Abstract
Background:
Treatment of postabstinence syndrome of alcohol is one of the major strategies of alcoholism treatment. Exercise can be modulated major brain pathways such as a reward system and pain perception centers. The aim of this study was to evaluation the effects of forced exercise in the management of alcohol dependence and pain perception alteration which induced by alcoholism.
Methods:
72 adult male rats were divided into 2 major groups: (1) 40 of them was divided into groups of positive control (alcohol dependent) negative control and alcohol dependent groups under treatment by forced exercise, diazepam (0.4 mg/kg) and forced exercise in combination with diazepam and alcohol withdrawal signs, and blood cortisols, were measured in this groups. (2) 32 rats were divided into control, alcohol dependent (without treatment), and alcohol-dependent groups under treatment by forced exercise or indometacin (5 mg/kg) and then pain perception was assessed by using writhing test, tail-flick and hot plate test.
Results:
Forced exercise, diazepam, and their combinations significantly attenuates withdrawal syndrome to 20 ± 2, 22 ± 1.3 and 16 ± 2 and blood cortisol level to 6.8 ± 1.3,7.9 ± 1.2 and 5.8 ± 1.1, respectively, in comparison with the positive control group (P < 0.05 and P < 0.001). In alcohol dependent animal under treatment by forced exercise, pain response significantly inhibited with 37%, 57% and 38% decreases in writhing test, hot plate, and tail-flick test, respectively, in comparison with alcohol dependent (without treatment) group (P < 0.05).
Conclusions:
This study suggested that forced exercise can be useful as adjunct therapy in alcoholism patient and also can be effective in modulation of pain threshold reduction that was induced by alcohol dependency.
Keywords: Alcohol, forced exercise, pain, withdrawal syndrome
INTRODUCTION
Alcohol dependence is a substance related disorder in which an individual is addicted to alcohol either physically or mentally.[1] The exact mechanism that describes dependency and withdrawal symptoms of alcohol is not clear.[2] A variety of techniques exist for managing alcohol withdrawal, some that involve the pharmacotherapy with sedatives and some that do not. Drugs with a sedative effect such as diazepam, chlordiazepoxide, topiramate, and nalteroxone are used for pharmacotherapy of alcohol withdrawal syndrome. These alternative medications act for reduction of withdrawal syndrome severity.[3,4,5,6] Alcohol withdrawal is characterized by a stressful condition and increased activity adrenal gland and cortisol level.[7,8] Previous studies demonstrated that alcohol dependency will increase the expression of corticotropin releasing factor (CRF) mRNA leading to activation of the adrenal gland that cause increasing cortisol level as a stress hormone.[7] Previous data demonstrate that CRF receptors play an important role in the management of ethanol self-administration and attenuation of withdrawal syndrome in dependent rats. These data showed that CRF antagonists and attenuation of cortisol level can be useful as new pharmacotherapeutic targets for the treatment of alcoholism in humans.[9] Also, these study indicate that there was an increase in the basal serum cortisol level in the alcoholic subjects compared to the controls.[10,11] This study approved that serum cortisol level can be used as a marker of alcohol withdrawal syndrome severity.[11] On the other way, pain transmission and alcohol's reinforcing effects share overlapping neural substrates giving rise to the possibility that alcohol use states significantly affect pain patterns and promote the development of dependence and addiction.[12] Previous study demonstrates that long-term alcohol intoxication and alcohol dependence induce pain symptoms and may exacerbate chronic pain arising from other sources. Neuroanatomical regions involved in pain transmission have been shown to play an important role in the development of alcohol dependence and addiction.[12,13] The likelihood that alcohol dependency and pain transmission share overlapping neural substrates suggests that alcohol reinforcement would alter the pharmacology and neurochemistry of pain.[13] Exercise plays an important role in the prevention and treatment of addictive disorders. Exercise may benefit drug dependent patients attempting recovery from substance problems through a number of different mechanisms of action.[14] Recent study has also shown that exercise alleviate mood disturbance and morphine and nicotine withdrawal symptoms.[15,16] It has also been shown that exercise can counteract withdrawal symptoms, and physical exercise can reduce the risk of some type of drug addiction.[17] Studies in recent years have found exercise has been consistently associated with reductions in depressive symptoms, and thus exercise may reduce the risk for relapse of addictive disorder by reducing depressive symptom.[17] Recent studies have demonstrated the acute effects of exercise on decreased craving to nicotine and morphine consumption and their withdrawal syndrome.[15] In the present study, the effects of forced exercise on alcohol dependency, stress level of the withdrawal period, by measurement of cortisol, and alteration of pain perception in alcohol dependent animals was investigated.
METHODS
Seventy adult male Wistar rats (180-210 g) were purchased from Iran Razi Institute (Tehran, Iran). All the rats were maintained at standard condition (22°C ± 2°C and 12/12 h dark and light cycle), having free access to food and water. The protocol was approved as an undergraduate research and was projected by the Research Council of the Tehran University of medical science.
Drugs
Alcohol, Diazepam, and indomethacin were purchased from Sigma-Aldrich Inc (St Louis MO, USA).
Experimental design for alcohol withdrawal syndrome
40 animals were divided randomly into 5 groups:
Group I, as a negative control (independent) received normal saline (0.2 ml/rat. ip, once daily) for 21 days
Group II, as a positive control (dependent) received alcohol (2 g/kg/day. By gavage, once daily) for 21 days
Group III, IV, and V as treatment groups, respectively, received alcohol (2 g/kg/day) for first 7 days and after that were treated by diazepam (0.4 mg/kg, ip, once daily), forced exercise (as below protocol) and diazepam (0.4 mg/kg, ip, once daily) in combination with forced exercise (as below protocol) for 2 weeks. All doses mentioned about alcohol and diazepam were chosen based on previous studies.[18,19]
Evaluation of Alcohol withdrawal syndrome: In day 22, the rats were observed for 4 min at the 24 h of the ethanol-withdrawal period. All of the subjects groups were observed, and their 5 behaviors (Stereotyped behaviors, Agitation, Tail stiffness, abnormal posture, and abnormal gait) were recorded by camera. After computation of recorded data, behaviors were counted and analyzed, and a digit allocated to each one [Table 1]. The summation of these digits gives Alcohol Total Withdrawal Score (ATWS).[20,21,22]
Table 1.
Rating scale for some behaviors signs induced by ethanol withdrawal in rats

Treadmill forced exercise protocol
Rats were allowed to run on a motor-driven leveled treadmill (ModelT408E, Diagnostic and Research Instruments Co., Taoyuan, Tai). The animal of groups of 4 and 5 was trained by treadmill for 45 min/day, for 5 days/week. The training speed was 12 miles/min (for 1st week) and reached14 miles/min (in the second week) by the end of the experiments. The slope and Intensity of exercise were settled as 0° at the first 10 min, 5° for second 10 min and 15° for last 25 min.[23,24,25]
Measuring the blood cortisol
On the 22nd day after, the behavioral signs been recording whole blood of animals were collected, and their serum was separated and the fasting serum level of cortisol was measured based on μg/dL and by ELISA method.
Experimental design for nociception protocols
32animals were divided randomly into 4 groups:
Group I, as negative control received normal saline (0.2 ml/rat ip, once daily) for 21 days
Group II, as positive control received alcohol (2 g/kg/day by gavage, once daily) for 21 days and single dose of indometacin (5 mg/kg, ip) in test day
Group III, dependent group received alcohol (2 g/kg/day by gavage, once daily) for 21 days
Groups IV were treated by alcohol (2 g/kg/day by gavage, once daily) and forced exercise (as mentioned protocol) for 21 days. All doses mentioned about alcohol and indometacin were chosen based on previous studies.[18,26]
This treatment in 1st day two types of nociception test tail-flick and hot plate test was assessed and after this treatment in 22 days of treatment, three types of nociception method were applied for evaluation of pain.
2-1: Writhing test
In 22 days of treatment, all mentioned animal acetic acid (0.8%) was administrated in a volume of 10 ml/kg in rat. Nociceptive behavior is characterized by abdominal contraction known as writhing, described as an exaggerated extension of the abdomen combined with the out stretching of hind limbs. Total number of writhing following i.p. administration of acetic acid was recorded in 30 min after acetic acid injection. Percentage of inhibition of abdominal constrictions in each group was computed by following ratio: Treated mean-control mean × 100/control mean. This meted of nociception assessment was done as base of previous studies. In additions, the onset of the first writhing was recorded as latency time.[27]
2-2: Tail-flick test
Before the start of treatment, this test was done in all mentioned animal. In this test, radiant heat (Tail-Flick Apparatus Model P-162, Pouyaye Armaghan Co., Iran) was applied for measurement of acute nociception responses in rat. Intensity of the thermal stimulus was adjusted to produce 5-6 s latency in tail-flick response. Five millimeters of the tail was submitted to noxious heating. To avoid damage to the tail, if the response did not occur, trial was automatically terminated at 12 s (cutoff time). The tail-flick test was measured in 1st day and last day of mentioned treatment as exercise (for 21 days), single dose of indometacin (5 mg/kg, ip) and saline (0.2 ml/rat). The percentage of nociception for each animal was calculated, using the following ratio: ([Posttreatment–pretreatment]/[pretreatment]) ×100. This method of nociception assessment was done as base of previous studies.[28]
2-3: Hot plate test
In this test, analgesic activity was measured with a thermostatically heated surface maintained at 55 ± 2C. Time of reaction was revealed as the time period from the instant animal was put on the hot plate until the moment the animal licked its feet or jumped out. The hot plate test was done twice in 1st day and last day of mentioned treatment as exercise (for 21 days), single dose of indometacin (5 mg/kg, ip) saline (0.2 ml/rat), the reaction time was again evaluated, but only once, this value represented the reaction time after treatment. The percentage of nociception was measured by following ratio: (Reaction time after treatment - reaction time before treatment) ×100/reaction time after treatment.[29]
Statistical analysis
Normality of continuous variables (ATWS, blood cortisol and pain perception) was assessed using Kolmogorov–Smirnov test. Based on this test, all variables were normally distributed. We also used Leven's test and Bartlett's test to assess homogeneity of variances between two and more than two groups, respectively. The results showed that variances are homogenous between tested groups. As parametric assumptions were met, we described continuous data as means ± standard error of the mean, compared the differences between positive and negative control groups by unpaired Student's t-test, and the differences between treatment groups by one-way ANOVA. Bonferroni's test was then used for group-by-group comparisons. Results were considered to be significant at 0.05 levels.
RESULTS
3-1: Alcohol total withdrawal score results in control and treatment groups
Our data indicate that ATWS in negative control group that received saline during protocol process was 14 ± 2 while ATWS in positive control group (dependent group) increased significantly by 53% (P < 0.05) in comparison with negative control and was 30 ± 1.6 [Figure 1].
Figure 1.
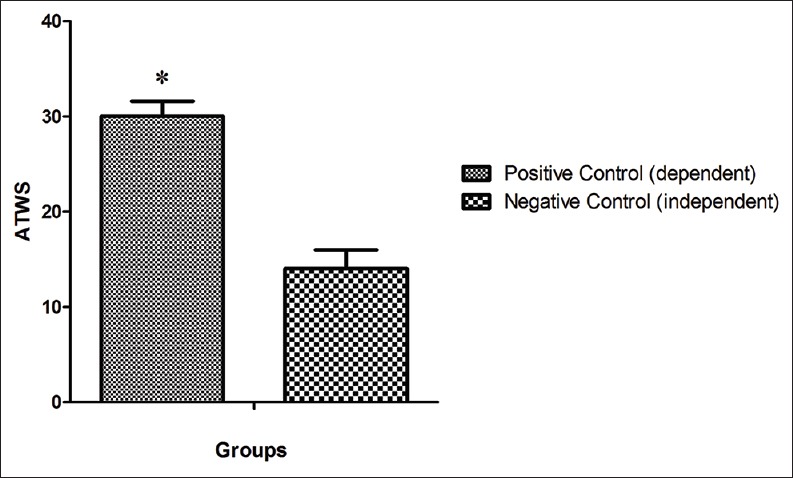
Comparison of severity of the alcohol withdrawal syndrome between the animal of the negative control group and positive control group. *Shows the significant difference (P<0.05) in comparison with the negative control. ATWS: Alcohol total withdrawal score
In different protocol and administration of diazepam caused, 33% of significantly decrease in ATWS 20 ± 2 (P < 0.05) in comparison with positive control group [Figure 2]. Also treatment of animal by treadmill forced exercise caused 26% decrease ATWS 22 ± 1.3 in comparison with positive control group and in the last treatment group, combination therapy with diazepam and treadmill forced exercise caused 46% decreasing in ATWS and reached 16 ± 2 (P < 0.05) [Figure 2].
Figure 2.
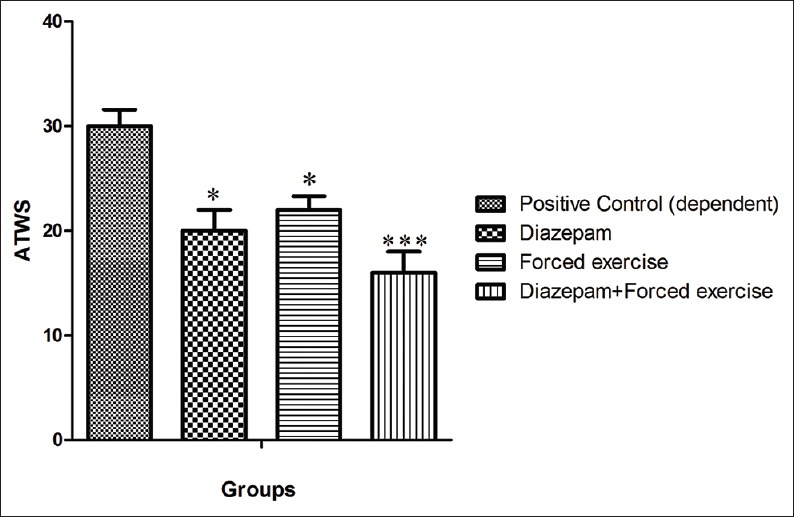
The severity of the alcohol withdrawal syndrome in the dependent animal of groups of under treatment by Diazepam, under treatment by forced exercise, and under treatment by combination of Diazepam and forced exercise, in comparison with the positive control group. ***Shows the significant difference (P<0.001) and *shows the significant difference (P<0.05) in comparison with group under treatment by Diazepam, forced exercise, and their combinations. ATWS: Alcohol total withdrawal score
3-2: Blood cortisol level in control and treatment groups
Blood cortisol level in the negative control group was 7.2 ± 1.3 μg/dL after withdrawal syndrome period, while in the positive control group was significantly higher, and was reached 16 ± 2.1 μg/dL (P < 0.05) [Figure 3].
Figure 3.
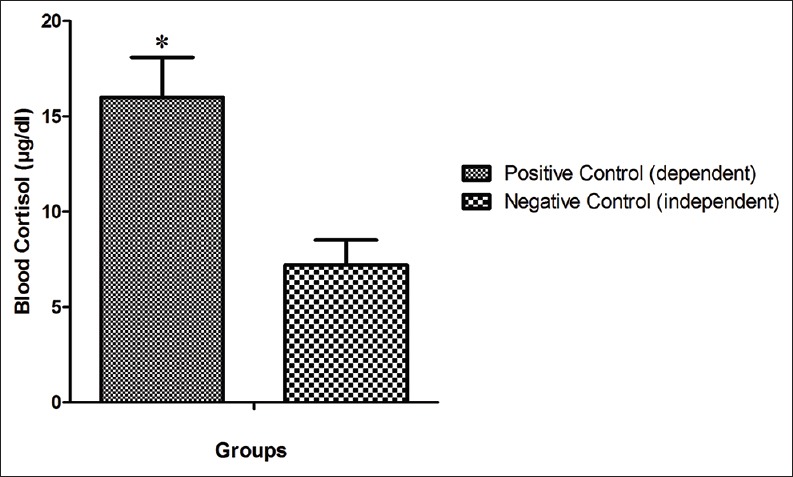
Comparison of blood cortisol levels between the animal of the negative control group and positive control group. *Shows the significant difference (P<0.05) in comparison with the negative control
Administration of diazepam significantly decreased cortisol level (from 16 ± 2.1 to 6.8 ± 1.3 μg/dL (P < 0.05), that is, 57%). Treatment of animals by forced exercise caused significantly attenuation of cortisol level (i.e. 50%) in comparison with positive control group and reached to 7.9 ± 1.2 μg/dL. Combination therapy of dependent animal by of diazepam and forced exercise caused 63% decrease in cortisol level and reached to 5.8 ± 1.1 μg/dL (P < 0.05) [Figure 4].
Figure 4.
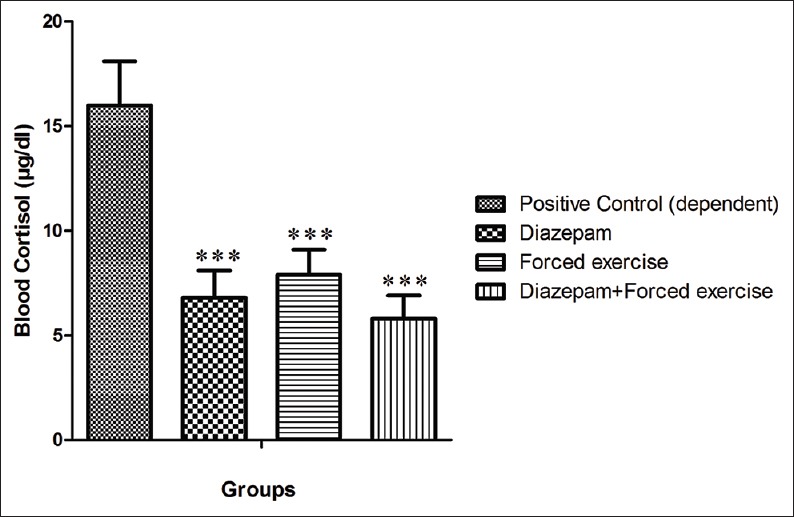
Comparison of blood cortisol levels in dependent animal of groups of under treatment by Diazepam, under treatment by forced exercise, and under treatment by combination of Diazepam and forced exercise, in comparison with the positive control group. ***shows the significant difference (P<0.001)
Effect of exercise and alcohol dependency in writhing test
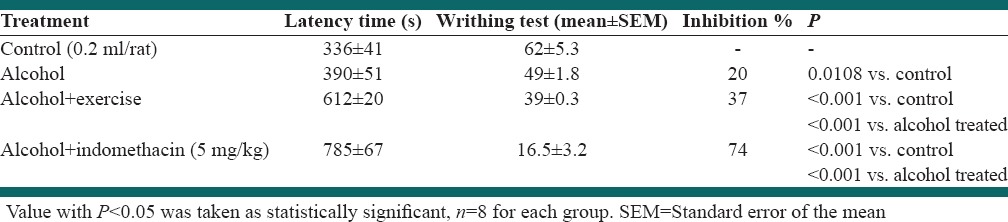
Administration of alcohol in dependent group induced significant alter in pain response when compared to negative control group (P < 0.05). Forced exercise inhibited the increase of pain perception which induced by alcohol dependency (P < 0.05). As well as indometacine significantly decrease the number of writhing as a reference drug (P < 0.05) [Table 2].
Table 2.
Effect of forced exercise in acetic acid-induced writhing test in alcohol dependent rat
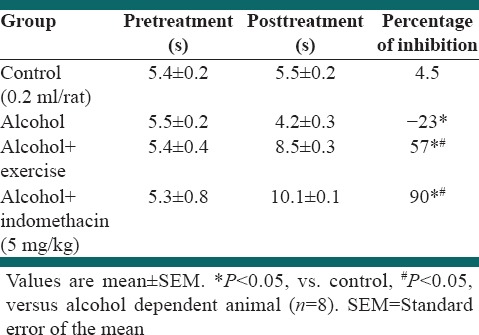
Effect of exercise and alcohol dependency tail-flick test
Table 3 indicates the effect of alcohol dependency on tail-flick test response in rat. Alcohol dependency significantly decreased the tail-flick test time compared to the control (P < 0.05). Forced exercise by treadmill significantly increased the tail-flick test time in alcohol dependent rat. Furthermore, indometacin (10 mg/kg) significantly increased the tail-flick test time (P < 0.05) [Table 3].
Table 3.
Effect of forced exercise in tail flick test in alcohol dependent rat
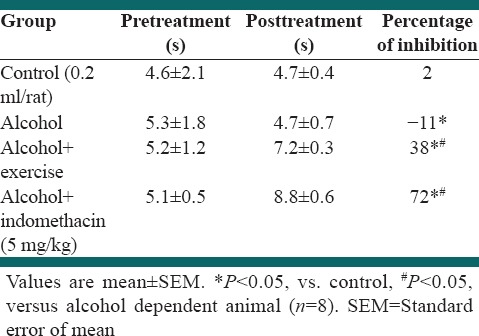
Effect of exercise and alcohol dependency on hot plate test
Table 3 indicates the effect of alcohol dependency on hot plate test response in rats. Alcohol dependency significantly decreased the hot plate test compared to the control (P < 0.05). Forced exercise by treadmill significantly increased the hot plate test time in alcohol dependent rat. Furthermore, Indometacin (10 mg/kg) significantly increased the hot plate test (P < 0.05) [Table 4].
Table 4.
Effect of forced exercise in hot plate test in alcohol dependent rat
DISCUSSION
Alcohol use disorders are a major public health concern. Despite the demonstrated efficacy of a number of different treatments for alcohol dependency, its withdrawal syndrome remains a major problem.[1] Healthy lifestyle changes and nonpharmacotherapy may contribute to long-term maintenance of recovery, and interventions targeting physical activity, in particular, may be especially valuable as an adjunct to alcohol treatment.[14] The role of exercise as an adjunct to alcohol treatment has been explored on participants receiving inpatient alcohol rehabilitation treatment.[14,30,31] Many previous studies were done in the field of treatment of alcohol dependency and management of withdrawal syndrome, all of this experimental study had been conducted by groups of sedative and hypnotic medications such as diazepam, chlordiazepoxide, and topiramate.[31,32] This study showed that forced exercise with treadmill and its combination with diazepam, as standard treatment of alcohol dependency, can be effective in alcohol withdrawal syndrome management. Our date showed that dependent groups under treatment by forced exercise with treadmill or dependent group under treatment by diazepam (0.5 mg/kg) alone showed significant decrease in alcohol abandonment sign and its severity (P < 0.05). Also dependent group under treatment by exercise in combination with diazepam significantly attenuate the withdrawal signs in comparison with positive control (dependent without treatment) (P < 0.001). Our results show that physical activity by treadmill can ameliorate severity of withdrawal symptom. Recent studies showed that exercise can abolish these symptoms by attenuating of pain perception, depression, and reducing anxiety probably by increasing release of the opioid like peptides such as endorphin.[33,34,35,36,37] These results can arguable with this concept that exercise plays an important role in the prevention and treatment of alcoholic addictive disorders. Furthermore, we can argue these findings by describing the possible mechanism of exercise on reducing the rewarding effects of drugs such as cocaine and morphine since recent study has demonstrated that exercise leads to an increase in the synthesis and release of some neurotransmitter such as dopamine, serotonin, and GABA.[37,38] On the other hand, exercise has been shown to result in acute improvements in positive-activated affect and alleviate mood disturbance and withdrawal symptoms in patient attempting to quit Alcohol.[30] These positive reinforcing properties may be mediated in part by exercise effects on the endogenous opioid system and potentiating of dopaminergic systems linked importantly to the experience of enhanced mood and experienced pleasure.[39,40] Furthermore, our previous study demonstrated that exercise can be effective in alleviation of morphine dependency and cortisol level of withdrawal syndrome periods.
The present study indicates that the chronic abuse of alcohol and its abstinence syndrome can increase the activity of the CRF-secreting cells and activates adrenal cortex.[7,9] Alcohol dependency increases the stress parameter and hypothalamic-pituitary-adrenal axis activity, by changes in gene expression of CRF in selective neurons of the para-ventricular nucleus.[9,41,42] The result of our experimental study indicate that alcohol doses in the dependent positive control group caused a significant increase in blood cortisol level in comparison with the independent negative control group during the withdrawal syndrome a cessation period (P < 0.05). We can argue this result with the basic concept that the alcohol abandonment cause increase in the level of stress in rats and consequently raising the cortisol secretion in the withdrawal period in rats.
On the other hand, by applying the treatment protocols with diazepam, exercise, and exercise in combination with diazepam, a significant reduction in the blood cortisol level was reached; in comparison with the dependent positive control group was statistically significant (P < 0.05). Generally our study results showed that treatment protocols decreased stress level in animal in the withdrawal syndrome period and consequently cortisol level. We conclude our results that there is significant difference in withdrawal syndrome, cortisol, levels between positive control group and the group treated by diazepam, exercise and diazepam in combination with exercise.
The consequences of the current study indicated that indometacin as typical antiinflammatory and pain killer drug reduce the acetic acid induced writing test) abdominal pain constriction (and increase the latency time of abdominal pain expression in comparison with control group. This study also confirmed that alcohol dependency alter the acetic acid induced writing test) abdominal pain constriction (and increase the latency time of abdominal pain expression in comparison with control group. We can discuss our study results with basic concept that neuroanatomical regions involved in pain transmission have been shown to play an important role in the development of alcohol dependence and addiction.[12] Our study showed that forced exercise ameliorated the acetic acid induced abdominal constriction in alcohol dependent group and increase the latency time of abdominal pain expression in comparison with control and alcohol dependent group.
The inhibitory action of exercise on pain perception of the dorsal horn may be mediated by activation of opioid-like peptide and releasing of dopamine and serotonin.[43] Previous study demonstrated that the exercise can change the pain signaling and alter and attenuate the inflammation induced pain in digestive systems.[44]
Present study indicated that the indometacin as standard antiinflammatory and pain killer drug increase the percent of pain inhibition in comparison with the control group in a hot plate and tail-flick test. Furthermore, our study demonstrated that alcohol dependency increase the percent of pain perception in comparison with the control group in a hot plate and tail-flick test. The probability that alcohol addiction and pain conduction share overlapping neural substrates suggests that alcohol abuse would alter the pharmacology and neurochemistry of pain perception.[12] Our study indicated that forced exercise increase the percent of pain inhibition in comparison with the control group in a hot plate and tail-flick test. Previous study demonstrated that the forced exercise has a neuroprotective effect, and is used for treatment of some neurodegenerative and central nervous system disease such as depression, neuropathic pain and neuroinflammation.[45] We can argue our data results with the basic concept that forced exercise can modulate opoidergic system and alter the pain perception level.
CONCLUSIONS
Our study showed that the forced exercise can be an effective adjunct therapy to reduce symptoms of alcohol withdrawal syndrome. Our study demonstrated that alcohol dependency can reduce pain perception threshold and forced exercise will assist this kind of pain perception alterations alcoholic.
ACKNOWLEDGMENTS
This research was financially supported by the Student Research Committee of Teheran University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: A HuGE gene-disease association review. Am J Epidemiol. 2008;167:125–38. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- 2.Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferri M, Amato L, Davoli M. Alcoholics Anonymous and other 12-step programmes for alcohol dependence. Cochrane Database Syst Rev. 2006;19:CD005032. doi: 10.1002/14651858.CD005032.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–9. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- 5.Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, et al. Baclofen in the treatment of alcohol withdrawal syndrome: A comparative study vs diazepam. Am J Med. 2006;119:276.e13–8. doi: 10.1016/j.amjmed.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Muzyk AJ, Leung JG, Nelson S, Embury ER, Jones SR. The role of diazepam loading for the treatment of alcohol withdrawal syndrome in hospitalized patients. Am J Addict. 2013;22:113–8. doi: 10.1111/j.1521-0391.2013.00307.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruijnzeel AW, Small E, Pasek TM, Yamada H. Corticotropin-releasing factor mediates the dysphoria-like state associated with alcohol withdrawal in rats. Behav Brain Res. 2010;210:288–91. doi: 10.1016/j.bbr.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–71. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, et al. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: Relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, et al. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol. 2010;85:357–60. doi: 10.1016/j.biopsycho.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Bokhan N, Gavrilova V, Gusev S, Ivanova S. 1581–Steroid hormones levels in alcohol dependent patients under conditions of social isolation. Eur Psychiatry. 2013;28:1. [Google Scholar]
- 12.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–92. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain. 1992;8:77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. A pilot study of aerobic exercise as an adjunctive treatment for drug dependence. Ment Health Phys Act. 2010;3:27–34. doi: 10.1016/j.mhpa.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MA, Lynch WJ. Exercise as a potential treatment for drug abuse: Evidence from preclinical studies. Front Psychiatry. 2011;2:82. doi: 10.3389/fpsyt.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DM, Dunsiger S, Whiteley JA, Ussher MH, Ciccolo JT, Jennings EG. Acute effects of moderate intensity aerobic exercise on affective withdrawal symptoms and cravings among women smokers. Addict Behav. 2011;36:894–7. doi: 10.1016/j.addbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: A neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–44. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 19.Gatch MB. Effects of benzodiazepines on acute and chronic ethanol-induced nociception in rats. Alcohol Clin Exp Res. 1999;23:1736–43. [PubMed] [Google Scholar]
- 20.Erden BF, Ozdemirci S, Yildiran G, Utkan T, Gacar N, Ulak G. Dextromethorphan attenuates ethanol withdrawal syndrome in rats. Pharmacol Biochem Behav. 1999;62:537–41. doi: 10.1016/s0091-3057(98)00175-0. [DOI] [PubMed] [Google Scholar]
- 21.Lal H, Prather PL, Rezazadeh SM. Anxiogenic behavior in rats during acute and protracted ethanol withdrawal: Reversal by buspirone. Alcohol. 1991;8:467–71. doi: 10.1016/s0741-8329(91)90153-n. [DOI] [PubMed] [Google Scholar]
- 22.Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–50. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168:345–8. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Saadipour K, Sarkaki A, Alaei H, Badavi M, Rahim F. Forced exercise improves passive avoidance memory in morphine-exposed rats. Pak J Biol Sci. 2009;12:1206–11. doi: 10.3923/pjbs.2009.1206.1211. [DOI] [PubMed] [Google Scholar]
- 25.Mello PB, Benetti F, Cammarota M, Izquierdo I. Effects of acute and chronic physical exercise and stress on different types of memory in rats. An Acad Bras Cienc. 2008;80:301–9. doi: 10.1590/s0001-37652008000200008. [DOI] [PubMed] [Google Scholar]
- 26.Golshani S, Karamkhani F, Monsef-Esfehani HR, Abdollahi M. Antinociceptive effects of the essential oil of Dracocephalum kotschyi in the mouse writhing test. J Pharm Pharm Sci. 2004;7:76–9. [PubMed] [Google Scholar]
- 27.Naddaf H, Najafzadeh H, Fallah H, Saremipoor P. Comparison the analgesic effect of electeroacupuncture and tramadol on writhing test in rat. Pharm Sci. 2013;18:205–7. [Google Scholar]
- 28.Silva ML, Silva JR, Prado WA. Retrosplenial cortex is involved in analgesia induced by 2-but not 100-Hz electroacupuncture in the rat tail-flick test. J Acupunct Meridian Stud. 2012;5:42–5. doi: 10.1016/j.jams.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Chavan M, Wakte P, Shinde D. Analgesic and anti-inflammatory activity of Caryophyllene oxide from Annona squamosa L. bark. Phytomedicine. 2010;17:149–51. doi: 10.1016/j.phymed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, et al. Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behav Modif. 2009;33:220–49. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato L, Minozzi S, Vecchi S, Davoli M. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010:CD005063. doi: 10.1002/14651858.CD005063.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Martinotti G, Di Nicola M, De Vita O, Tedeschi D, Guerriero L, Guglielmo R, et al. PW01-237-Low-dosage topiramate in alcohol dependence: A randomized, double-blind, placebo-controlled trial. Eur Psychiatry. 2010;25:1665. [Google Scholar]
- 33.Krogh J, Nordentoft M, Sterne JA, Lawlor DA. The effect of exercise in clinically depressed adults: Systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry. 2011;72:529–38. doi: 10.4088/JCP.08r04913blu. [DOI] [PubMed] [Google Scholar]
- 34.Tesarz J, Schuster AK, Hartmann M, Gerhardt A, Eich W. Pain perception in athletes compared to normally active controls: A systematic review with meta-analysis. Pain. 2012;153:1253–62. doi: 10.1016/j.pain.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Ströhle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009;116:777–84. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- 36.Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, et al. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20:364–76. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- 37.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41:15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 38.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217:354–62. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuckovic MG, Li Q, Fisher B, Nacca A, Leahy RM, Walsh JP, et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson's disease: In vivo imaging with [18F] fallypride. Mov Disord. 2010;25:2777–84. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boecker H, Tölle TR, Valet M, Sprenger T. Functional Neuroimaging in Exercise and Sport Sciences. New York: Springer Science/Business Media; 2012. Effects of Aerobic Exercise on Mood and Human Opioidergic Activation Measured by Positron Emission Tomography; pp. 499–510. [Google Scholar]
- 41.Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagen KB, Dagfinrud H, Moe RH, Østerås N, Kjeken I, Grotle M, et al. Exercise therapy for bone and muscle health: An overview of systematic reviews. BMC Med. 2012;10:167. doi: 10.1186/1741-7015-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Light AR, Bateman L, Jo D, Hughen RW, Vanhaitsma TA, White AT, et al. Gene expression alterations at baseline and following moderate exercise in patients with chronic fatigue syndrome and fibromyalgia syndrome. J Intern Med. 2012;271:64–81. doi: 10.1111/j.1365-2796.2011.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, et al. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 2010;1310:200–7. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]


