Abstract
Although oral iron is the initial treatment approach for iron deficiency anemia (IDA), some patients fail to respond to or cannot tolerate oral iron. This double-blind safety and efficacy study of the intravenous (IV) iron, ferumoxytol, randomized patients with a history of unsatisfactory oral iron therapy, or in whom oral iron could not be used, to ferumoxytol (n = 609) or placebo (n = 203). The proportion of patients achieving the primary endpoint (hemoglobin increase ≥2.0 g/dL at Week 5) was 81.1% with ferumoxytol versus 5.5% with placebo (P < 0.0001). The mean increase in hemoglobin from Baseline to Week 5, a secondary endpoint (also the alternative preplanned primary efficacy endpoint for other health authorities), was 2.7 versus 0.1 g/dL (P < 0.0001). Achievement of a hemoglobin ≥12 g/dL, time to a hemoglobin increase ≥2.0 g/dL, and improvement in the Functional Assessment of Chronic Illness Therapy Fatigue score also significantly favored ferumoxytol over placebo at Week 5 (P < 0.0001). Ferumoxytol treatment-emergent adverse events were mainly mild to moderate. Ferumoxytol was effective and well tolerated in patients with IDA of any underlying cause in whom oral iron was ineffective or could not be used. This trial was registered at http://www.clinicaltrials.gov as #NCT01114139. Am. J. Hematol. 89:7–12, 2014. © 2013 Wiley Periodicals, Inc.
Introduction
The World Health Organization estimates that anemia affects ∼1.6 billion people worldwide, with ∼50% of anemia cases caused by iron deficiency 1,2. In the United States and worldwide, IDA continues to be one of the most common types of anemia. Blood loss from chronic gastrointestinal (GI) bleeding is the most common cause of iron deficiency anemia (IDA) in men and postmenopausal women 3,4. Patients with abnormal uterine bleeding (AUB), postpartum blood loss, GI disorders, and cancer commonly present with IDA due to a number of pathophysiological processes, including blood loss, malabsorption, inadequate intake, and inflammatory disease 1,5–7.
Anemia impacts patients' quality of life and is associated with a variety of well-recognized clinical consequences, including fatigue, low energy, exercise intolerance, decreased physical functioning, decreased vitality, feeling cold, reduced cognitive abilities, and cardiac dysfunction 8–13, as well as increased hospitalizations, blood transfusions, operative morbidity, and child and maternal mortality 7,14–16.
Although oral iron therapy is the initial approach to IDA treatment, some patients fail to respond adequately to this therapy, or frequently they do not tolerate oral iron and withdraw from therapy 12,17. In addition, oral iron absorption is reduced in many patient populations, such as those with cancer, chronic inflammatory disease, or infection 18.
When oral iron therapy fails, treatment with intravenous (IV) iron should be considered. While several IV irons have been used in patients with chronic kidney disease (CKD), particularly those on dialysis, at the time of the study iron dextran was the only IV iron product indicated for the treatment of iron deficiency in patients for whom oral iron is unsatisfactory, including those without CKD. Unfortunately, healthcare providers may be reluctant to use the iron dextrans to treat these patients because of the risk of life-threatening anaphylactic reactions, the limitation to low individual doses (100 mg), and the requirement for a test dose 19–21. Thus, there is an unmet need for additional therapies to replenish iron stores in these patients with IDA who cannot tolerate oral iron.
Ferumoxytol (Feraheme®, AMAG Pharmaceuticals, Lexington, MA) is a colloidal iron oxide, coated with a semisynthetic carbohydrate specifically designed to minimize immunological reactivity. In addition, ferumoxytol has been formulated to be isotonic, eliminating the disadvantages of high osmolality and the need for a prolonged, diluted infusion 22.
Ferumoxytol was approved in June 2009 by the US Food and Drug Administration (FDA) for the treatment of IDA in adults with CKD 23. Two Phase 3 trials evaluating ferumoxytol for the treatment of IDA in adults who have a history of unsatisfactory oral iron therapy or in whom oral iron could not be used have been completed, and a supplemental new drug application has been submitted to the FDA. Here, we present the results of one of these trials comparing ferumoxytol to placebo. The objective of this study was to evaluate the efficacy and safety of a 1.02-g course of IV ferumoxytol administered as two doses for the treatment of IDA in patients with IDA of any cause. The effect of ferumoxytol on patient-reported outcomes (PROs) compared with placebo was also evaluated.
Patients, Materials, and Methods
Study design
This randomized, double-blind, placebo-controlled, multicenter global clinical study was conducted at 182 sites in Canada, Hungary, India, Latvia, Poland, and the United States between June 2010 and February 2012 (http://ClinicalTrials.gov identifier: NCT01114139). The study consisted of a screening period of up to 2 weeks followed by a 5-week treatment period. The 5-week treatment period consisted of six study visits: Day 1 (Baseline, dose 1), Week 1 (dose 2; 2–8 days postdose 1), and weekly thereafter to Week 5. The study was conducted according to Good Clinical Practice guidelines and in compliance with the ethical principles of the Declaration of Helsinki, and was approved by the ethics committee or institutional review board of each participating center before the commencement of the study. All patients provided written informed consent.
Patients
Eligible patients were men and women ≥18 years of age with a history of IDA, defined as a hemoglobin (Hgb) level <10.0 g/dL and a transferrin saturation (TSAT) <20%, and a history of unsatisfactory oral iron therapy or in whom oral iron could not be used. Serum ferritin was not utilized as an entry criterion, because, although indicative of iron deficiency if low, for example, <100 ng/mL, it is an acute-phase reactant and may be artifactually elevated in the face of inflammation. Patients were not eligible for participation if they had a history of allergy to IV iron, a Hgb level ≤7.0 g/dL1, serum ferritin >600 ng/mL, known causes of anemia other than iron deficiency, active infection, hematologic malignancies, were on dialysis or had an estimated glomerular filtration rate <30 mL/min/1.73 m2, or were pregnant, intended to become pregnant, or were breastfeeding. Patients who received another investigational agent or IV iron therapy within 4 weeks of screening or who had received oral iron therapy or blood transfusion within 2 weeks before screening were also excluded.
Study medication
Patients were randomized using an Interactive Voice Randomization System in a 3:1 ratio to receive either parenteral ferumoxytol (1.02-g course) or placebo, and were stratified by Baseline Hgb level (>7.0 to ≤8.5 g/dL; >8.5 to <10.0 g/dL) and by categories of underlying condition (AUB, cancer, GI disorders, postpartum anemia, and Other [included patients with nutritional iron deficiency, heart failure, and/or rheumatoid arthritis]).
Patients received an IV injection of either ferumoxytol 510 mg (17 mL) or normal saline, administered as a rapid IV injection in under 1 min at Baseline (Day 1), with a second dose 2–8 days later. Blinding was accomplished by having both ferumoxytol and normal saline administered in a shrouded manner by an unblinded Test Article Administrator, while both study participants and all other study staff including the investigator were blinded to what was administered. In addition, investigators and their staff were blinded to the results of laboratory test results that could potentially unblind treatment. Ferumoxytol for IV injection was supplied by AMAG Pharmaceuticals, (Lexington, MA) and normal saline for IV injection was supplied by Hospira, Inc. (Lake Forest, IL).
Study endpoints and assessments
Primary efficacy endpoints and assessments
Blood samples to assess Hgb were performed at Screening, Baseline, and Weeks 2, 3, 4, and 5 and were blinded to the study staff. The primary efficacy endpoint was the proportion of patients achieving a Hgb increase of ≥2.0 g/dL at any time from Baseline to Week 5. To meet the requests of different health authorities, an additional alternative efficacy analysis was conducted. The primary efficacy endpoint for the alternative efficacy analysis was the mean change in Hgb from Baseline to Week 5.
Secondary efficacy endpoints and assessments
Secondary efficacy endpoints included the proportion of patients achieving a Hgb level ≥12 g/dL at any time from Baseline to Week 5, the mean change in TSAT from Baseline to Week 5, and the time to Hgb increase of ≥2.0 g/dL or to ≥12 g/dL from Baseline. All efficacy endpoints were obtained at Baseline and at Weeks 2, 3, 4, and 5. PROs included the mean change from Baseline to Week 5 in the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale score (obtained at Baseline and Weeks 1, 2, 3, 4, and 5), and the Vitality domain of the Short-Form-General Health Survey (SF-36) 24 and the Energy domain of the Linear Analogue Scale Assessment (QOL LASA) (obtained at Baseline and Weeks 3 and 5).
Subgroup analyses included an assessment of efficacy (i.e., the proportion of patients achieving a ≥2.0 g/dL increase in Hgb at any time from Baseline to Week 5; mean change in Hgb from Baseline to Week 5) among patients with various underlying disorders (i.e., AUB, cancer, GI disorders, postpartum anemia, and Other).
Safety endpoints and assessments
Patients in both treatment groups were closely monitored for 60 min following IV study drug administration for adverse events (AEs). The primary safety analysis was a descriptive comparison of AEs experienced by patients in both the ferumoxytol and placebo groups. Safety data included AEs, clinical laboratory evaluations, physical examinations, and vital signs. AEs were assessed by the investigators for severity (mild, moderate, and severe) and potential relationship to study medication. AEs were considered “serious” if they resulted in death, were life-threatening, resulted in hospitalization or persistent or significant disability or incapacity, or were considered an important medical event, that is, one that was not immediately life-threatening but clearly jeopardized the subject and/or required intervention.
Determination of sample size
A sample size of 800 patients (600 exposed to ferumoxytol and 200 exposed to placebo) provided 99% power for the assessment of superiority of ferumoxytol to placebo, assuming a two-sided α of 0.05, a placebo efficacy rate of 20%, and a ferumoxytol efficacy rate of 60% for the difference between treatment groups. A sample size of 600 patients exposed to ferumoxytol was calculated as sufficient to identify possible key safety concerns.
Statistical analysis
The intent-to-treat (ITT) Population (the primary efficacy analysis population) included any randomized patients with exposure to study drug (ferumoxytol or placebo) by randomized treatment assignment. The Safety Population included all randomized patients who received study drug based on actual treatment. The two populations were identical in this study. For the primary efficacy endpoint and other categorical endpoints, the P value was calculated using a Cochran-Mantel-Haenszel test, adjusted for Baseline Hgb level and underlying condition. Statistical significance (and superiority) was established if the P value was ≤0.05. Continuous efficacy endpoints were calculated using an analysis of covariance model, adjusted for Baseline Hgb level and underlying condition. Statistical significance was established if the P value was ≤ 0.05.
All data were analyzed by representatives of the clinical and biostatistical groups of AMAG Pharmaceuticals. All authors had access to the clinical trial data.
Results
Patient characteristics
A total of 812 patients were randomized (ferumoxytol, n = 609; placebo, n = 203). The ITT and Safety Populations were the same and included 808 patients (ferumoxytol, n = 608; placebo, n = 200). Details of patient disposition and patient flow are summarized in the figure in the Supporting Information. Baseline patient demographic and clinical characteristics of the two treatment groups were comparable, including mean Baseline Hgb levels (ferumoxytol 8.8 g/dL and placebo 8.9 g/dL) and mean Baseline TSAT levels (ferumoxytol 6.6% and placebo 5.4%) (see Supporting Information table).
Primary endpoint
Ferumoxytol demonstrated superiority to placebo, with 81.1% (493/608) of ferumoxytol-treated patients achieving an increase in Hgb of ≥2.0 g/dL from Baseline to Week 5 compared with only 5.5% (11/200) in the placebo group (treatment difference: 75.6%; P < 0.0001) (Table 1; Fig. 1). Cumulative response analysis showed that a higher percentage of ferumoxytol-treated patients had a Hgb increase of ≥2.0 g/dL compared with those given placebo at each treatment timepoint examined (i.e., Weeks 2, 3, 4, and 5) ( Fig. 1).
Table 1.
Summary of Primary and Secondary Efficacy Endpoints (Intent-to-Treat Population)
| Efficacy endpoint | Treatment groups | ||
|---|---|---|---|
| Ferumoxytol (n = 608) | Placebo (n = 200) | P | |
| Primary: | |||
| Proportion of patients with ≥2.0 g/dL Hgb increase at any time from Baseline to Week 5, n (%) | 493 (81.1) | 11 (5.5) | <0.0001a |
| Secondary: | |||
| Mean (SD) change in Hgb (g/dL) from Baseline to Week 5 | 2.6 (1.5) | 0.1 (0.9) | <0.0001b |
| Proportion of patients with Hgb level ≥12.0 g/dL at any time from Baseline to Week 5, n (%) | 307 (50.5) | 6 (3.0) | <0.0001a |
| Mean (SD) change in TSAT (%) from Baseline to Week 5 | 11.4 (15.1) | 0.4 (5.8) | <0.0001b |
| Mean (SD) change in FACIT-Fatigue score from Baseline to Week 5 | 11.7 (11.7) | 6.8 (9.5) | <0.0001b |
| Mean time (days) to Hgb increase of ≥2.0 g/dL or to an Hgb level of ≥12.0 g/dL from Baseline | 23.5 | 42.5 | <0.0001c |
P value for the treatment difference was from the Cochran-Mantel-Haenszel test, adjusted for Baseline Hgb level and underlying condition.
P value was derived from the least-squares mean and an analysis of covariance model, adjusted for Baseline Hgb and underlying condition.
P value was derived from log-rank statistic comparing homogeneity of survival curves between treatment groups.
FACIT, Functional Assessment of Chronic Illness Therapy; Hgb, hemoglobin; SD, standard deviation; TSAT, transferrin saturation.
Figure 1.
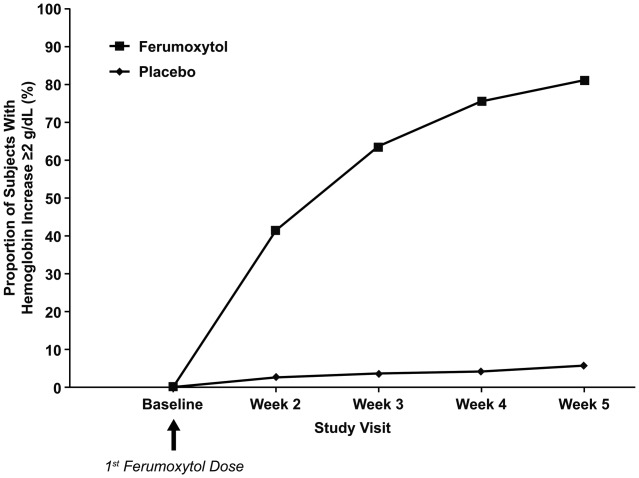
Proportion of patients with ≥2.0 g/dL increase in hemoglobin at any time from Baseline to Week 5 (intent-to-treat population).
Secondary efficacy endpoints
Secondary endpoint results are summarized in Table 1. Ferumoxytol was superior to placebo, with a clinically meaningful and statistically significantly greater increase in mean Hgb levels in the ferumoxytol group from Baseline to Week 5 (2.7 g/dL) compared with the placebo group (0.1 g/dL; treatment difference: 2.54 g/dL; P < 0.0001); this was also the predefined primary efficacy endpoint in the pre-defined alternative efficacy analysis. Ferumoxytol demonstrated superiority over placebo for the proportion of patients achieving a Hgb level ≥12 g/dL at any time from Baseline to Week 5 (50.5% vs. 3.0%, respectively; P < 0.0001). Ferumoxytol was also superior to placebo for the mean change in TSAT from Baseline (mean change: ferumoxytol, 11.0%; placebo, −0.1%; P < 0.0001). In addition, more ferumoxytol-treated patients achieved a ≥2.0 g/dL increase in Hgb or reached a Hgb level ≥12 g/dL over the course of the study than placebo-treated patients (82.4% vs. 6.0%, respectively; P < 0.0001). Among patients in the ferumoxytol group, the mean time from Baseline to achieve a Hgb increase of ≥2.0 g/dL or Hgb ≥12.0 g/dL was 23.5 days, compared with 42.5 days among those in the placebo group (P < 0.0001).
Patient-reported outcomes
Ferumoxytol was superior to placebo in the mean change in FACIT-Fatigue scores from Baseline to Week 5. Mean FACIT-Fatigue scores were similar between treatment groups at Baseline. Increases from Baseline in FACIT-Fatigue scores were observed in both treatment groups at Weeks 1 and 2, with larger increases seen for ferumoxytol-treated patients (P < 0.05; Fig. 2). At Weeks 3 through 5, FACIT-Fatigue scores continued to increase in the ferumoxytol group, while there was no further increase in the placebo group. By Week 5, the mean increase in FACIT-Fatigue scores for ferumoxytol-treated patients was 11.7, compared with an increase of only 6.8 for those receiving placebo (P < 0.0001) (Table 1). The correlation between FACIT-Fatigue scores and Hgb across the treatment period was high (r = 0.97; P = 0.002). Ferumoxytol was also associated with significantly greater improvements in the scores from Baseline to Week 5 in the Vitality domain of the SF-36 (10.6 vs. 5.4; P < 0.0001) and the Energy domain of the QOL LASA (19.7 vs. 10.3; P < 0.0001).
Figure 2.
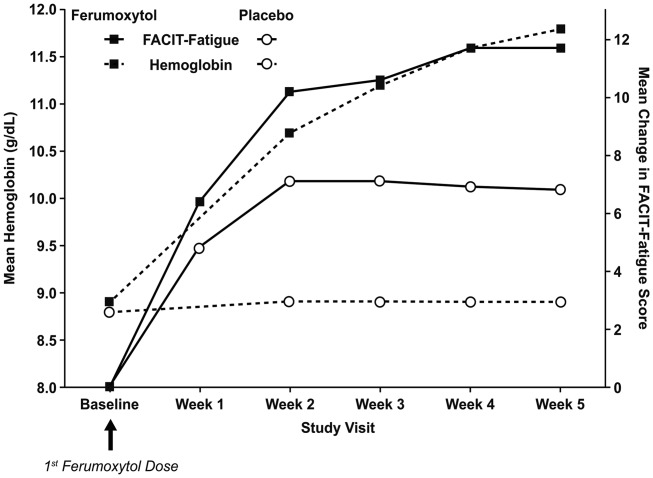
Mean change in hemoglobin and Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-Fatigue) score from Baseline to Week 5 by treatment group (intent-to-treat population).
Effect of underlying condition on hemoglobin increase
When patients were stratified according to underlying condition, ferumoxytol treatment demonstrated superiority over placebo in the proportion of patients achieving a ≥2.0 g/dL increase in Hgb at any time from Baseline to Week 5 in the AUB (ferumoxytol 87.3% vs. placebo 3.6%; P < 0.0001), GI disorders (ferumoxytol 82.1% vs. placebo 1.7%; P < 0.0001), and Other subgroups (ferumoxytol 73.9% vs. placebo 8.3%; P < 0.0001). A positive trend in favor of ferumoxytol was also demonstrated in the Cancer subgroup compared with placebo (ferumoxytol 51.7% vs. placebo 30.0%; P = 0.2478), although the difference was not statistically significant. Insufficient numbers of patients with postpartum anemia were enrolled to allow for subgroup analysis.
Safety
Overall, ferumoxytol was well tolerated in this study, with the majority of treatment-emergent AEs (TEAEs) being mild-to-moderate in intensity. The rates of AEs and treatment-related AEs were, as expected, higher in the ferumoxytol treatment group (49.2%) compared with the placebo group (43.0%) (Table 2), but no pattern or trend was observed to suggest a specific safety signal. The most common TEAEs reported in ≥2% of patients are summarized in Table 2, with no single AE exceeding 6%. The only TEAEs that were considered by the investigators to be treatment-related that occurred in >1% of patients in the ferumoxytol group were nausea (2.3%), headache (1.8%), hypersensitivity/drug hypersensitivity (1.3%), and dizziness (1.3%). AEs resulting in discontinuation occurred in 2.0% of ferumoxytol recipients and 0.5% of placebo recipients.
Table 2.
Summary of TEAEs by Treatment Group (Safety Population)
| AE category | Treatment group | |||||
|---|---|---|---|---|---|---|
| Ferumoxytol (n = 608) | Placebo (n = 200) | Total (N = 808) | ||||
| Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | |
| All TEAEs | 718 | 299 (49.2) | 206 | 86 (43.0) | 924 | 385 (47.6) |
| Treatment-related AEs | 176 | 89 (14.6) | 25 | 15 (7.5) | 201 | 104 (12.9) |
| SAEs | 23 | 16 (2.6) | 6 | 6 (3.0) | 29 | 22 (2.7) |
| Treatment-related SAEs | 4 | 4 (0.7) | 0 | 0 (0.0) | 4 | 4 (0.5) |
| Protocol-defined AEs of special interesta | 26 | 22 (3.6) | 2 | 2 (1.0) | 28 | 24 (3.0) |
| Cardiovascular AE composite endpointb | 6 | 5 (0.8) | 0 | 0 (0.0) | 6 | 5 (0.6) |
| AEs resulting in temporary discontinuation of study drug | 4 | 3 (0.5) | 0 | 0 (0.0) | 4 | 3 (0.4) |
| AEs resulting in permanent discontinuation of study drug | 17 | 12 (2.0) | 2 | 1 (0.5) | 19 | 13 (1.6) |
| AEs resulting in study discontinuation | 5 | 3 (0.5) | 3 | 2 (1.0) | 8 | 5 (0.6) |
| Death3 | 2 | 2 (0.3) | 1 | 1 (0.5) | 3 | 3 (0.4) |
| Treatment-emergent AEs reported in ≥2% of patients | ||||||
| Headache | 41 | 35 (5.8) | 13 | 12 (6.0) | 54 | 47 (5.8) |
| Nausea | 32 | 28 (4.6) | 5 | 5 (2.5) | 37 | 33 (4.1) |
| Dizziness | 28 | 24 (3.9) | 7 | 7 (3.5) | 35 | 31 (3.8) |
| Diarrhea | 20 | 17 (2.8) | 6 | 6 (3.0) | 26 | 23 (2.8) |
| Urinary tract infection | 19 | 17 (2.8) | 7 | 6 (3.0) | 26 | 23 (2.8) |
| Nasopharyngitis | 17 | 16 (2.6) | 4 | 4 (2.0) | 21 | 20 (2.5) |
| Vomiting | 15 | 13 (2.1) | 2 | 2 (1.0) | 17 | 15 (1.9) |
| Fatigue | 16 | 12 (2.0) | 3 | 3 (1.5) | 19 | 15 (1.9) |
| Rash | 12 | 12 (2.0) | 0 | 0 (0.0) | 12 | 12 (1.5) |
| Abdominal pain | 11 | 11 (1.8) | 7 | 5 (2.5) | 18 | 16 (2.0) |
| Arthralgia | 11 | 9 (1.5) | 5 | 5 (2.5) | 16 | 14 (1.7) |
| Dyspnea | 10 | 10 (1.6) | 7 | 4 (2.0) | 17 | 14 (1.7) |
| Anemia | 4 | 4 (0.7) | 4 | 4 (2.0) | 8 | 8 (1.0) |
Percentages are based on the number of patients in each treatment group and in total.
Related AEs are those classified by the investigator as related to the study drug.
AEs of special interest include hypotension and hypersensitivity as defined in the protocol; not all protocol-defined AEs of special interest were identified by the investigators.
Cardiovascular AE composite endpoint includes myocardial infarction, heart failure, moderate-to-severe hypertension, and hospitalization due to any cardiovascular cause.
Reported by the investigator to be unrelated to the study drug.
AE, adverse event; SAE, serious AE; TEAE, treatment-emergent AE.
The incidence of serious AEs (SAEs) was comparable between the two treatment groups (ferumoxytol, 2.6%; placebo, 3.0%). Most SAEs were assessed to be not related to study drug; many were attributable to comorbid disease, and all but one of the individual SAEs, hypersensitivity (three [0.5%] patients) in the ferumoxytol group and anemia (three [1.5%] patients) in the placebo group, occurred in single subjects. There was no clustering of SAEs to suggest a safety signal. Reported treatment-related SAEs were those known to be associated with the class of IV iron products, and included one (0.2%) ferumoxytol-treated patient with an anaphylactic reaction and three (0.5%) ferumoxytol-treated patients with hypersensitivity; all of these SAEs resolved.
Protocol-defined AEs of special interest (AESIs), which included moderate-to-severe signs/symptoms of hypotension or hypersensitivity associated with IV iron use, were identified in both treatment groups. As expected, AESIs were noted at a higher rate in ferumoxytol-treated patients (ferumoxytol, 3.6%; placebo, 1.0%); most were mild-to-moderate in intensity, with two meeting serious criteria. Most of these AESIs were considered related to study medication and all resolved with treatment. A Composite Cardiovascular Adverse Event Endpoint was predefined and specifically included nonfatal myocardial infarction, heart failure, moderate-to-severe hypertension, and hospitalization due to any cardiovascular event. Six events were reported in five (0.8%) ferumoxytol-treated patients, all of which were considered by the investigators to be unrelated to the study drug; the only event in the Composite Cardiovascular Adverse Event Endpoint reported in more than a single ferumoxytol-treated patient was hypertension (two events in two [0.3%] patients).
Two deaths were reported in the ferumoxytol group (disease progression and septic shock) and one in the placebo group (malignant lung neoplasm). All deaths occurred more than 35 days post-treatment and none were considered by investigators to be related to the study drug. Evaluation of clinical chemistries and vital signs did not demonstrate any relevant clinical differences between the two treatment groups.
Discussion
Oral iron is the traditional first-line treatment for patients with IDA. However, oral iron is poorly absorbed and some patients have an inadequate response or intolerance to oral iron, resulting in treatment noncompliance and discontinuation due to adverse effects. IV iron therapy is therefore an important treatment option for such patients. However, the only currently approved products for patients with IDA who do not have CKD are the IV iron dextrans, which have a boxed warning 25,26. Although off-label administration of the dextrans is at times accomplished by means of a slow infusion over 1–4 hr 27, the individual daily dose of the dextrans is limited, per the prescribing information, to 100 mg or less because of their safety profiles, thereby requiring multiple doses (≥10) to administer a full 1-g treatment course. The need for repeated dosing with the iron dextrans potentially increases patients' exposure risk 28, lowers treatment compliance 29, and leads to greater nursing time and higher administration costs 30. This has likely further limited their use in patients in whom oral administration is unsatisfactory or impossible. Thus, there is an unmet need for additional therapies to replenish iron stores in these patients with IDA who cannot tolerate oral iron.
In this Phase 3, randomized, double-blind, placebo-controlled study, ferumoxytol 1.02 g, delivered as two doses of 510 mg, was shown to be well tolerated and effective in correcting anemia in a relatively short time in adults with IDA and a history of unsatisfactory response to oral iron therapy or in whom oral iron could not be used. That ferumoxytol was superior to placebo was demonstrated by the fact that >80% of ferumoxytol-treated patients achieved the primary efficacy endpoint (proportion of patients with a ≥2.0 g/dL in Hgb at any time from Baseline to Week 5), which was ∼15-fold that seen with placebo (5.5%). The ferumoxytol group also had a superior increase in mean Hgb levels from Baseline to Week 5 (2.7 g/dL) compared with the placebo group (0.1 g/dL). Superiority was consistently demonstrated for ferumoxytol at all secondary endpoints, providing further corroboration of its therapeutic efficacy and treatment benefit for patients with IDA with a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used.
The clinical benefit of ferumoxytol treatment was further supported by the consistent, positive results from multiple PRO tools. Ferumoxytol reduced fatigue (FACIT-Fatigue) and increased both vitality (SF-36-Vitality) and energy (LASA-Energy) in parallel with the rise in Hgb levels. The improvements from Baseline to Week 5 were clinically meaningful and exceeded the minimall important differences (MID) previously reported for these measures 31,32. For the LASA Energy domain, a previously estimated value for the MID was 9.81 32. For the SF-36 Vitality domain, a difference of 5.0 points has been indicated as the MID, and for the SF-36 Vitality domain, a decrease of 5–10 points has been correlated with an increased risk of negative outcomes 33,34.
Together, these results suggest that the therapeutic usefulness of ferumoxytol may extend beyond its currently approved indication (i.e., the treatment of IDA in adults with CKD) to the broader population of patients with IDA with a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used 17,35–37.
Overall, this study demonstrated that ferumoxytol was well tolerated in this population of patients with IDA, with the types and incidence rates of TEAEs consistent with those reported in previous studies. No new safety signals were identified and the majority of reported TEAEs were mild-to-moderate in intensity. The safety profile of ferumoxytol was shown to be generally comparable to placebo. As with all IV iron products, serious hypersensitivity (0.3%) and anaphylactic reactions (0.2%) were reported. However, these responded to standard medical therapy and resolved without sequelae. No serious hypotensive reactions were reported with ferumoxytol.
In this study, a full 1.02-g treatment course of ferumoxytol was administered with only two injections of 510 mg each in 17 mL over a short time frame (each injection took 17–60 sec), with no requirement for the administration of a test dose and no need for premedication. In contrast, boxed safety warnings for iron dextran require the administration of a test dose and there is a limitation on the daily therapeutic dose that can be administered (≤100 mg/day). This results in the need for multiple office visits (as many as 10 visits) and repeated IV placements to administer the typical 1-g therapeutic dose of iron dextran. In contrast, ferumoxytol offers the ability to deliver the total 1.02-g dose with two clinic visits, possibly improving treatment compliance 29, efficiency, and cost savings 30. Being able to deliver the full therapeutic course in two doses also has the potential to reduce patient exposure to the risk of AEs that exist with each individual IV administration 28. The ability to administer 510 mg of iron in a single dose and the low rate of AEs observed in this study suggest that ferumoxytol may offer advantages over IV iron dextrans, which are the only IV iron formulations currently approved in the US for the treatment of IDA of any cause. This study further demonstrated the symptomatic improvement of patients treated with ferumoxytol, with a reduction in fatigue and increases in vitality, energy, and quality of life as assessed with PRO instruments.
Conclusions
In this large Phase 3 study, ferumoxytol was shown to be well tolerated and effective in patients with IDA of any underlying cause, increasing Hgb, reducing fatigue, increasing energy and vitality, and could provide an important treatment option and help meet the unmet medical need for patients with IDA and a history of unsatisfactory oral iron therapy or in whom oral iron cannot be used.
Author Contributions
S.V.-R. contributed patients; performed the clinical trial; wrote, edited, and proofread the manuscript; provided input on the study; and agreed upon the data presented.
W.S. designed and oversaw the execution of the trial; analyzed the data; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
D.F. contributed patients; performed the clinical trial; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
K.B. designed and oversaw the execution of the trial; analyzed the data; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
R.B. contributed patients; performed the clinical trial; discussed the outline; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
J.L. designed and oversaw the execution of the trial; analyzed the data; performed statistical analysis; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
L.F.A. designed and oversaw the execution of the trial; analyzed the data; wrote, edited, and proofread the manuscript; and agreed upon the data presented.
Acknowledgments
The authors thank Maria McGill, RPh, CMPP, and Mary Hines of inScience Communications and Bret Fulton, RPh, who provided medical writing support funded by AMAG Pharmaceuticals, Inc.
Footnotes
Given the potential confounding of the study results by subjects with Hgb ≤7.0 g/dL, who frequently have multiple anemia-related side effects, these subjects were excluded and were managed by their treating physicians' standard of care.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
References
- 1.Bergamaschi G, Markopoulos K, Albertini R, et al. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica. 2008;93:1785–1791. doi: 10.3324/haematol.13255. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Worldwide prevalence of anaemia 1995–2005. Geneva, Switzerland: World Health Organization; 2008. pp. 1–40. WHO global database on anaemia. [Google Scholar]
- 3.Niv E, Elis A, Zissin R, et al. Iron deficiency anemia in patients without gastrointestinal symptoms—A prospective study. Fam Pract. 2005;22:58–61. doi: 10.1093/fampra/cmh705. [DOI] [PubMed] [Google Scholar]
- 4.Croker JR, Beynon G. Gastro-intestinal bleeding—A major cause of iron deficiency in the elderly. Age Ageing. 1981;10:40–43. doi: 10.1093/ageing/10.1.40. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Kaffes AJ. Iron deficiency anaemia: A review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24:109–116. doi: 10.1097/MEG.0b013e32834f3140. [DOI] [PubMed] [Google Scholar]
- 6.Goodhand JR, Kamperidis N, Rao A, et al. Prevalence and management of anemia in children, adolescents, and adults with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:513–519. doi: 10.1002/ibd.21740. [DOI] [PubMed] [Google Scholar]
- 7.Tsiolakidou G, Koutroubakis IE. Stimulating erythropoiesis in inflammatory bowel disease associated anemia. World J Gastroenterol. 2007;13:4798–4806. doi: 10.3748/wjg.v13.i36.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson AJ, Brown WJ, Powers JR, Roberts DC. Iron deficiency, general health and fatigue: Results from the Australian Longitudinal Study on Women's Health. Qual Life Res. 2000;9:491–497. doi: 10.1023/a:1008978114650. [DOI] [PubMed] [Google Scholar]
- 9.Hinton PS, Giordano C, Brownlie T, Haas JD. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol. 2000;88:1103–1111. doi: 10.1152/jappl.2000.88.3.1103. [DOI] [PubMed] [Google Scholar]
- 10.Haas JD, Brownlie Tt. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–688S. doi: 10.1093/jn/131.2.676S. discussion 688S–690S. [DOI] [PubMed] [Google Scholar]
- 11.Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197. doi: 10.1136/gut.2003.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton JC, Barton EH, Bertoli LF, et al. Intravenous iron dextran therapy in patients with iron deficiency and normal renal function who failed to respond to or did not tolerate oral iron supplementation. Am J Med. 2000;109:27–32. doi: 10.1016/s0002-9343(00)00396-x. [DOI] [PubMed] [Google Scholar]
- 13.Cella D, Kallich J, McDermott A, Xu X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: Results from five randomized clinical trials. Ann Oncol. 2004;15:979–986. doi: 10.1093/annonc/mdh235. [DOI] [PubMed] [Google Scholar]
- 14.Diez-Lobo AI. Preoperative intravenous iron administration corrects anemia and reduces transfusion requirement in women undergoing abdominal hysterectomy. Trans Alt Trans Med. 2007;9:114–119. [Google Scholar]
- 15.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 16.Stoltzfus RJ. Iron-deficiency anemia: Reexamining the nature and magnitude of the public health problem. Summary: Implications for research and programs. J Nutr. 2001;131:697S–700S; discussion 700S-701S. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- 17.Provenzano R, Schiller B, Rao M, et al. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:386–393. doi: 10.2215/CJN.02840608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavill I, Auerbach M, Bailie GR, et al. Iron and the anaemia of chronic disease: A review and strategic recommendations. Curr Med Res Opin. 2006;22:731–737. doi: 10.1185/030079906X100096. [DOI] [PubMed] [Google Scholar]
- 19.Dexferrum [package insert] Shirley, NY: American Regent Laboratories, Inc; 2009. [Google Scholar]
- 20.INFed Prescribing Information. Morristown, NY: Watson Pharma, Inc; 2009. [Google Scholar]
- 21.Auerbach M, Rodgers GM. Intravenous iron. N Engl J Med. 2007;357:93–94. doi: 10.1056/NEJMc070203. [DOI] [PubMed] [Google Scholar]
- 22.McCormack PL. Ferumoxytol: In iron deficiency anaemia in adults with chronic kidney disease. Drugs. 2012;72:2013–2022. doi: 10.2165/11209880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Rosner MH, Auerbach M. Ferumoxytol for the treatment of iron deficiency. Expert Rev Hematol. 2011;4:399–406. doi: 10.1586/ehm.11.31. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 25.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant. 2004;19:1571–1575. doi: 10.1093/ndt/gfh185. [DOI] [PubMed] [Google Scholar]
- 26.Wysowski DK, Swartz L, Borders-Hemphill BV, et al. Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol. 2010;85:650–654. doi: 10.1002/ajh.21794. [DOI] [PubMed] [Google Scholar]
- 27.Auerbach M, Pappadakis JA, Bahrain H, et al. Safety and efficacy of rapidly administered (one hour) one gram of low molecular weight iron dextran (INFeD) for the treatment of iron deficient anemia. Am J Hematol. 2011;86:860–862. doi: 10.1002/ajh.22153. [DOI] [PubMed] [Google Scholar]
- 28.MacDougall IC, McGlaughlin J, Fortin GS, et al. 2011. FIRST: Head-to-head comparison study (Ferumoxytol compared to IRon Sucrose Trial) of the safety and efficacy of ferumoxytol with iron sucrose for the treatment of iron deficiency anemia (IDA) in patients with chronic kidney disease (CKD). Presented at the American Society of Nephrology 44th annual meeting, Philadelphia, PA, USA, 2011.
- 29.Rawal A, Katsulis P, Stutz L, et al. 2013. Comparison of intravenous iron therapy for the treatment of anemia in CKD. Presented at the National Kidney Foundation Spring Meeting (SCM13), Orlando, FL, USA, 2013.
- 30.Meyers M, Erdal E, Khan C, et al. 2011. Efficiency outcomes associated with increased ferumoxytol use in an infusion clinic. Presented at the American Society of Health System Pharmacists Midyear Clinical Meeting, New Orleans, LA, USA. Poster No. 5-004. 2011.
- 31.Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 32.Patrick DL, Gagnon DD, Zagari MJ, et al. Assessing the clinical significance of health-related quality of life (HrQOL) improvements in anaemic cancer patients receiving epoetin alfa. Eur J Cancer. 2003;39:335–345. doi: 10.1016/s0959-8049(02)00628-7. [DOI] [PubMed] [Google Scholar]
- 33.Bjorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 Vitality scale: Using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23:731–739. doi: 10.1185/030079907x178757. [DOI] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 35.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinowitz BS, Schwenk MH, Jacobs PM, et al. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int. 2005;68:1801–1807. doi: 10.1111/j.1523-1755.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Patel T, Hertel J, et al. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis. 2008;52:907–915. doi: 10.1053/j.ajkd.2008.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


