009.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yatomi Y, Ozaki Y, Satoh K, Kume S, Ruan F, Igarashi Y. N,N-Dimethylsphingosine Phosphorylation in Human Platelets. Biochemical and Biophysical Research Communications. 1997;231:848–851. doi: 10.1006/bbrc.1997.6207. [DOI] [PubMed] [Google Scholar]
- 35.Ren Q, Ye S, Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Current Opinion in Hematology. 2008;15:537–541. doi: 10.1097/MOH.0b013e328309ec74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung S-H, Polgár J, Reed GL. Protein Kinase C Phosphorylation of Syntaxin 4 in Thrombin-activated Human Platelets. Journal of Biological Chemistry. 2000;275:25286–25291. doi: 10.1074/jbc.M004204200. [DOI] [PubMed] [Google Scholar]
- 37.Hirling H, Scheller RH. Phosphorylation of synaptic vesicle proteins: modulation of the alpha SNAP interaction with the core complex. Proceedings of the National Academy of Sciences. 1996;93:11945–11949. doi: 10.1073/pnas.93.21.11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmsen Platelet metabolism and activation. Semin Hematol. 1985;22:219–240. [PubMed] [Google Scholar]
- 39.Wartiovaara U, Salven P, Mikkola H, Lassila R, Kaukonen J, Joukov V, Orpana A, Ristimäki A, Heikinheimo M, Joensuu H, Alitalo K, Palotie A. Peripheral Blood Platelets Express VEGF-C and VEGF which Are Released during Platelet Activation. Thrombosis and Haemostasis. 1998;80:171–175. [PubMed] [Google Scholar]