Abstract
Background:
Antispasmodic and vasorelaxant effects of Teucrium polium L. (TP) were mentioned in former studies, so we attempted to evaluate the eventual preventive effect of TP in an acute experimental model of hypertension induced by angiotensin II (Ang II).
Methods:
Forty-eight male Wistar rats were divided randomly into six groups (n = 8); control Group (C), which received only saline, group Ang II; which received Ang II (300 ng/min, IV), group losartan (Los); which received Los (10 mg/kg, IV) before Ang II injection, three groups of TP 100, TP 200, and TP 400; which received different doses of TP extract (100, 200 and 400 mg/kg, IP, respectively) before Ang II application. After cannulation of the femoral artery, mean arterial blood pressure (MAP) and heart rate (HR) was continuously measured and recorded during the experiments. Comparisons were performed using t-test with SPSS software, version 16 (SPSS, Chicago, IL).
Results:
MAP and HR in Ang group were significantly higher than the control group (P < 0.001), MAP in group Los significantly was lower than Ang group (P < 0.001) and pretreatment with three doses of TP extract also inhibited increasing of MAP after Ang II injection (P < 0.001). Los also inhibited the increase of HR due to Ang II (P < 0.001), but none of three doses of TP extract had a protective effect on tachycardia induced by Ang II.
Conclusions:
It seems TP extract could be effective in preventing of high blood pressure induced by Ang II pathway activation but could not have remarkable efficacy for improving the created tachycardia.
Keywords: Angiotensin II, hypertension, rat, Teucrium polium
INTRODUCTION
Hypertension is a highly prevalent cardiovascular risk factor worldwide. High blood pressure can induce coronary artery disease, congestive heart failure, stroke, impaired vision, and kidney disease; untreated hypertension affects all organ systems and can shorten people's life expectancy.[1]
Common clinical strategies to achieve a lowering of blood pressure include the use of beta adrenergic antagonists, calcium channel blockers, diuretics, angiotensin II (Ang II) receptor antagonists and Ang converting enzyme (ACE) inhibitors.[2]
Activation of the renin-Ang system (RAS) both systemically and locally seems to be of importance for cardiovascular function. The octapeptide Ang II definitively plays a central role. In a reversal, for example, of left ventricular hypertrophy, so far the most important independent risk factor for an adverse outcome, blocking of the RAS with ACE inhibition has been shown to be particularly effective. A more complete blockade of the Ang II Type 1 receptor would offer more effective attenuation of the unfavorable effect of Ang II.[3]
In recent years, there has been a growing interest and demand in using medicinal plants for treating and preventing various diseases including cardiovascular diseases. Traditional medicines of plants origin have received much attention due to several factors such as easy availability, affordable cost, safety, and efficacy as well as cultural acceptability. Teucrium polium L. (TP) is a flowering plant belonging to the family Labiate and is found abundantly in South Western Asia, Europe, and North Africa. Teucrium species have been used as medicinal herbs for over 2000 years as diuretic, diaphoretic, tonic, antipyretic, antispasmodic, antidiabetic, and many of them are used in folk medicine.[4] Antiinflammatory,[5] antinociceptive,[6] and anorexic[7] effects are other reported activities of TP.
There is increasing evidence of cardiovascular effects of TP such as positive inotropic and chronotropic,[8] decreasing of blood pressure,[9,10,11] and lowering blood lipid.[12] Nevertheless, the exact effect of TP extract on the vascular system has not been clarified. Therefore, in the present study, we aimed to investigate the effects of the hydroalcholic extract of TP on Ang II-induced hypertension and related heart rate (HR) variability.
METHODS
Plant material and preparation of the extract
Stems and leaves of TP were collected in October 2012 from Khorasan Province, Ferdows, Iran, and identified by Ferdowsi University Herbarium (Voucher No. 152-2016-4) and then dried at room temperature. Aerial parts (300 g) of the plant were soaked in ethanol (50%) for 48 h and paper filter was used to filter the solute after mixing. The solution was then dried using a 40°C oven for 72 h. The dried extract was dissolved in the distilled water to make desired doses.
Experimental animals
Male Wistar rats, 200-250 g were housed in colony rooms with 12/12 h light/dark cycle at 21°C ± 2°C and had free access to food and water. The experimental protocol was approved by Ethical Committee at Mashhad University of Medical Sciences (Process Number 900559).
Drugs and reagents
The following reagents were used: Ang II and urethane (Sigma, USA), losartan (Los) (a gift from Daru pakhsh, I.R. Iran).
Experimental groups
Forty-eight male Wistar rats were divided into six groups as following order (n = 8 in each group)
Control group; received saline (intravenous [I.V])
Ang group; received Ang II (300 ng, I.V)
Los group; received Los (10 mg/kg, I.V 0.5 ml) 30 min before injection of Ang II
TP 100 group; received 100 mg/kg of TP extract (i.p.) 30 min before injection of Ang II
TP 200 group; received 200 mg/kg of TP extract (i.p.) 30 min before injection of Ang II
TP 400 group; received 400 mg/kg of TP extract (i.p.) 30 min before injection of Ang II.
Experimental procedure
Rats were anesthetized with urethane (1.4 g/kg, i.p. with 0.7 g/kg as a supplementary dose). Temperature was kept at 37.5°C with a heating lamp. A polyethylene catheter-50 filled by heparinized saline was inserted in the femoral artery. The catheter connected to a pressure transducer then mean arterial pressure (MAP), and HR, were continuously recorded by a power lab system (ID instrument, Australia). Another similar catheter was inserted in the jugular vein for injection of Ang II. Volume of injection in I.V and i.p. methods was 0.5 ml.
Data analysis
The data of the blood pressure and HR were expressed as mean ± standard error of the mean time course alterations of HR and arterial pressure was plotted. The maximum change was compared with the control or Ang group (independent t-test) values. Repeated measures ANOVA were used to compare the time course changes between groups. P < 0.05 were considered to be statistically significant.
RESULTS
Effects of injection of saline on blood pressure and heart rate
Baseline MAP and HR before injection of saline were recorded then saline injected into the jugular vein. Injection of saline had no significant effects on MAP (before: 95 ± 1.6 mmHg and after: 99 ± 2.17 mmHg) or HR (before: 264 ± 7.32 beats/min and after: 262 ± 7.1 beats/min).
Cardiovascular responses to injections of angiotensin II
Injection of 300 ng Ang II significantly increased MAP and HR. The time course changes of MAP and HR after injection of Ang are shown in Figure 1. Maximal change in MAP was statistically significant compared with the control group (Δ: 32.37 ± 3.37, P < 0.001). Moreover, significant HR changes were shown after injection of Ang (Δ: 72.79 ± 5.1, P < 0.001) [Figure 2].
Figure 1.
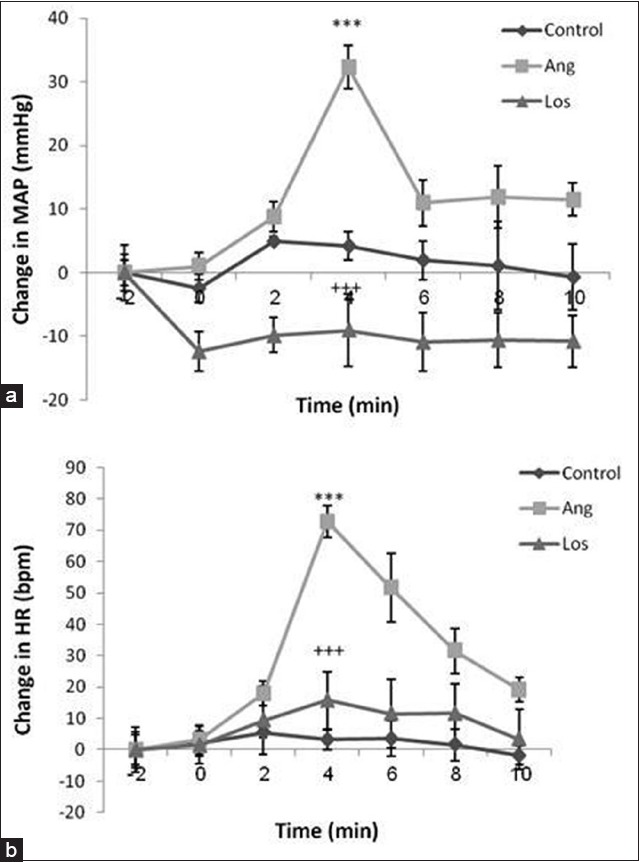
Time courses of changes in mean arterial pressure (MAP) and heart rate (HR) in response to injection of angiotensin II (Ang II) (300 ng) in groups of control, Ang II and losartan. For each group, n = 8. (a) MAP, ***P < 0.001 compared to control and +++P < 0.001 compared to Ang group. (b) HR, ***P < 0.001 compared to control and +++P < 0.001 compared to Ang group
Figure 2.
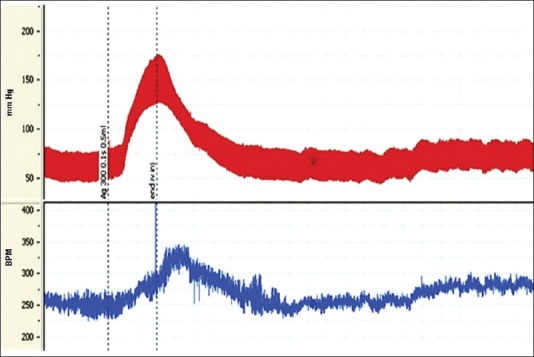
Typical recording of blood pressure and heart rate in response to angiotensin II injection. The vertical lines indicate the injection time
Effect of systemic intravenous pretreatment with losartan on the cardiovascular effects of angiotensin II
Losartan pretreatment (10 mg/kg, I.V) inhibited cardiovascular effects induced by Ang II so the cardiovascular responses to Ang II after pretreatment with Los did not differ significantly with those observed before injection of Ang II (ΔMAP: –9.05 ± 5.63 mmHg and ΔHR: 15.76 ± 5.7 beats/min). In 4th min after injection of Ang II, maximum significant changes of MAP and HR were observed in Los group compared with simultaneous changes in other two groups, independent t-test, P < 0.001. Meanwhile, MAP changes in Los group were significantly lower than those of control and Ang groups (repeated measures ANOVA, P < 0.001 for both) [Figure 1a]. As well as HR changes in Los group were lower compared to Ang group (repeated measures ANOVA, P < 0.001) [Figure 1b].
Effect of systemic i.p. pretreatment with different doses of Teucrium polium on the cardiovascular effects of angiotensin II
Pretreatment of normotensive rats with different doses of TP (100, 200, and 400 mg/kg, i.p.) reduced significantly MAP compared to baseline (paired t-test, P < 0.001 for all three doses, data not shown) but had no effect on HR.
The cardiovascular responses to Ang II injection antagonized by TP so that in these groups the maximum changes of MAP were significantly lower than those of induced in Ang group (ΔMAP: 14.73 ± 3.94, 13.44 ± 4.93, 14.19 ± 1.9 compared to 32.37 ± 3.37 mmHg, respectively, t-test, P < 0.001 for all three groups). Moreover, MAP changes in all three groups of TP extract were significantly lower than Ang group (repeated measures ANOVA, P < 0.001) [Figure 3a] and HR changes in two groups of TP 100 and TP 200 showed a significant reduction compared to Ang group (repeated measures ANOVA, P < 0.001 and P < 0.01, respectively) [Figure 3b].
Figure 3.
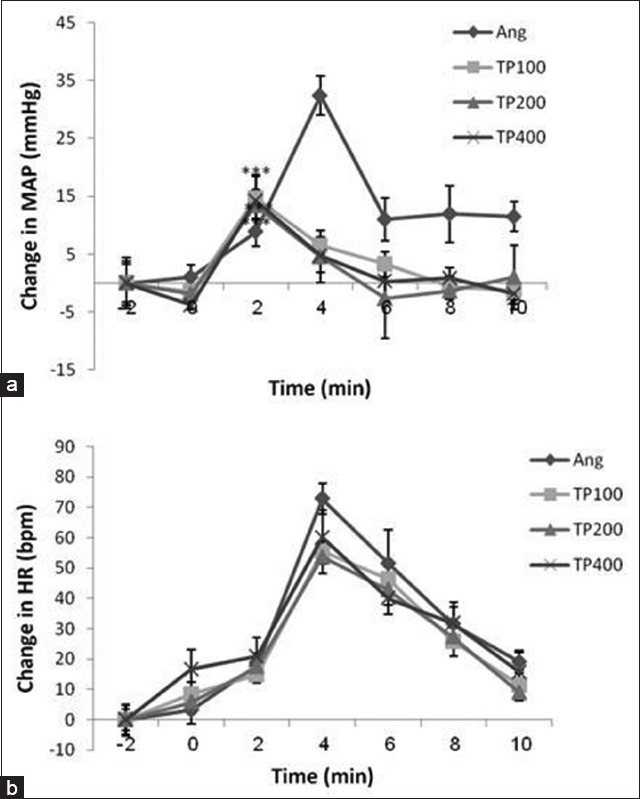
Time courses of changes in mean arterial pressure (MAP) and heart rate (HR) in response to injection of angiotensin II (Ang II) (300 ng) in groups of Ang II and three doses of Teucrium polium L. (TP) extract (TP 100, TP 200 and TP 400). For each group, n = 8. (a) MAP, ***P < 0.001 compared to Ang group. (b) HR
However, no significant HR changes were observed between these pretreated groups and Ang group in the maximum level of effect [ΔHR: 55.6 ± 3.8, 53.62 ± 5.25, 60.12 ± 8.87 compared to 72.79 ± 5.10 beats/min, respectively; Figure 3].
DISCUSSION
In the present study, the injection of normal saline with the same volume of TP extract had not significant effects on blood pressure and HR of the control group. All three doses of TP extract (100, 200, and 400 mg/kg) significantly reduced MAP in normotensive condition as well as inhibited of increase in blood pressure after injection of Ang II which indicated a relaxation effect on vascular smooth muscles.
Angiotensin II regulates blood pressure and plasma volume via aldosterone-regulated sodium excretion, sympathetic nervous activity, and thirst responses.[13]
As previous studies have been shown, Ang II-mediated hypertension could be induced through different signaling events; stimulation of phospholipase C and phosphatidylinositol hydrolysis, increased intracellular free calcium concentration, activation of protein kinase C, increased intracellular free concentrations of Na+, and decreased intracellular free concentrations of Mg2+, activation of tyrosine kinases, implication of mitogen-activated protein kinase pathways or phospholipase D activation,[13,14] so in our study the hypotensive effect of TP extract could be attributed to affect on each of these pathways. Besides, former reports have also demonstrated that TP extract had relaxation effects on ileum[15] and vascular smooth muscles.[11] Moreover, TP contains important ingredients such as salvigenin, cirsiliol, pinen-α and β, sabinene, myrcene, germacrene D, limonene, β-caryophyllene, spathulenol which can influence on vascular smooth muscle tone. For instance, in many studies, the antispasmodic effect of some components such as α- and β-pinen,[16,17] cirsiliol,[18,19] spathulenol,[20] limonene,[21] and salvigenin[22] from other members of Lamiacee family have been demonstrated. Altogether, it seems that the TP ingredients can induce the hypotensive effects in rats.
On the other hand, abundant evidence now suggests that a key mechanism by which Ang II influences blood pressure is via its ability to produce reactive oxygen species (ROS).[23,24] Most investigations of Ang II hypertension and oxidant stress have focused on the vasculature as a key player, and in particular the notion that increased levels of O2 lead to diminished bioactivity of nitric oxide and thus vasoconstriction.[25]
An important role for ROS-mediated vascular smooth muscle hypertrophy and remodeling in Ang II-dependent hypertension has also received considerable attention so considering to known antioxidant properties of TP,[26,27,28,29] it can impress on hypertension through this mechanism.
Our data showed that in spite of the effect of TP on lowering of blood pressure it could not improve tachycardia induced by Ang, but even if TP could reduce HR, it seems activation of baroreflex, which may be had a role in blood pressure reduction conceal impact of TP on HR, in addition, since the baroreflex response is impaired in anesthesia[30] therefore, no changes of HR may at least in part, be due to the blunted of baroreflex activity.
CONCLUSIONS
Taking together upon to our findings TP can prevent hypertension induced by activation of RAS through antagonizing the effects of Ang II and according to its various ingredients as well as different implicated pathways involve in this type of hypertension, the mechanisms of TP action are different.
ACKNOWLEDGMENTS
The authors would like to thank Research Affairs of Mashhad University of Medical Sciences for their financial support as well as Dr. H. Rakhshandeh and Mrs. Aghaei (Pharmacological Research Center of Medicinal Plants, School of Medicine, Mashhad University of Medical Sciences, Iran) for their help in providing the plant extract.
Footnotes
Source of Support: Research Affairs of Mashhad University of Medical Sciences financialy supported this work
Conflict of Interest: None declared.
REFERENCES
- 1.Xie Y, Zhang W. Antihypertensive activity of Rosa rugosa Thunb. Flowers: Angiotensin I converting enzyme inhibitor. J Ethnopharmacol. 2012;144:562–6. doi: 10.1016/j.jep.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 2.Gasparotto A, Junior, Gasparotto FM, Lourenço EL, Crestani S, Stefanello ME, Salvador MJ, et al. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J Ethnopharmacol. 2011;134:363–72. doi: 10.1016/j.jep.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Dahlöf B. Effect of angiotensin II blockade on cardiac hypertrophy and remodelling: A review. J Hum Hypertens. 1995;9(Suppl 5):S37–44. [PubMed] [Google Scholar]
- 4.Galati EM, Mondello MR, D’Aquino A, Miceli N, Sanogo R, Tzakou O, et al. Effects of Teucrium divaricatum Heldr. ssp. Divaricatum decoction on experimental ulcer in rats. J Ethnopharmacol. 2000;72:337–42. doi: 10.1016/s0378-8741(00)00280-4. [DOI] [PubMed] [Google Scholar]
- 5.Tariq M, Ageel AM, al-Yahya MA, Mossa JS, al-Said MS. Anti-inflammatory activity of Teucrium polium. Int J Tissue React. 1989;11:185–8. [PubMed] [Google Scholar]
- 6.Abdollahi M, Karimpour H, Monsef-Esfehani HR. Antinociceptive effects of Teucrium polium L total extract and essential oil in mouse writhing test. Pharmacol Res. 2003;48:31–5. [PubMed] [Google Scholar]
- 7.Gharaibeh MN, Elayan HH, Salhab AS. Anorexic effect of Teucrium polium in rats. Int J Crude Drug Res. 1989;27:201–7. [Google Scholar]
- 8.Niazmand S, Erfanian Ahmadpoor M, Moosavian M, Derakhshan M. The positive inotropic and chronotropic effects of Teucrium polium L. Extract on Guinea pig isolated heart. Pharmacol Online. 2008;2:588–94. [Google Scholar]
- 9.Bello R, Calatayud S, Moreno L, Beltran B, PrimoYufera E, Esplugues J. Effects on arterial blood pressure of the methanol extracts from different Teucrium species. Phytother Res. 1997;11:330–1. [Google Scholar]
- 10.Niazmand S, Esparham M, Hassannia T, Derakhshan M. Cardiovascular effects of Teucrium polium L. Extract in rabbit. Pharmacogn Mag. 2011;7:260–4. doi: 10.4103/0973-1296.84244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suleiman MS, Abdul-Ghani AS, Al-Khalil S, Amin R. Effect of Teucrium polium boiled leaf extract on intestinal motility and blood pressure. J Ethnopharmacol. 1988;22:111–6. doi: 10.1016/0378-8741(88)90236-x. [DOI] [PubMed] [Google Scholar]
- 12.Rasekh HR, Khoshnood-Mansourkhani MJ, Kamalinejad M. Hypolipidemic effects of Teucrium polium in rats. Fitoterapia. 2001;72:937–9. doi: 10.1016/s0367-326x(01)00348-3. [DOI] [PubMed] [Google Scholar]
- 13.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- 14.Touyz RM. Recent advances in intracellular signalling in hypertension. Curr Opin Nephrol Hypertens. 2003;12:165–74. doi: 10.1097/00041552-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Parsaee H, Shafiee-Nick R. Anti-Spasmodic and Anti-nociceptive effects of Teucrium polium aqueous extract. Iran Biomed J. 2006 [Google Scholar]
- 16.Câmara CC, Nascimento NR, Macêdo-Filho CL, Almeida FB, Fonteles MC. Antispasmodic effect of the essential oil of Plectranthus barbatus and some major constituents on the guinea-pig ileum. Planta Med. 2003;69:1080–5. doi: 10.1055/s-2003-45186. [DOI] [PubMed] [Google Scholar]
- 17.Sadraei H, Asghari GR, Hajhashemi V, Kolagar A, Ebrahimi M. Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine. 2001;8:370–6. doi: 10.1078/0944-7113-00052. [DOI] [PubMed] [Google Scholar]
- 18.Kohno S, Ohata K. Resting tonus of isolated airway smooth muscles. Nihon Yakurigaku Zasshi. 1993;102:1–10. doi: 10.1254/fpj.102.1. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa EH, Abu Zarga M, Abdalla S. Effects of cirsiliol, a flavone isolated from Achillea fragrantissima, on rat isolated ileum. Gen Pharmacol. 1992;23:555–60. doi: 10.1016/0306-3623(92)90127-6. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Hernandez N, Ponce-Monter H, Medina JA, Joseph-Nathan P. Spasmolytic effect of constituents from Lepechinia caulescens on rat uterus. J Ethnopharmacol. 2008;115:30–5. doi: 10.1016/j.jep.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 21.de Sousa DP, Júnior GA, Andrade LN, Calasans FR, Nunes XP, Barbosa-Filho JM, et al. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Z Naturforsch C. 2008;63:808–12. doi: 10.1515/znc-2008-11-1205. [DOI] [PubMed] [Google Scholar]
- 22.Uydes-Dogan BS, Takir S, Ozdemir O, Kolak U, Topçu G, Ulubelen A. The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. Vascul Pharmacol. 2005;43:220–6. doi: 10.1016/j.vph.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Braga VA, Medeiros IA, Ribeiro TP, França-Silva MS, Botelho-Ono MS, Guimarães DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: Implications in neurogenic hypertension. Braz J Med Biol Res. 2011;44:871–6. doi: 10.1590/s0100-879x2011007500088. [DOI] [PubMed] [Google Scholar]
- 24.de Queiroz TM, Monteiro MM, Braga VA. Angiotensin-II-derived reactive oxygen species on baroreflex sensitivity during hypertension: New perspectives. Front Physiol. 2013;4:105. doi: 10.3389/fphys.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–6. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 26.Ardestani A, Yazdanparast R, Jamshidi SH. Therapeutic effects of Teucrium polium extract on oxidative stress in pancreas of streptozotocin-induced diabetic rats. J Med Food. 2008;11:525–32. doi: 10.1089/jmf.2006.0230. [DOI] [PubMed] [Google Scholar]
- 27.Kadifkova Panovska T, Kulevanova S, Stefova M. In vitro antioxidant activity of some Teucrium species (Lamiaceae) Acta Pharm. 2005;55:207–14. [PubMed] [Google Scholar]
- 28.Ljubuncic P, Dakwar S, Portnaya I, Cogan U, Azaizeh H, Bomzon A. Aqueous extracts of Teucrium polium possess remarkable antioxidant activity in vitro. Evid Based Complement Alternat Med. 2006;3:329–38. doi: 10.1093/ecam/nel028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samec D, Gruz J, Strnad M, Kremer D, Kosalec I, Grubesic RJ, et al. Antioxidant and antimicrobial properties of Teucrium arduini L. (Lamiaceae) flower and leaf infusions (Teucrium arduini L. Antioxidant capacity) Food Chem Toxicol. 2010;48:113–9. doi: 10.1016/j.fct.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Fluckiger JP, Sonnay M, Boillat N, Atkinson J. Attenuation of the baroreceptor reflex by general anesthetic agents in the normotensive rat. Eur J Pharmacol. 1985;109:105–9. doi: 10.1016/0014-2999(85)90545-x. [DOI] [PubMed] [Google Scholar]


