Abstract
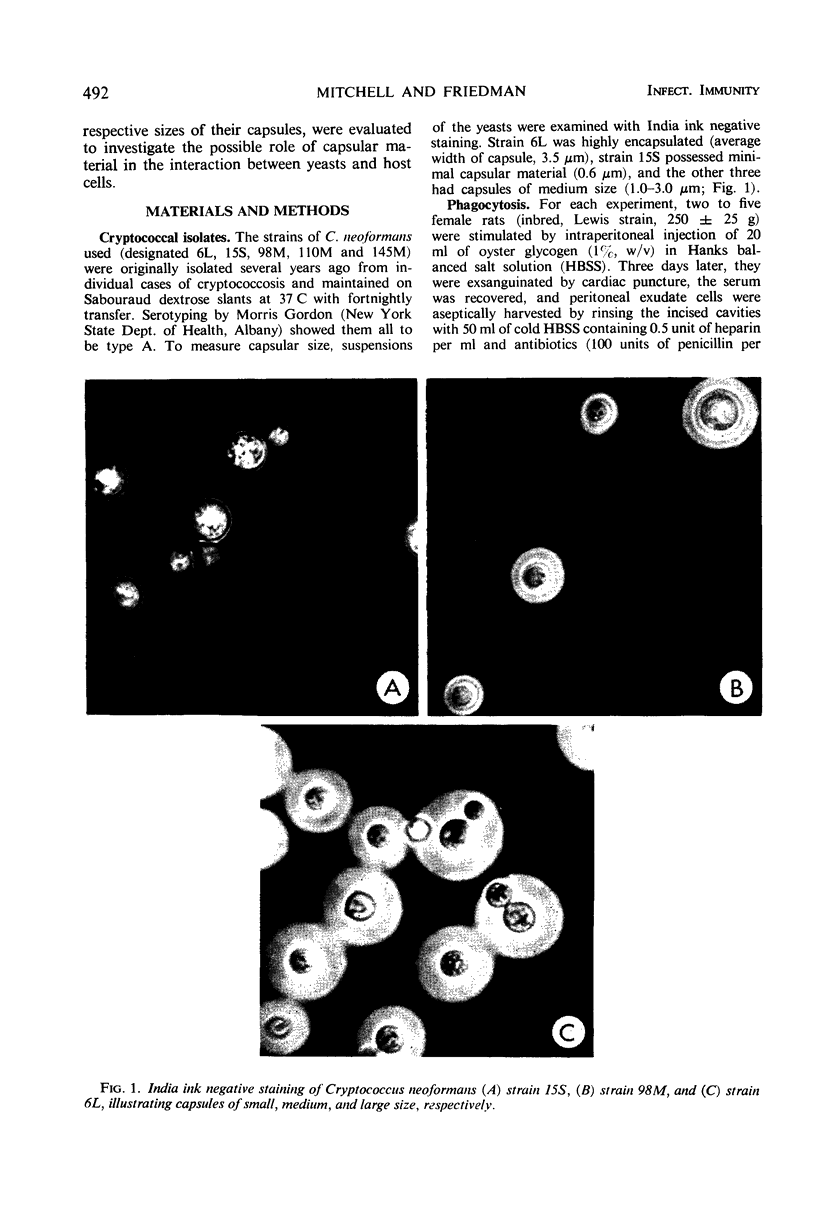
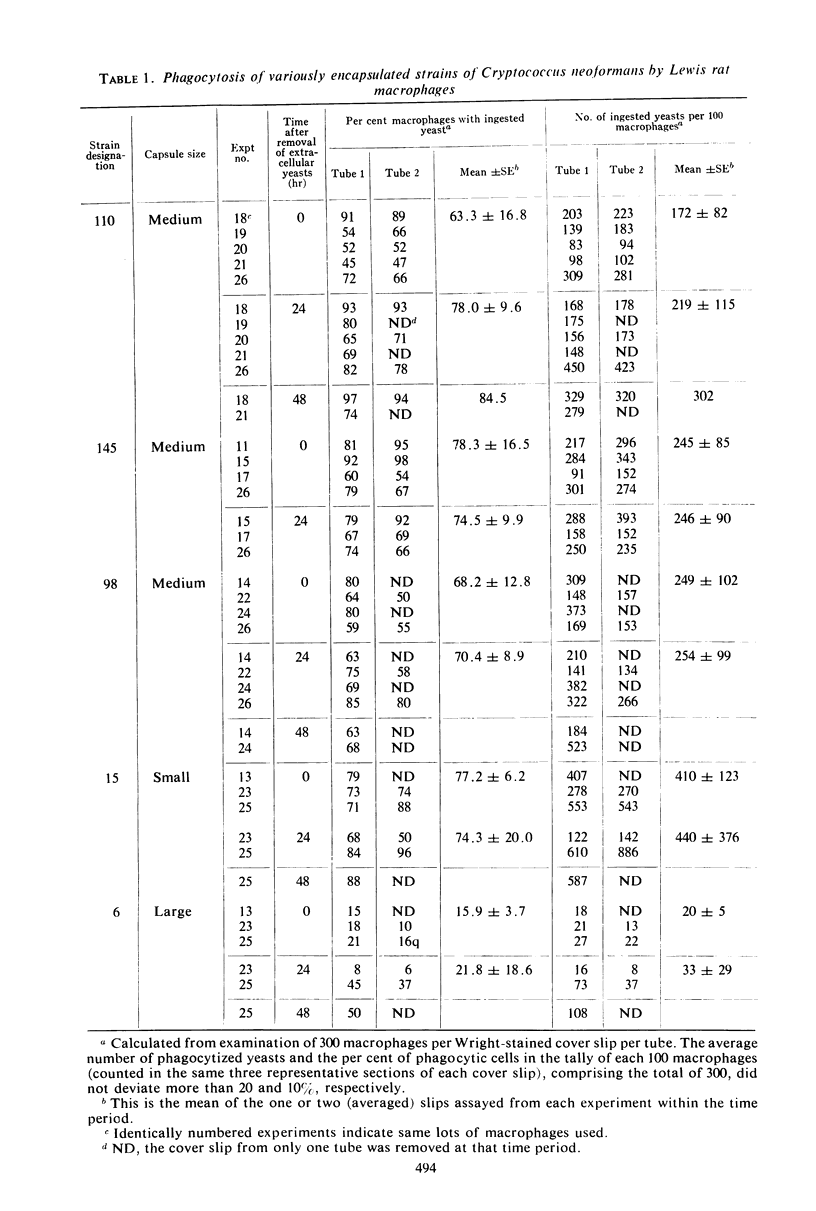
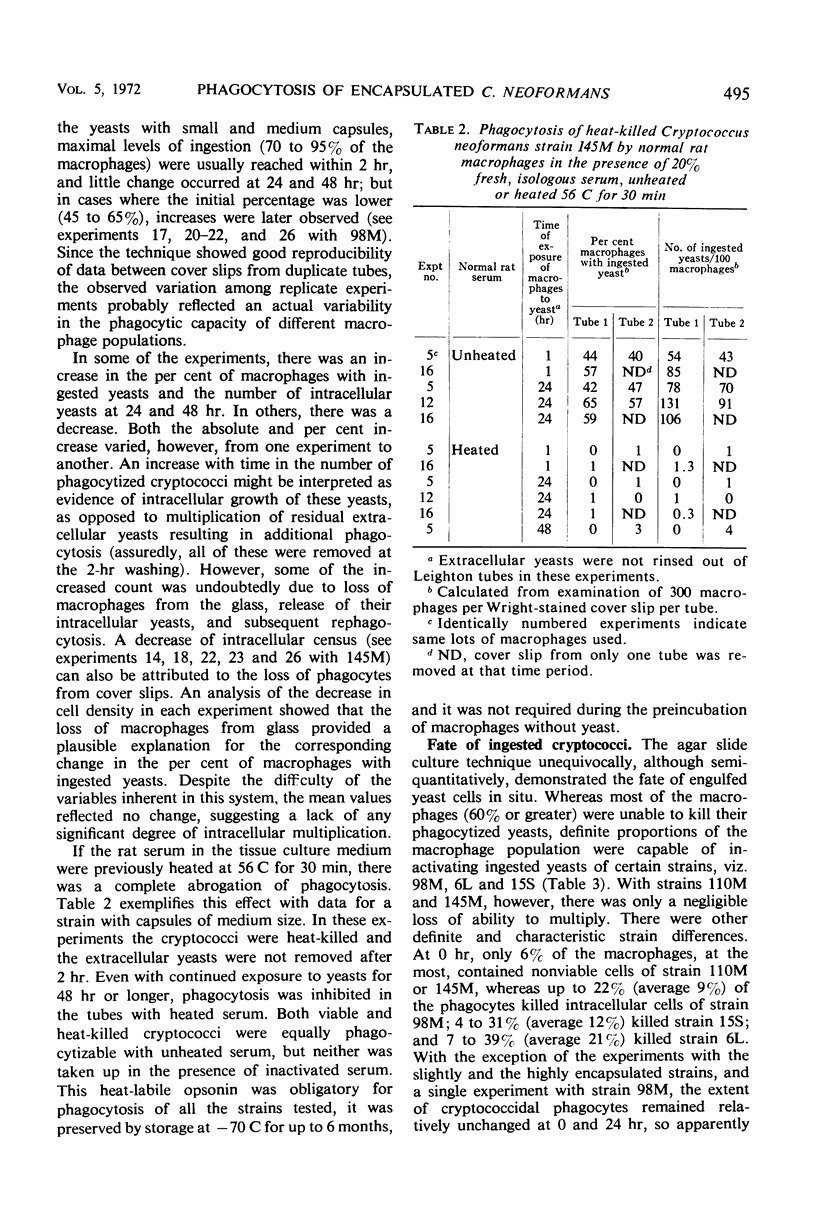
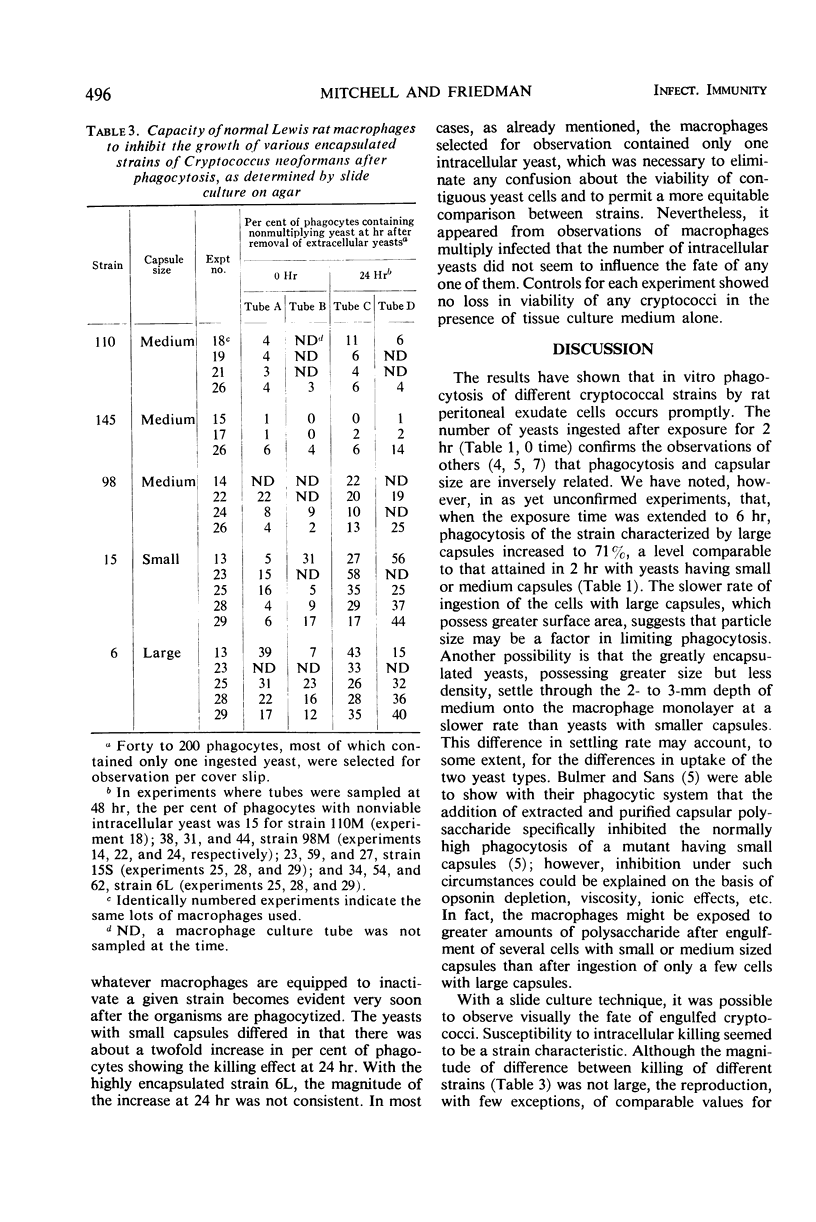
Five isolates of Cryptococcus neoformans type A with stable capsular thicknesses were used. Three of the isolates had capsules of medium size, one had a minimal capsule, and the other, a large capsule. Peritoneal exudate cells from Lewis rats were cultured on cover slips in Leighton tubes containing medium 199 and 20% fresh, isologous normal rat serum. Yeast cells were added to the Leighton tube cultures, and, 2 hr later, the extracellular yeasts were rinsed out. Cover slips were removed from some tubes for Wright staining and measurement of both phagocytosis and loss of macrophages. The remaining tubes were reincubated and sampled at 24 or 48 hr. To determine fate of yeast cells after ingestion, washed cover slips were inverted onto agar slide cultures, and specific macrophages were observed in situ for subsequent multiplication of their intracellular yeasts. More than half of the macrophages survived 24 to 48 hr of exposure to different strains of C. neoformans, with small, medium, or large capsules. Phagocytic activity was dependent upon a heat-labile factor in normal rat serum. The number of yeast ingested by macrophages was inversely proportional to the capsular size. Although most of the ingested yeasts were resistant to intracellular killing, the agar culture technique clearly demonstrated that many were unable to multiply, presumably dead. Three of the isolates were more susceptible than the other two, and the fate of these yeasts after engulfment was not correlated with their capsular size.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER R. D., HAUGEN R. K. Tissue changes and tissue diagnosis in cryptococcosis; a study of 26 cases. Am J Clin Pathol. 1955 Jan;25(1):14–24. doi: 10.1093/ajcp/25.1.14. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Migration inhibitory factor associated with delayed-type hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):13–15. [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. 3. Inhibition of phagocytosis. J Bacteriol. 1968 Jan;95(1):5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. II. Phagocytosis by human leukocytes. J Bacteriol. 1967 Nov;94(5):1480–1483. doi: 10.1128/jb.94.5.1480-1483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Gadebusch H. H., Johnson A. G. Natural host resistance to infection with Cryptococcus neoformans. V. The influence of cationic tissue proteins upon phagocytosis and on circulating antibody synthesis. J Infect Dis. 1966 Dec;116(5):566–572. doi: 10.1093/infdis/116.5.566. [DOI] [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Vedder D. K. Serologic tests in diagnosis and prognosis of cryptococcosis. JAMA. 1966 Sep 19;197(12):961–967. [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Littman M. L., Walter J. E. Cryptococcosis: current status. Am J Med. 1968 Dec;45(6):922–932. doi: 10.1016/0002-9343(68)90190-3. [DOI] [PubMed] [Google Scholar]
- Louria D. B., Kaminski T. Passively-acquired immunity in experimental cryptococcosis. Sabouraudia. 1965 Jun;4(2):80–84. doi: 10.1080/00362176685190211. [DOI] [PubMed] [Google Scholar]
- Ralston D. J., Elberg S. S. Medium-dependent activity of immune serum on Brucella-infected macrophages. Infect Immun. 1971 Feb;3(2):361–362. doi: 10.1128/iai.3.2.361-362.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson-Porat S., Shahar A., Aronson M. Formation of histiocyte rings in response to Cryptococcus neoformans infection. J Reticuloendothel Soc. 1965 Sep;2(3):249–255. [PubMed] [Google Scholar]
- Shahar A., Kletter Y., Aronson M. Granuloma formation in cryptococcosis. Isr J Med Sci. 1969 Nov-Dec;5(6):1164–1172. [PubMed] [Google Scholar]
- Weir D. M., Elson C. J. Heat labile antibody in rat serum. Clin Exp Immunol. 1968 Sep;3(7):725–731. [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN L. E., RAPPAPORT H. Occurrence of Cryptococcosis in patients with malignant disease of reticuloendothelial system. Am J Clin Pathol. 1954 Sep;24(9):1050–1072. doi: 10.1093/ajcp/24.9.1050. [DOI] [PubMed] [Google Scholar]




