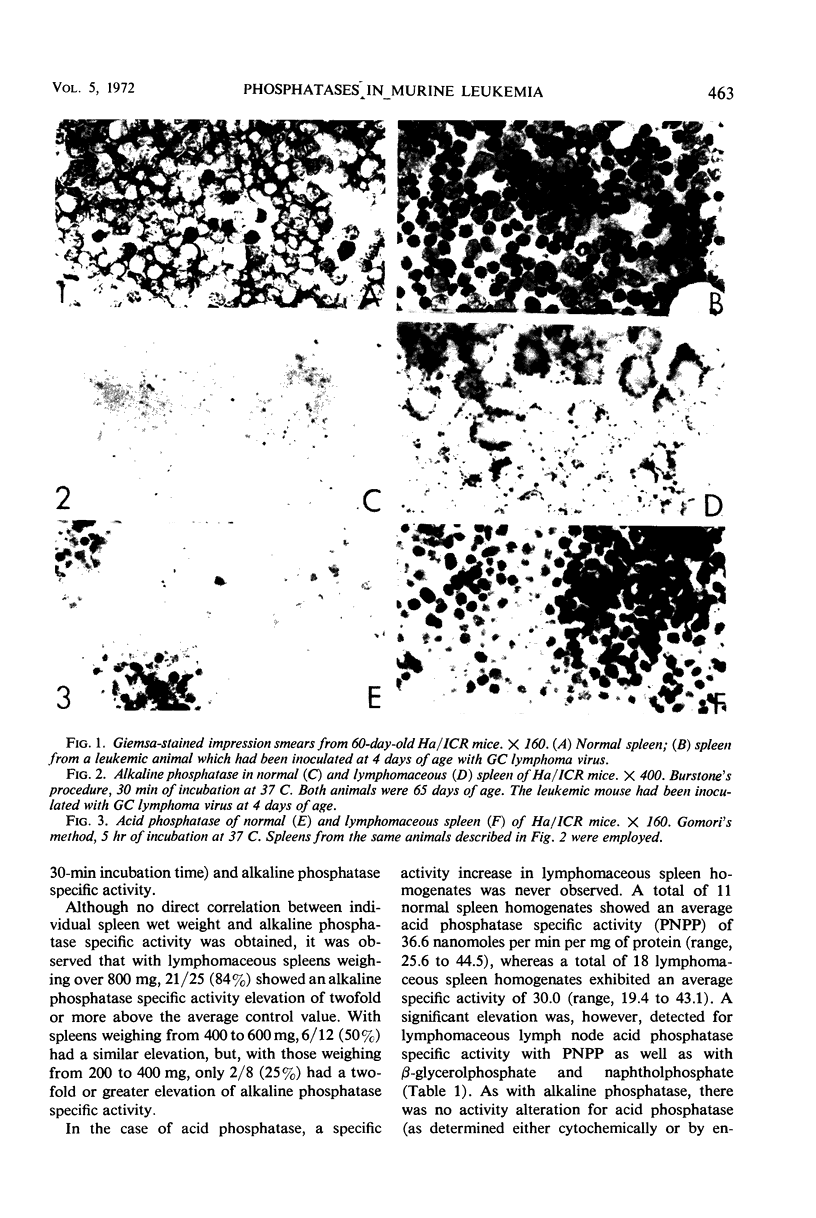
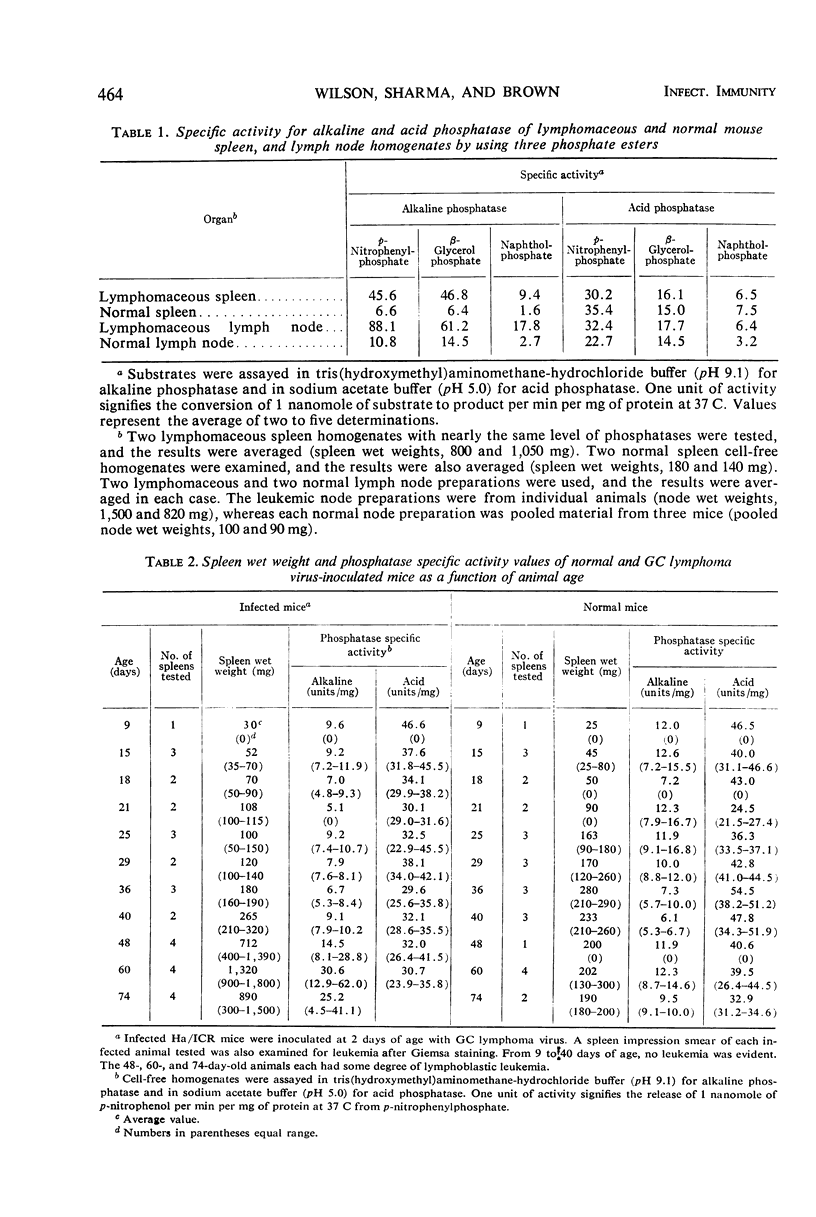
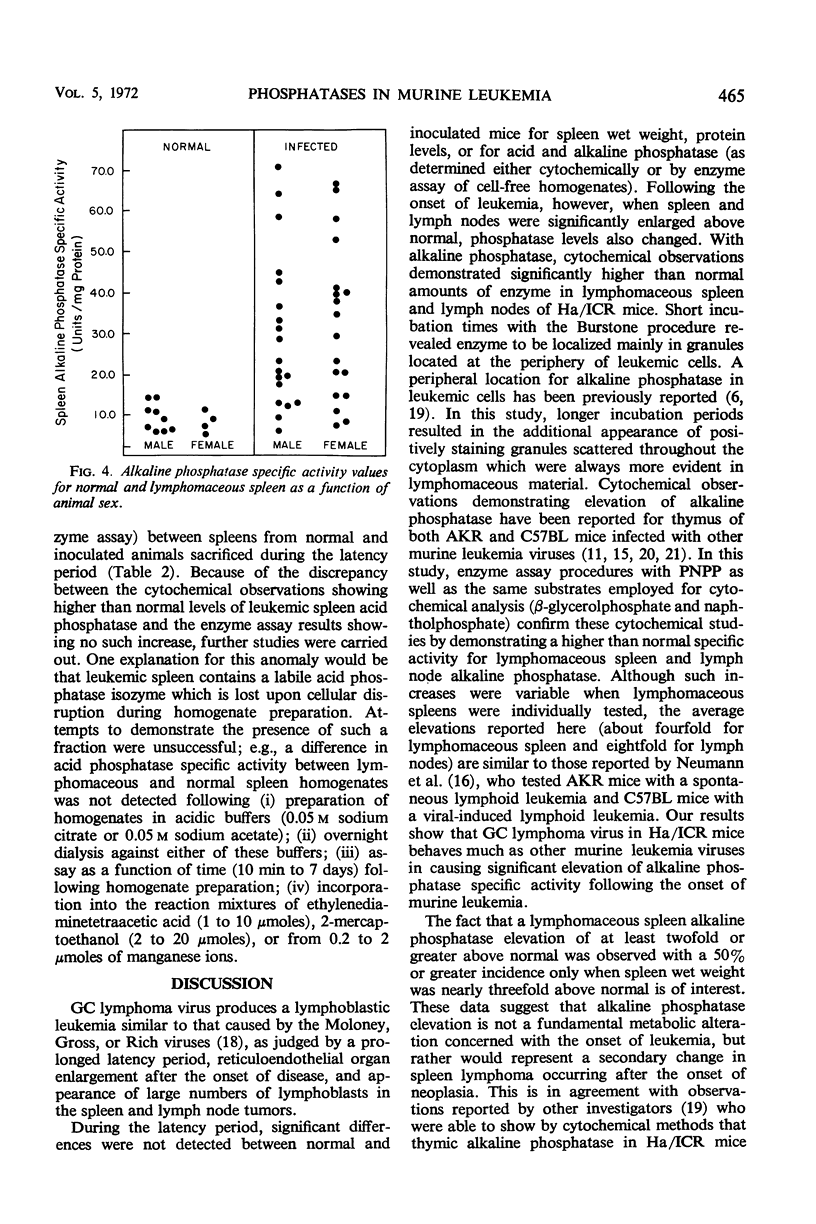
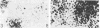Abstract
Alterations for acid and alkaline phosphatase levels and their pattern of splenic and lymph node activity in normal and virus-induced lymphoblastic leukemia were studied. Enzyme levels were examined by using both cytochemical and biochemical procedures. The GC leukemia virus, a ribonucleic acid murine virus antigenically related to the Rauscher-Moloney viruses, was used to stimulate acid and alkaline phosphatase by producing lymphomaceous disease in Ha/ICR mice. With the Burstone and Gomori cytochemical procedures, both enzymes were found in higher than normal levels in lymphomaceous spleen and lymph nodes. Confirmation of the cytochemical studies was obtained by enzyme assay of cell-free homogenates in each case with the exception of spleen acid phosphatase. The discrepancy between the cytochemical tests which showed significant elevation of spleen acid phosphatase and the enzyme assays which failed to reveal such elevation could be due to a labile acid phosphatase isozyme which is lost on cellular disruption during homogenate preparation. A significant spleen alkaline phosphatase specific activity elevation above normal was found with a 50% incidence only when leukemic spleen wet weight increased nearly threefold its normal value. This result suggests that alkaline phosphatase elevation is a secondary event occuring after the onset of disease and is not a fundamental metabolic alteration concerned with the onset of murine lymphoblastic leukemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. The relationship between fixation and techniques for the histochemical localization of hydrolytic enzymes. J Histochem Cytochem. 1958 Sep;6(5):322–339. doi: 10.1177/6.5.322. [DOI] [PubMed] [Google Scholar]
- Bowers W. E., Finkenstaedt J. T., de Duve C. Lysosomes in lymphoid tissue. I. The measurement of hydrolytic activities in whole homogenates. J Cell Biol. 1967 Feb;32(2):325–337. doi: 10.1083/jcb.32.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. R., Buinauskas P., Schwartz S. O. Certain physical and chemical properties of two murine leukemia viruses. Can J Microbiol. 1967 Aug;13(8):957–961. doi: 10.1139/m67-128. [DOI] [PubMed] [Google Scholar]
- Brown E. R., Rohlfing S. R., Schwartz S. O. An immunologic comparison of five murine leukemia viruses grown in tissue culture. Can J Microbiol. 1968 Dec;14(12):1347–1348. doi: 10.1139/m68-225. [DOI] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- FEY F., SCHNEIDER E. J. ALKALISCHE PHOSPHATASE BEI VIRUSINDUZIERTEN LEUKAEMIEN DER MAUS. Acta Biol Med Ger. 1963;11:264–273. [PubMed] [Google Scholar]
- GREENSPAN I., BROWN E. R., SCHWARTZ S. O. ANTIBODY RESPONSE OF MICE TO A LEUKAEMOGENIC VIRUS. Nature. 1964 May 30;202:916–917. doi: 10.1038/202916b0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagerlöf B. A., Kaplan H. S. Specificity of the relationship between thymic alkaline phosphatase activity and lymphoma development in strain C57BL mice. J Natl Cancer Inst. 1967 Apr;38(4):437–447. [PubMed] [Google Scholar]
- Lawrence S. A., Mass R. E., Siegel B. V. Cytochemical demonstration of acid phosphatase in peripheral blood cells in Rauscher virus infection. Proc Soc Exp Biol Med. 1968 Oct;129(1):175–179. doi: 10.3181/00379727-129-33277. [DOI] [PubMed] [Google Scholar]
- Lumb J. R., Doell R. G. The biochemical characterization of alkaline phosphatase from chemicaland viral-induced thymic lymphomas of C57BL mice. Cancer Res. 1970 May;30(5):1391–1396. [PubMed] [Google Scholar]
- METCALF D., SPARROW N., WYLLIE R. Alkaline phosphatase activity in mouse lymphoma tissue. Aust J Exp Biol Med Sci. 1962 Jun;40:215–224. doi: 10.1038/icb.1962.25. [DOI] [PubMed] [Google Scholar]
- Neumann H., Wilson K. J., Haran-Ghera N. Some physical properties of alkaline phosphatases found in various tissues of AKR and C57BL/6 mice, normal and leukemic. FEBS Lett. 1970 May 25;8(2):87–90. doi: 10.1016/0014-5793(70)80231-9. [DOI] [PubMed] [Google Scholar]
- Rich M. A., Siegler R. Virus leukemia in the mouse. Annu Rev Microbiol. 1967;21:529–572. doi: 10.1146/annurev.mi.21.100167.002525. [DOI] [PubMed] [Google Scholar]
- Siegler R., Rich M. A. Significance of increased alkaline phosphatase activity in viral-induced thymic lymphoma. Proc Soc Exp Biol Med. 1967 Jul;125(3):868–871. doi: 10.3181/00379727-125-32226. [DOI] [PubMed] [Google Scholar]