Abstract
Despite a high incidence of calcific aortic valve disease in metabolic syndrome, there is little information about the fundamental metabolism of heart valves. Cell metabolism is a first responder to chemical and mechanical stimuli, but it is unknown how such signals employed in valve tissue engineering impact valvular interstitial cell (VIC) biology and valvular disease pathogenesis. In this study porcine aortic VICs were seeded into three-dimensional collagen gels and analysed for gel contraction, lactate production and glucose consumption in response to manipulation of metabolic substrates, including glucose, galactose, pyruvate and glutamine. Cell viability was also assessed in two-dimensional culture. We found that gel contraction was sensitive to metabolic manipulation, particularly in nutrient-depleted medium. Contraction was optimal at an intermediate glucose concentration (2 g l−1) with less contraction with excess (4.5 g l−1) or reduced glucose (1 g l−1). Substitution with galactose delayed contraction and decreased lactate production. In low sugar concentrations, pyruvate depletion reduced contraction. Glutamine depletion reduced cell metabolism and viability. Our results suggest that nutrient depletion and manipulation of metabolic substrates impacts the viability, metabolism and contractile behaviour of VICs. Particularly, hyperglycaemic conditions can reduce VIC interaction with and remodelling of the extracellular matrix. These results begin to link VIC metabolism and macroscopic behaviour such as cell–matrix interaction.
Keywords: aortic valvular interstitial cell, collagen gel, valve metabolism
1. Introduction
There is increasing recognition of the importance of valvular cell biology in the pathogenesis of disease states such as aortic valve calcification [1]. The current standard of care for valvular diseases, however, relies on mechanical replacement or repair and not on aspects that address cell biology [2]. In particular, there is a paucity of information about the metabolism of heart valves (with the exception of oxygen consumption by valve tissues [3]), which is noteworthy given the reported correlations between metabolic syndrome and aortic valve calcification [4]. Furthermore, it has been shown in mouse models that hyperglycaemia and mild hyperlipidaemia from a high fat, high carbohydrate diet can induce aortic valve disease [5]. As the supplier of energy and building blocks for the cell, metabolism is involved in virtually all cellular processes and would provide a solid foundation upon which to build an understanding of heart valve cell biology to ultimately identify biological treatment options for valvular diseases. Valvular interstitial cell (VIC) interactions with their pericellular environment are also of great interest to the tissue engineering community. Mechanical and chemical stimulation have been widely used in the study and development of tissue-engineered heart valves [6,7]. Metabolism, however, which is a first responder and fundamental mediator in such processes, has rarely been studied for optimization of valve tissue engineering.
VICs in the aortic valve have been attributed as the cells primarily responsible for the calcification of the valve as a result of VIC interaction with and remodelling of the extracellular matrix (ECM) [8,9]. Metabolic activity underlies such processes. Energy and nutrients available in the system dictate the level and type of biosynthesis in the cell [10], including the synthesis of ECM, the production of integrins that interact with the ECM and the amount of energy available to maintain cell viability and undergo cytoskeletal remodelling [11]. In vitro, such VIC activity has been demonstrated when VICs are seeded into engineered three-dimensional gels and interact with and remodel the matrix to extrude water, resulting in gel contraction [12].
Pathological ECM remodelling has been closely linked to human aortic valve disease [13]. This remodelling has been specifically studied in VIC–collagen interaction, which suggests that disruptions in collagen homeostasis can predispose the valve to pathological disease states, including calcific aortic valve disease [14]. One method of investigating VIC–collagen interactions is the collagen gel contraction assay. This assay has been used in a variety of other cell types to monitor responses to cell signalling and subsequent effects on integrin regulation [15]. Compared with two-dimensional culture, suspension of fibroblasts in three-dimensional collagen gels can more closely mimic in vivo behaviour in terms of elongation, projections and collagen synthesis [16,17]. Collagen gels have often been used as scaffolds for VICs in mechanobiological investigations [18,19] and results have suggested that VICs are less activated in three-dimensional collagen gels than in two-dimensional culture on tissue culture plastic [20]. We hypothesized therefore that collagen gel contraction would be sensitive to metabolic manipulation and serve as a gross indication of a number of cell functions that include cell viability, cytoskeletal remodelling, integrin regulation and cell–ECM interactions. Consequently, metabolic conditions that negatively affect VIC collagen gel remodelling could be thought to predispose or contribute to aortic valve pathology.
In this study, we monitored contraction of three-dimensional collagen gels seeded with porcine VICs—as well as VIC lactate production and two-dimensional cell viability—in response to the manipulation of metabolic substrates in the culture medium, namely glucose, galactose, pyruvate, glutamine and Hams F-12 nutrient mixture. Porcine VICs have been frequently used as a model for aortic valve research, as they are readily available, fast-growing and exhibit similar properties to human valve cells, including calcification in long-term culture [21]. The range of metabolic substrates selected offers tools for analysing macroscopic changes in response to fundamental changes in metabolism through glycolysis and the Krebs citric acid cycle. To the best of our knowledge, this study is the first to investigate metabolism of VICs and macroscopically observe changes in VIC activity in an attempt to characterize a highly understudied phenomenon.
2. Material and methods
2.1. Cell. culture materials
Liquid Dulbecco's modified Eagle medium (DMEM) with glucose (1 g l−1) for cell culture and antibiotic–antimycotic solution were purchased from Cellgro (Manassas, VA). Liquid glucose-free DMEM was purchased from Invitrogen and powdered DMEM (−glucose, −pyruvate, −glutamine, −phenol red, −bicarbonate) was purchased from Sigma to prepare the experimental media. HEPES buffer, Hams F-12 nutrient mixture and bovine growth serum (BGS) were purchased from Hyclone (Logan, UT).
2.2. Harvesting. and cell culture
Aortic valves were dissected from porcine hearts obtained from a local abattoir (Fisher Ham and Meat, Spring, TX) within 6 h postmortem. In total, cells were obtained from four harvests with one or two hearts used per harvest. Valves were first soaked in serum-free medium (2% antibiotic–antimycotic, 1% HEPES buffer, 1 : 1 F-12 : DMEM) containing 2 mg ml−1 collagenase type II (Worthington Biochemical Corp, Lakewood, NJ) for 30 min in an incubated shaker (37°C, 2.3 Hz). Valves were subsequently wiped with cotton swabs to remove the endothelial cells from the surface, minced, immersed in serum-free medium containing 1 mg ml−1 collagenase type III and 0.1 mg ml−1 hyaluronidase (both from Worthington), and returned to the same incubated shaking conditions for 16 h. Afterwards, the cell suspension was strained through a 70 µm cell strainer (BD Falcon, San Jose, CA), centrifuged at 750g for 5 min at room temperature and cultured in DMEM : F-12 (1 : 1, DMEM containing 1 g l−1 glucose, +pyruvate, +glutamine) with 10% BGS, 2% antibiotic–antimycotic and 1% HEPES buffer at 37°C, 5% CO2. After this initial cell plating (P0) reached 80–90% confluence, cells were passaged using 0.25% trypsin (Cellgro). All passages after P0 were cultured in the same formulation of medium, with the exception of a 1%, rather than 2%, antibiotic concentration. For all experiments, cells were from passages 2 or 3.
2.3. Glucose starvation pre-treatment
Because prior work with cardiac fibroblasts suggested that culture pre-treatment can increase cell contraction of collagen gels [22], cells were starved from glucose and pyruvate 24 h prior to gel preparation by replacing the medium with a (−glucose, −pyruvate, +glutamine) DMEM solution containing 10% BGS, 1% antibiotic–antimycotic and 1% HEPES. Although our initial studies showed that depriving VICs of glucose for 24 h before collagen gel preparation did not appear to affect their collagen gel contraction behaviour, the withholding of glucose and pyruvate was performed prior to all experiments to avoid confounding results because those factors were being investigated. Afterwards, cells were trypsinized, seeded into a gel or two-dimensional monolayer culture, and supplemented with experimental media as described in §2.4.
2.4. Experimental media preparation
Overall, there were five variables in the type of media tested: concentration of sugar, type of sugar, presence of pyruvate, concentration of glutamine and presence of F-12 (table 1). All groups were prepared with 10% BGS, 1% antibiotic–antimycotic solution and 1% HEPES buffer.
Table 1.
Variables tested in this paper and their relevant functions within the cell.
| variable | parameters tested | function in cell |
|---|---|---|
| DMEM sugar concentration (g l−1) | 0, 0.5, 1, 2, 4.5 | input for glycolysis and subsequent metabolic pathways |
| DMEM sugar type | glucose, galactose | input for glycolysis with variable conversion costs |
| nutrient mixture | F-12 present in 1 : 1 dilution, F-12 absent | provides a variety of amino acids, vitamins, and metabolic cofactors for the cell; contains glucose |
| DMEM pyruvate concentration (g l−1) | 0, 1.1 | input for the Krebs cycle and other metabolic pathways |
| DMEM glutamine concentration (mM) | 0, 4, 8 | converted to α-ketoglutarate, feeding into the Krebs cycle |
2.4.1. Variable sugar and pyruvate
For the variable sugar and pyruvate studies, base media were prepared using liquid DMEM (−glucose, −pyruvate, +glutamine). d-Glucose (Invitrogen) or d-galactose (Sigma) was added in various experiments at concentrations of 0, 0.5, 1, 2 or 4.5 g l−1. In order to minimize variability, media formulations were prepared via serial dilutions. Experimental groups containing pyruvate were supplemented with 1.1 g l−1 pyruvate (Gibco).
2.4.2. Variable glutamine
For the glutamine trials, DMEM was prepared from powder (−glucose, −pyruvate, −glutamine, −phenol red, −bicarbonate) and supplemented with 0, 4 or 8 mM glutamine (Cellgro) as well as 15 mg ml−1 phenol red (Sigma), and 3.7 g l−1 sodium bicarbonate (Fisher).
2.4.3. F-12 presence
In the F-12 studies, DMEM without glucose was diluted 1 : 1 with a solution of F-12 nutrient mixture. The F-12 used contained 1.8 g l−1 glucose and, following dilution, the solution was supplemented with glucose to reach a final concentration of 1, 2 or 4.5 g l−1.
Each group was tested with at least three replicates (n = 3–6), and experiments were repeated at least twice from different VIC harvests.
2.5. Two-dimensional culture
In order to assess their viability, cells were seeded into 24-well plates at approximately 20 000 cells cm−2 with 500 µl of the above experimental media. After 24, 48 and 96 h, triplicates of these wells were washed with phosphate-buffered saline (PBS) and incubated for 30 min with 2 µg ml−1 calcein AM (Invitrogen, OR) in PBS. Plates were subsequently measured for fluorescence intensity in a SpectraMax M2 plate reader (Molecular Devices, Sunnyvale, CA) with excitation at 485 nm and emission at 538 nm.
2.6. Three-dimensional collagen gel preparation
Collagen gels were prepared using one part 10 × DMEM, eight parts collagen solution and one part 1 × DMEM culture medium (containing VICs). 10 × DMEM was prepared from powder (−glucose, −pyruvate, −glutamine, −phenol red, −bicarbonate) at 83 g l−1 in ultra-pure water and supplemented with 150 mg l−1 phenol red, 10 mM pyruvate, 37 g l−1 sodium bicarbonate and 40 mM l-glutamine. Collagen solution was prepared from a stock of rat tail collagen type I (BD Biosciences, Bedford, MA) diluted to 2 mg ml−1 using 0.02 M acetic acid, giving a collagen concentration of 1.6 mg ml−1 in the final gel. On ice, 10 × DMEM was mixed with collagen solution and neutralized by adding 1 M NaOH dropwise until the solution turned a peach colour (approximately physiological pH). Afterwards, pelleted cells were resuspended to produce a final concentration of 1 × 106 cells ml−1 in 1 × DMEM without sugar and thoroughly mixed into the solution containing the 10 × DMEM and collagen. A 500 µl volume of this solution was immediately placed in each well of a 24-well plate.
2.7. Gel maintenance and quantification of lactate and glucose
Gels were not manually detached from well sides, but were left to detach and contract spontaneously. Media were changed every 3 days in gels. This change frequency was determined based on initial observations of changes in the media colour (from red to yellow) with accumulation of acidic metabolites. Media were collected and stored at −80°C at each change. Lactate and glucose concentrations were analysed in the media using a YSI 2300 STAT plus glucose and lactate analyser (YSI Life Sciences, Yellow Springs, OH).
The individual wells were periodically imaged with a 3 megapixel digital camera over the course of the contraction period. Contraction was quantified digitally using ImageJ by measuring the area of the gel and normalizing it to the area of the base of the well. Following full contraction, gels were blotted dry, weighed and frozen for future analysis (not included in this report).
2.8. Statistical analysis
Data are reported as mean with standard error of the mean (s.e.m.). Statistical analysis to detect differences in gel contraction was performed via two-factor ANOVA of normalized gel size over each of the time points during the contraction period. Thus, a significant difference in contraction would be detected if either the contraction rate was slower between groups or the onset of contraction was delayed between groups. A single-factor ANOVA between groups was performed for comparisons at a single time point, such as for cell viability or lactate concentration at a point in time. Tukey's tests were used for post hoc analysis to identify significant differences between groups. Statistical analysis was performed in Microsoft Excel and Minitab v. 16.
3. Results
3.1. Variable glucose, F-12 present
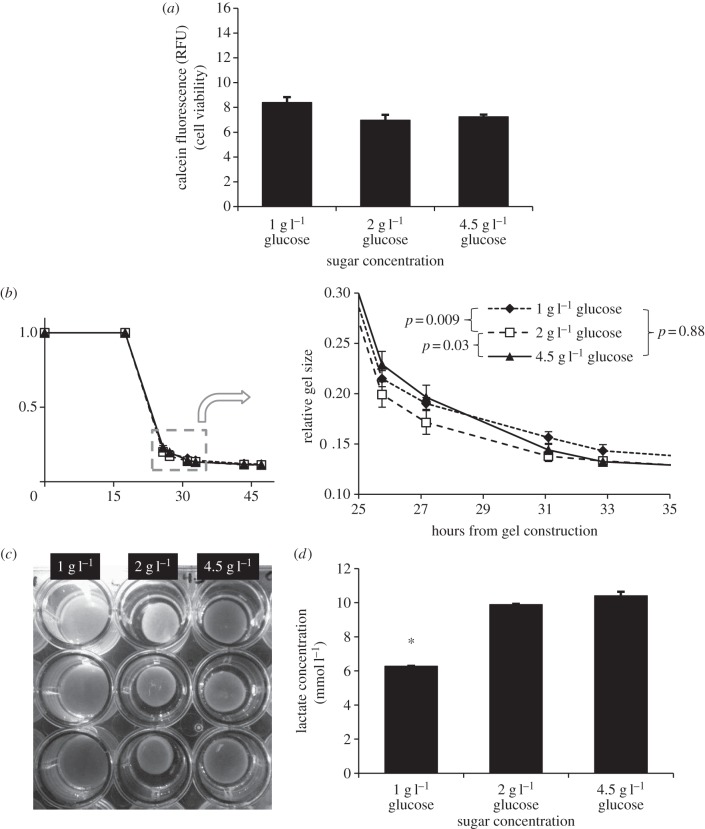
When F-12 was present in the culture medium, cells seeded in two-dimensional wells exhibited similar viability, regardless of the concentration of glucose present even up to 96 h after cell seeding (figure 1a).
Figure 1.
(a) Two-dimensional cell viability at 96 h of VICs supplemented with media containing one part DMEM and one part F-12 at variable levels of glucose. Differences in cell viability were not significant even after 96 h (p = 0.054). (b) Contraction of VICs in collagen gels with the same formulations of media. Contraction with 2 g l−1 glucose was slightly, but significantly faster than that with 1 or 4.5 g l−1 glucose (two-factor ANOVA, 26–47 h). (c) Stereotypical example of gels in wells with variable glucose media, illustrating fastest contraction with 2 g l−1 glucose. (d) Lactate concentration in media following contraction. Media with 1 g l−1 glucose contained significantly less lactate than did media with 2 or 4.5 g l−1 glucose (p < 0.001). There was no significant difference in lactate concentration observed between 2 and 4.5 g l−1 glucose (p = 0.07). Data represent mean ± s.e.m.
In three-dimensional culture, VICs began contracting collagen gels within 24 h of gel construction. Control gels not containing cells did not contract over the course of the experiment. Gels containing 2 g l−1 glucose media exhibited slightly greater contraction at the various time points than those with 1 or 4.5 g l−1 glucose (figure 1b). Differences were small, but significant (p = 0.007, two-factor ANOVA, 26–47 h).
Following contraction, lactate concentration in the media was significantly reduced in 1 g l−1 glucose media compared with groups with 2 and 4.5 g l−1 glucose (p < 0.001), but a significant difference was not observed in lactate concentration between the 2 and 4.5 g l−1 glucose groups (p = 0.07; figure 1d). Glucose was depleted in the initial 1 g l−1 glucose media (0.007 ± 0.002 g l−1, s.e.m.) and in the 2 g l−1 media (0.15 ± 0.01 g l−1, s.e.m.), with some remaining in the 4.5 g l−1 media (1.38 ± 0.04 g l−1, s.e.m.; p < 0.001, single-factor ANOVA).
3.2. Variable sugar, F-12 absent
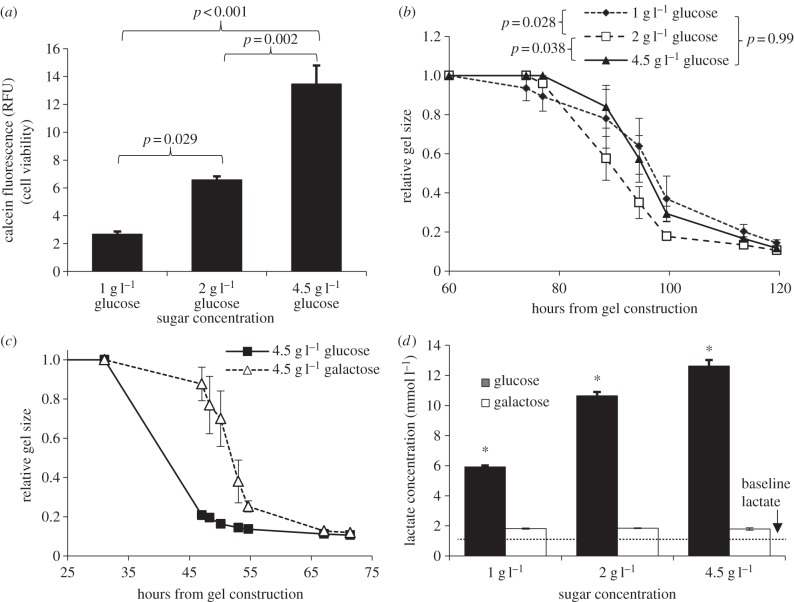
When F-12 and pyruvate were removed, a reduction in two-dimensional cell viability was observed in groups containing less glucose. Differences were not significant at 24 (p = 0.83) or 48 h (p = 0.17), but became significant after 96 h (p < 0.001, single-factor ANOVA; figure 2a). The cell viability at 96 h was not reduced by using 1 g l−1 galactose instead of 1 g l−1 glucose (data not shown).
Figure 2.
(a) Calcein viability assay of VICs after 96 h in two-dimensional cell culture with media composed of DMEM with variable glucose and no F-12. Differences between groups were significant with reduced viability in groups with less glucose (p < 0.001, single-factor ANOVA). (b) Contraction of VICs in collagen gels in the same formulations of media. Contraction with 2 g l−1 glucose was significantly faster than that with 1 or 4.5 g l−1 glucose. (c) Gel contraction comparing 4.5 g l−1 glucose and 4.5 g l−1 galactose DMEM media. Contraction with galactose was significantly delayed (p < 0.001, two-factor ANOVA, 47–71 h). (d) Lactate concentration in media following contraction in variable sugar DMEM. Differences were significant between all concentrations of glucose (p < 0.001), but not between concentrations of galactose (p = 0.49, single-factor ANOVA). The dotted line indicates the baseline level of lactate in the culture medium as measured from media supplied to collagen gels without any cells. Data represent mean ± s.e.m.
In collagen gels lacking F-12, VICs typically did not begin contracting gels until 2–5 days after gel construction. Groups with 0 and 0.5 g l−1 glucose did not contract at all during the experiment (data not shown). Similar to the experiments containing F-12, there was faster contraction for the groups containing 2 g l−1 glucose than for the other two groups, but the difference was more pronounced (p = 0.016 overall, two-factor ANOVA, 77–120 h; figure 2b).
Substitution of glucose with galactose generally slowed gel contraction, and this was especially pronounced at higher concentrations of sugar (p < 0.001 for 4.5 g l−1 glucose versus galactose, two-factor ANOVA, 47–71 h; figure 2c). Following contraction, significantly more lactate was found in the culture media in groups containing greater concentrations of glucose (p < 0.001, single-factor ANOVA). With galactose, however, there was no significant difference in lactate produced at any concentration of galactose (p = 0.49; figure 2d).
3.3. Variable pyruvate
The absence of pyruvate reduced gel contraction at the various time points compared with groups with pyruvate present, particularly at lower concentrations of sugar (figure 3a,b). Differences were statistically significant in 1 g l−1 glucose (p = 0.026) and in 2 g l−1 glucose (p = 0.035), but not in 4.5 g l−1 glucose (p = 0.226, two-factor ANOVA, 74–120 h). Likewise, lactate production was slightly but significantly reduced when pyruvate was absent and media contained 1 g l−1 galactose (p < 0.001) or 1 g l−1 glucose (p = 0.034, Tukey's test; figure 3c).
Figure 3.
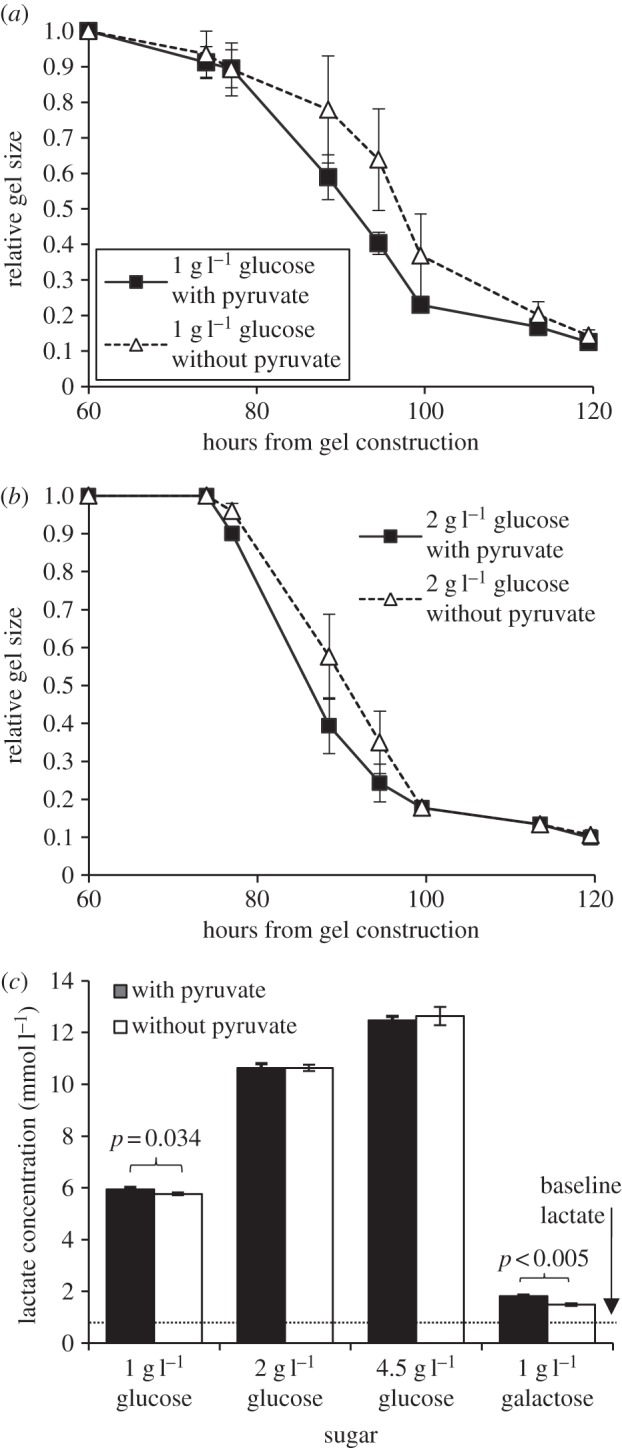
Contraction of collagen gels with and without pyruvate in DMEM media containing (a) 1 g l−1 glucose and (b) 2 g l−1 glucose. Differences in contraction between groups with and without pyruvate were significant at 1 g l−1 glucose (p = 0.026) and 2 g l−1 glucose (p = 0.035), but not at 4.5 g l−1 glucose (p = 0.226; two-factor ANOVA, 74–120 h). (c) Lactate concentration following contraction in gels of groups with variable sugar and pyruvate. Lactate production was slightly, but significantly reduced when pyruvate was absent in 1 g l−1 glucose and 1 g l−1 galactose media. The dotted line indicates the baseline level of lactate in the culture medium as measured from media supplied to collagen gels without any cells. Data represent mean ± s.e.m.
3.4. Variable glutamine
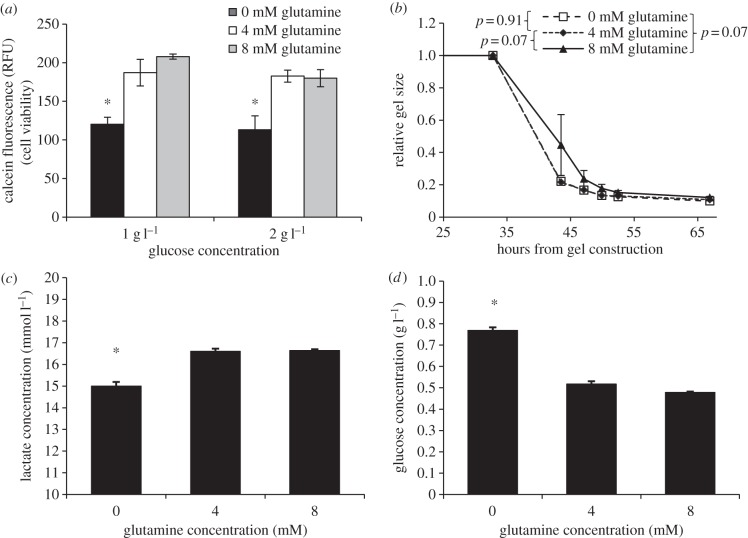
When glutamine was removed from the media, a significant reduction in cell viability was observed as early as 24 h later (p = 0.004 with 1 g l−1 glucose, p = 0.015 with 2 g l−1 glucose, single-factor ANOVA; figure 4a). Differences in two-dimensional cell viability were not significant between 4 and 8 mM glutamine at 1 and 2 g l−1 glucose. Lactate production was consistent with this trend, and a significantly lower concentration of lactate was observed when glutamine was removed (figure 4c). Likewise, glucose consumption was significantly reduced at 0 mM glutamine (p < 0.001, single-factor ANOVA; figure 4d). However, no significant differences in gel contraction were consistently observed, though there was a tendency for groups with low glucose (1 or 2 g l−1) to exhibit reduced contraction with 8 mM glutamine (p = 0.071, two-factor ANOVA, 4 mM glutamine versus 8 mM glutamine, 1 g l−1 glucose; figure 4b).
Figure 4.
(a) Calcein cell viability assay of VICs after 24 h in two-dimensional cell culture with 0, 4 and 8 mM glutamine in 1 or 2 g l−1 glucose DMEM. Groups with 0 mM glutamine had significantly reduced two-dimensional viability compared with either of the groups with 4 or 8 mM glutamine. (b) Contraction of VICs in collagen gels with 1 g l−1 glucose and variable glutamine. No significant differences were observed between groups. (c) Lactate concentration following contraction of collagen gels with variable glutamine in 4.5 g l−1 glucose. Groups with 0 mM glutamine produced significantly less lactate in collagen gels than either of the groups with 4 or 8 mM glutamine. (d) Concentration of glucose remaining in media after contraction with variable glutamine and an initial 4.5 g l−1 glucose. Significantly more glucose remained in groups lacking glutamine. Data represent mean ± s.e.m. Asterisk indicates p < 0.001, compared with groups with glutamine.
4. Discussion
In this study, we analysed gel contraction, lactate production and glucose consumption by VICs in three-dimensional collagen gels under different concentrations of sugar, nutrients, pyruvate and glutamine. We also examined viability of VICs seeded in two-dimensional culture under these conditions. Overall, gel contraction was sensitive to media formulation, particularly when fewer metabolic substrates were present, and greater cell viability did not necessarily correlate with an increase in gel contraction. To the best of our knowledge, no previous studies have analysed such an effect of metabolic changes on the macroscopic properties of VICs in vitro.
4.1. Glucose
Despite trends in viability, glucose was observed to have an optimal concentration for VIC contraction, with glucose excess or deprivation resulting in reduced contraction. In the experiments with F-12, cell viability was not significantly reduced in groups with lower glucose concentrations, indicative of the metabolic robustness of healthy cells, which can metabolize the variety of nutrients and cofactors present in F-12 through other metabolic pathways [23]. When these nutrients were removed, cells exhibited reduced viability in groups with less glucose, yet three-dimensional collagen gel contraction was greatest with an intermediate level of glucose around 2 g l−1. A low concentration of glucose is thought to have reduced the energy available for processes such as actin filament remodelling, biosynthesis of integrins and ECM components, and assembly of integrin-based focal adhesions linking the ECM to the cytoskeleton, which may have caused the reduction in gel contraction [10,11]. The detrimental effect of excess glucose may have been caused by increased production of metabolic by-products that adversely affected cell activity. This includes the generation of lactic acid or toxic reactive oxygen species that are products of active respiration [24]. Prior research in other cell types has implicated the activation of other processes, including hexosamine biosynthesis involving glutamine : fructose-6-phosphate amidotransferase, as contributors to the adverse effects of excess glucose [25,26]. Furthermore, metabolism—as measured by lactic acid production—plateaued when increasing the concentration of glucose in the media. Specifically, increasing glucose concentration from 1 to 2 g l−1 nearly doubled lactate production, but there was no significant difference in final lactate concentration between 2 and 4.5 g l−1 glucose when F-12 was present in the media (figure 1d). This finding supports our proposed interpretation of an optimal range for glucose concentration for VIC metabolism and gel contraction.
The collagen results comparing 1 with 2 g l−1 glucose in VICs are similar to those observed with cardiac fibroblasts, in which increased gel contraction was observed with an increase in glucose concentration from 1 to 4.5 g l−1 [22]. Results were attributed to an observed increase in the expression of α1 and β1 integrins, which account for increased cell–ECM interaction and adhesion. In cardiac fibroblasts, increased glucose concentration also increased collagen production, which has been proposed to promote a profibrotic response in diabetes [27–29], and may also be relevant to valvular dysfunction in patients with diabetes.
4.2. Galactose
Substitution of glucose with galactose reduced the metabolic activity of VICs as suggested by decreased metabolite production and was consequently observed to delay contraction. Galactose is metabolized by the cell through the Leloir pathway, which first requires a conversion to glucose (figure 5) [30]. This conversion process requires approximately the same amount of energy as that produced by glycolysis. It was as expected then that galactose substitution reduced the rate of gel contraction. More interesting was the observation that nearly a fivefold increase in galactose concentration did not significantly alter lactate production (figure 2d). Lactate is produced by the cell through the reduction of pyruvate, particularly when metabolism exceeds oxygen availability. The lack of significant difference in lactate production with an increased concentration of galactose—in contrast to the increased lactate production with increased glucose—may be an indication that galactose conversion is rate-limiting, perhaps with UDP–galactose-4-epimerase, which is speculated as being the rate-limiting enzyme in galactose metabolism [31]. This may be a factor in reducing toxicity of a high sugar concentration.
Figure 5.
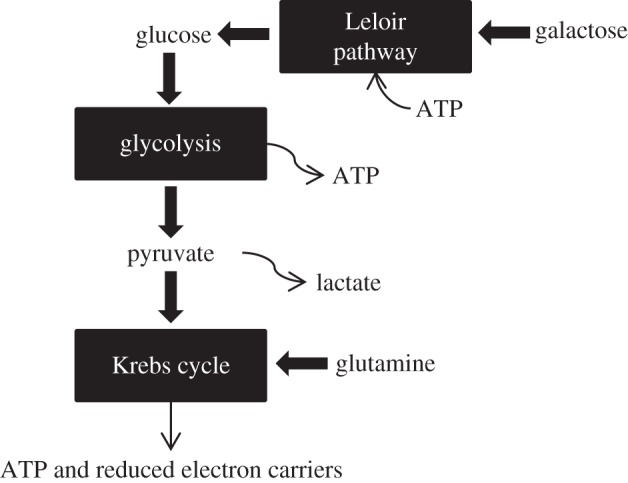
A condensed illustration of the main metabolic pathways in this study. Galactose is converted to glucose via the Leloir pathway, which requires ATP. Glucose is broken down to pyruvate with a net yield of ATP. Pyruvate proceeds into the Krebs cycle, producing ATP and reduced electron carriers for oxidative phosphorylation. Glutamine feeds into the Krebs cycle as α-ketoglutarate.
4.3. Pyruvate
Pyruvate absence was observed to significantly reduce gel contraction and lactate production, but only when glucose concentrations were low. Pyruvate, an end product of glycolysis, is typically metabolized through the Krebs cycle to supply energy via oxidative phosphorylation. Pyruvate absence was significant when less energy was available to the cell through sugar metabolism, as was the case with 1 g l−1 glucose media, or when additional energy was required to metabolize the sugar, as with 1 g l−1 galactose. At higher concentrations of glucose, glucose metabolism was sufficient to compensate for the lack of pyruvate.
4.4. Glutamine
Absence of glutamine in the culture media significantly decreased two-dimensional cell viability and metabolic activity in collagen gels. Glutamine, in addition to a large set of other cellular functions, enters the Krebs cycle in the form of α-ketoglutarate [32,33]. Glutamine participates in an anaplerotic pathway to feed the cycle by participating as a direct intermediate that can be recycled. When glutamine was absent in the experimental media, the supply of Krebs cycle intermediates was limited, reducing the cells' ability to undergo cellular respiration and consequently decreasing metabolism (figure 4c) and cell viability (figure 4a). Because Krebs cycle intermediates can be recycled and are maintained at a relatively stable level, it follows that doubling the glutamine concentration did not have a significant effect on cell viability or metabolism.
4.5. Clinical implications
These results have implications in clinical research related to diabetes. It has been shown that persistently elevated serum glucose levels in diabetes are directly linked to the development of the microvascular complications of retinopathy, nephropathy and neuropathy [34]. If it is the case that persistent hyperglycaemia is additionally a direct cause of valvulopathy through pathological collagen remodelling, the pathogenesis of the disease may advocate for strict glycaemic control. Intensive insulin regulation could prove to potentially reduce the development of valvular complications that have been associated with metabolic syndrome [4]. Perhaps even dietary modifications in terms of the type of sugar consumed (glucose versus galactose) may be beneficial in a diabetic diet.
Furthermore, there is active research in optimizing tissue-engineered heart valves that can be used in place of mechanical valves or cadaveric transplants [35]. While studies have investigated factors such ideal oxygen conditions [3] and conditioning [36] in engineering the valves, few studies have investigated ideal metabolic conditions, which would be essential to optimize bioreactor conditions.
4.6. Study limitations
Several factors need to be considered when extending these results to a physiological context. In healthy valves in vivo, aortic VICs express a quiescent fibroblast-like phenotype. When isolated and cultured, VICs have been shown to spontaneously differentiate into a myofibroblastic contractile phenotype, often characterized by the expression of α-smooth muscle actin. This phenotype is considered to be activated and is associated with pathological changes in vivo [37,38]. Three-dimensional free-floating collagen gels have been shown to be superior in maintaining the quiescent VIC phenotype over two-dimensional culture and stressed three-dimensional models under standard media preparations similar to baseline experiments in this study [18,20]. However, prior studies have suggested that various types of stress—be it metabolic or otherwise—may precipitate pathological VIC activation. For example, hypercholesterolaemia, both in vivo and in vitro, can modulate phenotypic behaviour of VICs by inducing endoplasmic reticulum stress [39]. Perhaps the starved or hyperglycaemic states may induce similar phenotypic changes that may have mediated the results that were observed in this study. Furthermore, the activation of VICs is expected to increase metabolic activity and demand. Future study would analyse whether metabolic changes such as nutrient deprivation or excess glucose availability can induce the activated state in VICs and whether such activation alters VIC metabolism.
Furthermore, while glucose concentrations of 1–4.5 g l−1 are common practice for in vitro culture, physiological blood sugar typically does not reach a concentration as high as 4.5 g l−1 in normoglycaemic patients, with fasting levels maintained at less than 1 g l−1, and any random sugar more than 2 g l−1 classified as diabetes [40]. The cyclic change in the concentration of glucose in diabetic conditions in vivo as well as commonly comorbid dyslipidaemia may also have an important effect on metabolism in addition to the increased magnitude of the serum glucose concentration. As with any in vitro study, there are a variety of other pathways and factors that can affect metabolism, but were not considered in this study, including, but not limited to, the aortic valve microenvironment [41], lymphocytic and macrophage infiltration of the valve [42] and the variety of infectious, congenital and systemic factors that have been shown to contribute to valvulopathy. However, our results suggest that metabolism may play a role in disease progression and may lead to clinical studies investigating the link between diabetes and aortic valve disease. Future study may also use fibrin—as opposed to collagen—gels, which offer less suppression of ECM production and may better mimic in vivo conditions [43]. Our preliminary tests with fibrin gels have shown similar trends to those observed in collagen gels.
5. Conclusion
These results begin to link VIC metabolism with macroscopic behaviour such as cell–matrix interaction and contraction. They demonstrate that an alteration in metabolic substrate availability can affect valvular phenotypic properties such as contractility and establish the collagen gel contraction assay as a novel and viable option in investigating metabolism through tissue engineering approaches. The data provide an initial understanding of the metabolic behaviour of heart valve cells and the potential clinical relevance. Inhibition of gel contraction by higher concentrations of glucose is particularly relevant to physiological conditions like diabetes, where elevated blood sugar levels are observed. This line of investigation opens the door for future experimentation including analysis of heart valve calcification in response to diabetic metabolic conditions as well as the incorporation of biologically relevant factors such as mechanical stimulation or disease states, to ultimately improve our understanding of the link between metabolism and valvular diseases.
Acknowledgements
Special thanks go to Dr Daniel Harrington for his critical evaluation of the research, Shannon Burke for assistance in experimentation, and Dr Melissa McHale for her review of the manuscript.
Data accessibility
Data from this study can be accessed at http://hdl.handle.net/1911/72186.
Funding statement
This research was supported by the Rice University Century Scholars Programme, a Hamill Innovation Award from the Rice University Institute for Biosciences and Bioengineering, and NIH R03EB011576.
References
- 1.Rajamannan NM, et al. 2011. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute aortic stenosis working group. Circulation 124, 1783–1791. ( 10.1161/circulationaha.110.006767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cribier A, et al. 2006. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J. Am. Coll. Cardiol. 47, 1214–1223. ( 10.1016/j.jacc.2006.01.049) [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Wilshaw S-P, Korossis S, Fisher J, Jin Z, Ingham E. 2009. Factors influencing the oxygen consumption rate of aortic valve interstitial cells: application to tissue engineering. Tissue Eng. C Methods 15, 355–363. ( 10.1089/ten.tec.2008.0415) [DOI] [PubMed] [Google Scholar]
- 4.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O'Brien KD. 2006. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation 113, 2113–2119. ( 10.1161/CIRCULATIONAHA.105.598086) [DOI] [PubMed] [Google Scholar]
- 5.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. 2006. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J. Am. Coll. Cardiol. 47, 850–855. ( 10.1016/j.jacc.2005.09.049) [DOI] [PubMed] [Google Scholar]
- 6.Masoumi N, Howell MC, Johnson KL, Niesslein MJ, Gerber G, Engelmayr GC. 2014. Design and testing of a cyclic stretch and flexure bioreactor for evaluating engineered heart valve tissues based on poly(glycerol sebacate) scaffolds. Proc. Inst. Mech. Eng. H, J. Eng. Med. 228, 576–586. ( 10.1177/0954411914534837) [DOI] [PubMed] [Google Scholar]
- 7.Jana S, Tefft BJ, Spoon DB, Simari RD. 2014. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 10, 2877–2893. ( 10.1016/j.actbio.2014.03.014) [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez KJ, Masters KS. 2009. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J. Biomed. Mater. Res. A 90, 1043–1053. ( 10.1002/jbm.a.32187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yip CYY, Chen JH, Zhao R, Simmons CA. 2009. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 29, 936–942. ( 10.1161/ATVBAHA.108.182394) [DOI] [PubMed] [Google Scholar]
- 10.Spiegelman S, Sussman M. 1952. Energy metabolism of biosynthesis at the cellular level. Annu. Rev. Physiol. 14, 97–114. ( 10.1146/annurev.ph.14.030152.000525) [DOI] [PubMed] [Google Scholar]
- 11.Sunyer R, Ritort F, Farré R, Navajas D. 2009. Thermal activation and ATP dependence of the cytoskeleton remodeling dynamics. Phys. Rev. E 79, 1–7. ( 10.1103/PhysRevE.79.051920) [DOI] [PubMed] [Google Scholar]
- 12.Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. 2003. The cardiac valve interstitial cell. Int. J. Biochem. Cell Biol. 35, 113–118. ( 10.1016/S1357-2725(02)00100-0) [DOI] [PubMed] [Google Scholar]
- 13.Fondard O, et al. 2005. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 26, 1333–1341. ( 10.1093/eurheartj/ehi248) [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez KJ, Piechura LM, Porras AM, Masters KS. 2014. Manipulation of valve composition to elucidate the role of collagen in aortic valve calcification. BMC Cardiovasc. Disord. 14, 29 ( 10.1186/1471-2261-14-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess ML, Carver WE, Terracio L, Wilson SP, Wilson Ma, Borg TK. 1994. Integrin-mediated collagen gel contraction by cardiac fibroblasts. Effects of angiotensin II. Circ. Res. 74, 291–298. ( 10.1161/01.RES.74.2.291) [DOI] [PubMed] [Google Scholar]
- 16.Tomasek JJ, Hay ED. 1984. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J. Cell Biol. 99, 536–549. ( 10.1083/jcb.99.2.536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta V, Werdenberg JA, Mendez JS, Grande-Allen KJ. 2008. Influence of strain on proteoglycan synthesis by valvular interstitial cells in three-dimensional culture. Acta Biomater. 4, 88–96. ( 10.1016/j.actbio.2007.08.009) [DOI] [PubMed] [Google Scholar]
- 18.Gould RA, Chin K, Santisakultarm TP, Dropkin A, Richards JM, Schaffer CB, Butcher JT. 2012. Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater. 8, 1710–1719. ( 10.1016/j.actbio.2012.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta V, Werdenberg JA, Blevins TL, Grande-Allen KJ. 2007. Synthesis of glycosaminoglycans in differently loaded regions of collagen gels seeded with valvular interstitial cells. Tissue Eng. 13, 41–49. ( 10.1089/ten.2006.0091) [DOI] [PubMed] [Google Scholar]
- 20.Butcher JT, Nerem RM. 2004. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J. Heart Valve Dis. 13, 478–485. [PubMed] [Google Scholar]
- 21.Johnson CM, Hanson MN, Helgeson SC. 1987. Porcine cardiac valvular subendothelial cells in culture: cell isolation and growth characteristics. J. Mol. Cell. Cardiol. 19, 1185–1193. ( 10.1016/S0022-2828(87)80529-1) [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Stewart J, Kane I, Massey E. 2007. Effects of elevated glucose levels on interactions of cardiac fibroblasts with the extracellular matrix. In Vitro Cell. Dev. Biol. Anim. 43, 297–305. ( 10.1007/s11626-007-9052-2) [DOI] [PubMed] [Google Scholar]
- 23.Kitano H, Oda K, Kimura T, Matsuoka Y, Csete M, Doyle J, Muramatsu M. 2004. Metabolic syndrome and robustness tradeoffs. Diabetes 53, S6–S15. ( 10.2337/diabetes.53.suppl_3.S6) [DOI] [PubMed] [Google Scholar]
- 24.Inoguchi T, et al. 2000. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49, 1939–1945. ( 10.2337/diabetes.49.11.1939) [DOI] [PubMed] [Google Scholar]
- 25.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. 1998. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Invest. 101, 160–169. ( 10.1172/JCI119875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crook ED, Crenshaw G, Veerababu G, Singh LP. 2000. Overexpression of glutamine: fructose-6-phosphate amidotransferase in Rat-1 fibroblasts enhances glucose-mediated glycogen accumulation via suppression of glycogen phosphorylase activity. Endocrinology 141, 1962–1970. ( 10.1210/en.141.6.1962) [DOI] [PubMed] [Google Scholar]
- 27.Chi J, Guo H, Peng F, Yang B, Hiroyasu U. 2011. Profibrotic influence of high glucose on human cardiac fibroblast functions. Heart 97, A113 ( 10.1136/heartjnl-2011-300867.333) [DOI] [Google Scholar]
- 28.Benazzoug Y, Borchiellini C, Labat-Robert J, Robert L, Kern P. 1998. Effect of high-glucose concentrations on the expression of collagens and fibronectin by fibroblasts in culture. Exp. Gerontol. 33, 445–455. ( 10.1016/S0531-5565(98)00015-1) [DOI] [PubMed] [Google Scholar]
- 29.Tang M, Zhang W, Lin H, Jiang H, Dai H, Zhang Y. 2007. High glucose promotes the production of collagen types I and III by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Mol. Cell. Biochem. 301, 109–114. ( 10.1007/s11010-006-9401-6) [DOI] [PubMed] [Google Scholar]
- 30.Kohn P. 1963. A study of the mechanism of conversion of galactose to glucose in the intact rat. J. Biol. Chem. 238, 1193–1195. [PubMed] [Google Scholar]
- 31.Wasilenko J, Fridovich-Keil JL. 2006. Relationship between UDP-galactose 4′-epimerase activity and galactose sensitivity in yeast. J. Biol. Chem. 281, 8443–8449. ( 10.1074/jbc.M600778200) [DOI] [PubMed] [Google Scholar]
- 32.Curthoys NP, Watford M. 1995. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 15, 133–159. ( 10.1146/annurev.nu.15.070195.001025) [DOI] [PubMed] [Google Scholar]
- 33.Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P. 2005. Molecular mechanisms of glutamine action. J. Cell. Physiol. 204, 392–401. ( 10.1002/jcp.20339) [DOI] [PubMed] [Google Scholar]
- 34.Fowler MJ. 2008. Microvascular and macrovascular complications of diabetes. Clin. Diab. 26, 77–82. ( 10.2337/diaclin.26.2.77) [DOI] [Google Scholar]
- 35.Rippel RA, Ghanbari H, Seifalian AM. 2012. Tissue-engineered heart valve: future of cardiac surgery. World J. Surg. 36, 1581–1591. ( 10.1007/s00268-012-1535-y) [DOI] [PubMed] [Google Scholar]
- 36.Flanagan TC, Cornelissen C, Koch S, Tschoeke B, Sachweh JS, Schmitz-Rode T, Jockenhoevel S. 2007. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 28, 3388–3397. ( 10.1016/j.biomaterials.2007.04.012) [DOI] [PubMed] [Google Scholar]
- 37.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. 2004. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 13, 841–847. [PubMed] [Google Scholar]
- 38.Liu AC, Joag VR, Gotlieb AI. 2007. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 171, 1407–1418. ( 10.2353/ajpath.2007.070251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Z, et al. 2013. Endoplasmic reticulum stress participates in aortic valve calcification in hypercholesterolemic animals. Arterioscler. Thromb. Vasc. Biol. 33, 2345–2354. ( 10.1161/ATVBAHA.112.300226) [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association. 2004. Screening for type 2 diabetes. Diab. Care 27, S11–S14. ( 10.2337/diacare.27.2007.S11) [DOI] [PubMed] [Google Scholar]
- 41.Yip CYY, Simmons CA. 2011. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc. Pathol. J. Soc. Cardiovasc. Pathol. 20, 177–182. ( 10.1016/j.carpath.2010.12.001) [DOI] [PubMed] [Google Scholar]
- 42.Freeman RV, Otto CM. 2005. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111, 3316–3326. ( 10.1161/circulationaha.104.486738) [DOI] [PubMed] [Google Scholar]
- 43.Ross JJ, Tranquillo RT. 2003. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 22, 477–490. ( 10.1016/S0945-053X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study can be accessed at http://hdl.handle.net/1911/72186.