Abstract
Objective
Magnetic resonance (MR) diffusion-weighted imaging (DWI) is sensitive to small acute ischemic lesions and might help diagnose transient ischemic attack (TIA). Reclassification of patients with TIA and a DWI lesion as “stroke” is under consideration. We assessed DWI positivity in TIA and implications for reclassification as stroke.
Methods
We searched multiple sources, without language restriction, from January 1995 to July 2012. We used PRISMA guidelines, and included studies that provided data on patients presenting with suspected TIA who underwent MR DWI and reported the proportion with an acute DWI lesion. We performed univariate random effects meta-analysis to determine DWI positive rates and influencing factors.
Results
We included 47 papers and 9,078 patients (range = 18–1,693). Diagnosis was by a stroke specialist in 26 of 47 studies (55%); all studies excluded TIA mimics. The pooled proportion of TIA patients with an acute DWI lesion was 34.3% (95% confidence interval [CI] = 30.5–38.4, range = 9–67%; I2 = 89.3%). Larger studies (n > 200) had lower DWI-positive rates (29%; 95% CI = 23.2–34.6) than smaller (n < 50) studies (40.1%; 95% CI = 33.5–46.6%; p = 0.035), but no other testable factors, including clinician speciality and time to scanning, reduced or explained the 7-fold DWI-positive variation.
Interpretation
The commonest DWI finding in patients with definite TIA is a negative scan. Available data do not explain why ⅔ of patients with definite specialist-confirmed TIA have negative DWI findings. Until these factors are better understood, reclassifying DWI-positive TIAs as strokes is likely to increase variance in estimates of global stroke and TIA burden of disease. ANN NEUROL 2014;75:67–76
Stroke is the third commonest cause of death worldwide and the commonest cause of dependency in adults.1 There are nearly 6 million deaths, 15 million strokes, and 7.5 million transient ischemic attacks (TIAs) worldwide per year. Patients with TIA are at high risk of early recurrent stroke.2 Rapid diagnosis and prompt treatment of underlying risk factors are essential to prevent stroke and reduce its global disease burden.
The diagnosis of TIA is mainly based on the clinical history, because neurological signs usually disappear quickly.3 Forty-five percent of referrals to TIA clinics are nonvascular mimics,4,5 but the observer agreement for diagnosis of TIA versus mimic is imperfect.6 For all these reasons, a confirmatory test for TIA would be helpful.
Magnetic resonance (MR) with diffusion-weighted imaging (DWI) is very sensitive to small ischemic lesions,7 and TIA patients with an acute ischemic lesion on DWI are at increased risk of recurrent stroke.8–10 Thus, DWI could both improve diagnosis of TIA and predict short-term stroke risk. National stroke guidelines now recommend use of DWI in TIA patients.11,12 Additionally, a change from a time-based to a tissue-based definition of TIA/stroke using DWI has been proposed13 and is now under consideration by the World Health Organization (http://apps.who.int/classifications/icd11/browse/f/en) and recommended by the American Stroke Association.14
Stroke is both common and associated with a huge global disease burden; thus, identification should be based on the most robust epidemiological data. In previous reviews (2,457 patients, 26 studies), DWI showed an acute ischemic lesion in 13 to 67% of patients (mean = 37%, standard deviation = ±12%), but the apparently low rate of positive scans and reasons for the large variance were not explored.15,16 We analyzed all available literature to obtain the best estimate of the proportion of TIA patients with an acute ischemic lesion on DWI, and to determine what factors influence that proportion, so as to inform use of DWI in TIA/stroke diagnosis.
Patients and Methods
We used the PRISMA guidelines for systematic reviews of observational studies.17 We aimed to identify all published studies in which TIA patients were assessed by DWI irrespective of aims, study design, or clinical setting. A detailed study protocol is available upon request.
Study Identification
We searched MEDLINE (Ovid, New York, NY) from January 1995 to July 2012, covering the time period from the introduction of DWI to clinical practice. We did not apply any language restrictions. The MEDLINE search strategy included both subject headings (MeSH terms) and text words for the target condition (eg, stroke, TIA, minor stroke) and the imaging modality under investigation (magnetic resonance imaging [MRI], DWI). We searched EMBASE, translating the MEDLINE MeSH terms into the corresponding terms available in the Emtree vocabulary. The searches were initially run in November 2010 and updated in July 2012 (details see online supplement). We also hand-searched proceedings of the International and European Stroke Conferences (2011, 2012), contacted experts in the field, and searched reference lists and the most recent issues of Stroke, Cerebrovascular Diseases, and European Neurology (not yet indexed in MEDLINE or EMBASE) to identify further relevant studies.
Inclusion/Exclusion Criteria
One review author examined the titles and abstracts and retrieved all potentially relevant citations in full. A second reviewer double-checked the search results. Doubtful papers were discussed with a third reviewer. We retained full-text articles that focused on primary studies of suspected TIA patients investigated with MR DWI. We excluded studies that did not assess patients with DWI, studies that did not report the proportion of patients with positive DWI lesions, and duplicate data.
Data Extraction
Two review authors independently extracted data; disagreements were resolved by discussion or referred to a third author. We recorded data on study methods (eg, setting, study design), characteristics of patients (TIA vs minor stroke), and imaging findings (DWI-positive brain lesions) as the proportion of patients with a recent ischemic lesion on DWI. We also collected information on study design: quality, including blinding, prospective versus retrospective, consecutive or not, definition of TIA (time or tissue based), timing of the imaging assessment, field strength, who read the imaging, definition of positive DWI, specialty of the evaluating clinicians, inclusion of TIA alone or also minor stroke, and study setting (specialist neurology clinic, emergency department, other). To assess study quality, we followed best practice18 by identifying methodological characteristics that were most likely to explain any heterogeneity (listed in Supplementary Tables 1 and 2).
Data Synthesis
We calculated the pooled proportion of TIA patients with a positive DWI lesion using univariate random effects meta-analysis performed in R 2.8.0, with within-study variance modeled as binomial (DiagMeta package for analysis) because outcomes were proportions. Confidence intervals (CIs) were 95%. Heterogeneity between studies was assessed with I2 statistics,19 using the statistical software R 2.14.2 (http://cran.r-project.org). We assessed potential sources of heterogeneity using SAS 9.3 PROC NLMIXED (http://www.sas.com) including: specialist versus nonspecialist units, evaluation by neurologist/stroke physician versus other specialties, retrospective versus prospective studies, study size, time from TIA to imaging, and inclusion of patients with minor stroke as well as TIA. Study size was categorized (necessary due to difficulties with model fit when using individual study sizes) into small (<50 patients), medium (50–99), large (100–199), and very large (>200).
Results
Number of Studies
The electronic searches identified 7,983 citations (Supplementary Fig 1); 185 potentially relevant studies underwent detailed assessment of the full-text articles. Hand-searching identified 4 more includable papers. We excluded 127 reports, mostly because they did not provide the number of patients with positive DWI lesions. Forty-five full-text studies (published in 58 reports) and 2 abstracts met the inclusion criteria, for a total of 47 studies.
Critical Appraisal
The 47 studies included 18 prospective and 17 retrospective cohort studies (12 did not state their design; see Supplementary Table 1). Twenty-six studies recruited consecutive patients, but the rest were not consecutive or did not give the information. All studies used a time-based definition of TIA, except 2 that did not give a definition; none used a tissue-based definition. Two studies assessed population-based cohorts, 7 assessed hospital-based cohorts, 8 assessed patients from emergency departments, 27 assessed cohorts from specialist stroke or neurology units, and 3 did not specify the source. The TIA was diagnosed by a neurologist or stroke specialist in a total of 27 of 47 (57%) studies; the remaining 20 did not report who made the diagnosis but were authored by stroke specialists. Virtually all imaging was performed on 1.5T scanners. In 21 studies a neuroradiologist was involved in scan reading, but most studies did not state who read the images, or the criteria used for determining when DWI was positive (see Supplementary Table 2). Timing of DWI assessment after symptom onset varied (see Supplementary Table 1); 18 studies assessed patients within 24 hours, 6 within 48 hours, 12 within 7 days, 3 within 2 weeks, and 2 within 3 weeks of symptom onset; 6 did not provide this information. Thirty-nine studies only included TIA patients, and 820–27 included some patients with minor stroke as well as TIA, but only 424–27 reported the results for minor stroke separately from TIA.
Main Findings
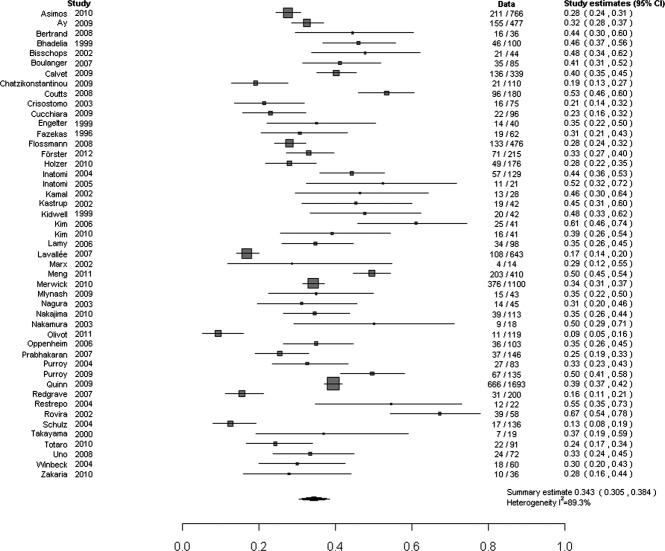
The 47 studies included 9,078 (median = 91, interquartile range = 42–161, range = 18–1,693) TIA patients; 16 studies (34%) included <50 patients (Supplementary Fig 2). An acute DWI lesion was present in 3,048 of 9,078 patients (pooled proportion with a positive DWI = 34.3%, 95% CI = 30.5–38.4%; Fig 1), with substantial between-study heterogeneity (I2 = 89.3%). The frequency of positive DWI findings varied from 9 to 67% (ie, 7-fold) between studies (see Supplementary Table 1).
FIGURE 1.
Proportion of patients with transient ischemic attack and visible ischemic lesion on diffusion-weighted imaging. Note that Ay et al 2009 includes 2 earlier studies by Ay et al; see Supplementary Table 1. CI = confidence interval.
Sources of Heterogeneity
Studies varied in several respects (see Supplementary Tables 1 and 2). We explored reasons for heterogeneity using comparisons with adequate data and that were the most clinically relevant: study size, year of publication, inclusion of minor stroke and TIA versus just TIA, time to scanning, retrospective versus prospective studies, study setting, and specialty of the evaluating clinician. Only study size influenced heterogeneity; the DWI-positive proportion decreased significantly as study size increased ( Fig 2) from 40.1% (95% CI = 33.5–46.6) in studies of <50 patients to 28.9% (95% CI = 23.2–34.6) in studies of >200 patients, a 4% (95% CI = 0.3–8; p = 0.035) significant reduction in the DWI-positive proportion per size category. Studies published up to 2007 had similar heterogeneity (I2 = 78.8%) to those published from 2007 onward (I2 = 92.5%), but note that earlier studies were smaller, thus confounding this comparison (Supplementary Fig 3).
FIGURE 2.
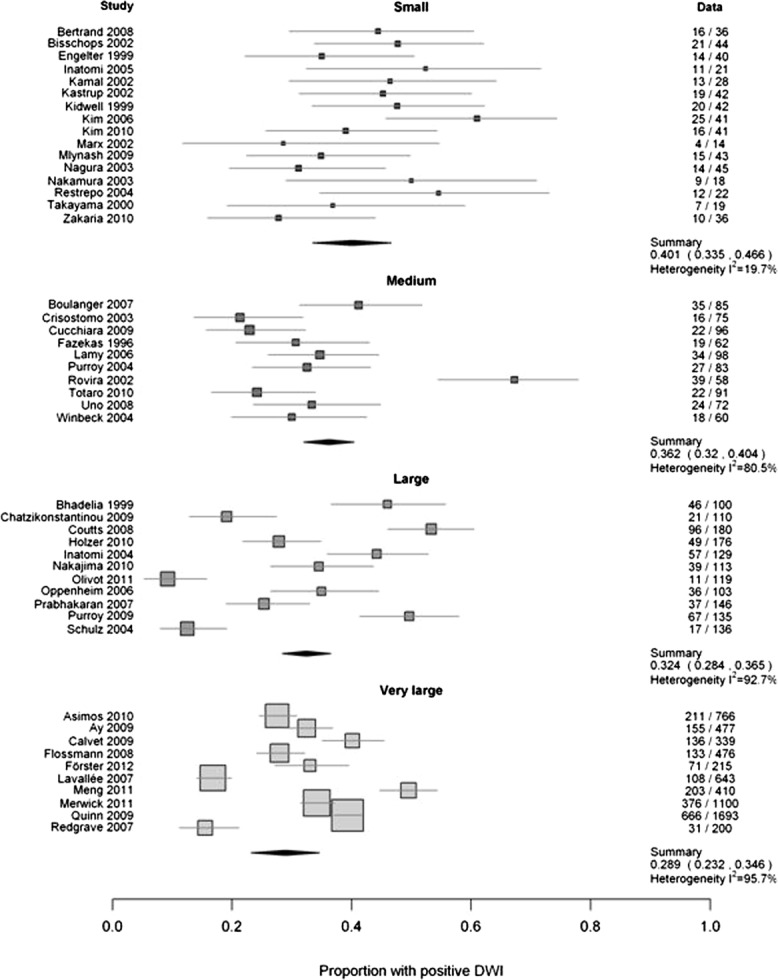
Comparison of study size and proportions with a positive lesion on diffusion-weighted imaging (DWI). Small, ≤50; medium, 50 to 99; large, 100 to 200; very large, ≥200. The numbers in the right hand column are the number of patients with a DWI+ve TIA / total patients in that study.
Four studies provided data separately for patients with TIA and minor stroke24–27; the proportion of patients with minor stroke and a DWI ischemic lesion was 47%,25 70%,26 and 100%.24,27 The 2 studies in which all minor stroke patients showed a positive DWI lesion were very small, and 1 focused on patients with high-grade symptomatic carotid stenosis.24 Four other studies included patients with minor stroke and TIA but did not report the results separately20–23; the proportion of patients with visible ischemic lesions on DWI (53%,23 28%,20 39%,21 39%22) overlapped with the 47 to 100% in patients with minor stroke listed above and was not noticeably higher than in the studies reporting on TIA alone.
There was no obvious effect of increasing time from symptom onset on the DWI-positive proportion using the available aggregate data ( Fig 3, details in Supplementary Table 2). Most studies scanned patients within 8 days, when the proportion with a DWI lesion ranged from 9 to 68%. There was also no readily apparent difference between studies that reported average times and those that reported maximum times to imaging. Among individual studies' analyses, 11 reported no association between DWI positivity and time, 11 reported an association, and 25 did not report whether there was an association. Further attempts to investigate the relationship between the proportion that were DWI positive and time to imaging failed to meet statistical modeling assumptions.
FIGURE 3.
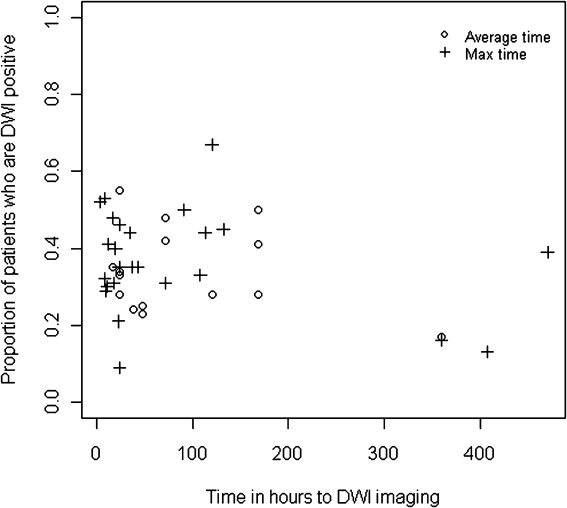
Scatter plot of time to imaging versus proportion of patients with a positive diffusion-weighted imaging (DWI) scan. Average time may indicate either a mean or median time.
The other potential sources did not explain heterogeneity. The DWI-positive rate was just as varied among the prospective as in the retrospective studies (difference = 1%, 95% CI = 0.0–11%; p = 0.84; I2 = 94.1% and 85.5%, respectively; Fig 4), among the 27 studies where all patients were examined and diagnosed by a neurologist/stroke physician as in the 20 studies where the examination was not by a stroke specialist or the specialty was not stated (difference = 6%, 95% CI = 0.0–14; p = 0.15; I2 92.6% and 78.1%, respectively; Fig 5), and in specialist units versus all other settings (difference = 4%, 95% CI = 0–12%; p = 0.29; I2 90.6% and 87.5%, respectively; Supplementary Fig 4). It was not possible to examine population versus nonpopulation studies, as there were only 2 of the former. Most studies did not provide information on DWI-positive TIA rates by individual patient characteristics, thus precluding further assessment or meta-analysis of patient characteristics that influence DWI-positive rates.
FIGURE 4.
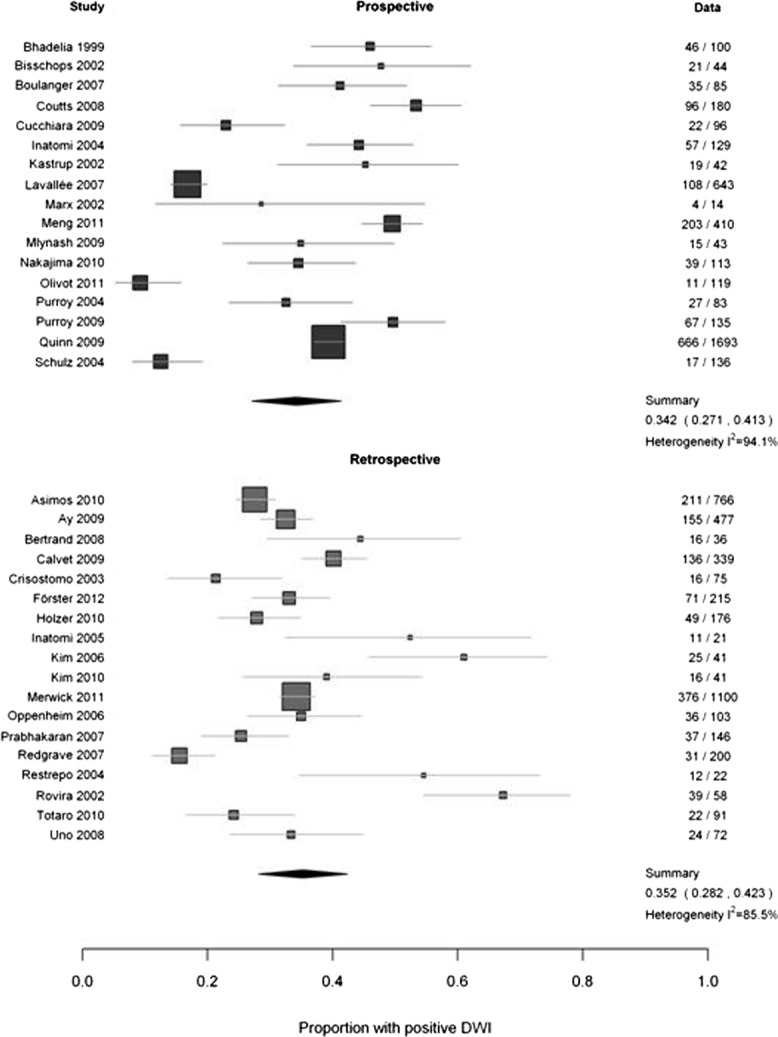
Comparison of prospective versus retrospective studies. DWI = diffusion-weighted imaging. The numbers in the right hand column are the number of patients with a DWI+ve TIA / total patients in that study.
FIGURE 5.
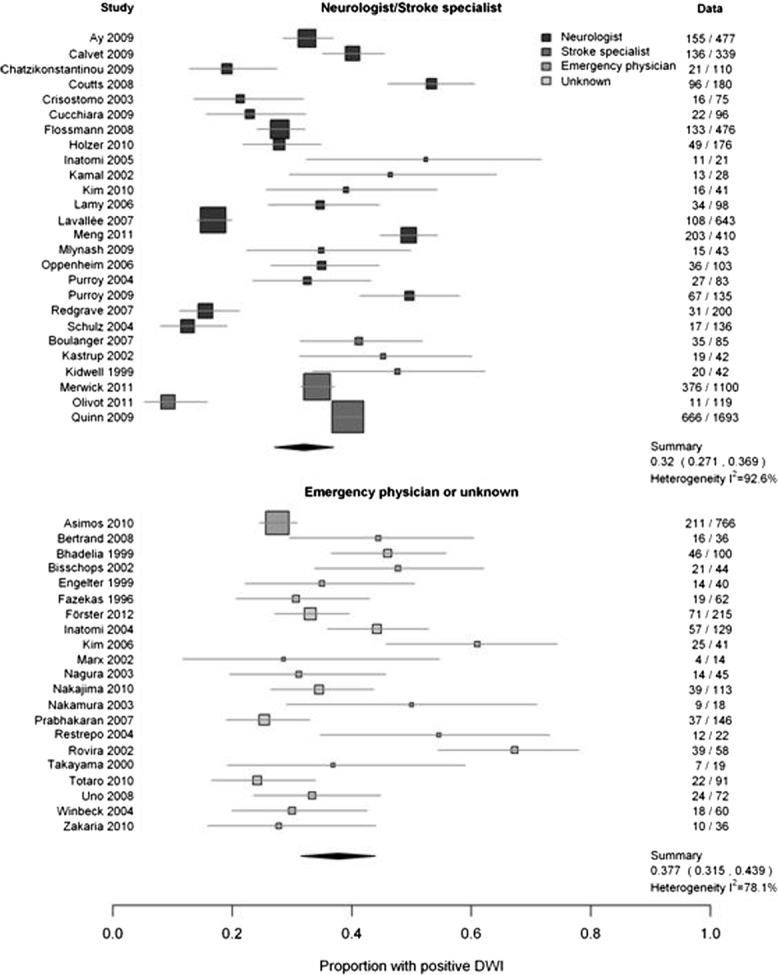
Comparison of evaluating physician's specialty and proportion with positive lesion on diffusion-weighted imaging (DWI). The numbers in the right hand column are the number of patients with a DWI+ve TIA / total patients in that study.
Discussion
The 9,078 patients in 47 studies show that on average only 34.3% of patients had a DWI lesion; thus, the commonest DWI finding in TIA is a negative scan. The largely unexplained 7-fold variation in positivity suggests that DWI does not provide a consistent or predictable basis for defining stroke. Adding a highly variable test (DWI) to clinical TIA diagnosis (also variable) is likely to increase rather than reduce variability of TIA/stroke diagnosis, as it is mathematically impossible for both sensitivity and specificity to increase if the results of 2 tests are merged, whether they are combined using “and” or “or”;28 instead, sensitivity or specificity will decrease, thus increasing diagnostic variability.
Only study size explained heterogeneity, large studies (n ≥ 200 patients) having 25% fewer DWI-positive patients (29%) than small studies (n ≤ 50, 40%), pointing to an undue influence of study methods on DWI-positive rates (see Fig 2).29 Two papers published since the end of our search describe DWI-positive rates in a population-based study involving the same 15 hospitals.30,31 One reported on 1,862 patients recruited over 36 months from November 2007, of whom 11% were DWI positive;30 the other extended the sample to 3,724 patients recruited over 54 months from November 2007, of whom 32.2% were DWI positive.31 No reason was given for this 3-fold difference, the recruitment methods, definitions, and assessments being identical in all other respects. These DWI-positive rates are very consistent with our meta-analysis and confirm the variability of DWI in TIA by demonstrating that a 3-fold difference in DWI-positive rate can be made to occur within 1 region simply by changing the recruitment period. If the DWI-positive TIAs were classified as strokes, then a substantial fluctuation in stroke would have been recorded in this region.
We found no evidence that the DWI-positive rate varied with time between TIA and scanning (see Fig 3), either on summary analysis of several time parameters or in individual studies' reports (see Supplementary Table 2). DWI lesions may disappear very rapidly, for example, being present at 4 hours after symptom onset but resolving completely from DWI and other MR sequences by 24 hours, or not being visible on hyperacute imaging but becoming visible at 24 to 48 hours,32 indicating some instability of DWI. Eleven studies reported no association between symptom duration and DWI positivity, and 11 other studies reported a positive association; this 50:50 split suggests that symptom duration is a less consistent factor than might be expected. The 7-fold variation in DWI-positive rate was not explained by prospective or retrospective study design (see Fig 4), specialist versus nonspecialist doctors/services ( Fig 5), inclusion of minor stroke and TIA versus TIA alone, older versus recent publication (heterogeneity was worse in recent publications; see Supplementary Fig 3), study setting (see Supplementary Fig 4), or inclusion of mimics (mimics were excluded).
The work has strengths. We included 3× more patients (9,000 vs 2,500) and 80% more studies than previous reviews,16,33 including 35 studies published within their time period. We used rigorous methods and multiple overlapping searches; several authors double-checked the data. We assessed the most clinically relevant sources of heterogeneity for which there were data (having 47 studies allows 4 factors to be examined reliably; http://handbook.cochrane.org/chapter_9/9_6_5_1_ensure_that_there_are_adequate_studies_to_justify.htm). There are also weaknesses. Analysis of original data in an individual patient data meta-analysis might be more informative but would be limited by the primary data quality. We were not able to assess the impact of the proportion of TIA patients in each study who did not have DWI, as this information was largely missing; most studies reported only on patients who did have DWI and the findings. Not all patients can have MRI anyway, due to claustrophobia or contraindications. If variation in the proportion scanned were a major source of variation in DWI positive rate, then we might have seen more difference between retrospective and prospective, or between expert-assessed and non–expert-assessed patients, but this was not the case. We did not perform complete duplicate assessments but used multiple cross checks between reviewers to ensure validity. That we found more data in the time frame than 2 previous reviews suggests that our methods were sensitive. We provide study quality markers in Supplementary Tables 1 and 2; these were used to assess heterogeneity, there being no valid tool for assessment of nonrandomized studies.18
What are the implications? There were no data for calculating sensitivity or specificity of DWI in TIA/minor stroke, all patients with mimics or hemorrhage being excluded, although TIA mimics make up about 45% of clinic referrals, and occasional small intracerebral hemorrhages can present with TIA. The proportion of TIA patients without a visible ischemic lesion on DWI (65.7%) would be even higher if the denominator were all patients attending the clinic including the mimics rather than only those with a definite TIA. Assuming that the 45% of patients attending stroke prevention clinics with a final diagnosis of mimic did not have a DWI-visible lesion (which would be incorrect, as some with migraine, postictal states, hypoglycemia,34 multiple sclerosis, etc might have DWI-positive lesions) would result in much larger proportions of DWI-negative scans. As an approximation, DWI would be positive in 19% of unselected patients referred with suspected TIA, leaving a large proportion (81%) of negative, noncontributory scans.
DWI may be useful to identify patients at increased risk of recurrent stroke, although it does not reliably identify patients with established risk factors for recurrent stroke, for example, atrial fibrillation or carotid stenosis, and although DWI-negative TIAs might be at lower risk of recurrent stroke, they still have some risk.31,35 Therefore, DWI-negative patients still require carotid and cardiac investigations and should be offered effective risk-factor modification.
The use of MR in stroke is rising rapidly, increasing by 235% in 10 states in the USA from 28% of strokes in 1999 to 66% in 2008.36 The cost of diagnostic imaging rose by 213% between 1999 and 2007 in the USA,36 making it the single fastest growing component of hospital costs. Despite this, a recent study found no data to justify the increased use of MR.37 A statistically significant association between a new marker and cerebrovascular risk is not enough to conclude that its use in clinical practice will improve clinical outcomes.38 The impact of diagnostic strategies, particularly costly ones, should be tested on clinically important outcomes38 prior to changing practice.
There is wide geographical variation in use of MR in stroke, from 55% of stroke patients in Oregon to 79% in Arizona.36 A change in the definition of TIA/stroke14 would result in more TIAs being converted to strokes in high MR user states like Arizona than in low MR user states like Oregon. This would decrease the severity of “stroke” and appear to improve stroke outcomes in Arizona by diluting the clinically based strokes with these DWI-positive TIAs, compared with regions with less MR usage like Oregon.39 The prognosis of TIA would improve where MRI rates were high, because DWI-positive TIAs (known to have a higher rate of recurrent stroke) would be reclassified as stroke, leaving the DWI-negative TIAs (with a better prognosis) classified as TIAs. Even with high availability of MR, a delay in access for 1 patient group, for example, the elderly or women,40 would result in more TIA patients having negative DWI and fewer being reclassified as stroke, so the severity of stroke and stroke outcomes would appear worse in these low MR access groups. The effect would be even greater in high versus low income countries, rendering cross-sectional surveys of global disease burden1 or risk factors, and longitudinal studies of trends in global disease burden, highly unpredictable and difficult to interpret. In many patients (about ⅓), the DWI-positive lesion if present soon after the TIA does not convert to a visible lesion on other sequences when scanning is repeated later (ie, a tissue diagnosis of stroke would become a diagnosis of TIA).41 The totality of the data on DWI in TIA suggest that it will be important to retain an awareness of the variability of DWI positivity in patients with TIA (and that we lack a clear understanding of what drives that variability) in countries or hospitals were the time-based definition is replaced by a tissue-based definition, at least until the heterogeneity is better understood, and MR with DWI is universally and immediately accessible for all patients with suspected TIA or stroke.
Acknowledgments
This project was funded by the National Institute for Health Research Health Technology Assessment Programme (project number 09/22/169). J.M.W. and K.M. received support from the SINAPSE Collaboration, which is funded by the Scottish Funding Council. The Brain Research Imaging Centre is supported by the Nation Health Service Lothian Research and Development Office, Scottish Funding Council, and Scottish Executive Chief Scientist Office.
Department of Health Disclaimer:
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA, NIHR, NHS or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
M.B.: literature search, literature evaluation, data extraction, tabulation, drafting of paper; F.M.C.: statistical analysis, editing of paper, creation of forest plots and other figures; H.M.: evaluation of literature, data extraction, tabulation of data, editing of paper; K.S.: data management, searching, data preparation, editing of paper; M.D.: concept, design, editing of paper, interpretation of data; P.A.G.S.: design, interpretation of data, editing of paper; K.M.: design, interpretation of data, editing of paper; J.M.W.: concept, obtaining funding, design, evaluation of papers, oversight of project, interpretation of data, drafting and editing of paper, and overall guarantor of the work. J.M.W. takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full unrestricted access to all the study data.
Potential Conflicts of Interest
Nothing to report.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 3.Warlow C, van Gijn J, Dennis M, et al. Is it a vascular event and where is the lesion? Identifying and interpreting the symptoms and signs of cerebrovascular disease. In: Stroke: practical management. 3rd ed. Malden, MA: Blackwell Publishing; 2008. pp. 35–130. [Google Scholar]
- 4.Miranda H, Brazzelli M, Chappell F, et al. Frequency, main causes and long term prognosis in patients presenting with TIA and diagnosed as non-vascular (TIA mimics) http://kenes.com/stroke2012/abstractcd/pdf/1154.pdf.
- 5.Giles MF, Rothwell PM. Substantial underestimation of the need for outpatient services for TIA and minor stroke. Age Ageing. 2007;36:676–680. doi: 10.1093/ageing/afm088. [DOI] [PubMed] [Google Scholar]
- 6.Ferro JM, Falcao I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. 1996;27:2225–2229. doi: 10.1161/01.str.27.12.2225. [DOI] [PubMed] [Google Scholar]
- 7.Gass A, Ay H, Szabo K, et al. Diffusion-weighted MRI for the “small stuff”: the details of acute cerebral ischaemia. Lancet Neurol. 2004;3:39–45. doi: 10.1016/s1474-4422(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 8.Ay H, Arsava EM, Johnston SC, et al. Clinical- and imaging-based prediction of stroke risk after transient ischemic attack. The CIP model. Stroke. 2009;40:181–186. doi: 10.1161/STROKEAHA.108.521476. [DOI] [PubMed] [Google Scholar]
- 9.Coutts SB, Simon JE, Eliasziw M, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol. 2005;57:848–854. doi: 10.1002/ana.20497. [DOI] [PubMed] [Google Scholar]
- 10.Purroy F, Montaner J, Rovira A, et al. Higher risk of further vascular events among transient ischemic attack patients with diffusion-weighted imaging acute ischemic lesions. Stroke. 2004;35:2313–2319. doi: 10.1161/01.STR.0000141703.21173.91. [DOI] [PubMed] [Google Scholar]
- 11.National Institute for Health and Clinical Excellence (NICE) Stroke. The diagnosis and acute management of stroke and transient ischaemic attacks. CG68, 1–37. London, UK: National Institute for Health and Clinical Excellence; 2008. [Google Scholar]
- 12.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 13.Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713–1716. doi: 10.1056/NEJMsb020987. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redgrave JNE, Coutts SB, Schulz UG, et al. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke. 2007;38:1482–1488. doi: 10.1161/STROKEAHA.106.477380. [DOI] [PubMed] [Google Scholar]
- 16.Engelter ST, Wetzel SG, Bonati LH, et al. The clinical significance of diffusion-weighted MR imaging in stroke and TIA patients. Swiss Med Wkly. 2008;138:729–740. doi: 10.4414/smw.2008.12249. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.0.0. [updated February 2008]. The Cochrane Collaboration, 2008. Available from http://www.cochrane-handbook.org http://www.cochrane-handbook.org.
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Flossmann E, Redgrave JN, Briley D, et al. Reliability of clinical diagnosis of the symptomatic vascular territory in patients with recent transient ischemic attack or minor stroke. Stroke. 2008;39:2457–2460. doi: 10.1161/STROKEAHA.107.511428. [DOI] [PubMed] [Google Scholar]
- 21.Quinn TJ, Cameron AC, Dawson J, et al. ABCD2 scores and prediction of noncerebrovascular diagnoses in an outpatient population. A case-control study. Stroke. 2009;40:749–753. doi: 10.1161/STROKEAHA.108.530444. [DOI] [PubMed] [Google Scholar]
- 22.Kim JT, Kim HJ, Yoo SH, et al. MRI findings may predict early neurologic deterioration in acute minor stroke or transient ischemic attack due to intracranial atherosclerosis. Eur Neurol. 2010;64:95–100. doi: 10.1159/000315138. [DOI] [PubMed] [Google Scholar]
- 23.Coutts SB, Eliasziw M, Hill MD, et al. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke. 2008;3:3–10. doi: 10.1111/j.1747-4949.2008.00182.x. [DOI] [PubMed] [Google Scholar]
- 24.Kastrup A, Schulz JB, Mader I, et al. Diffusion-weighted MRI in patients with symptomatic internal carotid artery disease. J Neurol. 2002;249:1168–1174. doi: 10.1007/s00415-002-0793-2. [DOI] [PubMed] [Google Scholar]
- 25.Marx JJ, Mika-Gruettner A, Thoemke F, et al. Diffusion weighted magnetic resonance imaging in the diagnosis of reversible ischaemic deficits of the brainstem. J Neurol Neurosurg Psychiatry. 2002;72:572–575. doi: 10.1136/jnnp.72.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz UG, Briley D, Meagher T, et al. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke. 2004;35:2459–2465. doi: 10.1161/01.STR.0000143455.55877.b9. [DOI] [PubMed] [Google Scholar]
- 27.Winbeck K, Bruckmaier K, Etgen T, et al. Transient ischemic attack and stroke can be differentiated by analyzing early diffusion-weighted imaging signal intensity changes. Stroke. 2004;35:1095–1099. doi: 10.1161/01.STR.0000125720.02983.fe. [DOI] [PubMed] [Google Scholar]
- 28.Metz CE. Constraints on the sensitivity and specificity of “logically” merged test results. J Nucl Med. 1991;32:1646–1647. [PubMed] [Google Scholar]
- 29.Ioannidis JPA. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 30.Al-Khaled M, Matthis C, Munte TF, et al. The incidence and clinical predictors of acute infarction in patients with transient ischemic attack using MRI including DWI. Neuroradiology. 2013;55:157–163. doi: 10.1007/s00234-012-1091-z. [DOI] [PubMed] [Google Scholar]
- 31.Al-Khaled M, Eggers J. MRI findings and stroke risk in TIA patients with different symptom durations. Neurology. 2013;80:1920–1926. doi: 10.1212/WNL.0b013e318293e15f. [DOI] [PubMed] [Google Scholar]
- 32.Lecouvet FE, Duprez TP, Raymackers JM, et al. Resolution of early diffusion-weighted and FLAIR MRI abnormalities in a patient with TIA. Neurology. 1999;52:1085–1087. doi: 10.1212/wnl.52.5.1085. [DOI] [PubMed] [Google Scholar]
- 33.Redgrave JN, Schulz UG, Briley D, et al. Presence of acute ischaemic lesions on diffusion-weighted imaging is associated with clinical predictors of early risk of stroke after transient ischaemic attack. Cerebrovasc Dis. 2007;24:86–90. doi: 10.1159/000103121. [DOI] [PubMed] [Google Scholar]
- 34.Yong AW, Morris Z, Shuler K, et al. Acute symptomatic hypoglycaemia mimicking ischaemic stroke on imaging: a systemic review. BMC Neurol. 2012;12:139. doi: 10.1186/1471-2377-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulanger JM, Coutts SB, Eliasziw M, et al. Diffusion-weighted imaging-negative patients with transient ischemic attack are at risk of recurrent transient events. Stroke. 2007;38:2367–2369. doi: 10.1161/STROKEAHA.106.475541. [DOI] [PubMed] [Google Scholar]
- 36.Burke JF, Kerber KA, Iwashyna TJ, et al. Wide variation and rising utilization of stroke magnetic resonance imaging: data from 11 states. Ann Neurol. 2012;71:179–185. doi: 10.1002/ana.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke JF, Gelb DJ, Quint DJ, et al. The impact of MRI on stroke management and outcomes: a systematic review. J Eval Clin Pract. doi: 10.1111/jep.12011. doi: 10.1111/jep.12011. [DOI] [PubMed] [Google Scholar]
- 38.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 39.Mullen MT, Cucchiara BL. Redefinition of transient ischemic attack improves prognosis of transient ischemic attack and ischemic stroke: an example of the Will Rogers phenomenon. Stroke. 2011;42:3612–3613. doi: 10.1161/STROKEAHA.111.627877. [DOI] [PubMed] [Google Scholar]
- 40.Mullen MT, Judd S, Howard VJ, et al. Disparities in evaluation at certified primary stroke centers: reasons for geographic and racial differences in stroke. Stroke. 2013;44:1930–1935. doi: 10.1161/STROKEAHA.111.000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreau F, Modi J, Almekhlafi M, et al. Early magnetic resonance imaging in transient ischemic attack and minor stroke: do it or lose it. Stroke. 2013;44:671–674. doi: 10.1161/STROKEAHA.111.680033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information