Summary
This study discusses improvement of popular rice varieties under drought through the identification and marker-assisted introgression of drought yield QTLs without any adverse effect on yield under normal conditions.
Key words: Direct selection, drought, grain yield, marker-assisted breeding, QTLs, rice.
Abstract
The increased occurrence and severity of drought stress have led to a high yield decline in rice in recent years in drought-affected areas. Drought research at the International Rice Research Institute (IRRI) over the past decade has concentrated on direct selection for grain yield under drought. This approach has led to the successful development and release of 17 high-yielding drought-tolerant rice varieties in South Asia, Southeast Asia, and Africa. In addition to this, 14 quantitative trait loci (QTLs) showing a large effect against high-yielding drought-susceptible popular varieties were identified using grain yield as a selection criterion. Six of these (qDTY 1.1, qDTY 2.2, qDTY 3.1, qDTY 3.2, qDTY 6.1, and qDTY 12.1) showed an effect against two or more high-yielding genetic backgrounds in both the lowland and upland ecosystem, indicating their usefulness in increasing the grain yield of rice under drought. The yield of popular rice varieties IR64 and Vandana has been successfully improved through a well-planned marker-assisted backcross breeding approach, and QTL introgression in several other popular varieties is in progress. The identification of large-effect QTLs for grain yield under drought and the higher yield increase under drought obtained through the use of these QTLs (which has not been reported in other cereals) indicate that rice, because of its continuous cultivation in two diverse ecosystems (upland, drought tolerant, and lowland, drought susceptible), has benefited from the existence of larger genetic variability than in other cereals. This can be successfully exploited using marker-assisted breeding.
Introduction
Around 90% of rice is grown and consumed in Asia. The semi-aquatic nature of rice and high water requirements for its cultivation make it much more prone to losses from drought than other cereals such as wheat and maize, which are better adapted to be grown with less water. As a result of the reduction in water availability and recent climate change scenarios, rice production is likely to be more severely affected by drought in Asia. Prior to the Green Revolution, traditional varieties adapted to the respective rice-growing ecosystems were cultivated across these areas. However, in the post-Green Revolution era, these varieties were replaced by a few fertilizer-responsive high-yielding varieties adapted to the irrigated ecosystem. These varieties were never screened for tolerance of drought and they suffer heavy yield losses even under mild stress conditions (Kumar et al., 2008). On the other hand, the large and variable area under rice cultivation as well as different methods of rice cultivation (direct-seeded upland, transplanted lowland) make the crop unique in terms of its inherent variability available for tolerance of drought compared with other cereals.
Dry spells can occur at almost any time during the rice growth period in rain-fed areas, leading to drought stress of varying intensity. However, rice is highly sensitive to water stress at the reproductive stage (O’Toole 1982; Venuprasad et al., 2007) as floral fertility in rice is extremely sensitive to water stress. Improving resilience to drought during floral development and anthesis is an important target (Richards et al., 2010). This scenario has long been realized, and efforts have been made to understand the mechanisms related to drought tolerance as well as to develop varieties tolerant of drought. In the past, the major focus in breeding rice for drought tolerance was on secondary traits such as root architecture, water use efficiency, etc (Babu et al., 2003; Lanceras et al., 2004). It has also been believed that grain yield as a selection criterion is not suitable in breeding rice for drought tolerance. This has been attributed to the high complexity of genetic control of this trait, which leads to its low heritability under drought. Several experiments to standardize the procedures for uniform screening of segregating populations for grain yield under reproductive-stage drought (Venuprasad et al., 2007, 2008; Kumar et al., 2008, 2009) showed moderate heritability of grain yield under drought, thereby confirming the suitability of grain yield as a selection criterion. It was also reported that, in large mapping populations, the correlation between high yield potential and good yield under drought was low but always positive (Kumar et al., 2008), suggesting the possibility to combine high yield potential and good yield under drought successfully.
Once screening protocols were standardized, large-scale conventional breeding and quantitative trait locus (QTL) identification programmes were started, using yield as a selection criterion. This manuscript reports on the progress achieved in developing drought-tolerant varieties through new conventional breeding approaches based on direct selection for grain yield under drought, the identification of large-effect QTLs for grain yield under drought, and marker-assisted breeding using the identified QTLs to improve the grain yield of popular high-yielding but drought-susceptible varieties under drought.
Materials and methods
Donors, recipients, and segregating populations
Before the initiation of any breeding programme or mapping experiments, drought-tolerant donors were identified through screening of germplasm material. A majority of this material involved traditional Aus, Indica, and Basmati accessions. These accessions were evaluated for drought tolerance along with popular high-yielding varieties such as IR64, Swarna, and Sambha Mahsuri as checks, using grain yield as the selection criterion. Drought-tolerant lines identified through these experiments were evaluated for rice blast disease (caused by Magnaporthe oryzae) to identify lines tolerant of both drought and blast. These tolerant lines were crossed to popular high-yielding varieties to develop segregating populations for conventional breeding programmes and for developing mapping populations. Three main kinds of mapping populations were commonly used for the identification of QTLs for grain yield under drought. The first of these were recombinant inbred lines (RILs) developed by crossing two parents contrasting for the trait of interest followed by subsequent selfing and advancement through the single seed descent (SSD) method to achieve nearly homozygous lines (Kumar et al., 2013). Backcross inbred lines (BILs) were developed through backcrossing (n=1–3) followed by self-pollination through the SSD method to develop BCnF3:4 populations. Advanced backcross (AB) populations proved to be specifically advantageous as they allowed the identification of lines with high yield potential and good plant and grain type because of the high percentage of the recipient parent. These could be used directly for testing and release in the target environment or could be used as parents for further backcross programmes to develop near-isogenic lines (NILs) of the recipient parent.
Phenotyping of donors and mapping and segregating populations
Experimental designs and crop maintenance
Screening of donors and mapping and segregating populations was conducted under upland and/or lowland reproductive-stage drought stress (RS) and irrigated non-stress (NS) conditions in dry season (DS) experiments at the International Rice Research Institute (IRRI). The experiments were planted in an α-lattice design with two replications in single or two-row plots with 5 m row length in lowland and 2.0–3.0 m row length in upland. Lowland experiments were carried out under transplanted conditions in which 21-day-old seedlings were transplanted in the field with a single seedling per hill. However, upland experiments were dry direct seeded. The row-to-row and plant-to-plant spacing of 20 cm×20cm in lowland and 25 cm×25cm in upland was maintained. Nitrogen, phosphorus, and potassium (NPK) were applied at the rate of 120:30:30 and 100:40:40kg ha–1 in lowland and upland, respectively. P and K were applied as basal, and N was applied in three splits, the first as basal, the second at maximum tillering, and the third at panicle initiation, in both lowland and upland. In order to control snails, Bayluscide (niclosamide, 0.25kg a.i. ha–1) was sprayed just after transplanting. At 4 days after transplanting (DAT; based on medium-duration lines), Sofit (pretilachlor±safener, 0.3kg a.i. ha–1) was sprayed to control weeds, followed by Furadan (carbofuran, 1kg a.i. ha–1) at 5 DAT and Cymbush (cypermethrin, 1 litre ha–1)±Dimotrin (cartap hydrochloride, 0.25kg a.i. ha–1) at 16 DAT to control insect pests.
Drought screening in upland conditions
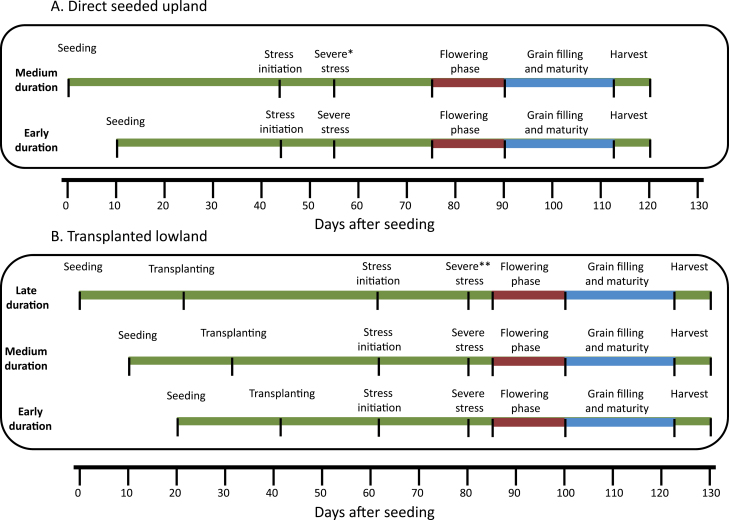
In upland conditions, donors and mapping populations were screened in sprinkler-irrigated dry direct-seeded trials. Up to 45 days after sowing (DAS; based on medium-duration lines), the trials were irrigated by sprinkler twice a week during establishment and early vegetative growth (Fig. 1A). Stress was initiated after this period by withholding irrigation, and plots were irrigated only when the soil water tension fell below –50 kPa at 30cm soil depth. At this soil water potential, most lines wilted and exhibited leaf drying. This type of cyclic stress is considered to be efficient in screening for drought tolerance in populations consisting of genotypes with a broad range of growth duration (Lafitte et al., 2004) and it ensures that all lines receive adequate stress during reproductive development. Upland non-stress trials received the same cultural practices as the stress trials, except that irrigation was continued twice a week up to 10 d before harvest.
Fig. 1.
Protocol used for screening under reproductive-stage drought: (A) direct-seeded upland conditions and (B) transplanted lowland conditions. *Upland direct seeded: irrigated when the soil tensiometer shows a reading of –70 kPa at 30cm depth at 10:00h. **Lowland transplanted: irrigated when the water table depth recorded was below 90cm and susceptible checks showed severe leaf rolling and a leaf rolling score of 9 at 10:00h. (This figure is available in colour at JXB online.)
Drought screening in lowland conditions
In lowland conditions, transplanted experiments were drained at 30 DAT and irrigation was withheld to impose drought stress at the reproductive stage (Fig. 1B). Stress was continued until severe leaf rolling (LR) was observed in at least 75% of the population lines and water table depth remained below 100cm for >2 weeks. Life-saving irrigation was provided thereafter through flash flooding, and water was drained after 24h to impose a second cycle of drought stress. Water table depth was measured by inserting a 1.1 m polyvinyl chloride (PVC) pipe in the experimental fields at regular intervals. Pipes were inserted to 1.0 m depth and 10cm of pipe remained above the soil surface. Depletion in the water table was measured through a meter scale daily after the onset of the stress.
Data recorded
Data for days to 50% flowering (DTF), plant height (PHT), and grain yield (GY) were recorded. DTF was recorded when 50% of the panicles of the plants of each plot were exerted. PHT (cm) was measured at maturity from the soil surface to the tip of the panicle on the main tiller from three random plants of each plot and then the mean was calculated. Harvesting for GY was done at physiological maturity. Samples were harvested and dried to 12% moisture before weighing, and weights were converted to kg ha–1.
Genotyping approaches, and QTL identification and validation
In the drought grain yield QTL identification as well as introgression programme, rice microsatellite [simple sequence repeat (SSR)] markers were widely used (Bernier et al., 2007; Venuprasad et al., 2009; Vikram et al., 2011; Dixit et al., 2012a ; Mishra et al., 2013; Yadaw et al., 2013) for their easy PCR amplification and electrophoresis (Kumar et al., 2013), the abundance of these markers across the genome that allows elaborate coverage, and their co-dominance nature that allows the detection of heterozygotes, making them suitable for genotyping all kinds of mapping populations. Whole-genome genotyping (WGG), selective genotyping (SG), and bulk segregant analysis (BSA) were used in different studies to identify QTLs for grain yield under drought. Each of these approaches has its own advantages and disadvantages. The selection of an approach was based on the type of mapping population and the aim of the study. The WGG approach was used in some QTL mapping studies (Vikram et al., 2011). A full population was genotyped in this approach, with polymorphic markers spread evenly across the genome. Although the approach allowed the identification of major- and minor-effect QTLs as well as interaction between different loci, it was a relatively expensive and time-consuming approach. BSA (Michelmore et al., 1991) that involved DNA pooling of lines based on the phenotypic extremes for developing high- and low-yielding bulks was used in several studies (Venupradsad et al., 2009; Vikram et al., 2011; Ghimire et al., 2012; Mishra et al., 2013; Yadaw et al., 2013; Dixit et al. 2014). These bulks were genotyped along with the parents with all polymorphic markers. The markers having bulk bands corresponding clearly to the parents were considered as candidates for full population genotyping and subsequent QTL analysis. BSA has proven to be a cost-effective approach although it does not allow the identification of minor QTLs and interaction between loci. Selective genotyping (Lebowitz et al., 1987) that combines the advantages of both WGG and BSA but has some limitations was also used in some studies (Bernier et al., 2007). A subset of the mapping population constituting 12% of the lines from the phenotypic extremes was selected for genotyping in this study. QTLs identified in different populations were evaluated at the IRRI across seasons for testing the consistency of effect. Whole populations or their subsets were evaluated in the target environment to test the effect of the QTLs.
QTL identification
Linkage maps for the populations were developed using MapManager QTX (Manly et al., 2001). To detect the relationship between markers and trait value, QTL cartographer 2.5.009 (Churchill and Doerge, 1994), Q gene 4.3.10 (Joehanes and Nelson, 2008), and QTLNetwork 2.1 (Yang et al., 2008) were used. Single marker analysis (SMA) followed by composite interval mapping (CIM) were conducted to identify significant QTLs. All three software programs allow permutation tests to determine the significance threshold to identify significant QTLs. Some 500–1000 permutation tests were conducted to determine the threshold level. QTL cartographer and Q gene provided the LOD score to describe the significance of a QTL, while QTLNetwork used mixed model analysis for detecting QTLs and the significance of the QTLs was provided in terms of F-value.
Introgression of QTLs
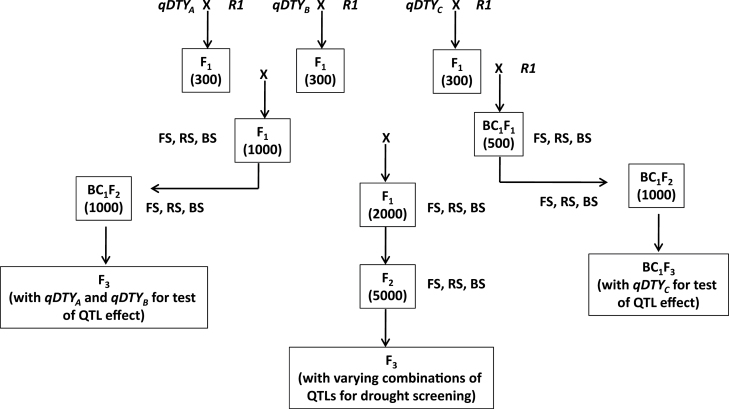
Efforts were made to identify at least three major QTLs in the background of popular high-yielding varieties from Asia. The initial introgression approach was to pyramid 2–3 QTLs in the background of a popular high-yielding variety for which they had been identified to obtain an economic yield advantage of 1.0–1.2 t ha–1 under drought in farmers’ fields. As many of the identified QTL regions were not fine mapped, all markers within the identified regions were used in the genotyping of the segregating generations to avoid a loss of candidate genes governing drought tolerance in the region due to crossover. To achieve this with reduced cost and minimum efforts, the sequential genotyping approach was followed. The backcrossed F2 segregants were first genotyped with a peak marker for each QTL, and lines possessing the donor allele at the peak marker were genotyped with flanking markers, followed by all markers within the QTL region. Further, because loci governing early days to flowering, plant height, and reduced yield under irrigated conditions were also detected within some of these QTL regions, larger segregating populations were used to break such linkages and develop dwarf high-yielding introgressed plants with DTF similar to those of recipient parents. A QTL pyramiding plan for pyramiding three QTLs coming from different sources in a variety is presented in Fig. 2. The use of large backcross populations for the identification of QTLs for grain yield under drought has proven advantageous for speeding up marker-assisted selection (MAS) and the quick recovery of the recipient genome in the background. With this technique, QTL mapping studies can be conducted simultaneously in three or more backcross populations derived from the cross of a common popular recipient with different drought-tolerant donors. QTLs can be identified through the use of genotyping techniques such as BSA. Once identified, lines with QTLs and the highest phenotypic similarity to the recipient parent can be selected and intercrossed. In each intercross F1 generation, foreground selection can be practised to select for F1 plants segregating for the respective QTLs. The final set of F1 plants segregating for all QTLs can be selfed to develop a large F2 population segregating for all QTLs. Plants fixed for different combinations of QTLs can then be identified from this population of F2 plants and advanced to the F3 generation. F3 lines so identified can be tested under drought stress and non-stress conditions. In a majority of the cases, single plant selection has to be made in the F3 generation to develop pure lines. Screening under drought stress conditions allows selection of the drought-tolerant plants within each line. Selection for plant type and grain type can also be practised in these lines to achieve the maximum possible similarity to the recipient parent as well as to identify plants better than the recipient parent. For the success of a QTL pyramiding programme, the nature of interactions between different QTLs introgressed and pyramided needs to be known. In the absence of this information, efforts can be made to develop NILs with all possible combinations of the target QTLs.
Fig. 2.
Marker-assisted backcrossing strategy used to develop NILs of a high-yielding popular variety with three DTY QTLs from different sources (from Kumar A, Dixit S, Henry A. 2013. Marker-assisted introgression of major QTLs for grain yield under drought in rice. In: Varshney RK, Tuberosa R, eds. Translational genomics for crop breeding: abiotic stress, yield and quality, Vol. 2. ©2013 John Wiley and Sons, Inc. with permission).
Evaluation of introgressed lines
Selected NILs were screened under reproductive-stage drought (RS) and irrigated non-stress (NS) conditions. The introgressed lines along with the recipient high-yielding variety and drought-tolerant donors were evaluated first at IRRI for yield under RS and NS conditions. This was followed by evaluation of the selected lines for diseases—blast (caused by M. oryzae) and bacterial blight (caused by Xanthomonas oryzae pv. oryzae)—and grain and cooking quality traits. Selected lines from evaluation at IRRI for all these traits were tested under national/state trials in different countries. IR64 introgressed lines with qDTY 2.2 and qDTY 4.1—IR87707-445-B-B-B and IR87707-446-B-B-B—together with IR64 as a check were evaluated together at 51 locations in 2011 and 2012 under the All India Coordinated Rice Improvement Program in India and under the drought breeding programme in Nepal. IR84984-83-15-481-B, a qDTY 12.1 NIL in the Vandana background, along with Vandana as a check were evaluated in 12 experiments conducted at the IRRI and at three locations in India in 2011–2012.
Results and Discussion
Identification and use of donors in QTL mapping and breeding programmes
The evolution of rice cultivars in drought-prone rainfed areas has allowed the development of a large number of landraces that possess high drought tolerance. However, a majority of these donors are landraces with low yield potential, low tillering, tall plant height, and poor grain and eating quality. Despite being known to possess drought tolerance, very few of them have been systematically characterized for the trait. A list of some of the donors characterized under reproductive-stage drought is presented in Table 1. Improved donors with good grain type (medium to long slender) and improved plant type (medium height, higher tillering, and lodging resistance) and tolerance of blast, such as Basmati 370, PSBRc 80, Aus 257, IR77298-14-1-2, IR83614-1002-B-B, and IR83614-1005-B-B were selected for direct use in the breeding programme. Some of these donors (Basmati 370 N22, Kali Aus, Dular and Apo) were also used in the QTL identification studies.
Table 1.
Grain yield of drought-tolerant donors identified at the IRRI for use in conventional breeding and QTL mapping studies as compared with high-yielding susceptible varieties under reproductive-stage drought
| Designation | Parentage | PHT | DTF | GY | BS | Suitability for use |
|---|---|---|---|---|---|---|
| Basmati 370 | Traditional | 113 | 32 | 5041 | 5 | Conventional breeding |
| CT9993-5-10-1-M | CT 6241-2-2-1-3//Maravilha | 96 | 28 | 3686 | 6 | QTL mapping and pre-breeding |
| PSBRc 82 | IR47761-27-1-3-6/IRRI 108 | 96 | 31 | 4630 | 6 | QTL mapping and pre-breeding |
| PSBRc 68 | IR43581-57-3-3-6/IR26940-20-3-3-3-1/Khao Dawk Mali 105 | 88 | 27 | 4433 | 7 | QTL mapping and pre-breeding |
| PSBRc 80 | IR50401-77-2-1-3/IR42068-22-3-3-1-3 | 81 | 34 | 4189 | 0 | Conventional breeding |
| Aus Bak Tulsi | Traditional | 89 | 84 | 4995 | 6 | QTL mapping and pre-breeding |
| Kalia | Traditional | 77 | 90 | 4752 | 3 | QTL mapping and pre-breeding |
| Lal Aus | Traditional | 86 | 97 | 4510 | 8 | QTL mapping and pre-breeding |
| IR83614-1007-B-B | IR78875-131-B-1-2/IR64 | 79 | 86 | 4442 | 7 | QTL mapping and pre-breeding |
| Aus 257 | Traditional | 96 | 89 | 4185 | 0 | Conventional breeding |
| Kali Aus | Traditional | 67 | 85 | 4032 | 7 | QTL mapping and pre-breeding |
| IR77298-14-1-2 | IR64 (WH)/Aday sel//3*IR64 | 65 | 91 | 4028 | 5 | Conventional breeding |
| Dular | Traditional | 82 | 88 | 3980 | 4 | Conventional breeding and QTL mapping |
| IR83614-1002-B-B | IR78875-131-B-1-2/IR64 | 87 | 85 | 3179 | 4 | Conventional breeding |
| IR83614-1005-B-B | IR78875-131-B-1-2/IR64 | 83 | 87 | 3155 | 5 | Conventional breeding |
| IR57514-PMI-5-B-1-2 | IR43581-57-3-3-6/Khao Dawk Mali 105//IR21836-90-3 | 96 | 90 | 2656 | 6 | QTL mapping and pre-breeding |
| N22 | Traditional | 63 | 76 | 2249 | 0 | Conventional breeding and QTL mapping |
| Apo | UPL RI 5/IR12979-24-1 (Brown) | 66 | 100 | 1722 | 3 | Conventional breeding and QTL mapping |
| IR36 | IR1561-228-1-2/IR 1737//CR94-13 | 48 | 92 | 1331 | Susceptible check | |
| IR64 | IR5657-33-2-1/IR2061-465-1-5-5 | 63 | 98 | 1054 | 0 | Susceptible check |
| Sambha Mahsuri | RP 5/Mahsuri | 72 | 30 | 754 | Susceptible check | |
| Swarna | Vasistha/Mahsuri | 73 | 29 | 1073 | Susceptible check | |
| LSD0.05 | 5.24 | 12.2 | 643 |
PHT, plant height; DTF, days to 50% flowering; GY, grain yield (kg ha–1); BS, blast score (on a scale of 0–9, where 0=highly tolerant and 9=highly susceptible.
Most of the traditional drought-tolerant donors are not used directly in breeding because of several undesirable traits that they possess. Table 2 compares drought-tolerant traditional donors and improved high-yielding drought-susceptible varieties in relation to morphology, phenology, yield, and growth-related traits. The specific differences in these characters led to the adaptation of these sets of lines in their specific environments (Supplementary Fig. S1 available at JXB online). For example, a majority of the drought-tolerant landraces show early flowering, tall plant height, low tillering, and low yield compared with medium to late flowering, semi-dwarf plant height, high tillering, and high yield of the high-yielding popular varieties. Some of these landraces have also been known to possess deep roots up to 70cm below the soil surface. Greater root length density at depth has also been reported in drought-tolerant genotypes such as Dular, Azucena, and Rayada compared with high-yielding drought-susceptible varieties such as IR64 (Henry et al., 2011).
Table 2.
Major morphological and phenological differences between traditional drought-tolerant donors and modern high-yielding varieties
| Traits | Donors | Recipients |
|---|---|---|
| Lines | Landraces, improved tolerant varieties | High-yielding varieties |
| Yield potential | Low-medium (1.5–4.0 t ha–1) | Medium-high (>4 t ha–1) |
| Yield under drought | Low-medium (1.5–3.5 t ha–1) | Low (0–1.5 t ha–1) |
| Duration | Early-medium (DTF=60–85 d) | Medium-late (DTF=85–100 d) |
| Plant height | Semi-dwarf-tall | Dwarf |
| Tillering | Low-medium | High |
| Panicle length | Short-medium | Long |
| Root system | Shallow-deep rooted | Shallow rooted |
| Grain quality | Poor (landraces), good (improved donors) | Good (preferred) |
| Examples | N22, Moroberekan, Aus 276, Kali Aus | Swarna, IR64, TDK1, Sabitri |
Donors with coarse grain type, tall plant height, and susceptibility to blast were used in the mapping study to identify QTLs and develop pre-breeding lines with improved tolerance of reproductive-stage drought and appropriate plant height and grain type for use in the breeding programme. Some of the pre-breeding lines that are widely used in a conventional breeding programme are mentioned in Table 3. These pre-breeding lines possessed high yield under non-stress conditions similar to the high-yielding varieties used as susceptible checks and good yield under reproductive-stage drought similar to the drought-tolerant checks (Table 3).
Table 3.
Pre-breeding lines developed from mapping populations for use as improved donors in drought breeding programmes
| Donor | QTL | GY (kg ha–1) | |
|---|---|---|---|
| Non-stress | Stress | ||
| IR84984-83-15-185B | qDTY 12.1 | 4933 | 2526 |
| IR86918-B-92 | qDTY 1.1 | 4812 | 3831 |
| IR86931-B-400 | qDTY 1.1 | 4910 | 3367 |
| IR86929-B-482 | qDTY 1.1 | 5145 | 3517 |
| IR86929-B-45 | qDTY 1.1 | 5230 | 3414 |
| IR86929-B-320 | qDTY 1.1 | 5460 | 3185 |
| IR86929-B-101 | qDTY 1.1 | 6728 | 3033 |
| IR55419-04 (tolerant check) | 3465 | 1732 | |
| IR77298-14-1-2-10 (tolerant check) | 3036 | 1330 | |
| IR74371-54-1-1 (tolerant check) | 5537 | 2705 | |
| IR74371-70-1-1 (tolerant check) | 5327 | 2437 | |
| Apo (tolerant check) | 5163 | 1661 | |
| MTU1010 (moderately susceptible check) | 5451 | 624 | |
| Swarna (susceptible check) | 3677 | 0 | |
| IR64 (susceptible check) | 4511 | 250 | |
| LSD0.05 | 2566 | 2699 | |
Direct selection for grain yield under reproductive-stage drought
The suitability of grain yield as a selection criterion allowed the initiation of large-scale breeding programmes aimed at developing high-yielding drought-tolerant varieties with good grain quality and tolerance of major diseases. The conventional breeding programme at IRRI included screening of a large F2 population of ~5000 plants for rice blast. The selected tolerant plants were transplanted in the field and evaluated for reaction to bacterial blight at ~30 DAT by clipping the leaves with scissors infested with PXO61 and PXO86, two strains of X. oryzae pv. oryzae. Plants showing scores >3 based on the Standard Evaluation System (SES) were rejected. Single-plant selections were screened under reproductive-stage drought stress conditions in the F3 generation, and tolerant plants were selected based on grain yield. In F4 and F5 generations, selection was carried out in non-stress conditions with grain quality traits evaluated in the F5 generation. In the F6 generation, lines were screened under both reproductive-stage drought stress and non-stress conditions, and lines with high yield under both conditions were advanced to an observational yield trial (OYT). All trials until the F6 generation were conducted as unreplicated trials. In the OYT, lines were divided into groups based on crop duration, and lines belonging to each group were planted with checks in replicated yield trials in larger plots. Selected lines from OYTs constituted an advanced yield trial (AYT). These trials were conducted at IRRI and in the target environments in an α-lattice design with large plot sizes. Lines performing well in the target environment were advanced for release by the respective national systems. Combining high yield under reproductive-stage drought stress and non-stress in one genotype is the goal that breeders have been targeting for a long time. This is mainly because, in the years with well-distributed rainfall in these areas, the drought-tolerant varieties should provide high yield comparable with that of the popular high-yielding varieties. This cannot be achieved through direct cultivation of drought-tolerant landraces because of their low yield potential. The breeding programme described above allowed the development of several high-yielding drought-tolerant lines. Multilocation testing of these lines has led to the release of 17 varieties across South and Southeast Asia and Africa over the past 6–7 years (Table 4). Multilocation testing of elite breeding lines has also allowed a better understanding of the genotype×environment (G×E) interactions related to grain yield under reproductive-stage drought. In general, it has been observed that a majority of these lines perform best in their specific environments. This is also evident from the list of varieties presented in Table 5. A majority of these varieties were released in specific countries where they turned out to be the best performers. However, lines IR74371-70-1-1 and IR74371-54-1-1 were released under three different names in three countries—India, Bangladesh, and Nepal, and Nepal, the Philippines, and Nigeria, respectively—showing the stability of performance of these lines across environments. Regardless of the complexity of grain yield under reproductive-stage drought, lines selected under managed dry-season field experiments at IRRI were able to perform well in different countries. The success of this breeding programme points to the adaptability of lines across regions in shallow lowland environments of different countries not seen before, and this validates the earlier prediction that G×E interactions can be handled more accurately within the different topography (shallow lowland, medium lowland, or deep lowland) in the rainfed ecosystem (Kumar et al., 2012).
Table 4.
High-yielding drought-tolerant varieties developed from IRRI’s drought breeding programm e and released in different countries of South and Southeast Asia and Africa
| Name | Designation | Days to maturity | Plant height (cm) | Country, release year, situation |
|---|---|---|---|---|
| Sahod Ulan 1 | IR74371-54-1-1 | 110 | 104 | Philippines 2009, RL, UP |
| Hardinath 1 | IR80411-B-49-1-1 | 115 | 100 | Nepal 2009, RL |
| Sahbhagi dhan | IR74371-70-1-1 | 110 | 104 | India 2010, RL, UP |
| BRRI dhan56 | IR74371-70-1-1 | 110 | 108 | Bangladesh 2011, RL |
| Sookha dhan 3 | IR74371-70-1-1 | 110 | 108 | Nepal 2011, RL |
| Sookha dhan 1 | IR74371-46-1-1 | 110 | 101 | Nepal 2011, RL |
| Sookha dhan 2 | IR74371-54-1-1 | 110 | 104 | Nepal 2011, RL |
| Katihan 1 | IR79913-B-176-B-4 | 105 | 90 | Philippines 2011, UP |
| Sahod Ulan 3 | IR81412-B-B-82-1 | 120 | 107 | Philippines 2011, RL |
| Sahod Ulan 5 | IR81023-B-116-1-2 | 115 | 130 | Philippines 2011, RL |
| Sahod Ulan 6 | IR72667-16-1-B-B-3 | 115 | 100 | Philippines 2011, RL |
| Sahod Ulan 8 | IR74963-262-5-1-3-3 | 125 | 100 | Philippines 2011, RL |
| Inpago LIPI Go 1 | IR79971-B-191-B-B | 110 | 115 | Indonesia 2011, UP |
| Inpago LIPI Go 2 | IR79971-B-227-B-B | 113 | 114 | Indonesia 2011, UP |
| Sahod Ulan 12 | IR81047-B-106-2-4 | 105 | 119 | Philippines 2013, RL, DS |
| M’ZIVA | IR77080-B-B-34-3 | 120 | 130 | Mozambique 2013, RL |
| UPIA3 | IR74371-54-1-1 | 110 | 104 | Nigeria 2013, RL |
RL, rainfed lowland; UP, rainfed upland, DS, direct seeded.
Table 5.
Major QTLs reported for high grain yield under upland and lowland reproductive-stage drought stress
| QTL | Donor | Recipient | Ecosystem | Chr | Interval | R 2 | Reported by | |
|---|---|---|---|---|---|---|---|---|
| P | G | |||||||
| qDTY 1.1 | Dhagad deshi | Swarna | Lowland | 1 | RM431–RM104 | 32 | Ghimire et al. (2012) | |
| qDTY 1.1 | Dhagad deshi | IR64 | Lowland | 1 | RM104–RM12091 | 9 | Ghimire et al. (2012) | |
| qDTY 1.1 | N22 | Swarna | Lowland | 1 | RM11943–RM12091 | 13 | Vikram et al. (2011) | |
| qDTY 1.1 | N22 | IR64 | Lowland | 1 | RM11943–RM12091 | 17 | Vikram et al. (2011) | |
| qDTY 1.1 | N22 | MTU1010 | Lowland | 1 | RM11943–RM12091 | 13 | Vikram et al. (2011) | |
| qDTY 1.1 | Apo | IR64 | Upland | 1 | RM486–RM472 | 58 | Venuprasad et al. (2012a ) | |
| qDTY 1.3 | Kali Aus | IR64 | Upland | 1 | RM488–RM315 | 5 | Sandhu et al. (2014) | |
| qDTY 1.2 | Kali Aus | MTU1010 | Upland | 1 | RM259–RM315 | 7 | Sandhu et al. (2014) | |
| qDTY 2.1 | Apo | Swarna | Lowland | 2 | RM327–RM262 | 16 | Venuprasad et al. (2009) | |
| qDTY 2.2 | Aday Sel. | IR64 | Lowland | 2 | RM236–RM279 | 11 | Swamy et al. (2013) | |
| qDTY 2.2 | Aday Sel. | IR64 | Lowland | 2 | RM236–RM555 | 3 | Swamy et al. (2013) | |
| qDTY 2.2 | Aday Sel. | IR64 | Lowland | 2 | RM236–RM555 | 9 | Swamy et al. (2013) | |
| qDTY 2.2 | Kali Aus | MTU1010 | Upland | 2 | RM211–RM263 | 6 | Sandhu et al. (2014) | |
| qDTY 2.2 | Kali Aus | MTU1010 | Lowland | 2 | RM211–233A | 16 | Palanog et al. (2014) | |
| qDTY 2.3 | Kali Aus | IR64 | Upland | 2 | RM263–RM573 | 6 | Sandhu et al. (2014) | |
| qDTY 2.3 | Kali Aus | IR64 | Lowland | 2 | RM573–RM250 | 9 | Palanog et al. (2014) | |
| qDTY 3.1 | Apo | Swarna | Lowland | 3 | RM520–RM16030 | 31 | Venuprasad et al. (2009) | |
| qDTY 3.1 | IR55419-04 | TDK1 | Lowland | 3 | RM168–RM468 | 8 | Dixit et al. (2014) | |
| qDTY 3.1 | IR55419-04 | TDK1 | Upland | 3 | RM168–RM468 | 15 | Dixit et al. (2014) | |
| qDTY 3.2 | Aday Sel. | Sabitri | Lowland | 3 | RM569–RM517 | 23 | Yadav et al. (2013) | |
| qDTY 3.2 | N22 | Swarna | Lowland | 3 | RM60–RM22 | 19 | Vikram et al. (2011) | |
| qDTY 3.2 | Moroberekan | Swarna | Lowland | 3 | id3000019–id3000946 | 8 | Dixit et al. (unpublished) | |
| qDTY 3.2 | Moroberekan | Swarna | Upland | 3 | id3000019–id3000946 | 19 | Dixit et al. (unpublished) | |
| qDTY 4.1 | Aday Sel. | IR64 | Lowland | 4 | RM551–RM16368 | 11 | Swamy et al. (2013) | |
| qDTY 6.1 | Vandana | IR72 | Upland | 6 | RM589–RM204 | 40 | Venuprasad et al. (2012b ) | |
| qDTY 6.1 | Apo | IR72 | Upland | 6 | RM589-RM204 | 63 | Venuprasad et al. (2012b ) | |
| qDTY 6.1 | IR55419-04 | TDK1 | Lowland | 6 | RM586-RM217 | 9 | Dixit et al. (2014) | |
| qDTY 6.1 | IR55419-04 | TDK1 | Upland | 6 | RM586-RM217 | 36 | Dixit et al. (2014) | |
| qDTY 6.2 | IR55419-04 | TDK1 | Lowland | 6 | RM121-RM541 | 9 | Dixit et al. (2014) | |
| qDTY 6.2 | IR55419-04 | TDK1 | Upland | 6 | RM121-RM541 | 20 | Dixit et al. (2014) | |
| qDTY 9.1 | Aday Sel. | IR64 | Lowland | 9 | RM105-RN434 | 13 | Swamy et al. (2013) | |
| qDTY 9.1 | Aday Sel. | IR77298-5-6-B-11 | Lowland | 9 | RM105-RM434 | 19 | Swamy et al. (2013) | |
| qDTY 10.1 | MTU1010 | N22 | Lowland | 10 | RM216–RM304 | 5 | Vikram et al. (2011) | |
| qDTY 10.2 | Aday Sel. | IR64 | Lowland | 10 | RM269–G2155 | 17 | Swamy et al. (2013) | |
| qDTY 11.1 | Moroberekan | Swarna | Upland | 11 | id11002304–id11006765 | 25 | Dixit et al. (unpublished) | |
| qDTY 12.1 | IR74371-46-1-1 | Sabitri | Lowland | 12 | RM28166–RM28199 | 24 | Mishra et al. (2013) | |
| qDTY 12.1 | Way Rarem | Vandana | Upland | 12 | RM28048–RM28166 | 33 | 51 | Bernier et al. (2007) |
Modified from from Kumar A, Dixit S, Henry A. 2013. Marker-assisted introgression of major QTLs for grain yield under drought in rice. In: Varshney RK, Tuberosa R, eds. Translational genomics for crop breeding: abiotic stress, yield and quality, Vol. 2. ©2013 John Wiley and Sons, Inc. with permission.
QTLs for high grain yield under reproductive-stage drought
The objective of any QTL identification programme should be the ultimate use of the identified QTLs for marker-assisted breeding. However, in the case of drought tolerance, the proportion of QTLs identified and used for MAS differs greatly. Although the term marker-assisted selection was first used in the literature more than two decades ago (Beckmann and Soller, 1986), very few studies have been able to use the identified QTLs for MAS and report them in publically available literature. A large proportion of QTL identification studies have targeted secondary traits related to drought tolerance (Babu et al., 2003; Lanceras et al., 2004). Some of the studies in the past have also focused on grain yield (Kumar et al., 2007). However, very few studies have actually been able to use the identified QTLs for MAS.
In the case of rice, the choice of parents to develop mapping populations in a majority of these studies has been based on the trait of interest targeted for the study. For example, the selection of highly drought-susceptible parents adapted to the lowland ecosystem as recipient parents for a QTL identification study under direct-seeded upland conditions may allow the identification of large-effect QTLs but will definitely limit the future use of the identified QTLs. On the other hand, a desirable QTL allele with a large effect in a non-elite genetic background may not offer any improvement in the elite genetic background because the allele may already be ubiquitous in current varieties (Collins et al., 2008).
The lack of repeatability of QTL effects across different populations—QTL×genetic background interaction (Q×G)—and across environments—QTL×environmental interaction (Q×E)—has been another factor limiting the use of QTLs in molecular breeding (Price et al., 2002; Courtois et al., 2003; Lafitte et al., 2004; Bernier et al., 2008). This demands that donor and recipient varieties be selected with appropriate consideration. The recipient variety for QTL studies should be an improved, high-yielding, drought-susceptible variety popular in the drought-prone environment. Due consideration for growth duration in addition to its drought tolerance, as well as resistance to insects and diseases, should be given when selecting a donor.
Using grain yield under reproductive-stage drought as a selection criterion, a number of large-effect QTLs for grain yield under reproductive-stage drought for both upland and lowland conditions have been identified. Table 5 presents a summary of such QTLs reported in rice. qDTY 12.1 was the first reported large-effect QTL for grain yield under reproductive-stage drought (Bernier et al., 2007). This QTL was identified in a population of 436 random F3-derived lines from a cross between upland rice cultivars Vandana and Way Rarem. Located between RM28048 and RM28166, this QTL explained an R 2 of 33% under severe upland reproductive-stage drought conditions. Later on, qDTY 12.1 was also identified to show a similar high effect in lowland reproductive-stage drought in an IR74371-46-1-1/Sabitri population (Mishra et al., 2013).
qDTY 2.1 and qDTY 3.1, two large-effect QTLs affecting grain yield under lowland reproductive-stage drought, were identified in a BIL population derived from a cross of high-yielding lowland rice variety Swarna and upland rice variety Apo. Both QTLs showed a very high effect under severe lowland reproductive-stage drought (R 2=16.3% and 30.7%). The effect of both these QTLs was also seen on other traits such as DTF and PHT. BSA was successfully used for the first time to identify such large-effect QTLs for grain yield under reproductive-stage drought (Venuprasad et al., 2009). qDTY 6.1, another large-effect QTL for grain yield under favourable aerobic and irrigated lowland conditions, was identified in this population (Venuprasad et al., 2012a ). This QTL explained an R 2 of up to 66% and 39%, respectively, under upland and lowland non-stress conditions.
A series of experiments began on F3-derived populations developed from the cross of drought-tolerant donor N22 with high-yielding mega-varieties Swarna, IR64, and MTU1010 that resulted in the identification of qDTY 1.1, a large-effect QTL having an effect on grain yield under severe lowland reproductive-stage drought across these three populations. This QTL showed an R 2 of 13.4, 16.9, and 12.6% across two seasons of screening under severe lowland drought in N22/Swarna, N22/IR64, and N22/MTU1010 populations, respectively (Vikram et al., 2011). QTLs for grain yield under reproductive-stage drought at this locus have also been reported in other populations derived from crosses of CT9993-5-10-1-M/IR62266-42-6-2 and Apo/IR64 (Kumar et al., 2007; Venuprasad et al., 2012a ). In an IR64 background, four large-effect QTLs, qDTY 2.2, qDTY 4.1, qDTY 9.1, and qDTY 10.1, were identified in an Aday Sel/*4 IR64 BIL population (Swamy et al., 2013). Similarly, qDTY 3.2 was identified to show a large effect in an IR77298-14-1-2-10/Sabitri population (Yadaw et al., 2013).
In terms of QTLs, qDTY 1.1 showed an effect against three genetic backgrounds (Swarna, IR64,and MTU1010) from donor N22 (Vikram et al., 2011) and against two genetic backgrounds (Swarna and IR64) from donor Dhagaddeshi (Ghimire et al., 2012) in the lowland ecosystem and in the background of IR64 from donor Apo in the upland ecosystem (Venuprasad et al., 2012a ). qDTY 3.1 showed an effect in lowland against Swarna (Venuprasad et al., 2009) and BR11 (IRRI, unpublished) from donor Apo. qDTY 2.2 showed an effect in IR64 from donor Aday Sel (Swamy et al., 2013) in lowland and from Kali Aus in upland. qDTY 3.2 showed an effect against Sabitri from donor IR77298-14-1-2 in lowland (Yadaw et al., 2013) and in upland against Way Rarem from donor Vandana (Bernier et al., 2007). qDTY 6.1 showed an effect against Swarna in upland from donor Apo (Venuprasad et al., 2012b ) and in lowland against recipient variety TDK1 from donor IR55419-04 9 (Dixit et al., 2014). qDTY 12.1 showed an effect against Vandana from donor Way Rarem (Bernier et al., 2007) in lowland and against recipient variety Sabitri in lowland from donor IR74371-46-1-1 (Mishra et al., 2013).
In summary, the studies identified four QTLs (qDTY 1.1, qDTY 2.1, qDTY 3.1, and qDTY 6.1) to show an effect against Swarna, a popular variety in India, Nepal, and Bangladesh; six QTLs (qDTY 1.1, qDTY 2.2, qDTY 3.2, qDTY 4.1, qDTY 9.1, and qDTY 10.1) to show an effect in the background of IR64, a popular variety in many countries of South and Southeast Asia; two QTLs (qDTY 3.2 and qDTY 12.1) to show an effect against the background of Sabitri, a popular variety from Nepal; three QTLs (qDTY 3.1, qDTY 6.1, and qDTY 6.2) to show an effect against TDK1, a popular variety from Laos; and one QTL (qDTY 3.1) to show an effect against BR11, a popular variety from Bangladesh.
QTL interactions with genetic background and environment
G×E interactions have always played a major role in the development of drought-tolerant crop varieties. The complexity of genetic control of these traits leads to large differences in the performance of lines across variable environments. However, for MAS to be worthwhile, it is important that the identified QTLs show large and consistent effects under varying environmental conditions and across a wide range of genetic backgrounds (Bernier et al., 2009; Vikram et al., 2011). It is therefore important that the QTLs have a genetic effect large enough to be effective across a variety of environmental conditions and drought intensities. One way to overcome this could be to choose a recipient parent suitable for the target environment to develop the mapping population and screen the mapping population in the target environment under naturally occurring drought stress conditions. However, the surety of achieving the desired level of drought stress in the field in the rainy season is much less. In such cases, it is often advantageous to screen mapping populations under managed drought stress conditions to identify QTLs and to validate the QTL effect by screening the full set or a subset of the mapping population in the target environment. Bernier et al. (2009), from 21 experiments conducted at the IRRI and in eastern India, confirmed that qDTY 12.1 showed an increased effect with increasing severity of drought stress. Similarly, two large-effect QTLs (qDTY 12.1 and qDTY 3.2) identified in two different populations were validated for their effect in Nepal by phenotyping the full mapping population in Nepal in the second season (Mishra et al., 2013; Yadaw et al., 2013).
Another major limitation in the use of QTLs in MAS despite their large effects is their specificity to genetic backgrounds. It may be very advantageous if QTLs with large effects show an effect across multiple genetic backgrounds. QTL studies for grain yield under drought have allowed the identification of at least seven QTLs that have shown an effect across multiple genetic backgrounds: qDTY 1.1, qDTY 2.2, qDTY 2.3, qDTY 3.1, qDTY 3.2, qDTY 6.1, and qDTY 12.1. Genotyping strategies such as BSA also make it possible to screen a large number of mapping populations simultaneously for the presence of a QTL affecting grain yield in more than one background. Table 5 summarizes the effect of QTLs identified in a particular genetic background from different donors under different ecosystems. It has been observed that the effect of the same QTL varies with donors and recipients, as well as with the environment in which it is detected. For example, one of the most consistent QTLs, qDTY 1.1, contributed by donor N22, was identified in the background of mega-varieties MTU1010, IR64, and Swarna (Vikram et al., 2011). This QTL was also contributed by another donor, Dhagaddeshi, to IR64 and Swarna (Ghimire et al., 2012). In both studies, the QTL was identified using BSA.
The interaction of QTLs with genetic backgrounds has been a major limitation in the use of QTLs for MAS. Epistatic interactions play an important role in determining the level of effect of a QTL across genetic backgrounds. This phenomenon is also observed with DTY QTLs. The effect of genetic background can most clearly be observed in the case of qDTY 12.1. Despite being one of the largest QTLs reported for grain yield under reproductive-stage drought, explaining 51% of the genetic variation, a study of epistatic interaction in a Vandana/Way Rarem population showed two loci (qDTY 2.3 and qDTY 3.2) to be interacting with qDTY 12.1 and significantly enhancing the yield of qDTY 12.1-positive lines (Dixit et al., 2012b ).
Marker-assisted breeding with DTY QTLs and products developed
The rapid development of drought-tolerant versions of popular varieties can be one of the strategies to ensure rice production under reproductive-stage drought without compromising on yield potential and the preferences of farmers and consumers. Moreover, because of the low positive correlation between high yield potential and grain yield under reproductive-stage drought, marker-assisted breeding using well-defined QTLs allows precise combining of high yield potential and good yield under reproductive-stage drought. Apart from this, marker-assisted breeding also allows rapid product development with reduced efforts and with relatively smaller segregant populations. However, marker-assisted breeding for drought tolerance requires careful planning from the start of the QTL identification process.
Large-scale QTL identification and introgression programmes in different popular drought-susceptible varieties showed the specific compatibility of QTLs in terms of yield under reproductive-stage drought. Some of these large-effect QTLs showed an effect in a majority of the genetic backgrounds, stress severities, and ecosystems, while others showed more specificity for these factors. It therefore becomes important to characterize the compatibility of these QTLs in different genetic backgrounds and environments. For example, the combination of qDTY 12.1 with qDTY 2.3 and qDTY 3.2 led to a higher yield advantage than in lines with qDTY 12.1 alone under upland stress conditions in a Vandana/Way Rarem F3-derived population (Dixit et al., 2012b ). This combination of QTLs also led to an advantage under lowland stress conditions in which the effect of qDTY 12.1 alone was not observed. Similarly, in an IR64 background, lines with qDTY 2.2 and qDTY 4.1 showed a higher yield advantage under reproductive-stage drought than lines with four QTLs (qDTY 2.2, qDTY 4.1, qDTY 9.1, and qDTY 10.1) under lowland stress conditions (Swamy et al., 2013). The combination of qDTY 1.1, qDTY 2.1, and qDTY 3.1 together has been found more advantageous than having one or two QTL combinations in a Swarna–Sub1 background. Efforts are being made to bring these QTLs together in one genetic background to understand their interactive effects on grain yield under reproductive-stage drought.
QTL identification studies at the IRRI identified a set of QTLs with large effects in the background of rice varieties Swarna, IR64, TDK1, Sabitri, and BR11, and enlisted the set of QTLs that should be used to improve varieties for grain yield under reproductive-stage drought in lowland (qDTY 1.1, qDTY 2.2, qDTY 3.1, qDTY 3.2, and qDTY 12.1) and upland (qDTY 2.3, qDTY 3.2, and qDTY 12.1). Although the set of identified QTLs mentioned above will bring about yield improvement under reproductive-stage drought in a majority of the high-yielding backgrounds, it is not necessary that they be the best combination of QTLs for every background. This is due to the interaction between the QTLs and the genetic background of the recipient varieties. In such cases, the development of a BC1F3 BIL population to identify the best QTL combination before proceeding further in the pyramiding programme could be an appropriate strategy.
In many cases, DTY QTLs link tightly to traits such as plant height and earliness. It becomes important to develop large BCnF1 (n being the number of backcrosses) populations in each cycle of backcrossing to allow enough recombination to break these linkages. It is also required that a large number of BCnF2 segregants with different QTL combinations be selected and precisely phenotyped under reproductive-stage drought as against the selection of fewer plants practised for traits with simpler genetic control. Proper drought phenotyping of different combinations of QTLs allows the selection of lines with a positive interaction between different QTLs and the genetic background of the recipient variety, allowing breeders to capture a high yield advantage under reproductive-stage drought.
Table 6 presents a list of varieties improved or being improved by the introgression of DTY QTLs at the IRRI. A marker-assisted breeding programme for seven popular varieties, Swarna, IR64, Vandana, Sabitri, TDK1, Anjali, and Sambha Mahsuri, was undertaken. For Swarna, a combination of three QTLs (qDTY 1.1, qDTY 2.1, and qDTY 3.1) was pyramided along with Sub1, the large-effect QTL for tolerance of submergence. Lines tolerant of both drought and submergence are at the final stages for testing in the target environment. Similarly, IR64 introgression lines with qDTY 2.2 and qDTY 4.1 were developed and have been tested in a wide range of environments for tolerance of drought. Two upland rice varieties were also improved through marker-assisted introgression of DTY QTLs: Vandana and Anjali. qDTY 12.1 was introgressed in Vandana, and qDTY 12.1 and qDTY 3.1 were introgressed in Anjali. Three large-effect QTLs (qDTY 3.1, qDTY 6.1, and qDTY 6.2) were identified and introgressed in TDK1, a popular variety from Lao PDR. Similar to Swarna, these QTLs were pyramided along with Sub1 to confer tolerance of both drought and submergence. Two other varieties, Sabitri and Sambha Mahsuri, that are popular in Nepal and in India, respectively, are also in the marker-assisted breeding pipeline. Sabitri is being introgressed with qDTY 3.2 and qDTY 12.1. The effect of these two QTLs in Nepal has already been validated in two separate QTL identification programmes (Mishra et al., 2013; Yadaw et al., 2013). The combination of qDTY 2.2 and qDTY 4.1 is being introgressed into Sambha Mahsuri. A large-scale QTL introgression programme is also underway to introgress six DTY QTLs from different sources along with Sub1 in IR64.
Table 6.
List of DTY QTLs pyramided in the background of popular rice varieties through marker-assisted breeding along with QTLs for tolerance of other stresses
| Variety | Target ecosystem | DTY QTLs used | Other QTLs | Current stage |
|---|---|---|---|---|
| IR64 | Rainfed lowland | qDTY 2.2, qDTY 4.1 | Released in Nepal, identified for release in India, tested for release in Bangladesh | |
| Swarna | Rainfed lowland | qDTY 1.1, qDTY 2.1, qDTY 3.1 | Sub1 | Testing and validation in progress |
| Vandana | Rainfed upland | qDTY 12.1 | Testing and validation in progress | |
| Sabitri | Rainfed lowland | qDTY 3.2, qDTY 12.1 | Introgression ongoing | |
| Anjali | Rainfed upland | qDTY 3.1, qDTY 12.1 | Testing and purification in progress | |
| TDK1 | Rainfed lowland | qDTY 3.1, qDTY 6.1, qDTY 6.2 | Sub1 | Testing and purification in progress |
| Sambha Mahsuri | Rainfed lowland | qDTY 2.2, qDTY 4.1 | Testing and purification in progress | |
| IR64 | Rainfed lowland | qDTY 1.1, qDTY 1.2, qDTY 2.2, qDTY 12.1, qDTY 2.3, qDTY 3.2 | Sub1 | Testing and purification in progress |
The performance of DTY QTL introgressed lines in South Asia
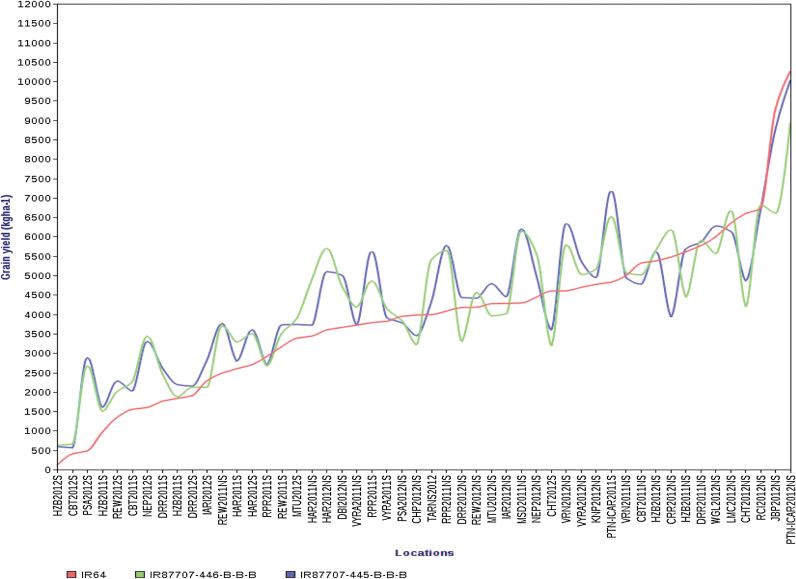
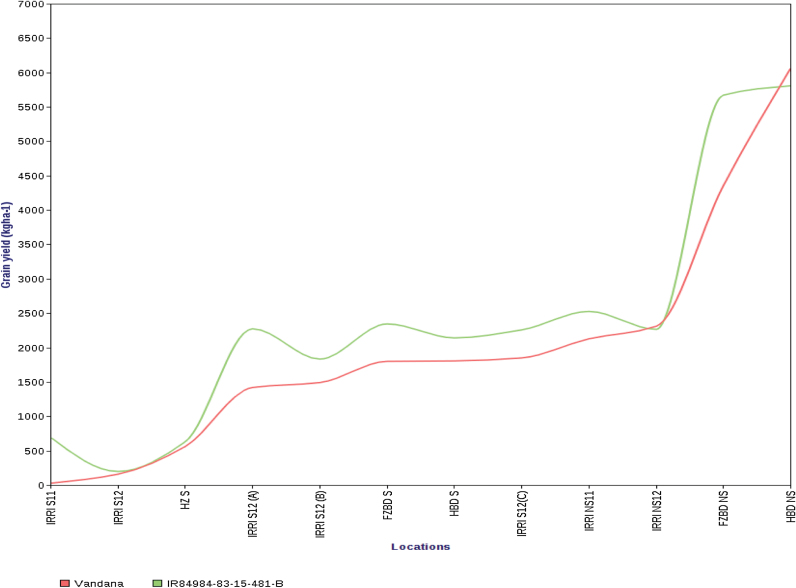
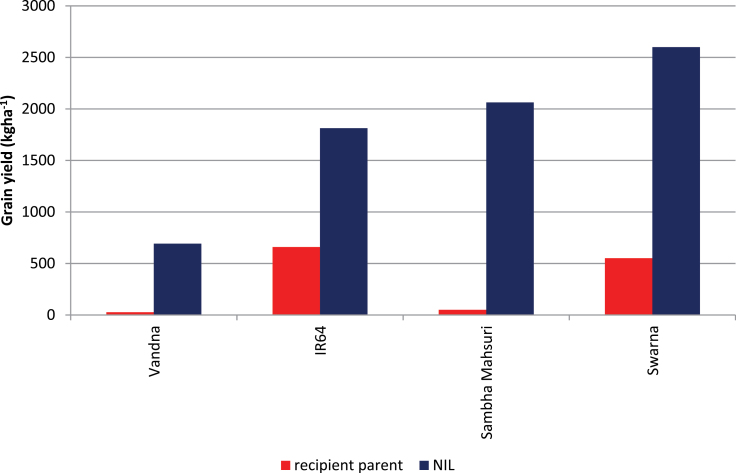
IR64 lines introgressed with qDTY 2.2 and qDTY 4.1 (IR87707-445-B-B-B and IR87707-446-B-B-B) were tested in 51 experiments conducted across India and Nepal. Both lines showed increased yield at most of the sites, where drought conditions varied from irrigated conditions with no drought to mild drought and moderate drought, to very severe drought (Fig. 3). IR87707-445-B-B-B has been identified for release in India and IR87707-446-B-B-B has been identified for release in Nepal (see mean and LSD0.05 details in Supplementary Table S1 at JXB online). Under severe reproductive-stage drought stress conditions in a rainout shelter as well as in the field, the IR64 NILs showed a yield advantage of 100–500 percentage points over the recurrent parent IR64 (Table 7). In a farmers’ preference score conducted in Nepal, the IR64 introgressed lines showed a higher preference score of +0.34 and +0.43 for IR87707-446-B-B-B and +0.15 and +0.27 for IR87707-445-B-B-B as against –0.03 and –0.05 for IR64 in 2011 and 2012, respectively. This is the first product developed through marker-assisted breeding of DTY QTLs released for commercial cultivation. A Vandana introgressed line with qDTY 12.1, IR84984-83-15-481-B, outperformed Vandana under all trials in upland conditions and had a high yield similar to that of Vandana under lowland irrigated non-stress conditions (Fig. 4; Supplementary Table S2). The introgression of different combinations of DTY QTLs has also allowed researchers to understand the effect of specific combinations of the QTLs and the yield improvement achieved in different cultivars. For example, the introgression of one QTL in Vandana led to an increase in yield of 0.5 t ha–1 under drought, and the introgression of two QTLs led to an increase in yield of >1.0 t ha–1 in IR64 and Sambha Mahsuri, whereas the introgression of three QTLs in Swarna showed a yield advantage of >1.5 t ha–1 under drought (Fig. 5).
Fig. 3.
Performance of NILs IR87707-445-B-B-B and IR87707-446-B-B-B compared with IR64 in 51 experiments conducted under varying levels of drought stress and non-stress conditions across India and Nepal. Experiments are arranged in order of increasing mean yield of IR64, classifying HZB2012S–DRR2012S as severe stress experiments, IARI2012S–RPR2011S as moderate stress experiments, REW2011S–TARNS2012 as mild stress experiments, and RPR2011NS–PTN-ICAR2012NS as non-stress experiments. (This figure is available in colour at JXB online.)
Table 7.
Percentage yield advantage of IR64 NILs over recurrent parent IR64 under severe and moderate drought in rainout shelter and field conditions across eight locations in India in 2012
| Location | Stress intensity | Percentage advantage over IR64 | |
|---|---|---|---|
| IR87707-445-B- B-B | IR87707- 446-B-B-B | ||
| Hazaribagh | Severe | 396.6 | 362.2 |
| Coimbatore | Severe | 38.6 | 61.1 |
| Pusa | Severe | 500.0 | 433.3 |
| Hyderabad | Severe | 12.9 | 13.2 |
| New Delhi | Moderate | 22.6 | 2.4 |
| Patna | Moderate | 48.8 | 13.0 |
| Rewa | Moderate | 69.0 | 82.6 |
| Maruteru | Moderate | 10.4 | 3.9 |
Fig. 4.
Performance of IR84984-83-51-481-B compared with Vandana in 12 experiments conducted under varying levels of drought stress and non-stress conditions at the IRRI and in India. Experiments are arranged in order of increasing mean yield of Vandana, classifying IRRIS11–HZS as severe stress experiments, IRRIS12(A)–IRRIS12(C) as moderate stress experiments, and IRRINS11–HBDNS as non-stress experiments. (This figure is available in colour at JXB online.)
Fig. 5.
Yield gains under drought obtained in rice through introgression and pyramiding of QTLs with additive effects in high-yielding genetic backgrounds. (This figure is available in colour at JXB online.)
Concluding comments
Parallel cultivation of rice in two diverse ecosystems, upland and lowland, has allowed the evolution of the crop in two very diverse environments. On the one hand, some upland-adapted drought-tolerant rice varieties are characterized by traits such as early flowering and root systems suitable for dry conditions, whereas, on the other hand, some drought-susceptible lowland-adapted rice varieties are characterized by medium to late maturity, high input responsiveness, and specificity to anaerobic growing environments. The existence of such large diversity for drought tolerance puts rice in a unique position, with much higher genetic diversity available for drought tolerance. The presence of conserved regions conferring drought tolerance in upland rice and the high susceptibility of high-yielding post-Green Revolution varieties provide unique opportunities for plant breeders to move drought tolerance alleles from upland drought-tolerant donors to lowland drought-susceptible rice varieties. Swamy et al. (2011), through a study on a panel of random drought-tolerant donors for the identified drought yield QTLs, reported the presence of qDTY 12.1 in 85% of the lines, followed by qDTY 4.1 in 79% of the lines and qDTY 1.1 in 64% of the lines, thus validating the high presence of these identified QTLs in drought-tolerant donors. Advances in molecular biology have provided new opportunities for breeders to identify such regions, refine these regions through fine mapping, and move those regions into drought-susceptible varieties, an opportunity that was not available a few years back to break the yield improvement barrier under drought.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Difference in plant types and drought response of upland-adapted and lowland-adapted cultivars under severe drought.
Table S1. Mean grain yield (kg ha–1) of IR87707-445-B-B-B, IR87707-446-B-B-B, and IR64, and LSD0.05 values for experiments conducted across India and Nepal.
Table S2. Mean grain yield (kg ha–1) of IR84984-83-15-481-B and Vandana, and LSD0.05 values for experiments conducted across India and at IRRI.
Acknowledgements
The authors thank the Generation Challenge Program (GCP), Mexico; the Bill & Melinda Gates Foundation (BMGF); and BMZ, Germany, for financial support for QTL identification and introgression work. We thank the All India Coordinated Rice Improvement Program (AICRIP) and associated scientists for the evaluation of IR64 NILs at AICRIP locations in India.
Glossary
Abbreviations:
- AB
advanced backcross
- BIL
backcross inbred line
- BSA
bulk segregant analysis
- CIM
composite interval mapping
- DAS
days after sowing
- DAT
days after transplanting
- DTF
days to 50% flowering;
- GY
grain yield
- NS
irrigated non-stress
- LR
leaf rolling
- MAS
marker-assisted selection
- NIL
near-isogenic line
- PHT
plant height
- PVC
polyvinyl chloride
- Q×E
QTL×environment
- Q×G
QTL×genetic background
- RIL
recombinant inbred line
- RS
reproductive-stage drought stress
- SG
selective genotyping
- SIM
simple interval mapping
- SMA
single marker analysis
- SSD
single seed descent
- WGG
whole-genome genotyping.
References
- Babu RC, Nguyen BD, Chamarerk V, et al. 2003. Genetic analysis of drought resistance in rice by molecular markers: association between secondary traits and field performance. Crop Science 43, 1457–1469. [Google Scholar]
- Beckmann JS, Soller M. 1986. Restriction fragment length polymorphisms and genetic improvement of agricultural species. Euphytica 35, 111–124. [DOI] [PubMed] [Google Scholar]
- Bernier J, Atlin GN, Serraj R, Kumar A, Spaner D. 2008. Breeding upland rice for drought resistance. Journal of the Science of Food and Agriculture 88, 927−–939. [Google Scholar]
- Bernier J, Kumar A, Venuprasad R, et al. 2009. Characterization of the effect of a QTL for drought resistance in rice, qtl12.1, over a range of environments in the Philippines and eastern India. Euphytica 166, 207–217. [Google Scholar]
- Bernier J, Kumar A, Venuprasad R, Spaner D, Atlin GN. 2007. A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Science 47, 507–516. [Google Scholar]
- Churchill GA, Doerge RW. 1994. Empirical threshold values for quantitative trait mapping. Genetics 138, 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Tardieu F, Tuberosa R. 2008. Quantitative trait loci and crop performance under abiotic stress: where do we stand? Plant Physiology 147, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois B, Shen L, Petalcorin, Carandang S, Mauleon R, Li ZK. 2003. Locating QTLs controlling constitutive root traits in the rice population IAC 165×Co39. Euphytica 134, 335–345. [Google Scholar]
- Dixit S, Mallikarjuna Swamy BP, Vikram P, Ahmed HU, Sta Cruz MT, Amante M, Atri D, Leung H, Kumar A. 2012a. Fine mapping of QTLs for rice grain yield under drought reveals sub-QTLs conferring a response to variable drought severities. Theoretical and Applied Genetics 125, 155–169. [DOI] [PubMed] [Google Scholar]
- Dixit S, Singh A, Sta Cruz MT, Maturan PT, Amante M, Kumar A. 2014. Multiple major QTL lead to stable yield performance of rice cultivars across varying drought intensities. BMC Genetics 15, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit S, Swamy BPM, Vikram P, Bernier J, Sta Cruz MT, Amante M, Atri D, Kumar A. 2012b. Increased drought tolerance and wider adaptability of qDTY12.1 conferred by its interaction with qDTY 2.3 and qDTY 3.2 . Molecular Breeding 30, 1767–1779. [Google Scholar]
- Ghimire KH, Quiatchon LA, Vikram P, Mallikarjuna Swamy BP, Dixit S, Ahmed H, Hernandez JE, Borromeo TH, Kumar A. 2012. Identification and mapping of a QTL (qDTY 1.1) with a consistent effect on grain yield under drought. Field Crops Research 131, 88–96. [Google Scholar]
- Henry A, Gowda VRP, Torres RO, McNally KL, Serraj R. 2011. Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Research 120, 205–214. [Google Scholar]
- Joehanes R, Nelson JC. 2008. QGene 4.0, an extensible Java QTL-analysis platform. Bioinformatics 24, 2788–2789. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN. 2008. Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crops Research 107, 221–231. [Google Scholar]
- Kumar A, Dixit S, Henry A. 2013. Marker-assisted introgression of major QTLs for grain yield under drought in rice. In: Varshney RK, Tuberosa R, eds. Translational genomics for crop breeding: abiotic stress, yield and quality, Vol. 2 Chichester, UK: Wiley, 47. [Google Scholar]
- Kumar R, Venuprasad R, Atlin GN. 2007. Genetic analysis of rainfed lowland rice drought tolerance under naturally-occurring stress in eastern India: heritability and QTL effects. Field Crops Research 103, 42–52. [Google Scholar]
- Kumar A, Verulkar SB, Dixit S, Chauhan B, Bernier J, Venuprasad R, Zhao D, Shrivastava MN. 2009. Yield and yield-attributing traits of rice (Oryza sativa L.) under lowland drought and suitability of early vigor as a selection criterion. Field Crops Research 114, 99–107. [Google Scholar]
- Kumar A, Verulkar SB, Mandal NP, et al. 2012. High-yielding, drought-tolerant, stable rice genotypes for the shallow rainfed lowland drought-prone ecosystem. Field Crops Research 133, 37−–47. [Google Scholar]
- Lafitte HR, Price AH, Courtois B. 2004. Yield response to water deficit in an upland rice mapping population: associations among traits and genetic markers. Theoretical and Applied Genetics 109, 1237–1246. [DOI] [PubMed] [Google Scholar]
- Lanceras JC, Pantuwan G, Jongdee B, Toojinda T. 2004. Quantitative trait loci associated with drought tolerance at reproductive stage in rice. Plant Physiology 135, 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz RJ, Soller M, Beckmann JS. 1987. Trait-based analyses for the detection of linkage between marker loci and quantitative trait loci in crosses between inbred lines. Theoretical and Applied Genetics 73, 556–562. [DOI] [PubMed] [Google Scholar]
- Manly KF, Cudmore Robert H, Jr, Meer JM. 2001. Map Manager QTX, cross-platform software for genetic mapping. Mammalian Genome 12, 930–932. [DOI] [PubMed] [Google Scholar]
- Michelmore RW, Paran I, Kesseli RV. 1991. Identification of markers linked to disease resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proceedings of the National Academy of Sciences, USA 88, 9828–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra KK, Vikram P, Yadaw RB, Swamy BPM, Dixit S, Sta Cruz MT, Maturan P, Marker S, Kumar A. 2013. qDTY 12.1: a locus with a consistent effect on grain yield under drought in rice. BMC Genetics 14, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole JC. 1982. Adaptation of rice to drought prone environments. In: Drought resistance in crops with emphasis on rice . IRRI, Los Baños, Philippines, 195–213. [Google Scholar]
- Palanog AD, Mallikarjuna Swamy BP, Shamsudin NAA, Dixit S, Hernandez JE, Boromeo TH, Sta. Cruz PC, Kumar A. 2014. Grain yield QTLs with consistent-effect under reproductive-stage drought stress in rice. Field Crops Research 161, 46–54. [Google Scholar]
- Price AH, Cairns JE, Horton P, Jones RGW, Griffiths H. 2002. Linking drought-resistance mechanisms to drought avoidance in upland rice during a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. Journal of Experimental Botany 53, 989–1004. [DOI] [PubMed] [Google Scholar]
- Richards RA, Rebetzke GJ, Watt M, Condon AG, Spielmeye W, Dolferus R. 2010. Breeding for improved water productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment. Functional Plant Biology 37, 85–97. [Google Scholar]
- Sandhu N, Singh A, Dixit S, Sta Cruz MT, Maturan PC, Jain RK, Kumar A. 2014. Identification and mapping of stable QTL with main and epistasis effect on rice grain yield under upland drought stress. BMC Genetics 15, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BPM, Ahmed HU, Henry A, et al. 2013. Genetic, physiological, and gene expression analyses reveal that multiple QTL enhance yield of rice mega-variety IR64 under drought. PLoS One 8, e62795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BPM, Vikram P, Dixit S, Ahmed HU, Kumar A. 2011. Meta-analysis of grain yield QTL identified during agricultural drought in grasses showed consensus. BMC Genomics 12, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuprasad R, Bool ME, Quiatchon L, Atlin GN. 2012a. A QTL for rice grain yield in aerobic environments with large effects in three genetic backgrounds. Theoretical and Applied Genetics 124, 323–332. [DOI] [PubMed] [Google Scholar]
- Venuprasad R, Bool ME, Quiatchon L, Sta Cruz MT, Amante M, Atlin GN. 2012b. A large effect QTL for rice grain yield under upland drought stress on chromosome 1. Molecular Breeding 30, 535–547. [Google Scholar]
- Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, Sta Cruz MT, Amante M, Kumar A, Atlin GN. 2009. Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theoretical and Applied Genetics 120, 177–190. [DOI] [PubMed] [Google Scholar]
- Venuprasad R, Lafitte HR, Atlin GN. 2007. Response to direct selection for grain yield under drought stress in rice. Crop Science 47, 285–293. [Google Scholar]
- Venuprasad R, Sta Cruz MT, Amante M, Magbanua R, Kumar A, Atlin GN. 2008. Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crops Research 107, 232–244. [Google Scholar]
- Vikram P, Mallikarjuna Swamy BP, Dixit S, Ahmed HU, Sta Cruz MT, Singh AK, Kumar A. 2011. qDTY 1.1, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genetics 12, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadaw RB, Dixit S, Raman A, Mishra KK, Vikram P, Swamy BPM, Sta Cruz MT, Maturan PT, Pandey M, Kumar A. 2013. A QTL for high grain yield under lowland drought in the background of popular rice variety Sabitri from Nepal. Field Crops Research 144, 281–287. [Google Scholar]
- Yang J, Hu CC, Hu H, Yu R, Xia Z, Ye X, Zhu J. 2008. QTL Network: mapping and visualizing genetic architecture of complex traits in experimental populations. Bioinformatics 24, 721–723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.