Abstract
Materials chemistry is making a fundamental impact in regenerative sciences providing many platforms for tissue development. However, there is a surprising paucity of replacements that accurately mimic the structure and function of the structural fabric of tissues or promote faithful tissue reconstruction. Methodologies in biomimetic materials chemistry have shown promise in replicating morphologies, architectures and functional building blocks of acellular mineralized tissues dentine, enamel and bone or that can be used to fully regenerate them with integrated cell populations. Biomimetic materials chemistry encompasses the two processes of crystal formation and mineralization of crystals into inorganic formations on organic templates. This review will revisit the successes of biomimetics materials chemistry in regenerative medicine, including coccolithophore simulants able to promote in vivo bone formation. In-depth knowledge of biomineralization throughout evolution informs the biomimetic materials chemist of the most effective techniques for regenerative framework construction exemplified via exploitation of liquid crystals (LCs) and complex self-organizing media. Therefore, a new innovative direction would be to create chemical environments that perform reaction–diffusion exchanges as the basis for building complex biomimetic inorganic structures. This has evolved widely in biology, as have LCs, serving as self-organizing templates in pattern formation of structural biomaterials. For instance, a study is highlighted in which artificially fabricated chiral LCs, made from bacteriophages are transformed into a faithful copy of enamel. While chemical-based strategies are highly promising at creating new biomimetic structures there are limits to the degree of complexity that can be generated. Thus, there may be good reason to implement living or artificial cells in ‘morphosynthesis’ of complex inorganic constructs. In the future, cellular construction is probably key to instruct building of ultimate biomimetic hierarchies with a totality of functions.
Keywords: biomineralization, regenerative medicine, bioinorganic materials chemistry
1. Introduction
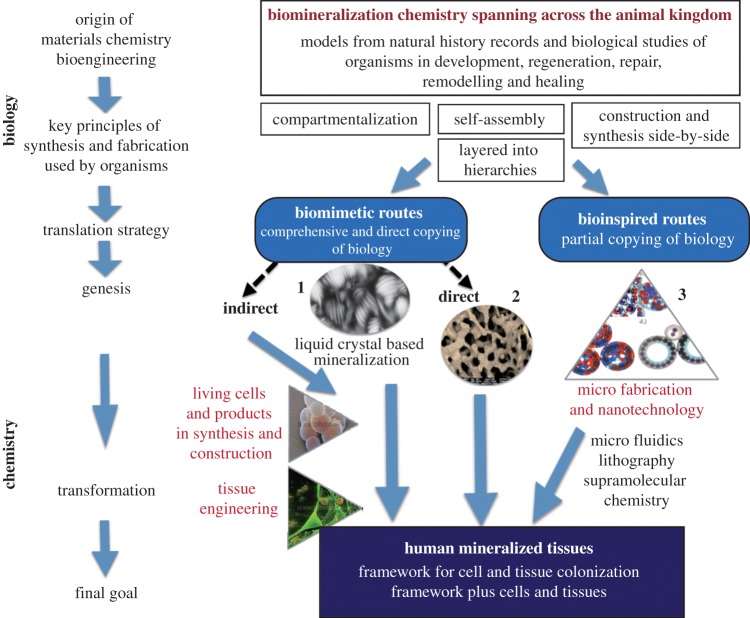
Living organisms are consummate innovators with powerful ingenuity to solve problems and conflicts that arise in the competition for all critical resources. Among Bacteria, single-cell eukaryotes and invertebrates, there is a rich variety of exquisite and intricate inorganic materials and structures that have gradually evolved by natural selection. They are featured with ultimate miniaturization, complex hierarchies, versatility, adaptability and optimization [1]. These biomaterials and biostructures have emerged with many constraints placed on them through historical legacies and the narrow availability of resources at the period of selection. Because of these restrictions, there has been convergence on sets of universal fabrication principles such as use of liquid crystals (LCs) to generate shapes and patterns with low energy usage. This has meant that inherited features, elements and characteristics have been adapted for many different roles and purposes. Using biomimetic logic, the aim is to transfer superior materials and structural design in nature into technologies. Regenerative sciences will be a prime beneficiary from proper biomimetic translation of the abstraction of ‘good design’ from natural biological sources and that goes beyond the human body [2] (figure 1). Having structures that match the performance of the host promotes integration, healing and reduces inflammation.
Figure 1.
Suggested pathways for the translation of organism biomineralization knowledge into innovative tissue-regeneration products. The topic of this review looks at one biomimetic pathway with emphasis on chemical-based approaches highlighted here by LC synthesis [1]. It must also be noted that bioinspired routes have become very important in providing innovative solutions for constructing materials and structures for regenerative sciences. (Online version in colour.)
At this current stage, there is a paucity of such frameworks to repair and build hard tissues either in the culture dish or in situ. Properties that make calcified biological tissues unique and adapted to function include toughening mechanisms in the materials, proper interfaces and a graded relief in properties. Artificial materials and structures with these devices are particularly rare. The key to this success could emerge from the principles and practices of biomimetic materials chemistry. The principles of the chemistry are self-organization, minimal energy inputs, self-replication and miniatuarization of components and at assembly [1]. This important cluster of chemical methods is closely related to crystallization events and mineralization. The methods are chemically engineered to simulate critically essential mineralization processes to achieve intricate bioinorganic formations that are either natural (biomimetic) or unnatural (bioinspired) in shape, architecture and form [3,4]. One of the major sets of objects in crystallization and mineralization are the packaging, transport and templating devices made from phospholipid bilayers. Materials chemists are aiming to generate chemical environments that lead to the intricate self-organization of these components into the complex hierarchical structures of natural bone. Also, there is equal validity in replicating skeletal structural forms from other organisms. These can also be used to support functions in the bone environment. There has been a reliance on emulsion chemistry (e.g. water-in-oil and oil-in-water mixtures) [5], Langmuir monolayers [6], organic frameworks (reused or those generated from bacteria, e.g. [7]) and reaction–diffusion effects [8] to create patterned materials and structures.
In this review article, we will highlight examples of how complex inorganic morphosynthesis has been used to produce biomimetic structures and materials for tissue regeneration. Examples include microscopic microsponges as a packing material in bone and chiral bioliquified crystals that self-assemble into structural replicates of acellular bone, dentine and enamel [9]. Still, the major difficulties outlined in this field are the reproduction of stiff, toughened materials in clinically significant quantities and their synthesis into fully integrated hierarchal structures. Nevertheless, the ability to build multi-layered ‘hierarchical’ structures that possess the form and function of bone, enamel or dentine is achievable by using biomimetic chemistry. Resolution of these problems is probable via the incorporation of selected peptides and proteins into a biomimetic chemical system. It is also likely by the use of living or artificial cells or viruses for synthesis. Also there is need to develop more effective chemical environments that freely evolve and transform in an adaptable, ever-changing flow to truly recreate a ‘synthesis-with-construction’ and development mode of calcified materials production chemistry.
2. An overview of biomimetic materials chemistry
In the vast expanse of biology, materials and structures are made with complexity, minimum energy budgets and with substrates that are invariably most freely available in the habitat or the more restricted space of a niche. Owing to evolution by natural selection, these materials and structures are riddled with historical aberrations derived from past adaptations. There is also a high degree of multitasking, convergence in form and function and conservation among organisms. For example, the biomineralization toolkit has similarities between the original ancestors and the successors [10]. Materials chemists are striving to replicate this and have superior performing materials for medicine, optics and electronics, etc. One of the finest examples has been the lesson derived from study of nacre, which incorporates structural and biochemical features that stop and contain the spread of fractures [11–14]. The core principles and processes from nanocrystal to macrostructure are at least known such as the need for phospholipid vesicles, molecular patterned for crystal organization and a special set of proteins to direct and organize construction [15–17]. Explanations of how biological materials and structures are fabricated by organisms had their beginnings in scientific descriptions made by Haeckel and later superceded by Thompson [18]. Pieter Harting was the first to recreate life-like inorganic forms and structures in the laboratory by experimenting with crystal growth in special ionic solutions. Later chemical discoveries in areas that converged with biological programming of its own materials chemistry led to replicated shapes and forms. Ideas such as, molecular cooperation, synergy and self-organization were harnessed. For example, amphiphillic molecules associate with themselves into collectives such as, micelles, platelet lamellae and bicontinuous phases of water, oil and surfactants. The architectural patterns reminiscent of Foraminifera and diatoms were faithfully copied with aluminophosphate substrates that are bounded by surfactant templates and organized by nature into identical self-assembly and self-organization activities [19,20].
2.1. Evolution from morphogenesis to biomimetic morphosynthesis
Research distilled from chemical synthesis of catalytic zeolites showed great promise in replicating microskeletons in nature and it emerged that the chemical syntheses converged on the same principles such as molecular recognition and template synthesis [21]. Synthetic vesicles were used to generate greater scale in pattern formations and these began to resemble the formations in Radiolaria and diatoms. Advances in phospholipid chemistry at the time provided clues to the development of biomimetic formations. This precipitated inorganic microstructures patterned into honeycombs, spheroids and hollow shells carved with pores, bowls, platelets, posts, etc. Adaptations to this line of chemical synthesis facilitated developments in building different shapes with a selected template, patterning via control of surface tension, shear and fabrication of foams, bubbles and nested bubbles. It was possible to create hexagonal, gradient, polydisperse, asymmetrically sheared and shrunken patterns through adjustments to the physical and chemical force fields [21]. This is the basis for morphological and architectural diversity in the beaker.
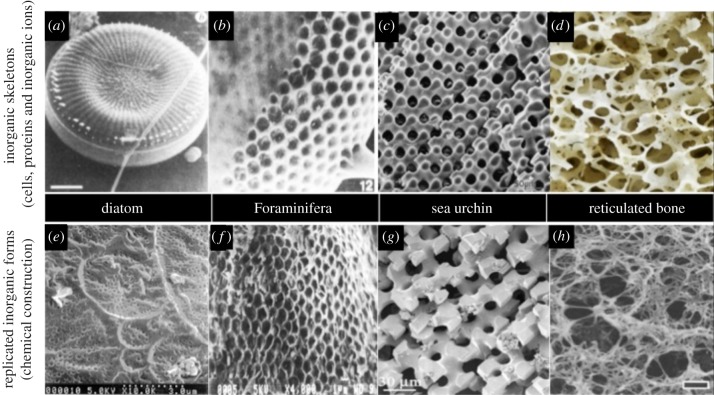
At 16 years of age, the theme of biomimetic materials chemistry is arguably still an unfulfilled branch of applied inorganic chemistry. Its focal point has been to simulate biomineralization of complex exoskeletons from relatively simple marine invertebrates [4]. There have been credible attempts also at fabricating dentine microstructures [22] and acellular bone equivalents [23–26]. Unfulfilled because only a few specified model organisms such as: choanoflagellates [27], radiolarians [27], foraminiferans, diatoms [27] and coccolithophores [28,29], Porifera (marine sponges) [30], Strongylocentrotus species (sea urchins), sand dollars (Scaphechinus mirabilis) [31], sea stars (Pisaster giganteus) [32] and Acantharia [33], representing a fraction of the total number of mineralizing organisms that have been studied to a level of detail of how the biomineral ions are acquired from the environment, confined, concentrated, safely transported, deposited, patterned, densified and displayed. This pathway of information is required for a smooth translation into a simulant. The details of these mineralizing events are also identified for bone, dentine and enamel formation. Only diatoms, Foraminifera, sea urchin spines and reticulated bone textures have been accurately emulated by chemical methods alone, free from inputs of proteins and cell engagement, as highlighted in figure 2.
Figure 2.
The only natural skeletons that have been faithfully copied using chemical construction, free from participation of proteins and genetic programming. Panels (a–d) represent the natural inorganic skeletons. They are each paired with their artificially produced copies laid out in panels (e–h) [21]. ((a,b,e,f) Adapted from American Chemical Society; (c) adapted from Wiley-VCH; (d) adapted from Wikipedia, Patrick Siemer; (h) adapted from Macmillan; (g) adapted from Wiley-VCH.) (Online version in colour.)
2.2. Biomineralization understanding
There are many thousand more organisms that can be studied as a wellspring of ideas for fabricating new materials either via biomimetic materials chemistry or bioinspired routes [34]. Studying natural history collections, using zoo resources of live animals and reading historical natural history publications is a major starting point for biomimetic and bioinspired discoveries [34,35]. The enterprise of making materials akin to natural counterparts is learnt from a breadth of knowledge about the processes of mineralization in many different living organisms both extant and sometimes fossilized [36]. The natural archetypes on which to carry through mimicry are simple in complexity and include: Foraminifera, coccolithophores and bacterial threads [29,37,38]. These natural inorganic formations are confined to two levels of hierarchy and usually lack an intrinsic organic component and a genetic and metabolic code of autonomous instruction [39]. Adaptable and shape shifting vesicular templates, held in place with internal microtubules are given shape and architecture by the endoplasmic reticulum and Golgi apparatus. These represent the conduits and piping that shift the cargoes of minerals and precursors and in providing the energy to power the processes and the intricate boundaries and frameworks for mineralization to interface against [40,41]. Inside the cell, the different organelles, sometimes unified into groups and tethered together with cytoskeletal guy-ropes, carry out these functions in an assembly line fashion. The cell itself can do the same encapsulation and transport of mineral precursor or crystals at the next higher level of scale: by creating enclosed vesicle compartments inside the cell and differently shaped templates excreted and woven outside the cell into delineated spaces for mineralization. The intracellular vesicles must exert tight control over the attributes of the enclosed mineral products such as concentration, which can be toxic to the host cell. There is conflicting evidence as to what products are used by mineralizing cells. Evidence obtained in fixed cells under transmission electron microscopy and scanning electron microscopy (SEM) shows that the product is nanocrystals aggregated into amorphous phases. Whereas, live cell imaging of oyster haemocytes tends to show that it is crystalline already [42]. There is clear evidence that exosomes compartmentalize small nucleated biomineral crystals within the cell and are then carried out of the cell, secreted, assembled and finally densified in place [43]. Clearly, the crystallization processes operating inside the cell are a vital component in biomineralization events. There is yet stronger evidence that calcium phosphate is formed inside bone cells, stored and transferred to permeate the pre-formed organic matrix [44]. Inside the osteoblast, cell cytoplasmic vesicles with calcium have been observed to migrate and merge with mitochondria that also store calcium phosphate particles. This exchange is a prelude to ejection and permeation of organic frameworks outside the cell.
In fact, every material and structure in nature is defined by the most specific molecule-by-molecule organization of matter into exquisitely intricate morphologies. This biologically organized matter is under continual re-adjustment in response to the ever-changing flow of the environment. Adaptations are made in real-time during the lifetime of the organism and between generations via genetic, epigenetic and environmental influences. This is hard to replicate using the current tools in inorganic and organic chemistry, but there have been notable successes [16], Lipids (phospholipids) [15], amphiphile sheets [45], peptide amphiphile nanofibres [23,24], charged macromolecules [46] and nature-derived nano-, micro and macroscopic templates (viruses and pollen) [47] have been assembled to create surfaces for the development of inorganic materials synthesis [41]. We can begin to generate inorganic complex forms by following common core principles in biomineralization [4]. Biomineralization can be used as the blueprint for producing complex mineralized tissues, such as dentine [39]. There are four guiding principles in order of timing towards the end-product: supramolecular pre-organization, which provides a scaffold to create the proper space filling environment and a mould for amorphous biomineral exchange to crystals; interfacial molecular recognition to seed the crystals at regular positions in the framework; ‘vectorial crystallization’ to shape the biomineral crystal plates into spaces over time and ‘pattern evolution’ in which the mineral is fabricated into large diverse formations principally by involvement of cells via gene and protein regulators and biologically controlled contributions of LCs and reaction–diffusion chemistry (figure 3a–f) [50]. Both are fundamental originators of pattern formation in biological systems.
Figure 3.
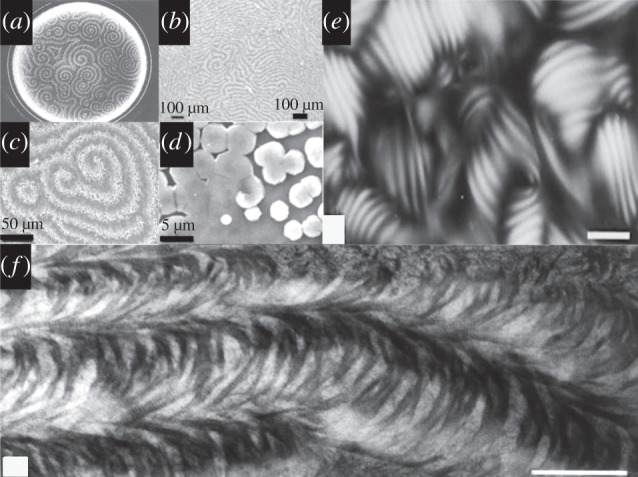
LCs in reaction–diffusion chemical systems (illustrated in a) showing ‘diffusive instability’ are a major source for the emergence of intricately structured macromolecular frameworks in mollusc shell (b–d) and bone (f). (a) An intricate pattern generated in a solution by the classic process discovered by Belousov–Zhabotinsky. This was the fundamental basis for explaining complex pattern formation in biology predicted at the same time by the mathematician Alan Turing. Biological LCs are prevalent in nature as a physical framework for development and pattern formation. Diffusion instabilities in diffusion reaction systems are the beginnings of complex pattern formation in biological patterning and development [8]. Plates (a) reproduced with kind permission from Princeton University Press; (b–d) adapted from Proceedings of the National Academy of Science [48]; plates (e,f) adapted from Elsevier [49].
Use of bioinspired and biomimetic principles and concepts in the production of advanced biomedical materials and structures has increased the flexibility, diversity and utility of frameworks for tissue engineering. The benefits are simpler and cleaner ‘green’ synthesis and increased biological interactivity and function [51–54].
2.3. Bioinorganic materials and influences on cell regeneration
An explanation is necessary of the effects of biomimetic and nature-inspired bioinorganic materials on cells in regeneration and why such materials are an important advantage in hard-tissue regeneration [55–57]. New research is investigating the potential of nanometric internal and external modulation of cells, viruses, bacteria and embryos with biomimetic inorganic mineralization strategies. The effects of inorganic materials on the living cell are profound. There is a historical and fundamental synergy between cells, environment and inorganic chemistry. During cell evolution and transformation into complex organisms, there has been an intimate connection with chemical evolution in the environment [58]. Evolution was promoted, driven and directed by the relationship with inorganic minerals and chemistry, inevitable given the exclusive availability of these chemicals on the early Earth. Mineralized substrates therefore influence many cell responses and activities related to regenerative transformation into hard tissues. In particular, the exchanges in proteins and ions at mineralized surfaces are important in conditioning the microenvironment for bone formation [59]. Mineral ions become hydrated at the surface and influence cells very heavily. As do proteins, their type and conformation. Mineralized substrates partially dissolve at surfaces and the availability of free mineral ions influences cell biochemistry. This is related to the mineral ion composition of the substrate and one parameter in particular, the calcium and phosphate ratio [59]. In addition, the surface topography at the nanoscale and microscale as well as surface energy values have strong influences on cell behaviour in regeneration.
3. Chemistry-based biomimetic materials
Chemical-based biomimicry of inorganic materials can be fabricated via chemical assembly. Typically, inorganic materials are made by physical methodologies. Through chemical processes, inorganic organized matter can emerge in different ways with time and space, increasing volume as the chemical environment reacts and changes [60]. It is conceptually feasible to derive tooth enamel using these biomimetic principles [39]. The most profound difficulty would be to engineer nanocrystals, millimetres in length and nanometres wide, with the available conceived templates [39]. At some stage in the construction process, there must be an intervention by cells and hormones, unless perfectly true mimics can be synthesized particularly with many layered hierarchies.
It is feasible to combine pure chemical routes to synthesis of complex inorganic morphologies with organic molecules and even living cells, either to adapt crystal tectonics or to add therapeutic functions such as, drug protein delivery. A key hurdle in the translation of biomineralization concepts into the ‘beaker’ is a completed blueprint showing all the molecular processes involved in making complex inorganic formations [61]. Many organisms with life histories that include abundant mineralizing do not have a full genetic profile, and there are just a handful of protein sequence databases to interrogate [62]. Thus, harnessing microorganisms to do the work for the biomimetic materials engineer or the construction of autonomous artificial cells are appealing routes to follow [63].
3.1. Growing inorganic materials with viruses, bacterial and living cell templates and liquid crystals
Bacterial cells and viruses have been implemented in building materials, including gold nanoparticles and cadmium-selenide nanostructures, [48] as moulds for packing nanoparticles into tight, secured arrangements that line the template. Bacteriophages have been genetically engineered to present peptides on their outer casings (capsid) that promulgate crystal binding [49,64]. Decoration of capsids with cell signalling or adhesion peptides offers a chance to create ordered structures that may order cell adhesion density and cell distribution [65]. Intricately patterned chains of nanocrystals have been organized onto naturally fabricated protein films derived from bacterial cell envelopes (surface-layers or S-layers) in cadmium sulfide [66]. The promise and advantage of using S-layers, as templates, results from their structural diversity and the possibility of easily changing and adding proteins and other molecules to their surface [66]. These characteristics apply to biomimetic lipid bi-layer membranes in eukaryotic cells [7]. Helical-shaped nanofibre networks assembled from M13 phages have been developed as small elemental building blocks with advantages of LC tendencies [49].
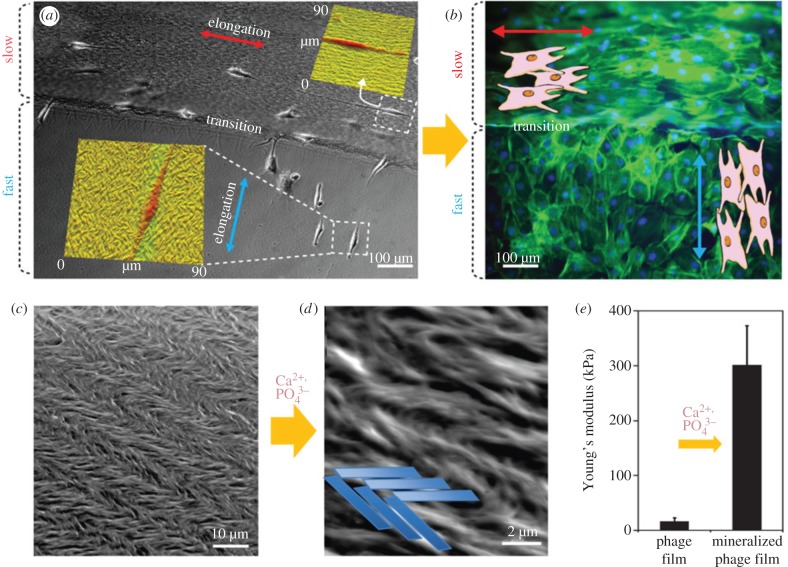
LC materials and LC versions of DNA, polypeptides, collagen solutions are vital, fundamental and prevalent in biology behind organization and function of biological matter [67]. LCs undergo rapid transitions into a variety of structures via pH, temperature, pressure and inorganic ions, which is one reason they are employed in biology. Another reason is that the energy needed to convert them into exquisite structures in phase transitions is lower compared with cellular construction using adenosine tri-phosphate [2]. Large helical structural biomolecules, chitin, collagen and cellulose are assembled via LC building blocks, which possess chirality, or left- and right-handedness. Evolution by natural selections has harnessed LCs in the making of exquisitely patterned and textured tissue architectures with high order of function such as cornea and bone [50,68]. These chiral fluids are able to self-assemble into patterned formations at the sub-micrometre scale and then layer into a micrometre scale (nematic with rod-shaped molecules in parallel strands, cholesteric with twisting of the molecules at right angles to the long axis of the molecules and smectic with layers of molecules that slip past each other) that are dictated by the nature of the environment and defined by geometrical barriers to growth, particle flow speed and surface tension at interfaces [49]. Therefore, fibre bundles built at the microscale level can be made to twist left or right and can be combined into elaborate rope structures. The added advantage of bacteriophage capsules as a building block for materials is that bioactive molecules can be covalently bound to the outer surface. In one instance, the prevalent cell adhesion tripeptide motif, RGD has been tethered onto M13 phages. In this study, it was explicitly shown that connective tissue fibroblast cells preferentially attached and aligned themselves in exact mirroring of the underlying textures made by the original twisted helical fibres (figure 4a,b). The intertwined ropework made up of phage proteins directly resembled enamel crystalline structure and architecture. When mineralized with calcium phosphate saturated solutions, the intertwined and twisted phage fibres truly mimicked the chemical composition and mechanical strength of natural tooth enamel (figure 4c,d). The functional efficacy of this artificial mineralized enamel texture was demonstrated by a 300-fold increase in Young's modulus (figure 4e). The phage virus capsule is a versatile materials building block. It has also been used to help build larger scale structures in organized parallel arrays via electrospinning [69]. The templates that serve as the constrained boundaries, compartments and foundations for mineralization are usually made artificially, from scratch, with either biological or synthetically derived macromolecules. Artificial biotemplating in the mirror image of simple, naturally occurring mineralization in tiny marine plankton is possible and yields morphologies reminiscent of coccolithophores, Foraminifera and diatoms.
Figure 4.
An individual demonstration of how chiral LC media can give rise to an exact structural mimic of the complete mineralized enamel framework. A common biology principle in tissue framework fabrication with complex structure is to harness LCs. Supramolecular structures such as collagen fibre bundles can arise from LC precursors. (a) A unique method devised by Chung et al. allows the operator to recreate four LC assemblages. In this image, two separately patterned collagens have been added next to each other. Fibroblast cells have been cultured on the textures and are shown to elongate in direct contact with the alignment of the fibre texture. (b) Cell growth allowed to proceed on the surface textures shows them to proliferate and spread in the alignment with the underlying fibres. (c) Intertwined ropework generated from a chiral media. Collagen fibres have organized themselves into a characteristic pattern, which is similar to the underlying framework in enamel as dictated by the LC phase structure. (d) An intertwined ropework of phage capsids generated from a chiral media was deployed into a film and is highly reminiscent of enamel superstructure. The structure was crystallized into calcium phosphate, which increased stiffness (e) (adapted from MacMillan Publishing Ltd. [49]). (Online version in colour.)
Another type of living object for inorganic templating has been mammalian and yeast cells [70–73]. There has been increasing interest in using fabricated microcoatings and nanocoatings to encapsulate individual cells and clusters of living cells. Specially coated cells could extend their use as biosensors, cryoprotectants, products to facilitate cell therapy and tissue engineering strategies. For instance, living HeLa cells have been coated in a silica nanolayer [71] and yeast cells enclosed inside a layer of crystallized calcium carbonate [72]. The modes of coating attachment vary between electrostatic attractions with the cell membrane [71], ionic charge pairing, direct precipitation and polyamine connectors. Another cited rationale is the need to protect individual cells from outside stresses such as heat, freezing and mechanical forces [71]. The protection given by coatings will have important utility in cell culture, husbandry and unique modifications for cell therapy. The force and magnitude of habitat stresses is the reason for evolutionary adaptations in many single-cell microorganisms. They grow shells for protection against predators and environmental extremes in temperature.
In some examples, the formation and synthesis of coating structures have mimicked actual processes in the biomineralization of single-celled organisms such as diatoms. There are two notable bioinspired approaches modelled on diatoms and Foraminifera. The first copies the biological role of polyamines in driving silicification reactions via silicic acids with a PEI equivalent [71]. In the second approach, titanium dioxide is deposited onto single cells of Chlorella algae via silaffin-mimicking polyamines, rich in arginine and lysine that catalyse condensation reactions with titanium. Coatings provide a convenient surface for decoration with non-native active molecules. In one example, catchecol molecules were attached to the reactive surface of a titania single-cell coating [73]. These can give rise to new pertinent functions and possibly new materials.
Inorganic nanocoatings have also been implemented with human primary mesenchymal stem cells facilitating induction towards specialization into a bone cell lineage and targeted gene transfer into human MSCs [65].
3.2. Coccolithophore analogues made in the beaker
An attractive approach to growing inorganic materials is to set up a chemical microenvironment that autonomously transforms into the final material via a number of linked steps involving cooperative reactions and metamorphosis. We know that production of amorphous mineral precursors is an important step that precedes the formation of the nanocrystals and their assembly into crystallites and plates [74]. Templates with intricate three-dimensional outward forms can be produced within ‘self-organizing’ media that mimic the templating with vesicles in single-celled mineralizing coccolithophores (figure 5a). One simple system is a ‘water-in-oil’ reverse microemulsion in which the water phase is loaded with the starting biomineral ions, such as calcium carbonate, while the changes in the external environment induce precipitation at the interface of the water and oil [37]. Both physical and chemical patternings are at play in the generation of porous microsponges; the mechanism is displayed in figure 5b and the types of structure that can be recreated shown in figure 5c,d. Such microsponges have been adapted into a delivery vehicle for targeted drug and gene product capture and release [9]. This was achieved by adding the gene or protein component into the water phase prior to synthesis. During the synthesis, these factors become integrated and entrapped within the crystals and between the crystals during phase transition (figure 5d) [9]. These same structures also showed utility in promoting osteoid bone formation when mixed with allograft and hBMSC within an animal model. The tissue production was dramatically increased when compared with tests for bone regeneration with just allograft and hBMSC (figure 5e,f). Compressive strength was correspondingly much more significant in the microsponge samples (figure 5g).
Figure 5.
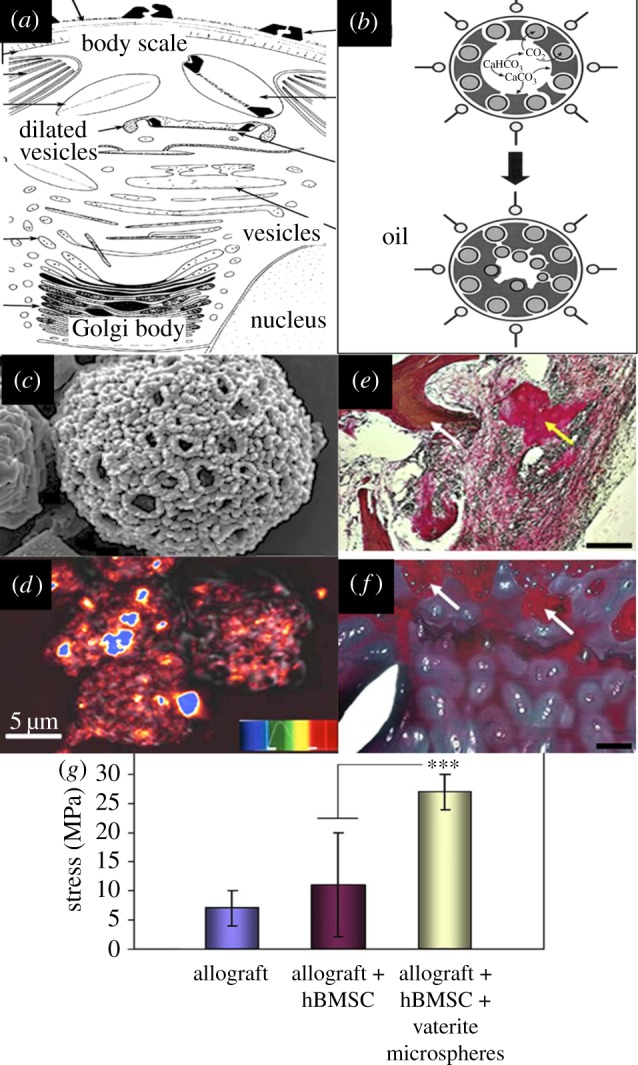
(a) Diagram illustrating biological formation of coccolithophore skeletal sections by cell organelles [29] (adapted from Mineralogical Society, America & Highwire). (b) A simple chemical system devised by Walsh et al. that emulates biomineral patterning in calcium carbonate reminiscent of the cuboid building blocks in coccolithophore species [37] (adapted from Wiley). The re-action field conditions were modified during evaporation of the oil phase during the last step of vaterite microsponge synthesis. (c) An SEM image showing an intricate biomimetic crystal architecture generated using this adaptable chemical system (DW Green 2008, unpublished data). It is also possible to add biological functions to the structures by doping the water phase with any desired protein additive which will lead to its entrapment between crystals and inside them [9]. (d) A laser confocal image of detected haemoglobin, highlighted in orange and red entrapped into vaterite microsponges (adapted from Royal Society of Chemistry) [9]. (e,f) Vaterite microspheres generated via biomimetic materials chemistry have shown to promote extensive immature bone formation in an animal model within composites of bone allograft and hBMSC. (e) Human bone allograft co-cultured with hBMSC in vivo gave rise to small segments of collagen type I positive bone tissue. When human allograft and hBMSC were co-cultured with vaterite microspheres, there was new bone tissue production almost throughout the implanted material (f). (g) The microsphere-laden tissue gave significantly enhanced compressive strength compared with the diffuse tissue generated without microspheres (Adapted from Elsevier) [75]. (Online version in colour.)
4. Extraordinary biomineralization systems
One can speculate on the future course of biomimetic materials chemistry. We have considered the possibilities of using living cells to synergistically construct and grow inorganic materials and automatically self-engineer clinical-grade human tissue replacements. So far this research theme has not been explored at all. Yeast cells have been complexed with clay nanotubes to form a stable living hybrid [76]. There have been other interesting developments such as construction of calcite nanocrystals into one type of coccolithophore microstructure (figure 6a, creation of semi-autonomous artificial cells—that could be adapted to biomineralization roles—and use of reaction–diffusion organizing media composed of stacked chemical gradients [8,79]).
Figure 6.
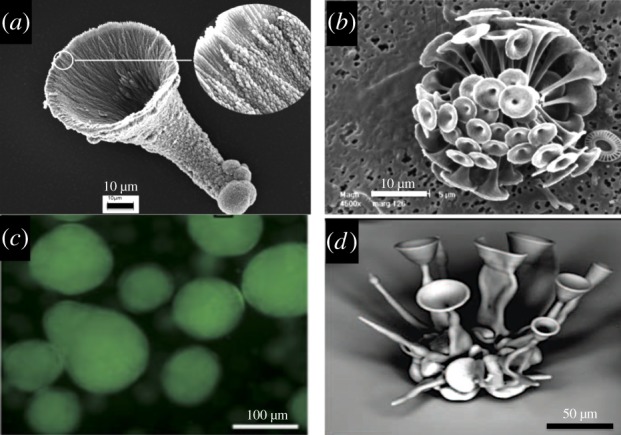
Directed construction of biomimicking microstructures. (a) Trumpets built from nanocrystalline calcium carbonate. (b) Original calcium carbonate ‘trumpet shapes’ made by coccolithophore species [77]. (c) Artificial cell glowing fluorescent green with GFP expressed by the added genetic machinery inside [78]. (d) Complex artificial microarchitectures made with biological principles harnessing reaction–diffusion systems and microenvironmental control of pH temperature and shear flow in self-organizing media [79]. (a,b) Reproduced with kind permission from Royal Society of Chemistry; (c) reproduced with kind permission from Macmillan; (d) reproduced with kind permission from AAAS & Highwire. (Online version in colour.)
Some chemists are creating synthetic cells although not as yet specially programmed for biomineralization functions [80,81]. Fascinating examples of this approach can be seen in investigations of the proto-biological world. Artificial protocells are simplified replicates of cells made with natural (proteins) and un-natural materials [82]. Artificial protocells are increasingly being made, not according to biological principles of construction but by self-assembly without a biological membrane (figure 6c,d) [83]. Artificial cells can include primitive gels and can be used as containers for biochemical reactions and gene expression [82] (figure 6c,d). There has been a need for facile gene expression systems without the disadvantages of interference from cellular protein secretions. In a study by Bawazer et al., vesicles (synthetic cell surrogates) were constructed around an internalized polymer bead onto which genes for silicatein—from marine sponge—were attached. Entrapment of a co-reactant within a vesicle bounded with water droplets in oil created the enclosed reaction environment wherein silicon dioxide was autonomously synthesized [82]. Artificial selection was imposed on the diversity of silicatein products obtained via cell sorting to isolate enzyme variants with different catalysis rates. Using this system, they could perform evolutionary selection of specific silica product variants. Therefore, it is conceivable to employ these artificial cells and synthetic cell surrogates to synthesize substrates for building new inorganic biomaterials. These simplified cells are a counterpoint to actual gene engineered cells that could do the same task, only without entering evolution. There is some concern about the ability to influence these processes in medical applications; therefore, there are further possibilities with this line of thinking. One possibility, founded on replicating the range of cell nanopieces (i.e. receptors and enzymes) is the creation of artificial life, made from equivalent polymer membranes and molecular motors that acts like their organic counterparts, to manufacture and simulate a living cellular machine [63,84].
Reaction–diffusion chemistry in a liquid media can produce highly elaborate and intricate patterns. This is one main way where complexity can emerge from simple mixtures. In §2 (figure 3), we highlighted that swirl and target patterns arising at the beginning of nacre biomineralization. The autonomous evolution of these templates leads to directed nucleation and defines the tectonic growth of crystals. Reaction–diffusion chemical systems are fundamental to pattern formation and generation of complex structures across nature. The vital and exploitable property of these chemical systems is that the final stable structures can be deliberately structurally designed by changing the environment at any stage during the metamorphosis. Noorduin et al. [79] recently showed how it was possible to judiciously control the development of forms and emerging in a system via stepwise adjustments to convoluted chemical gradients in pH, CO2 and the temperature (figure 6e).
5. Conclusion
In regenerative orthopaedics and dentistry (as there is throughout regenerative biological science), there is paucity of biomaterials and biostructures with broad clinical applicability and versatility. In this review, we have focused on chemical-based approaches to recreating mineralized structural biomaterials. Although, we considered the requirement to involve living entities in construction; juxtaposed with biomimetic chemical systems. It is dependent on whether the tissue engineering prefers cellular or acellular tissue replacements.
Pure chemical approaches to simulate and emulate the exquisitely controlled and precise construction of inorganic biomineral formations have shown utility in mimicking small skeletal structures and small components of hierarchical tissues such as dentine and bone. We have also shown that these replicated bioinorganic structures can be implemented in mineralized tissues of different origin and higher complexity. The point is that there are cases where the tissue engineer does not need to have a perfect copy of a framework to rebuild bone, enamel or dentine. Biostructures with complex form and function are also needed for targeted delivery of proteins and gene products.
Manipulating molecular and macromolecular biological molecules, such as peptides, carbohydrates and DNA, is to harness their recognition and docking efficiencies, their numerous permutations to organize into a diversity of conformations—fitting them together in the exact likeness of living biological structures—provides a thorough opportunity to directly mimic biological frameworks for the mineralization of intricate structures. Getting them to autonomously self-assemble and to generate large volumes of self-supporting structures remains a monumental challenge in both pure synthetic chemistry approaches and in molecule-by-molecule assembling approaches. Especially to build many-layered hierarchies that are tightly integrated.
We have just seen, with a few examples at a basic level, how concepts in biomineralization are transferred into a synthetic model and involve reactions that adapt and metamorphose in real-time according to local conditions, just as natural biomaterials do and have to. We believe that the field has not been thoroughly explored nor sufficiently advanced so far to be capable of the continual generation of fresh structures for implementation in medical therapeutics. Though it can be allotted to some restricted medical purposes. We showed that the coccolithophore mimics stimulate special bone formation. Nevertheless, it is probable that to grow a proper anatomically perfected bone, dentine or enamel framework in a beaker, it will require inputs from cells and genetic and metabolic coding over long periods to organize and construct a truly significant volume of bone. An ideal system to aim for would encompass self-organizing constituents in a liquid crystalline media—in order to truly generate complex form—in combination with living stem cells progenitors—in order to build living tissue—and have a synergy of growth between these two creative elements.
Further future progress in making protocells that harbour working genes enclosed within gated cell boundaries made from lipid or protein could create a route for the controlled autonomous synthesis and fabrication of mineralized tissue compartments. Artificial cells could be harnessed to recreate tissue environments and build biomimetic tissue frameworks. Our overall philosophical direction for the next round of biomimetic systems for regenerative medical materials and structures is to follow nature and biology much deeper and prepare liquid melt precursors of biominerals and liquid crystalline proteins and evolve them in complex self-organizing media, which have reaction–diffusion currents. The skill will be to design chemical systems and environments that are set up in such a way that they act autonomously to create highly ordered patterns which would fuse and mineralize into new structures with hierarchies of organized matter facilitated by living cells to complete a true ‘living biocomposite’. Only time and hard work in these directions will tell.
Funding statement
This work was supported by a grant for the Bio and Medical Technology Development Program (2012M3A9B4028738) funded by the National Research Foundation of Korea (NRF) of the Ministry of Education, Science and Technology (MEST), Republic of Korea.
References
- 1.Sanchez C, Arribart H, Guille MM. 2005. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 4, 277–288. ( 10.1038/nmat1339) [DOI] [PubMed] [Google Scholar]
- 2.Vincent JFV. 2002. Survival of the cheapest. Mater. Today 5, 28–41. ( 10.1016/S1369-7021(02)01237-3) [DOI] [Google Scholar]
- 3.Mann S. 2000. The chemistry of form. Angew. Chem. Int. Ed. Engl. 39, 3392–3406. () [DOI] [PubMed] [Google Scholar]
- 4.Mann S, Ozin GA. 1996. Synthesis of inorganic materials with complex form. Nature 382, 313–318. ( 10.1038/382313a0) [DOI] [Google Scholar]
- 5.Walsh D, Hopwood JD, Mann S. 1994. Crystal tectonics: construction of reticulated calcium phosphate frameworks in bicontinuous reverse microemulsions. Science 264, 1576–1578. ( 10.1126/science.264.5165.1576) [DOI] [PubMed] [Google Scholar]
- 6.Mann S, Archibald DD, Didymus JM, Douglas T, Heywood BR, Meldrum FC, Reeves NJ. 1993. Crystallization at inorganic–organic interfaces: biominerals and biomimetic synthesis. Science 261, 1286–1292. ( 10.1126/science.261.5126.1286) [DOI] [PubMed] [Google Scholar]
- 7.Schuster B, Sleytr UB. 2014. Biomimetic interfaces based on S-layer proteins, lipid membranes and functional biomolecules. J. R. Soc. Interface 11, 20140232 ( 10.1098/rsif.2014.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanag VK, Epstein IR. 2009. Pattern formation mechanisms in reaction–diffusion systems. Int. J. Dev. Biol. 53, 673–681. ( 10.1387/ijdb.072484vv) [DOI] [PubMed] [Google Scholar]
- 9.Green D, Walsh D, Yang X, Mann S, Oreffo ROC. 2004. Stimulation of human bone marrow stromal cells using growth factor encapsulated calcium carbonate porous microspheres. J. Mater. Chem. 14, 2206–2212. ( 10.1039/B400486H) [DOI] [Google Scholar]
- 10.Murdock DJE, Donoghue PCJ. 2011. Evolutionary origins of animal skeletal biomineralization. Cells Tissues Organs 194, 98–102. ( 10.1159/000324245) [DOI] [PubMed] [Google Scholar]
- 11.Barthelat F. 2010. Nacre from mollusk shells: a model for high-performance structural materials. Bioinspir. Biomim. 5, 035001 ( 10.1088/1748-3182/5/3/035001) [DOI] [PubMed] [Google Scholar]
- 12.Burghard Z, Zini L, Srot V, Bellina P, Aken PA, Bill J. 2009. Toughening through nature-adapted nanoscale design. Nano Lett. 9, 4103–4108. ( 10.1021/nl902324x) [DOI] [PubMed] [Google Scholar]
- 13.Espinosa HD, Juster AL, Latourte FJ, Loh OY, Gregoire D, Zavattieri PD. 2011. Tablet-level origin of toughening in abalone shells and translation to synthetic composite materials. Nat Commun. 2, 173 ( 10.1038/ncomms1172) [DOI] [PubMed] [Google Scholar]
- 14.Wang R, Gupta HS. 2011. Deformation and fracture mechanisms of bone and nacre. Annu. Rev. Mater. Res. 41, 41–73. ( 10.1146/annurev-matsci-062910-095806) [DOI] [Google Scholar]
- 15.Stephen M, John PH, Williams RJP. 1986. Phospholipid vesicles as a model system for biomineralization. Nature 324, 565–567. ( 10.1038/324565a0) [DOI] [PubMed] [Google Scholar]
- 16.Heuer AH, et al. 1992. Innovative materials processing strategies: a biomimetic approach. Science 255, 1098–1105. ( 10.1126/science.1546311) [DOI] [PubMed] [Google Scholar]
- 17.Mann S. 1993. Molecular tectonics in biomineralization and biomimetic materials chemistry. Nature 365, 499–505. ( 10.1038/365499a0) [DOI] [Google Scholar]
- 18.Thompson DAW. 1917. On growth and form, XV Cambridge, UK: University Press. [Google Scholar]
- 19.Oliver SRJ, Ozin GA. 1998. Phosphate liquid crystals: novel supramolecular template for the synthesis of lamellar aluminophosphates with natural form. J. Mater. Chem. 8, 1081–1085. ( 10.1039/A708598B) [DOI] [Google Scholar]
- 20.Scott O, Alex K, Neil C, Alan L, Geoffrey AO. 1995. Lamellar aluminophosphates with surface patterns that mimic diatom and radiolarian microskeletons. Nature 378, 47–50. ( 10.1038/378047a0) [DOI] [Google Scholar]
- 21.Ozin GA. 1997. Morphogenesis of biomineral and morphosynthesis of biomimetic forms. Acc. Chem. Res. 30, 17–27. ( 10.1021/ar960021r) [DOI] [Google Scholar]
- 22.Xu Z, Neoh KG, Lin CC, Kishen A. 2011. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentine modified with phosphorylated chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 98, 150–159. ( 10.1002/jbm.b.31844) [DOI] [PubMed] [Google Scholar]
- 23.Hartgerink JD, Beniash E, Stupp SI. 2001. Self-assembly and mineralization of peptide–amphiphile nanofibers. Science 294, 1684–1688. ( 10.1126/science.1063187) [DOI] [PubMed] [Google Scholar]
- 24.Hartgerink JD, Beniash E, Stupp SI. 2002. Peptide–amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc. Natl Acad. Sci. USA 99, 5133–5138. ( 10.1073/pnas.072699999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mata A, Geng Y, Henrikson KJ, Aparicio C, Stock SR, Satcher RL, Stupp SI. 2010. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 31, 6004–6012. ( 10.1016/j.biomaterials.2010.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. 2008. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 108, 4754–4783. ( 10.1021/cr8004422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leadbeater BSC, Riding R. 1986. Biomineralization in lower plants and animals. Systematics Association by the Clarendon Press Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Young JR, Davis SA, Bown PR, Mann S. 1999. Coccolith ultrastructure and biomineralisation. J. Struct. Biol. 126, 195–215. ( 10.1006/jsbi.1999.4132) [DOI] [PubMed] [Google Scholar]
- 29.Young JR, Henriksen K. 2003. Biomineralization within vesicles: the calcite of coccoliths. Rev. Mineral. Geochem. 54, 189–215. ( 10.2113/0540189) [DOI] [Google Scholar]
- 30.Cha JN, Shimizu K, Zhou Y, Christiansen SC, Chmelka BF, Stucky GD, Morse DE. 1999. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl Acad. Sci. USA 96, 361–365. ( 10.2307/46816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrlich H, et al. 2010. The spines of sand dollar Scaphechinus mirabilis (Agassiz 1863): analytical and structural study. J. Adv. Micro. Res. 5, 100–109. ( 10.1166/jamr.2010.1030) [DOI] [Google Scholar]
- 32.Gayathri S, Lakshminarayanan R, Weaver JC, Morse DE, Kini RM, Valiyaveettil S. 2007. In vitro study of magnesium-calcite biomineralization in the skeletal materials of the seastar Pisaster giganteus. Chemistry 13, 3262–3268. ( 10.1002/chem.200600825) [DOI] [PubMed] [Google Scholar]
- 33.Wilcock JR, Perry CC, Williams RJP, Mantoura RFC. 1988. Crystallographic and morphological studies of the celestite skeleton of the acantharian species Phyllostaurus siculus. Proc. R. Soc. Lond. B 233, 393–405. ( 10.2307/36226) [DOI] [Google Scholar]
- 34.Fratzl P, Weiner S. 2010. Bio-inspired materials—mining the old literature for new ideas. Adv. Mater. 22, 4547–4550. ( 10.1002/adma.201002127) [DOI] [PubMed] [Google Scholar]
- 35.Fratzl P, Weinkamer R. 2007. Nature's hierarchical materials. Prog. Mater. Sci. 52, 1263–1334. ( 10.1016/j.pmatsci.2007.06.001) [DOI] [Google Scholar]
- 36.Cuif J-P, Dauphin Y, Sorauf JE. 2011. Biominerals and fossils through time. Cambridge, NY: Cambridge University Press. [Google Scholar]
- 37.Walsh D, Lebeau B, Mann S. 1999. Morphosynthesis of calcium carbonate (vaterite) microsponges. Adv. Mater. 11, 324–328. () [DOI] [Google Scholar]
- 38.Zhang B, Davis SA, Mendelson NH, Mann S. 2000. Bacterial templating of zeolite fibres with hierarchical structure. Chem. Commun. 781–782. ( 10.1039/B001528H) [DOI] [Google Scholar]
- 39.Mann S. 1997. The biomimetics of enamel: a paradigm for organized biomaterials synthesis. Ciba Found. Symp. 205, 261–269. [PubMed] [Google Scholar]
- 40.Katchburian E. 1976. Initiation of mineral deposition in dentine. Calc. Tissue Res. 22, 179–184. ( 10.1007/BF02064061) [DOI] [PubMed] [Google Scholar]
- 41.Mann S. 2001. Biomineralization: principles and concepts in bioinorganic materials chemistry, XII New York, NY: Oxford University Press. [Google Scholar]
- 42.Mount AS, Wheeler AP, Paradkar RP, Snider D. 2004. Hemocyte-mediated shell mineralization in the eastern oyster. Science 304, 297–300. ( 10.1126/science.1090506) [DOI] [PubMed] [Google Scholar]
- 43.Weiner S, Addadi L. 2011. Crystallization pathways in biomineralization. Annu. Rev. Mater. Res. 41, 21–40. ( 10.1146/annurev-matsci-062910-095803) [DOI] [Google Scholar]
- 44.Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, Stevens MM. 2012. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl Acad. Sci. USA 109, 14 170–14 175. ( 10.1073/pnas.1208916109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavalli S, Popescu DC, Tellers EE, Vos MRJ, Pichon BP, Overhand M, Rapaport H, Sommerdijk NAJM, Kros A. 2006. Self-organizing β-sheet lipopeptide monolayers as template for the mineralization of CaCO3. Angew. Chem. Int. Edn. 45, 739–744. ( 10.1002/anie.200501654) [DOI] [PubMed] [Google Scholar]
- 46.Arias JL, Fernandez MS. 2008. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev. 108, 4475–4482. ( 10.1021/cr078269p) [DOI] [PubMed] [Google Scholar]
- 47.Hall SR. 2009. Biotemplating: complex structures from natural materials. Singapore: Imperial College Press. [Google Scholar]
- 48.Huang Y, Chiang C-Y, Lee SK, Gao Y, Hu EL, Yoreo JD, Belcher AM. 2005. Programmable assembly of nanoarchitectures using genetically engineered viruses. Nano Lett. 5, 1429–1434. ( 10.1021/nl050795d) [DOI] [PubMed] [Google Scholar]
- 49.Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, Wang E, Hexemer A, Lee SW. 2011. Biomimetic self-templating supramolecular structures. Nature 478, 364–368. ( 10.1038/nature10513) [DOI] [PubMed] [Google Scholar]
- 50.Weiner S, Traub W, Wagner HD. 1999. Lamellar bone: structure–function relations. J. Struct. Biol. 126, 241–255. ( 10.1006/jsbi.1999.4107) [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharya P, Du D, Lin Y. 2014. Bioinspired nanoscale materials for biomedical and energy applications. J. R. Soc. Interface 11, 20131067 ( 10.1098/rsif.2013.1067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopa S, Madry H. 2014. Bioinspired scaffolds for osteochondral regeneration. Tissue Eng. Part A 20, 2052–2076. ( 10.1089/ten.tea.2013.0356) [DOI] [PubMed] [Google Scholar]
- 53.Bruinink A, Bitar M, Pleskova M, Wick P, Krug HF, Maniura-Weber K. 2013. Addition of nanoscaled bioinspired surface features: a revolution for bone-related implants and scaffolds? J. Biomed. Mater. Res. A 102, 275–294. ( 10.1002/jbm.a.34691) [DOI] [PubMed] [Google Scholar]
- 54.Hudalla GA, Murphy WL. 2011. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv. Funct. Mater. 21, 1754–1768. ( 10.1002/adfm.201002468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi S, Yu X, Jongpaiboonkit L, Hollister SJ, Murphy WL. 2013. Inorganic coatings for optimized non-viral transfection of stem cells. Sci. Rep. 3, 1567 ( 10.1038/srep01567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drachuk I, Gupta MK, Tsukruk VV. 2013. Biomimetic coatings to control cellular function through cell surface engineering. Adv. Funct. Mater. 23, 4437–4453. ( 10.1002/adfm.201300038) [DOI] [Google Scholar]
- 57.Reis RL, Weiner S. 2004. Learning from nature how to design new implantable biomaterials: from biomineralization fundamentals to biomimetic materials and processing routes. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 58.Williams RJP, Rickaby REM. 2012. Evolution's destiny: co-evolving chemistry of the environment and life. Cambridge, UK: Royal Society of Chemistry. [Google Scholar]
- 59.Barrere F, van Blitterswijk CA, de Groot K. 2006. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 1, 317–332. [PMC free article] [PubMed] [Google Scholar]
- 60.Colfen H. 2010. Biomineralization: a crystal-clear view. Nat. Mater. 9, 960–961. ( 10.1038/nmat2911) [DOI] [PubMed] [Google Scholar]
- 61.Belcher AM, Hansma PK, Stucky GD, Morse DE. 1998. First steps in harnessing the potential of biomineralization as a route to new high-performance composite materials. Acta Mater. 46, 733–736. ( 10.1016/S1359-6454(97)00253-X) [DOI] [Google Scholar]
- 62.Profeta AC, Mannocci F, Foxton RM, Thompson I, Watson TF, Sauro S. 2012. Bioactive effects of a calcium/sodium phosphosilicate on the resin–dentine interface: a microtensile bond strength, scanning electron microscopy, and confocal microscopy study. Eur. J. Oral Sci. 120, 353–362. ( 10.1111/j.1600-0722.2012.00974.x) [DOI] [PubMed] [Google Scholar]
- 63.Mann S. 2012. Systems of creation: the emergence of life from nonliving matter. Acc. Chem. Res. 45, 2131–2141. ( 10.1021/ar200281t) [DOI] [PubMed] [Google Scholar]
- 64.Smalyukh II. 2011. Materials science: deft tricks with liquid crystals. Nature 478, 330–331. ( 10.1038/478330a) [DOI] [PubMed] [Google Scholar]
- 65.Merzlyak A, Indrakanti S, Lee SW. 2009. Genetically engineered nanofiber-like viruses for tissue regenerating materials. Nano Lett. 9, 846–852. ( 10.1021/nl8036728) [DOI] [PubMed] [Google Scholar]
- 66.Wayne S, Dietmar P, Uwe BS, Stephen M. 1997. Synthesis of cadmium sulphide superlattices using self-assembled bacterial S-layers. Nature 389, 585–587. ( 10.1038/39287) [DOI] [Google Scholar]
- 67.Rey AD. 2010. Liquid crystal models of biological materials and processes. Soft Matter. 6, 3402–3429. ( 10.1039/B921576J) [DOI] [Google Scholar]
- 68.Holmes DF, Gilpin CJ, Baldock C, Ziese U, Koster AJ, Kadler KE. 2001. Corneal collagen fibril structure in three dimensions: structural insights into fibril assembly, mechanical properties, and tissue organization. Proc. Natl Acad. Sci. USA 98, 7307–7312. ( 10.1073/pnas.111150598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flynn CE, Lee S-W, Peelle BR, Belcher AM. 2003. Viruses as vehicles for growth, organization and assembly of materials. Acta Mater. 51, 5867–5880. ( 10.1016/j.actamat.2003.08.031) [DOI] [Google Scholar]
- 70.Dzamukova MR, Zamaleeva AI, Ishmuchametova DG, Osin YN, Kiyasov AP, Nurgaliev DK, Ilinskaya ON, Fakhrullin RF. 2011. A direct technique for magnetic functionalization of living human cells. Langmuir 27, 14 386–14 393. ( 10.1021/la203839v) [DOI] [PubMed] [Google Scholar]
- 71.Lee J, Choi J, Park JH, Kim M-H, Hong D, Cho H, Yang SH, Choi IS. 2014. Cytoprotective silica coating of individual mammalian cells through bioinspired silicification. Angew. Chem. Int. Edn. 53, 8056–8059. ( 10.1002/anie.201402280) [DOI] [PubMed] [Google Scholar]
- 72.Fakhrullin RF, Minullina RT. 2009. Hybrid cellular–inorganic core–shell microparticles: encapsulation of individual living cells in calcium carbonate microshells. Langmuir 25, 6617–6621. ( 10.1021/la901395z) [DOI] [PubMed] [Google Scholar]
- 73.Yang SH, Ko EH, Choi IS. 2012. Cytocompatible encapsulation of individual Chlorella cells within titanium dioxide shells by a designed catalytic peptide. Langmuir 28, 2151–2155. ( 10.1021/la203667z) [DOI] [PubMed] [Google Scholar]
- 74.Gower LB. 2008. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 108, 4551–4627. ( 10.1021/cr800443h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green DW, Bolland BJ, Kanczler JM, Lanham SA, Walsh D, Mann S, Oreffo RO. 2009. Augmentation of skeletal tissue formation in impaction bone grafting using vaterite microsphere biocomposites. Biomaterials 30, 1918–1927. ( 10.1016/j.biomaterials.2008.12.052) [DOI] [PubMed] [Google Scholar]
- 76.Konnova SA, Sharipova IR, Demina TA, Osin YN, Yarullina DR, Ilinskaya ON, Lvov YM, Fakhrullin RF. 2013. Biomimetic cell-mediated three-dimensional assembly of halloysite nanotubes. Chem. Commun. 49, 4208–4210. ( 10.1039/C2CC38254G) [DOI] [PubMed] [Google Scholar]
- 77.Babu Mukkamala S, Powell AK. 2004. Biomimetic assembly of calcite microtrumpets: crystal tectonics in action. Chem. Commun. 918–919. ( 10.1039/B401754D) [DOI] [PubMed] [Google Scholar]
- 78.Huang X, Li M, Green DC, Williams DS, Patil AJ, Mann S. 2013. Interfacial assembly of protein–polymer nano-conjugates into stimulus–responsive biomimetic protocells. Nat. Commun. 4, 2239 ( 10.1038/ncomms3239) [DOI] [PubMed] [Google Scholar]
- 79.Noorduin WL, Grinthal A, Mahadevan L, Aizenberg J. 2013. Rationally designed complex, hierarchical microarchitectures. Science 340, 832–837. ( 10.1126/science.1234621) [DOI] [PubMed] [Google Scholar]
- 80.Noireaux V, Libchaber A. 2004. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl Acad. Sci. USA 101, 17 669–17 674. ( 10.1073/pnas.0408236101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solé RV, Rasmussen S, Bedau M. 2007. Introduction. Artificial protocells. Phil. Trans. R. Soc. B 362, 1725 ( 10.1098/rstb.2007.2084) [DOI] [Google Scholar]
- 82.Bawazer LA, Izumi M, Kolodin D, Neilson JR, Schwenzer B, Morse DE. 2012. Evolutionary selection of enzymatically synthesized semiconductors from biomimetic mineralization vesicles. Proc. Natl Acad. Sci. USA 109, E1705–E1714. ( 10.1073/pnas.1116958109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Z, Newcomb CJ, Zhou Y, Lei YP, Bringas P, Jr, Stupp SI, Snead ML. 2013. The role of bioactive nanofibers in enamel regeneration mediated through integrin signals acting upon C/EBPalpha and c-Jun. Biomaterials 34, 3303–3314. ( 10.1016/j.biomaterials.2013.01.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mann S. 2008. Life as a nanoscale phenomenon. Angew. Chem. Int. Edn Engl. 47, 5306–5320. ( 10.1002/anie.200705538) [DOI] [PubMed] [Google Scholar]