Abstract
Objective
Pituitary dysfunction is a recognized consequence of traumatic brain injury (TBI) that causes cognitive, psychological, and metabolic impairment. Hormone replacement offers a therapeutic opportunity. Blast TBI (bTBI) from improvised explosive devices is commonly seen in soldiers returning from recent conflicts. We investigated: (1) the prevalence and consequences of pituitary dysfunction following moderate to severe bTBI and (2) whether it is associated with particular patterns of brain injury.
Methods
Nineteen male soldiers with moderate to severe bTBI (median age = 28.3 years) and 39 male controls with moderate to severe nonblast TBI (nbTBI; median age = 32.3 years) underwent full dynamic endocrine assessment between 2 and 48 months after injury. In addition, soldiers had structural brain magnetic resonance imaging, including diffusion tensor imaging (DTI), and cognitive assessment.
Results
Six of 19 (32.0%) soldiers with bTBI, but only 1 of 39 (2.6%) nbTBI controls, had anterior pituitary dysfunction (p = 0.004). Two soldiers had hyperprolactinemia, 2 had growth hormone (GH) deficiency, 1 had adrenocorticotropic hormone (ACTH) deficiency, and 1 had combined GH/ACTH/gonadotrophin deficiency. DTI measures of white matter structure showed greater traumatic axonal injury in the cerebellum and corpus callosum in those soldiers with pituitary dysfunction than in those without. Soldiers with pituitary dysfunction after bTBI also had a higher prevalence of skull/facial fractures and worse cognitive function. Four soldiers (21.1%) commenced hormone replacement(s) for hypopituitarism.
Interpretation
We reveal a high prevalence of anterior pituitary dysfunction in soldiers suffering moderate to severe bTBI, which was more frequent than in a matched group of civilian moderate to severe nbTBI subjects. We recommend that all patients with moderate to severe bTBI should routinely have comprehensive assessment of endocrine function. Ann Neurol 2013;74:527–536
The use of improvised explosive devices (IEDs) has characterized the Iraq and Afghanistan conflicts, with blast traumatic brain injury (bTBI) a “signature injury.”1 More than 400 UK and 2,000 US soldiers have been fatally wounded by blast injuries in Afghanistan since 2001.2 Among survivors, it is estimated that 19.5% of 1.64 million US troops deployed in both conflicts have suffered a probable bTBI.3 Soldiers are usually young, so the long-term impact of consequent physical, cognitive, and psychological problems represents a significant health burden. There are no current pharmaceutical treatments that improve recovery following TBI.4
Nonblast TBI (nbTBI) is a recognized cause of pituitary dysfunction, in particular growth hormone (GH) deficiency.5 Reported prevalence rates of pituitary dysfunction following nbTBI vary between 2 and 68%.5,6 This variability is due in part to differences in the normal ranges and dynamic endocrine tests used, the time since injury, and injury severity.5–7 In addition to adverse metabolic consequences, hypopituitarism causes multiple symptoms impacting on physical and psychological well-being that will impair recovery after TBI, and thus hormone replacement represents an important therapeutic opportunity.8–11 It is unknown how often bTBI leads to pituitary dysfunction.12
Diffusion tensor imaging (DTI) is a sensitive magnetic resonance (MR) technique that can assess the presence and severity of white matter damage after TBI.13,14 TBI alters the pattern of water diffusion within white matter, resulting in abnormal diffusion measures, including fractional anisotropy (FA). DTI abnormalities in several brain regions have been reported in soldiers following mild bTBI.15 We hypothesized that DTI would reveal differences in white matter damage in those soldiers with pituitary dysfunction after bTBI.
Here we report findings from the UK BIOSAP (United Kingdom Blast Injury Outcome Study of Armed Forces Personnel). We investigated the prevalence and associations of pituitary dysfunction in soldiers after moderate to severe bTBI compared to a control group of patients after nbTBI.
Subjects and Methods
Recruitment
Nineteen military bTBI patients were recruited using the Academic Department of Military Emergency Medicine (Birmingham, UK) trauma database to identify soldiers injured between December 2009 and March 2012. This represents 10.4% of the 183 UK soldiers who had survived a moderate to severe bTBI in Afghanistan during this 27-month period, of what is now the 12th year of this conflict. Research ethics committee approval and informed consent were obtained.
Comparison was made with an age- and gender-matched control group of 39 patients after nbTBI. This represented all the patients seen in our multidisciplinary Traumatic Brain Injury clinic at Charing Cross Hospital, London, United Kingdom between August 2009 and March 2012 who met all inclusion/exclusion criteria and were within the age range of the bTBI group. These patients had identical endocrine assessment as part of their routine clinical care.
The inclusion criterion for bTBI was a moderate to severe brain injury caused directly by a single exposure to a blast. To better examine the effects of the primary blast wave only, exclusion criteria for bTBI were: (1) requirement for massive blood transfusion; (2) intracranial lesions causing mass effect; and (3) post-traumatic stress disorder (PTSD), because this has been linked with endocrine disturbance.16,17 PTSD was diagnosed on the basis of psychologist interview and, if suspected, subsequent self-reported symptom ratings from the PTSD Checklist–Military version derived from Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria.18 Although this includes symptoms present in many soldiers after bTBI, such as loss of memory of the event, anhedonia, social isolation, sleep disturbance, emotional lability, and poor concentration, subjects did not display additional symptoms required for the diagnosis of PTSD, such as “repeated, disturbing memories, thoughts, images or dreams of a previous stressful experience” or “physical reactions (such as heart pounding, trouble breathing or sweating) when reminded of a previous stressful experience.”
Inclusion criteria for both bTBI and nbTBI were: (1) male gender, (2) >2 and <48 months from a single TBI, (3) moderate to severe brain injury using the Mayo classification criteria,19 (4) ongoing cognitive and/or psychological symptoms, and (5) completion of all endocrine testing. Exclusion criteria for bTBI and nbTBI subjects were: (1) diabetes mellitus, (2) pre-TBI history of psychiatric disorder, (3) current or previous drug or excess alcohol use, (4) reversed sleep–wake cycle, and (4) craniotomy following injury (to avoid the difficulties in brain image registration resulting from gross changes in brain structure).
Both bTBI and nbTBI subjects underwent clinical assessment and calculation of Abbreviated Injury Score (AIS) and total Injury Severity Score (ISS), and completed quality of life (QoL) and symptom questionnaires (see Supplementary Methods).
Endocrine Testing
The algorithm used to define pituitary dysfunction is shown in Table 1 (see Supplementary Methods). All patients had measurement of basal serum anterior pituitary hormones followed by dynamic endocrine testing. Initial screening for GH and adrenocorticotropic hormone (ACTH) deficiency used the glucagon stimulation test (GST).20,21 The diagnosis of GH deficiency was confirmed with second-line growth hormone-releasing hormone (GHRH)–arginine test and/or insulin tolerance test (ITT).10,22,23 ACTH deficiency was confirmed with an ITT or metyrapone stimulation test, together with a cortisol day curve.21,24 Symptoms of diabetes insipidus were investigated further with a water deprivation test.
Table 1.
Diagnostic Algorithm for Pituitary Dysfunction
| Pituitary Axis | First Test | Confirmatory Test |
|---|---|---|
| GH deficiency | Glucagon stimulation test: peak GH < 5μg/l | GHRH–arginine test: GH < cutoff based on age and BMI;22 OR ITT: peak GH < 3μg/l |
| ACTH deficiency | Glucagon stimulation test: peak cortisol < 350nmol/l (<12.7μg/dl)21 | Metyrapone test: 11-DOC < 200nmol/l (<6.9μg/dl) OR if unavailable ACTH < 60ng/l despite cortisol < 200nmol/l (<7.2μg/dl); OR ITT: peak cortisol < 450nmol/l (<16.3μg/dl); supported by am cortisol < 100nmol/l (<3.62μg/dl) |
| Hyperprolactinemia | Prolactin > 375 mU/l (NR = 75–375) | Repeat prolactin > 375mU/l AND negative macroprolactin AND normal MRI pituitary with contrast |
| Gonadotrophin deficiency | Random testosterone < 10nmol/l (<2.9ng/ml) OR if SHBG low (<15nmol/l) FAI < 30; AND nonelevated LH (NR = 1.7–12.0 IU/l) and FSH (NR = 1.7–8.0 IU/l) | Repeat abnormal basal levels using morning (9–10 am) sample |
| TSH deficiency | Free T4 < 9.0pmol/l (<0.70ng/dl) OR free T3 < 2.5pmol/l (<0.16ng/dl); AND nonelevated TSH (NR = 0.30–4.22mU/l) | Repeat abnormal basal levels |
| ADH (vasopressin) deficiency (diabetes insipidus) | Symptoms of polyuria or polydipsia AND random urine osmolarity <750 mosmol/kg | Water deprivation test |
11-DOC = 11-deoxycorticosterone; ACTH = adrenocorticotropic hormone; ADH = antidiuretic hormone; BMI = body mass index; FAI = free androgen index (100 × testosterone/SHBG); FSH = follicle-stimulating hormone; GH = growth hormone; GHRH = growth hormone-releasing hormone; ITT = insulin tolerance test; LH = luteinizing hormone; MRI = magnetic resonance imaging; NR = normal range; SHBG = sex hormone-binding globulin; TSH = thyroid-stimulating hormone.
Cognitive Function Assessment
Each soldier with bTBI completed a standardized neuropsychological test battery previously shown to be sensitive to cognitive impairment after TBI.14 The tests looked at the cognitive domains of: (1) current verbal and nonverbal reasoning ability; (2) associative memory and learning; (3) executive functions of set shifting, inhibitory control, cognitive flexibility, and word generation fluency; and (4) information processing speed (see Supplementary Methods).
Structural Brain Imaging
Each soldier had standard T1, gradient-echo (T2*), and susceptibility-weighted MR imaging (MRI) to assess focal brain injury, microbleeds, superficial siderosis, gliosis, contusions, and DTI. Most patients with pituitary dysfunction also had a pituitary MRI with gadolinium contrast to look for more detailed hypothalamic–pituitary abnormalities. Patients with nbTBI had only computed tomography (CT) brain and/or standard T1/T2 brain MRI as part of routine clinical practice. DTI analysis of white matter tracts combined tract-based spatial statistics and region of interest (ROI) approaches (Functional Magnetic Resonance Imaging of the Brain Software Library, Oxford, UK), focusing on regions previously shown to be sensitive to damage in bTBI and nbTBI (Supplementary Fig S1 and Supplementary Methods).14,15 This allowed assessment of regional FA, a measure of traumatic axonal injury.
Statistical Analyses
Comparisons between groups (nbTBI vs bTBI; and bTBI with pituitary dysfunction vs bTBI without pituitary dysfunction) were made using Fisher exact test for prevalence data, and unpaired Student t test (FA and neurocognitive variables), or Mann–Whitney U test (other variables) for continuous data (SPSS v19.0; IBM, Armonk, NY). Significance was defined as p < 0.05. A group × ROI repeated measure analysis of variance was performed to assess the overall effect of pituitary dysfunction on FA.
Results
Patient Characteristics
All soldiers with bTBI had been injured by IEDs and had been wearing full personal protective equipment. All required immediate transfer to Camp Bastion for emergency medical treatment, and repatriation to the United Kingdom within 48 hours. We have detailed information about the blast exposure, but for operational security reasons these cannot be reported. In the control nbTBI group, injuries were secondary to road traffic accidents (RTAs; 43%), assaults (32%), falls (23%), and sporting injuries (2%). Three subjects in the nbTBI group had experienced previous TBI (1 subject had 2 mild TBIs from an RTA and an assault, 1 a mild TBI from a fall, and 1 a TBI of unknown severity from an assault).
The bTBI and nbTBI groups were well matched in most respects (Table 2). There were no significant differences in age, ISS whole body injury severity, skull/facial fractures (15.8 vs 15.4%), or post-traumatic seizures (10.5 vs 7.7%). The bTBI group had longer post-traumatic amnesia (PTA; median 5.5 days vs 0.5 days, p = 0.01); more injuries requiring surgery to or loss of function of major extracranial organs (57.9 vs 7.7%, p = 0.002); more amputations (36.8 vs 0%, p < 0.001); and, in keeping with this, more use of strong prescription opiates (47.3 vs 7.7%, p = 0.001). The time from TBI to endocrine testing was significantly longer in the bTBI group (median 15.2 vs 5.8 months, p = 0.001).
Table 2.
Patient Characteristics
| Characteristic | Maximum Score | All nbTBI | All bTBI | p | bTBI: No Pituitary Dysfunction | bTBI: Pituitary Dysfunction | p |
|---|---|---|---|---|---|---|---|
| No. | 39 | 19 | 13 | 6 | |||
| Age at TBI, yr | 31.3 [22.5–35.7] | 26.7 [26.1–30.9] | 0.40 | 26.6 [24.6–30.6] | 29.3 [25.8–36.6] | 0.48 | |
| 17.2–44.8 | 19.0–43.5 | 19.0–36.3 | 25.0–43.5 | ||||
| Age at testing, yr | 32.3 [23.1–36.7] | 28.3 [26.8–32.2] | 0.40 | 28.0 [25.3–31.4] | 30.3 [27.4–38.3] | 0.32 | |
| 19.9–45.1 | 19.6–44.7 | 19.6–37.6 | 26.3–44.7 | ||||
| Time since TBI, mo | 5.8 [3.1–11.0] | 15.2 [10.8–19.3] | 0.001a | 15.2 [8.8–16.6] | 17.6 [12.3–20.2] | 0.32 | |
| 1.9–41.2 | 4.1–23.7 | 4.1–23.7 | 4.9–21.9 | ||||
| ISS | 75 | 25.0 [16.0–32.0] 1–75 | 33.0 [20.0–45.0] 9–70 | 0.17 | 24.0 [14.5–40.5] 9–45 | 35.5 [27.0–51.3] 9–70 | 0.24 |
| AIS head | 6 | 5.0 [4.0–5.0] 1–6 | 4.0 [3.0–5.0] 0–6 | 0.04a | 4.0 [2.5–4.0] 0–5 | 5.0 [3.0–5.3] 0–6 | 0.06 |
| AIS chest | 6 | 0 [0–0] 0–6 | 0 [0–2] 0–4 | 0.11 | 0 [0–3] 0–4 | 0.5 [0–2.3] 0–3 | 0.83 |
| AIS abdomen | 6 | 0 [0–0] 0–3 | 0 [0–2] 0–3 | 0.02a | 0 [0–2] 0–2 | 0 [0–2.3] 0–3 | 0.97 |
| GCS | 15 | 14.0 [6.0–14.0]b 3–15 | 3.0 [3.0–14.5]c 3–15 | 0.24 | 14.0 [3.0–15.0]d 3–15 | 3.0 [3.0–3.0]e 3–3 | 0.19 |
| PTA, days | 0.5 [0–7.3]f 0–42 | 5.5 [0.8–22.8] 0–84 | 0.01a | 3.0 [0–19.3] 0–84 | 15.5 [6.3–31.5] 4–42 | 0.10 | |
| PTA > 24 hours | 20 (51.3%) | 13 (68.4%) | 0.27 | 7 (58.3%) | 6 (100%) | 0.11 | |
| BMI, kg/m2 | 24.7 [22.4–29.4] 17.0–33.4 | 26.7 [24.5–28.9] 21.7–33.7 | 0.28 | 26.6 [24.5–28.7]g 23.6–29.4 | 25.5 [22.4–32.0]h 21.7–33.7 | 0.79 | |
| Limb amputation | 0 (0%) | 8 (42.1%) | <0.001a | 6 (46.1%) | 2 (33.3%) | 1.00 | |
| Major organ damage | 3 (7.7%) | 11 (57.9%) | <0.001a | 7 (53.9%) | 4 (66.7%) | 1.00 | |
| Skull/facial fracture | 6 (15.4%) | 3 (15.8%) | 1.00 | 0 (0%) | 3 (50.0%) | 0.02 | |
| Opiate use | 3 (7.7%) | 9 (47.3%) | 0.001a | 6 (46.2%) | 3 (50.0%) | 1.00 | |
| Antidepressant use | 5 (12.8%)i | 10 (52.7%)j | 0.003a | 7 (53.8%)k | 3 (50.0%)l | 1.00 | |
| Seizures post-TBI | 3 (7.7%)m | 2 (10.5%)n | 1.00 | 1 (7.7%)o | 1 (16.7%)p | 1.00 | |
| Primary hypogonadism | 1 (2.6%)q | 4 (21.1%)r | 0.04a | 4 (30.8%)r | 0 (0%)r | 0.26 |
Data are expressed as median [interquartile range], range, or No. (%). Probability values are from Mann–Whitney U test or Fisher exact test between groups.
aStatistically significant; p < 0.05.
Data available for bn = 16, cn = 9, dn = 5, en = 4, fn = 38, and due to amputations: gn = 7, hn = 4.
For analgesic purposes only in: in = 5 (12.8%), jn = 6 (31.6%), kn = 4 (30.8%), ln = 2 (33.3%).
For depression itself in: in = 0 (0%), jn = 4 (21.1%), kn = 3 (23.1%), ln = 1 (16.7%).
On antiepileptic drugs in mn = 3, nn = 1, on = 0, pn = 1.
qNot due to trauma.
rDue to perineal trauma.
AIS = Abbreviated Injury Score; BMI = body mass index; bTBI = blast traumatic brain injury; GCS = Glasgow Coma Scale; ISS = Injury Severity Score; nbTBI = nonblast TBI; PTA = post-traumatic amnesia.
Prevalence of Pituitary Function in bTBI and nbTBI Cohorts
Six of 19 soldiers with bTBI (31.6%) had anterior pituitary dysfunction, compared to only 1 of 39 (2.6%) subjects with nbTBI (p = 0.004; Fig 1, Supplementary Tables S1–S3). Two soldiers (10.5%) had monomeric hyperprolactinemia (without secondary hypogonadism), 1 (5.3%) had isolated ACTH deficiency, 2 (10.5%) had isolated GH deficiency, and 1 (5.3%) had combined ACTH, GH, and gonadotrophin deficiencies. The only pituitary dysfunction noted in 1 patient with nbTBI was isolated GH deficiency following a single TBI. No patients in either group had thyroid-stimulating hormone (TSH) deficiency or diabetes insipidus.
FIGURE 1.
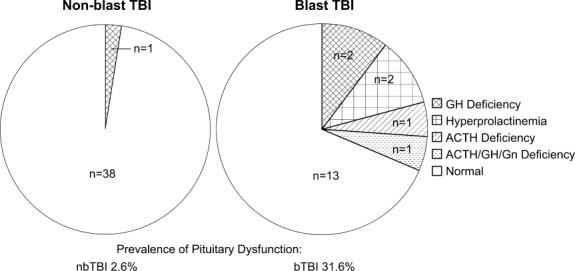
Prevalence of pituitary dysfunction in nonblast traumatic brain injury (nbTBI) and blast TBI (bTBI). Greater prevalence of anterior pituitary dysfunction was seen in subjects after bTBI (right) than nbTBI (left). No subjects had thyroid-stimulating hormone deficiency or diabetes insipidus. ACTH = adrenocorticotropic hormone; GH = growth hormone; Gn = gonadotrophin.
The 3 soldiers with GH deficiency had insulin-like growth factor-I (IGF-I) levels in the low normal range (see Supplementary Table S2), and the 2 soldiers with ACTH deficiency had normal early morning cortisol levels on initial assessment of 287 to 292nmol/l equivalent to 10.3 to 10.5μg/dl (normal, >150nmol/l, >5.4μg/dl, respectively; see Supplementary Table S3). However, on subsequent cortisol day curves, both subjects with ACTH deficiency had low cortisol levels (<100nmol/l, 3.62μg/dl) at either 9:00 AM or 12:00 pm on a day curve consistent with the diagnosis (see Supplementary Results, Supplementary Table S3). Thus, although the less commonly used metyrapone test was occasionally performed as the confirmatory test to diagnose or exclude ACTH deficiency instead of the gold standard ITT, findings were always compatible with the results of baseline or day curve cortisol levels. Furthermore, as with previous studies, we have found good specificity and concordance between the results of the metyrapone test compared to the ITT or ACTH stimulation test for diagnosing ACTH deficiency (see Supplementary Results). None of the soldiers with ACTH deficiency had any history of hypotension, hypoglycemia, or hyponatremia.
Primary hypogonadism due to perineal/testicular blast injury had been found in an additional 4 of 19 soldiers with bTBI (21.2%), none of whom had pituitary dysfunction, and all were already on testosterone replacement (see Supplementary Results, Supplementary Table S1).
Comparison of bTBI with versus bTBI without Pituitary Dysfunction
There was no significant difference in age at TBI, time since injury, ISS, abdominal AIS, body mass index (BMI), or prevalence of amputations, nonhead major organ damage, seizures, any use of antidepressants or specifically for depression, or opiate use between bTBI patients with versus those without pituitary dysfunction (see Table 2, Supplementary Tables S6 and S7). BMI could not be adequately assessed in the 8 soldiers with bTBI who had limb amputations, but none was morbidly obese on clinical examination.
There were trends for the AIS head injury scores to be higher (p = 0.06), and duration of PTA to be longer (median = 15.5 vs 3.0 days, p = 0.10) in those soldiers with pituitary dysfunction after bTBI than in those without.
The single soldier (M08) with multiple pituitary deficiencies was taking opiates at the time of diagnosis of gonadotrophin deficiency and initial dynamic endocrine testing with a GST. However, both GH and ACTH deficiency were subsequently confirmed using an ITT after opiates had been discontinued.
Neuroimaging Results
In the bTBI group, we investigated whether particular structural abnormalities were associated with pituitary dysfunction. Three of the 6 (50.0%) soldiers with pituitary dysfunction, compared to only 1 of the 13 (7.7%) soldiers without pituitary dysfunction, had contusions on brain MRI scans (p = 0.07). One soldier with pituitary dysfunction had 2 contusions, whereas the remainder had 1 contusion (Supplementary Fig S2). The total contusion volume was <10cm3 in all cases; the soldier without pituitary dysfunction had the smallest contusion volume. There was a greater prevalence of skull/facial fractures in the soldiers with pituitary dysfunction compared to those without (50 vs 0%, p = 0.02).
There were no significant differences in the prevalence of other abnormalities visible on acute CT brain scans following blast exposure or study structural MR scans, including presence of extracerebral, subarachnoid, or intraventricular hemorrhage, microbleeds, superficial siderosis, or gliosis, between those soldiers with versus without pituitary dysfunction (Supplementary Table S4). No hypothalamic–pituitary abnormalities were seen on MRI brain scans in any soldiers in the bTBI group, or in the 4 with pituitary dysfunction who had dedicated contrast-enhanced MRI pituitary scans (M01, M08, M10, M14). This included all those soldiers with hyperprolactinemia and multiple pituitary hormone deficiencies.
DTI analysis showed a reduction in FA depending on the ROI, indicating greater white matter damage, in those soldiers with pituitary dysfunction after bTBI compared to those without (p = 0.14 effect of group, p = 0.02 group × ROI interaction). Planned post hoc analysis showed significantly lower FA values for those soldiers with pituitary dysfunction within the cerebellum (p < 0.05), and body/genu (p < 0.05) and splenium (p = 0.01) of the corpus callosum ( Fig 2).
FIGURE 2.
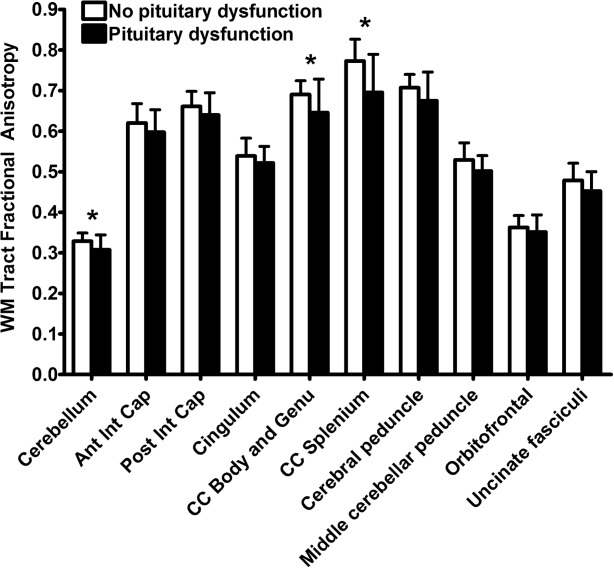
Pituitary dysfunction and white matter damage in blast traumatic brain injury. Lower fractional anisotropy was seen in a priori white matter tract regions of interest in soldiers with pituitary dysfunction after blast traumatic brain injury (black, n = 6) compared to those without pituitary dysfunction (white, n = 13). Data are expressed as mean ± standard deviation. *p < 0.05 (unpaired t test). Ant = anterior; CC = corpus callosum; Cap = capsule; Int = internal; Post = posterior; WM = white matter.
Symptoms, QoL, and Cognitive Function
Consistent with their higher prevalence of polytrauma and amputations, the soldiers with bTBI had significantly worse scores for physical activity and daily living problems than the control nbTBI group, but not in measures of depression and emotional well-being (see Supplementary Table S5, Supplementary Results).
In the bTBI group, soldiers with pituitary dysfunction had trends toward worse measures of QoL and symptom scores in several domains relating to emotional and social functioning, fatigue, and mood compared to those without pituitary dysfunction (see Supplementary Table S5, Supplementary Results).
The bTBI subjects with pituitary dysfunction had significantly worse average current verbal intellectual ability than those without pituitary dysfunction, despite there being no significant difference in their premorbid intelligence (Wechsler Test of Adult Reading; Table 3). The bTBI group with pituitary dysfunction also showed significantly worse cognitive impairment in the domains of visual/naming/reading processing speed, verbal fluency, and information processing (see Table 3).
Table 3.
Pituitary Dysfunction and Cognitive Function in Blast Traumatic Brain Injury
| Cognitive Domain | Cognitive Variable | No Pituitary Dysfunction, n = 13 | Pituitary Dysfunction, n = 6 |
|---|---|---|---|
| Premorbid intelligence: reading ability | WTAR raw score | 35.9 ± 11.7 | 34.7 ± 14.6 |
| Intellectual ability | WASI similarities (verbal) | 32.6 ± 6.2 | 27.0 ± 4.1a |
| WASI matrix reasoning (nonverbal) | 24.4 ± 7.5 | 24.2 ± 6.0 | |
| Memory: associative memory | People test immediate recall | 22.6 ± 8.1 | 25.0 ± 7.8 |
| Processing speed: visual search/complex | Trail Making Test trail A, seconds | 23.1 ± 5.7 | 28.7 ± 5.2a |
| Trail Making Test trail B, seconds | 47.9 ± 14.5 | 53.8 ± 12.2 | |
| Processing speed: naming/reading | Stroop color naming, seconds | 32.5 ± 9.1 | 51.0 ± 29.7a |
| Stroop word reading, seconds | 24.3 ± 6.7 | 37.2 ± 13.6b | |
| Executive function: alternating-switch cost | Trail Making Test trail B − A, seconds | 24.8 ± 13.5 | 25.2 ± 9.0 |
| Executive function: cognitive flexibility | Color word Stroop inhibition/switching, seconds | 70.5 ± 24.2 | 86.3 ± 30.8 |
| Inhibition/switching minus a baseline of color naming and word reading, seconds | 30.0 ± 18.8 | 26.5 ± 8.5 | |
| Word generation fluency | DKEFS letter fluency F + A + S total | 40.1 ± 12.9 | 28.8 ± 3.6a |
| Information processing | Choice reaction task median reaction time, milliseconds | 413 ± 38 | 473 ± 31a |
Worse cognitive function was seen in soldiers with pituitary dysfunction after blast traumatic brain injury (n = 6) compared to those without pituitary dysfunction (n = 13). Data are expressed as mean ± standard deviation. See Supplementary Methods for further details on cognitive tests.
p < 0.05,
p < 0.005 (unpaired t test).
DKEFS = Delis–Kaplan Executive Function System; WASI = Wechsler Abbreviated Scale of Intelligence Similarities and Matrix Reasoning subsets; WTAR = Wechsler Test of Adult Reading.
Discussion
We have demonstrated a high prevalence of pituitary dysfunction following moderate to severe blast TBI. Almost a third of soldiers with bTBI had anterior pituitary abnormalities, compared to only 2% of age- and gender-matched civilians with moderate to severe nbTBI. The most common pituitary abnormality in bTBI was GH deficiency, followed by hyperprolactinemia, ACTH, and gonadotrophin deficiency. One patient had multiple hormone deficiencies.
We carefully avoided overdiagnosis of pituitary dysfunction. We used identical diagnostic algorithms in the bTBI and nbTBI groups, excluded the presence of macroprolactin, applied strict normal ranges for diagnosing testosterone and TSH deficiency, performed 2 stimulation tests to confirm ACTH or GH deficiencies, and adjusted for the confounds of age and obesity in diagnosing GH deficiency.22 This allows us to be confident of our reported prevalence of pituitary dysfunction in both groups.6,7
Our results suggest that all patients after moderate to severe bTBI should undergo endocrine assessment. Unlike TSH and gonadotrophin deficiency, GH and ACTH deficiency cannot be excluded or always confirmed by basal IGF-I or cortisol measurements. Therefore, dynamic endocrine testing is required. The choice of tests needs to take into account contraindications for use of the ITT, such as seizures, as well as the advantages and disadvantages of each test, including their specificity/sensitivity, age/obesity-adjusted normal ranges, resource implications, local expertise, and drug availability.7,21,23
The presence of pituitary dysfunction after bTBI was not explicable by differences in age, gender, or obesity. The time to endocrine testing was longer in the bTBI than nbTBI group. However, this might be expected to reduce the prevalence of pituitary dysfunction, as it may resolve over time following TBI.25 Similarly, use of opiates or other medications does not explain our results. Opiates can have complex neuroendocrine effects, including induction of hypogonadotrophic hypogonadism, and potentially decreasing ACTH secretion but increasing GH secretion.26 Although there was greater use of opiates in the bTBI as a whole than in the nbTBI group, the individual pituitary dysfunction seen in each soldier within the bTBI group was not explicable by opiate use. The bTBI group did have more polytrauma than the nbTBI group, which may be a contributory factor, although the mechanism linking peripheral injury to hypothalamic–pituitary dysfunction is uncertain.
Blast appears to produce a distinct pattern of TBI,15,27 although the mechanism by which blast injury damages the brain remains unclear, limiting our ability to identify those patients at high risk of pituitary dysfunction. The primary blast wave or wind may cause direct injury, or secondary injuries from explosion debris or tertiary injuries from the impact of being thrown by the blast may occur.28,29 These injuries could affect the hypothalamus, pituitary gland, or pituitary stalk, resulting in damage to cell bodies or white matter connections as well as hypophyseal vessels, local superficial siderosis, inflammation, or hypovolemia/ischemia.
Our imaging results do not provide clear evidence about the precise mechanism of hypothalamic–pituitary damage. We did not see evidence of focal injury to the hypothalamus–pituitary or superficial siderosis, and we excluded bTBI subjects who needed massive blood transfusions. However, pituitary dysfunction may be related to the severity of brain injury after blast exposure, as suggested in nbTBI.5 This is supported in our study by the longer duration of PTA in the bTBI than in the nbTBI group (although interpretation may be complicated by sedation and anesthesia), and the presence of more white matter damage15 and more skull/facial fractures, and a trend for more cerebral contusions and longer PTA, in those soldiers with than in those without pituitary dysfunction after bTBI. Diffuse axonal injury is common in the corpus callosum after TBI in general,30 and posterior fossa white matter tracts are particularly damaged after mild bTBI.15 It remains unclear whether the more severe damage to these tracts in bTBI with pituitary dysfunction simply indicates a greater severity of brain injury, or is indicative of a particular injury pattern associated with hypothalamic–pituitary damage.
Our study focused on subjects with a single episode of moderate to severe bTBI. It remains to be determined whether pituitary dysfunction is a significant problem after single, or especially repeated, mild bTBI, because there is evidence that multiple bTBI may augment neurological deficits.31 A single previous study has suggested that repeated mild bTBI can produce endocrine disturbance.32 However, methodological issues with this study make it difficult to interpret, including their reliance on basal hormone measurements, the definition of normal ranges from a small cohort of control subjects, and the nonstandard assessment of posterior pituitary function.
The trend for worse fatigue, emotional symptoms, social problems, and mood in those soldiers with pituitary dysfunction after bTBI may be related to worse underlying brain injury and/or their endocrine problems. These are well-recognized features of GH deficiency, and lethargy is also seen in cortisol and testosterone deficiency.8,9,33 Similarly, cognitive impairment in soldiers with pituitary dysfunction after bTBI may be related to both greater brain/axonal injury and hormone deficiencies, including GH.14,34,35
Our findings led to substantial changes in clinical management. The soldier with hypogonadotrophic hypogonadism was treated with intramuscular long-acting testosterone. Both soldiers with ACTH deficiency were commenced on hydrocortisone replacement. All 3 soldiers with GH deficiency were >1 year after bTBI and have been started on GH replacement in view of persistent neuropsychological symptoms despite replacement of other pituitary hormones. The soldiers with sufficient follow-up data available have had a symptomatic improvement after 6 months of GH replacement, with adult growth hormone deficiency QoL assessment (AGHDA-QoL) score falling from 19 to 14 (of 25), and Beck Depression Inventory II (BDI-II) score from 36 to 18 (of 63) in 1 subject (M14), and AGHDA-QoL from 14 to 3, and BDI-II falling from 20 to 16 in another (M08) during this period. However, the other soldier receiving GH (M07) is still undergoing dose titration, and so it is too early to assess his symptomatic improvement. The soldiers with mild hyperprolactinemia did not require treatment, as secondary hypogonadism was absent.
In conclusion, this is the first study to demonstrate a high prevalence of anterior pituitary hormone abnormalities after moderate to severe bTBI. The prevalence was greater than in a matched group of civilian nbTBI, suggesting that pituitary dysfunction is a particular problem after blast exposure. Pituitary dysfunction following bTBI was associated with worse cognitive function and greater severity of head injury, including white matter damage. Given that there were no completely diagnostic predictors of pituitary dysfunction in bTBI, we recommend that in clinical practice all soldiers with moderate to severe bTBI undergo routine and comprehensive pituitary function testing during rehabilitation.
Acknowledgments
This study was supported by the UK Medical Research Council (MRC), National Institute for Health Research (NIHR, ref. NIHR-RP-011-048), Imperial College Healthcare Charity (ref. 7006/R21U), and the Royal Centre for Defence Medicine. D.J.S. is supported by the MRC (Clinician Scientist Fellowship) and NIHR, A.P.G. by the MRC, and C.F. by Imperial College Healthcare Charity.
We thank the trauma nurse coordinators, Academic Department of Military Surgery and Trauma, Birmingham, UK; doctors, nurses, and rehabilitation staff based at Defence Medical Rehabilitation Centre, Headley Court, Surrey, UK; E. Hughes and J. Allsop, Robert Steiner MRI Unit, MRC Clinical Sciences Centre, Hammersmith Hospital, London, UK for assistance with MRI; endocrinology colleagues at Imperial College Healthcare NHS Trust, London, and doctors and nursing staff, Patient Investigation Unit, Charing Cross Hospital, London and Metabolic Day Ward, St Mary's Hospital, London for assistance with endocrine testing; Department of Clinical Biochemistry, Imperial College Healthcare NHS Trust, London for performing hormone assays; and J. Monson for helpful comments. We especially thank the soldiers from the UK military who after suffering these life-changing injuries still had the enthusiasm and spirit to take part in this research.
The opinions expressed are those of the authors and not the UK Ministry of Defence.
Authorship
Patient recruitment: D.B., D.J.S., T.E.H., A.N.B., A.M., E.M., A.P.G.; study design: D.B., D.J.S., M.M., A.P.G.; data collection: D.B., D.J.S., D.P., T.E.H., S.J., A.P.G.; data analysis: D.B., D.J.S., C.F., T.E.H., S.J., P.J.H., M.C.P., A.P.G.; data interpretation: D.B., D.J.S., C.F., A.P.G.; writing the manuscript: D.B., D.J.S., C.F., A.P.G.; review and editing the manuscript: all authors.
Potential Conflicts of Interest
D.J.S., A.P.G.: grants/grants pending, Pfizer.
Supporting Information
Additional supporting information can be found in the online version of this article.
Supporting Information
References
- 1.Benzinger TL, Brody D, Cardin S, et al. Blast-related brain injury: imaging for clinical and research applications: report of the 2008 St. Louis workshop. J Neurotrauma. 2009;26:2127–2144. doi: 10.1089/neu.2009.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chesser GS. Afghanistan casualties: military forces and civilians. Washington, DC: Congressional Research Service; 2012. [Google Scholar]
- 3.Tanielian T, Jaycox HL. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Arlington, VA: RAND Center for Military Health Policy Research; 2008. [Google Scholar]
- 4.Ruff RL, Riechers RG. Effective treatment of traumatic brain injury: learning from experience. JAMA. 2012;308:2032–2033. doi: 10.1001/jama.2012.14008. [DOI] [PubMed] [Google Scholar]
- 5.Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, et al. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429–1438. doi: 10.1001/jama.298.12.1429. [DOI] [PubMed] [Google Scholar]
- 6.Kokshoorn NE, Smit JW, Nieuwlaat WA, et al. Low prevalence of hypopituitarism after traumatic brain injury: a multicenter study. Eur J Endocrinol. 2011;165:225–231. doi: 10.1530/EJE-11-0365. [DOI] [PubMed] [Google Scholar]
- 7.Kokshoorn NE, Wassenaar MJ, Biermasz NR, et al. Hypopituitarism following traumatic brain injury: prevalence is affected by the use of different dynamic tests and different normal values. Eur J Endocrinol. 2010;162:11–18. doi: 10.1530/EJE-09-0601. [DOI] [PubMed] [Google Scholar]
- 8.Salvatori R. Adrenal insufficiency. JAMA. 2005;294:2481–2488. doi: 10.1001/jama.294.19.2481. [DOI] [PubMed] [Google Scholar]
- 9.Cherrier MM. Testosterone effects on cognition in health and disease. Front Horm Res. 2009;37:150–162. doi: 10.1159/000176051. [DOI] [PubMed] [Google Scholar]
- 10.Molitch ME, Clemmons DR, Malozowski S, et al. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1587–1609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]
- 11.Bondanelli M, Ambrosio MR, Cavazzini L, et al. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J Neurotrauma. 2007;24:1687–1697. doi: 10.1089/neu.2007.0343. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero AF, Alfonso A. Traumatic brain injury-related hypopituitarism: a review and recommendations for screening combat veterans. Mil Med. 2010;175:574–580. doi: 10.7205/milmed-d-09-00189. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald CL, Dikranian K, Bayly P, et al. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnunen KM, Greenwood R, Powell JH, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pervanidou P, Chrousos GP. Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149–160. doi: 10.1016/S0079-6123(10)82005-9. [DOI] [PubMed] [Google Scholar]
- 17.van Liempt S, Vermetten E, Lentjes E, et al. Decreased nocturnal growth hormone secretion and sleep fragmentation in combat-related posttraumatic stress disorder; potential predictors of impaired memory consolidation. Psychoneuroendocrinology. 2011;36:1361–1369. doi: 10.1016/j.psyneuen.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28:596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malec JF, Brown AW, Leibson CL, et al. The Mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007;24:1417–1424. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- 20.Leong KS, Walker AB, Martin I, et al. An audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic-pituitary disease. Clin Endocrinol (Oxf) 2001;54:463–468. doi: 10.1046/j.1365-2265.2001.01169.x. [DOI] [PubMed] [Google Scholar]
- 21.Cegla J, Jones B, Seyani L, et al. Comparison of the overnight metyrapone and glucagon stimulation tests in the assessment of secondary hypoadrenalism. Clin Endocrinol (Oxf) 2013;78:738–742. doi: 10.1111/cen.12043. [DOI] [PubMed] [Google Scholar]
- 22.Colao A, Di SC, Savastano S, et al. A reappraisal of diagnosing GH deficiency in adults: role of gender, age, waist circumference, and body mass index. J Clin Endocrinol Metab. 2009;94:4414–4422. doi: 10.1210/jc.2009-1134. [DOI] [PubMed] [Google Scholar]
- 23.Yuen KC, Biller BM, Molitch ME, et al. Clinical review: Is lack of recombinant growth hormone (GH)-releasing hormone in the United States a setback or time to consider glucagon testing for adult GH deficiency? J Clin Endocrinol Metab. 2009;94:2702–2707. doi: 10.1210/jc.2009-0299. [DOI] [PubMed] [Google Scholar]
- 24.Grossman AB. Clinical review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95:4855–4863. doi: 10.1210/jc.2010-0982. [DOI] [PubMed] [Google Scholar]
- 25.Aimaretti G, Ambrosio MR, Di SC, et al. Residual pituitary function after brain injury-induced hypopituitarism: a prospective 12-month study. J Clin Endocrinol Metab. 2005;90:6085–6092. doi: 10.1210/jc.2005-0504. [DOI] [PubMed] [Google Scholar]
- 26.Vuong C, Van Uum SH, O'Dell LE, et al. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134ra60. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams JH, Graham DI, Murray LS, et al. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 31.Ruff RL, Riechers RG, Wang XF, et al. A case-control study examining whether neurological deficits and PTSD in combat veterans are related to episodes of mild TBI. BMJ Open. 2012;2:e000312. doi: 10.1136/bmjopen-2011-000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson CW, Pagulayan KF, Petrie EC, et al. High prevalence of chronic pituitary and target-organ hormone abnormalities after blast-related mild traumatic brain injury. Front Neurol. 2012;3:11. doi: 10.3389/fneur.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb SM. Measurements of quality of life in patients with growth hormone deficiency. J Endocrinol Invest. 2008;31:52–55. [PubMed] [Google Scholar]
- 34.van Dam PS. Neurocognitive function in adults with growth hormone deficiency. Horm Res. 2005;64(suppl 3):109–114. doi: 10.1159/000089326. [DOI] [PubMed] [Google Scholar]
- 35.Bonnelle V, Leech R, Kinnunen KM, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


