Summary
Background
In 2002–04, we did a randomised controlled trial in southern Nepal, and reported that children born to mothers taking multiple micronutrient supplements during pregnancy had a mean birthweight 77 g greater than children born to mothers taking iron and folic acid supplements. Children born to mothers in the study group were a mean 204 g heavier at 2·5 years of age and their systolic blood pressure was a mean 2·5 mm Hg lower than children born to mothers in the control group. We aimed to follow up the same children to mid-childhood (age 8·5 years) to investigate whether these differences would be sustained.
Methods
For this follow-up study, we identified children from the original trial and measured anthropometry, body composition with bioelectrical impedance (with population-specific isotope calibration), blood pressure, and renal dimensions by ultrasound. We documented socioeconomic status, household food security, and air pollution. Main outcomes of the follow-up at 8 years were Z scores for weight-for-age, height-for-age, and body-mass index (BMI)-for-age according to WHO Child Growth Standards for children aged 5–19 years, and blood pressure. This study is registered with the International Standard Randomised Controlled Trial register, number ISRCTN88625934.
Findings
Between Sept 21, 2011, and Dec 7, 2012, we assessed 841 children (422 in the control group and 419 in the intervention group). Unadjusted differences (intervention minus control) in Z scores were 0·05 for weight-for-age (95% CI −0·09 to 0·19), 0·02 in height-for-age (−0·10 to 0·15), and 0·04 in BMI-for-age (−0·09 to 0·18). We recorded no difference in blood pressure. Adjusted differences were similar for all outcomes.
Interpretation
We recorded no differences in phenotype between children born to mothers who received antenatal multiple micronutrient or iron and folate supplements at age 8·5 years. Our findings did not extend to physiological differences or potential longer-term effects.
Funding
The Wellcome Trust.
Introduction
Data for the developmental origins of health and disease suggest that early-life experience can have lasting effects on growth and physiology. The mechanisms include an interplay between environment, genes, and hormones, in which epigenetic regulation plays a part. How tractable this interplay is to environmental effects, how long the tractability lasts, and whether it is reversible, remain uncertain.1, 2, 3
Macronutrients and micronutrients are both important for the short-term and long-term health of mothers and children, especially in resource-poor settings in which women can have many deficiencies.2, 4, 5 Many women take iron and folic acid before or during pregnancy, and a supplement containing 15 vitamins and minerals developed by UNICEF, the United Nations University, and WHO has been considered for use in pregnancy.6 We previously did a double-blind randomised controlled trial7 with pregnant women in which we compared the effects of taking this multiple micronutrient supplement every day in the second and third trimesters of pregnancy with supplements of iron and folic acid. We found an increase of 77 g in birthweight (95% CI 24–130) in the intervention group, with a corresponding 25% relative reduction in low birthweight prevalence.7 Ours was one of several trials of this supplement,8, 9, 10, 11, 12, 13, 14, 15 and findings of meta-analyses of these and similar trials have shown an increase in birthweight of between 22 g and 54 g, a reduction in low birthweight and small-for-gestational age, but no other changes in anthropometry, gestation, or mortality.16, 17, 18, 19, 20
Children born in our trial were followed up at age 2·5 years. The multiple micronutrient group were a mean 204 g (95% CI 27–381) heavier, with small increases in body circumferences and a mean systolic blood pressure 2·5 mm Hg lower (0·5–4·6) than those in the control group.21 We have now followed up children at 8 years of age, hypothesising that the differences in weight and blood pressure recorded at 2·5 years would be maintained in relative terms into mid-childhood. We repeated the anthropometry and blood pressure measurements, and also examined body composition.
Methods
Study design and participants
The study was based in Dhanusha district in the Central Terai of Nepal, close to the Indian border. Nepal is one of the world's poorest countries. Gross domestic product is US$35·81 billion and per person income is $1160 (at purchasing power parity), which places Nepal 103rd in terms of wealth.22, 23 The Human Development Index is 0·458, ranked 157th worldwide.23
The trial has been described previously.7 1200 women attending Janakpur Zonal Hospital for antenatal care were randomly allocated to receive either a multivitamin supplement (containing 800 μg vitamin A, 10 mg vitamin E, 5 μg vitamin D, 1·4 mg vitamin B1, 1·4 mg vitamin B2, 18 mg niacin, 1·9 mg vitamin B6, 2·6 μg vitamin B12, 400 μg folic acid, 70 mg vitamin C, 30 mg iron, 15 mg zinc, 2 mg copper, 65 μg selenium, and 150 μg iodine) or a control supplement of 60 mg iron and 400 μg folic acid.6 Supplements were taken every day from 12 to 20 weeks' gestation (average 15·9 weeks) until delivery, and women were assessed every 2 weeks. Exclusion criteria included multiple pregnancies (ie, twins or more), fetal abnormalities on obstetric ultrasound, and maternal illness that could compromise the outcome of the pregnancy. 1069 mothers and infants completed the trial and were seen by the study team at birth and at 1 month of age. Participants, their families, and research staff were masked to trial allocation.
The follow-up study was approved by the UCL research ethics board (reference 2744/001) and the Nepal Health Research Council (reference 51/2011). Parents or guardians of children gave signed informed consent in their local language.
Procedures
We tried to find children from the original trial with location data from previous follow-up. We travelled anywhere in Dhanusha and adjoining districts, and also assessed 14 children who had moved to Kathmandu or the town of Hetauda.
A questionnaire was developed in Maithili, Nepali, and English, back-translated to ensure equivalence, piloted in the local population, and adapted before use. Modules covered socioeconomic status, food security, and parental recall of diarrhoeal and respiratory illnesses in the past week and year. We assessed food security with the Household Food Insecurity Access Scale (HFIAS)24 and the Household Dietary Diversity Score (HDDS),25 which have been used in other research in the specialty.26 We used the HFIAS to work out the level of food insecurity experienced by a household during the past 30 days, in terms of anxiety about food access, quality, and quantity of food. We used the HDDS to examine the breadth of the child's diet in the preceding week.
For anthropometric measurements, we followed guidelines by UCL Institute of Child Health (based on an anthropometric standardisation reference manual)27 and WHO.28 We attempted to minimise biological variation by taking measurements at a similar time of day. We did duplicate measures of standing height with a Leicester stadiometer (Invicta Plastics, UK), accurate to 1 mm. The child's footwear and hair accessories were removed and they were positioned with their head and back touching the stadiometer, with knees extended and feet together, heels touching the base of the stadiometer, and head in the Frankfort plane. For sitting height, the child was seated on a custom-made stool with the base of the spine touching the stadiometer and head in the Frankfort plane.
Weight and body composition were measured with a Tanita BC-418 scale (Tanita, Japan) accurate to 0·1 kg. Children were given a standard set of clothes (underwear, vest, and sarong) weighing 200 g, which they wore instead of their own. Before assessment, the child was asked to pass urine. Body composition was estimated with a bioelectrical impedance analysis, with a population-specific calibration study that used isotope dilution. Bioelectrical impedance analysis uses a calibration equation to convert electrical impedance to an estimate of total body water, and to in turn generate an estimate of lean mass, relying on the assumption that lean mass and fat mass have different and consistent impedance to current. We did isotope calibration by measuring total body water with 0·06 mg/kg deuterium oxide with 100 children aged 7–9 years. We converted total body water to lean mass by use of age-specific and sex-specific hydration values.29 We selectively sampled children of different weights to produce as accurate a prediction equation as possible: lean mass (kg)=1·95 + (0·68 × height [cm]2 / impedance [Ω]) + (0·21 × weight [kg]) – (0·36 × male sex); R2=0·93, root mean squared error=0·95 kg.
We measured body circumferences in duplicate with Seca 201 tapes (Seca, Germany) accurate to 1 mm with the child standing in the anatomical position. Maximum horizontal head circumference was measured around the forehead and occiput, under the hair if needed, in the Frankfort plane. Chest circumference was measured at the nipple line at the end of normal expiration. To measure the waist circumference, the child was asked to bend from one side to the other and the points of flexure were marked. We placed the measuring tape around the abdomen at these points and read when the child was relaxed at the end of normal expiration. Hip circumference was measured at the widest girth, mid-upper arm circumference midway between the olecranon process, and the tip of the acromion and upper leg circumference midway between the greater trochanter and the lateral femoral epicondyle. We calibrated instruments every 2 weeks.
Left biceps, triceps, subscapular and supra-iliac skinfold thicknesses were measured in triplicate with a Harpenden calliper (Assist Creative Resource, UK), accurate to 0·2 mm and calibrated against a Vernier calliper. We used an Omron M6 electronic blood pressure monitor (Omron Healthcare, Japan) with a paediatric cuff, or adult cuff if needed. Measurements followed Great Ormond Street Hospital for Children guidelines.30 Blood pressure was recorded after the child had been seated for at least 1 min with legs uncrossed. The child was told to relax with head back and right arm on the armrest at the level of the sternum. Two readings were taken, with the cuff deflated fully and 1 min between them, and the lowest value was recorded.
Measurements of kidney size were made to examine a potential mediator of blood pressure difference. Ultrasound measurements were made by a clinician trained in ultrasonography with an Aloka SDD-500 (Aloka, Japan) with a 2–8 MHz convex probe (Aloka, Japan), accurate to 1 mm. We recorded maximum renal length and anteroposterior dimensions, after visualising sinus and parenchyma using predefined landmarks.
Air pollution is a potential environmental confounder that has been associated with abnormal growth, either directly through, for example, lung damage, or indirectly through an increase in morbidity.31 Personal exposure to air pollution was estimated with both gravimetric and photometric sampling of respirable particle mass.32, 33 Briefly, sampling was done in the microenvironments in which children resided (eg, bedroom, kitchen during and outside cooking hours, outdoors, school, and verandah) three times per year. With time activity data from the questionnaire, we calculated a 24 h time-weighted-average. Estimates were restricted to children living in the plains.
Each child was given a T-shirt, refreshments, and a voucher to be seen by a paediatrician outside the research team, with costs of minor acute treatments covered as a gesture of thanks. Guardians were compensated for travel costs.
We entered data into a Base Open Office database (Apache Software foundation) and Microsoft Excel (version 14·3·2). Data files containing personal details were password protected. No analyses or shared data included the names of participants or families. Questionnaires were stored in a locked office with a security guard.
Statistical analysis
The study was powered at 81% to detect a difference of 0·2 Z scores between allocation groups with 400 children in each, at 5% significance level. This corresponded to the 0·2 Z score weight difference recorded in the trial and the previous follow-up, and would be roughly 800 g at 8 years of age. Analysis was done in Excel (version 14.3.2), Prism (version 6.0a), and Stata (version 12.1). We made Bland-Altman plots for all repeat measures to look at reproducibility34 and outliers identified. We calculated intra-observer and inter-observer technical error of measurement (TEM)35 and coefficient of reliability (R)28, 36, 37 and relative TEM (TEM%) allowed the comparison of variables.38
The WHO Child Growth Standards for children aged 5–19 were applied to generate Z scores for weight, height, and BMI for age.39 We assessed distributions for normality. The primary analysis compared allocation groups with t tests and univariable regression models by intention to treat.
Although allocation was balanced, we adjusted for potential confounders to increase precision. We constructed a causal diagram based on a priori assumptions and putative associations between variables (appendix). Based on this diagram, multivariable linear regression models included covariates describing air pollution (24 h time-weighted-average), dietary diversity (HDDS score), food security (HFIAS score), maternal education (no education, primary school, or secondary and above) and height, household asset score, and residence (urban or rural). Adjustment for maternal height was not deemed essential, but was included in the model to augment information about diet and socioeconomic status. We included covariates for maternal education and residence to offset the potential effects of differential loss to follow-up.40 Model assumptions were tested for linearity by plotting residuals against each covariate, for normality by examining a kernel density plot of residuals, for multi-co-linearity by calculating variance inflation factor, and for heteroskedasticity with the Breusch-Pagan test and plotting residuals against predicted values. The multivariable models seemed linear, had normally distributed residuals, and showed no evidence of co-linearity. Outcomes were usually heteroskedastic and robust standard errors were used.41
For the main outcomes, we investigated the direct effects of antenatal multiple micronutrient supplementation, not mediated by birth size. We included anthropometric data at birth in multivariable regression models developed as above.
We excluded 34 children with major or chronic illness and looked at the effect of antenatal supplementation on girls and boys separately. Illnesses were self-reported and classified by a member of the research team trained in paediatrics.
We examined conditional growth to see if growth in a given time differed from the growth expected on the basis of previous measurements. The indicative variable was calculated as the standardised residual on the basis of previous Z scores using the WHO reference ranges.39, 42 A positive value represented growth faster than expected and a negative value, slower. Conditional growth was calculated for two timepoints—2·5 years and 8·5 years. We regressed current size on previous measures with the method described by Adair and colleagues.43 Conditional height took into account previous height and weight, whereas conditional relative weight accounted for these and current height.
Role of the funding source
The funder played no part in the study design, data collection, analysis, interpretation of results, writing the report, or the decision to submit for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
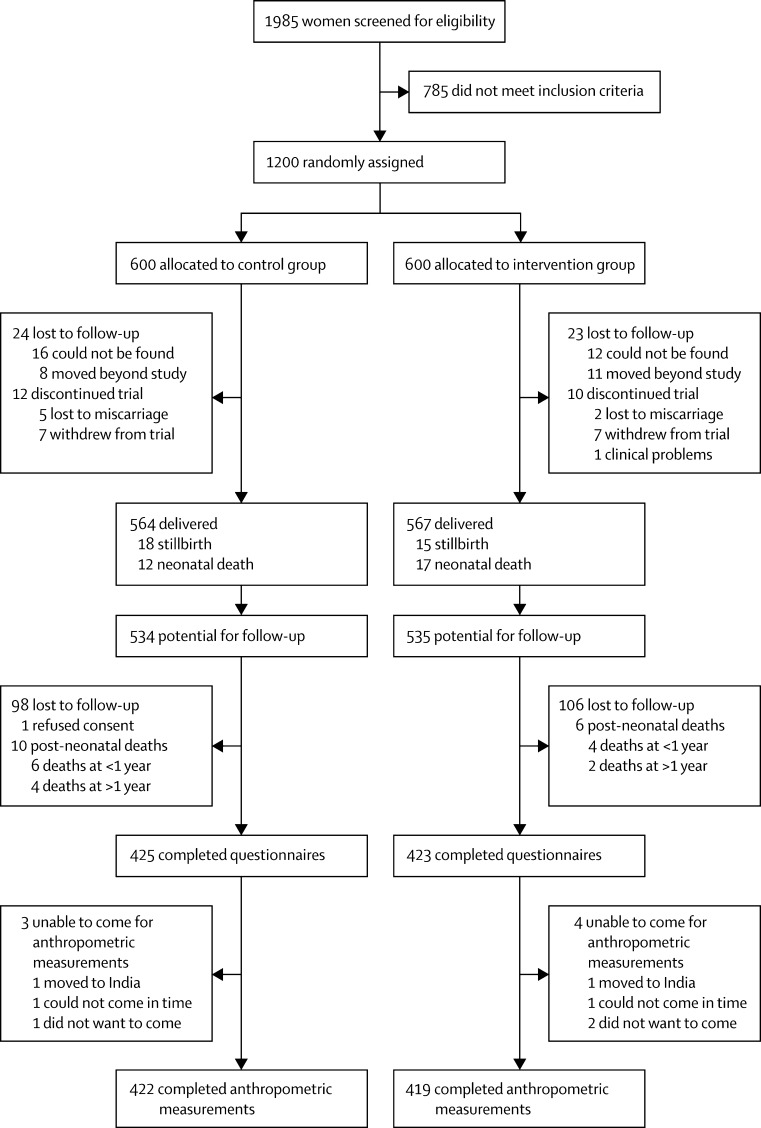
We visited 852 families between Sept 21, 2011, and Dec 7, 2012. One guardian declined consent and three completed questionnaires only. Retention rates were 81% for the control group and 80% for the intervention group. The figure shows the trial profile. Mean ages at follow up were 8·44 years in the intervention group and 8·47 in the control group. Since the previous follow-up, one child had died in the intervention group and two in the control group. Neonatal mortality had been non-significantly greater in the intervention group,7 but mortality rates were similar over the entire follow-up period (appendix). Other than for fever (in 81% of children in the past year; appendix), illness rates were low and similar in intervention and control groups. Children lost to follow-up were similar for most indicators to those retained in the study (table 1). They were more likely to be urban residents (p<0·0001) and their mothers to have some education (p<0·0001).
Figure.
Study profile
Table 1.
Characteristics of children retained at 8 years and of those lost to follow-up
|
8 year follow-up |
Lost to follow-up |
||||
|---|---|---|---|---|---|
| Control group (n=422) | Intervention group (n=419) | Before end of trial* (n=69) | After end of trial† (n=290) | ||
| Residence | |||||
| Urban | 199 (47%) | 197 (47%) | 47 (68%) | 184 (64%) | |
| Rural | 223 (53%) | 222 (53%) | 22 (32%) | 106 (37%) | |
| District | |||||
| Dhanusha | 348 (83%) | 336 (80%) | 59 (86%) | 245 (85%) | |
| Mahotari | 74 (18%) | 79 (19%) | 10 (15%) | 43 (15%) | |
| Siraha | 0 | 2 (1%) | 0 | 1 (0%) | |
| Sarlahi | 0 | 2 (1%) | 0 | 1 (0%) | |
| Mother's age at enrolment | |||||
| <20 years | 126 (30%) | 130 (31%) | 20 (29%) | 85 (29%) | |
| 20–29 years | 273 (65%) | 272 (65%) | 44 (64%) | 196 (68%) | |
| ≥30 years | 23 (6%) | 17 (4%) | 5 (7%) | 9 (3%) | |
| Maternal education | |||||
| None | 206 (49%) | 210 (50%) | 27 (39%) | 101 (35%) | |
| Primary | 37 (9%) | 33 (8%) | 16 (23%) | 37 (13%) | |
| Secondary or higher | 179 (42%) | 176 (42%) | 26 (38%) | 152 (52%) | |
| Mean (SD) maternal height mean (cm) | 151·0 (6) | 150·4 (5) | 150·4 (5) | 151·0 (6) | |
| Ethnic origin | |||||
| Terai Brahmin or Chhetri | 62 (15%) | 65 (16%) | 13 (19%) | 62 (21%) | |
| Terai Middle Madeshi | 296 (70%) | 276 (66%) | 38 (55%) | 176 (61%) | |
| Terai Janjati or Dalit | 10 (2%) | 14 (3%) | 2 (3%) | 3 (1%) | |
| Hill Brahmin or Chhetri | 24 (6%) | 23 (6%) | 6 (9%) | 19 (7%) | |
| Terai Muslim | 23 (6%) | 29 (7%) | 8 (12%) | 17 (6%) | |
| Janjati (hill) | 7 (2%) | 11 (3%) | 2 (3%) | 12 (4%) | |
| Other | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | |
| Main household livelihood | |||||
| No work | 49 (12%) | 46 (11%) | 1 (2%) | 34 (12%) | |
| Farming | 72 (17%) | 72 (16%) | 7 (10%) | 34 (12%) | |
| Salaried | 153 (36%) | 178 (43%) | 34 (49%) | 148 (51%) | |
| Small business | 82 (19%) | 76 (18%) | 19 (28%) | 46 (16%) | |
| Waged labour | 53 (13%) | 43 (10%) | 5 (7%) | 18 (6%) | |
| Student | 7 (2%) | 3 (1%) | 3 (4%) | 4 (1%) | |
| Out of country | 6 (1%) | 5 (1%) | 0 (0%) | 6 (2%) | |
| Land ownership | |||||
| 0 | 47 (11%) | 44 (11%) | 8 (12%) | 30 (10%) | |
| <30 dhur (about 500 m2) | 290 (69%) | 294 (70%) | 45 (65%) | 196 (68%) | |
| >30 dhur | 85 (21%) | 81 (19%) | 16 (23%) | 64 (22%) | |
| Appliance score | |||||
| Motor vehicle, TV, or refrigerator | 217 (51%) | 214 (51%) | 36 (53%)‡ | 146 (50%) | |
| Sewing machine, cassette player, camera, fan, bullock cart, wall clock, radio, iron, or bicycle | 147 (35%) | 138 (33%) | 21 (31%) | 95 (33%) | |
| None of the above | 58 (14%) | 67 (16%) | 11 (16%) | 49 (17%) | |
| Parity | |||||
| 0 | 185 (44%) | 182 (43%) | 33 (48%) | 140 (48%) | |
| 1-2 | 183 (43%) | 202 (48%) | 28 (41%) | 124 (43%) | |
| ≥3 | 54 (13%) | 35 (8%) | 8 (12%) | 26 (9%) | |
| Preterm (<37 weeks' gestation by ultrasound assessment) | 29 (7%) | 28 (7%) | ‡ | 41 (14%) | |
| Delivery site | |||||
| Hospital | 218 (51·7) | 252 (60·1) | ‡ | 176 (60·7) | |
| Home | 194 (46·0) | 165 (39·4) | ‡ | 100 (34·5) | |
| On the way | 10 (2·4) | 2 (0·5) | ‡ | 14 (4·8) | |
| Child sex and weight | |||||
| Girl | 210 (50%) | 196 (47%) | ‡ | 152 (54%)‡ | |
| Boy | 212 (50%) | 223 (53%) | ‡ | 132 (46%) | |
| Mean (SD) birthweight (kg) | 2·74 (0·41) | 2·81 (0·43) | ‡ | 2·75 (0·50) | |
Data are frequency (%) unless otherwise stated.
14 women withdrew from the trial, one dropped out with a clinical problem, 19 moved beyond study area, 28 were untraceable, and seven had miscarriages.
Four refused to attend, two moved beyond study area, two not assessable within follow-up, 204 untraceable, 33 stillbirths, 29 neonatal deaths, and 16 post-neonatal deaths.
Incomplete data at delivery.
Intraobserver TEM% was less than 0·25% (most values <0·1%) and TEM values were less than 0·6 mm, with the exception of skinfold thickness, which was 1–2% (TEM ≤0·1 mm), and renal anteroposterior dimensions at about 5% (TEM <2 mm). With the exception of head, waist, and chest circumferences, interobserver variability was low, with R values of 0·93 or higher. Controlling for the effect of observer made little difference and did not change the inferences.
We took anthropometric measurements for 841 children. At 8·5 years of age, roughly 50% of children had low weight-for-age, and a third had low height-for-age and low BMI-for-age (table 2). Weight-for-age scores fell consistently with age, but height-for-age showed some recovery after age 2·5 years. The mean fat mass proportion was 14·2%. Some children were overweight (BMI-for-age >1 SD): 0·5% of girls and 2·3% of boys. We recorded no difference between allocation groups in the main anthropometric outcomes at 8·5 years (table 3). We recorded no difference in systolic and diastolic blood pressure between the groups.
Table 2.
Anthropometric indices at birth, 2·5 years, and 8·5 years, by allocation
| Number of children | Frequency ≤2 SD (%) |
Mean Z score (95% CI) |
||
|---|---|---|---|---|
| Control group | Intervention group | |||
| Birth* | ||||
| Weight-for-age | 1044 | 186 (18%) | −1·28 (−1·35 to −1·19) | −1·08 (−1·17 to 1·00) |
| Height-for-age | 1016 | 91 (9%) | −0·41 (−0·52 to −0·30) | −0·34 (−0·45 to −0·24) |
| BMI-for-age | 1002 | 348 (35%) | −1·63 (−1·74 to −1·52) | −1·42 (−1·53 to −1·31) |
| 2·5 years | ||||
| Weight-for-age | 915 | 340 (37%) | −1·76 (−1·85 to −1·67) | −1·62 (−1·72 to −1·53) |
| Height-for-age | 915 | 537 (59%) | −2·29 (−2·39 to −2·19) | −2·20 (−2·31 to −2·10) |
| BMI-for-age | 915 | 54 (6%) | −0·40 (−0·50 to −0·30) | −0·29 (−0·39 to −0·18) |
| 8·5 years | ||||
| Weight-for-age | 841 | 444 (53%) | −2·08 (−2·18 to −1·98) | −2·03 (−2·13 to −1·93) |
| Height-for-age | 841 | 242 (29%) | −1·51 (−1·59 to −1·42) | −1·48 (−1·57 to −1·39) |
| BMI-for-age | 841 | 293 (35%) | −1·67 (−1·76 to −1·58) | −1·63 (−1·72 to −1·53) |
BMI=body-mass index.
Excluding birth data ≤4.5 and >3 Z scores.
Table 3.
Child anthropometric indices by allocation, showing mean values, unadjusted, and adjusted differences at 8·5 years
| Control group | Intervention group | Unadjusted difference (95% CI) | Adjusted difference*(95% CI) | Adjusted difference restricted to children without major or chronic illness (95% CI) | ||
|---|---|---|---|---|---|---|
| Weight (kg) | ||||||
| Weight | 20·04 (3·31) | 20·14 (3·35) | 0·10 (−0·35 to 0·55) | 0·30 (−0·08 to 0·67) | 0·25 (−0·12 to 0·63) | |
| Lean mass | 17·34 (2·44) | 17·30 (2·49) | −0·05 (−0·43 to 0·34) | 0·10 (−0·23 to 0·43) | 0·08 (−0·25 to 0·42) | |
| Fat mass | 3·01 (1·6) | 2·94 (1·58) | −0·07 (−0·32 to 0·18) | 0·02 (−0·21 to 0·25) | 0·05 (−0·18 to 0·27) | |
| Height (cm) | ||||||
| Standing | 120·72 (5·91) | 120·73 (6·06) | 0·00 (−0·81 to 0·81) | 0·35 (−0·31 to 1·01) | 0·27 (−0·38 to 0·93) | |
| Trunk length | 64·14 (2·94) | 64·16 (2·96) | 0·02 (−0·38 to 0·42) | 0·14 (−0·20 to 0·48) | 0·07 (−0·28 to 0·41) | |
| Anthropometric scores | ||||||
| Weight-for-age | −2·08 (1·01) | −2·03 (1·06) | 0·05 (−0·09 to 0·19) | 0·09 (−0·03 to 0·22) | 0·07 (−0·06 to 0·19) | |
| Height-for-age | −1·51 (0·92) | −1·48 (0·96) | 0·02 (−0·10 to 0·15) | 0·06 (−0·05 to 0·17) | 0·05 (−0·06 to 0·16) | |
| BMI-for-age | −1·67 (0·96) | −1·63 (0·98) | 0·04 (−0·09 to 0·18) | 0·07 (−0·06 to 0·20) | 0·05 (−0·08 to 0·17) | |
| Skinfold thickness (mm) | ||||||
| Triceps | 7·39 (2·56) | 7·36 (2·41) | −0·03 (−0·37 to 0·31) | 0·07 (−0·24 to 0·38) | 0·10 (−0·20 to 0·37) | |
| Biceps | 3·95 (1·34) | 3·95 (1·40) | −0·01 (−0·19 to 0·12) | 0·06 (−0·11 to 0·23) | 0·08 (−0·10 to 0·25) | |
| Subscapular | 4·91 (1·29) | 4·93 (1·51) | 0·01 (−0·18 to 0·20) | 0·06 (−0·12 to 0·24) | 0·07 (−0·11 to 0·26) | |
| Supra–iliac | 5·76 (2·54) | 5·57 (2·35) | −0·19 (−0·52 to 0·14) | −0·11 (−0·42 to 0·21) | −0·06 (−0·36 to 0·25) | |
| Body circumference (cm) | ||||||
| Head | 49·19 (1·48) | 49·37 (1·47) | 0·18 (−0·02 to 0·38) | 0·19 (0·02 to 0·37) | 0·15 (−0·02 to 0·32) | |
| Chest | 55·59 (3·39) | 55·74 (3·64) | 0·15 (−0·33 to 0·63) | 0·28 (−0·14 to 0·71) | 0·27 (−0·16 to 0·70) | |
| Waist | 49·01 (3·76) | 49·20 (3·96) | 0·19 (−0·33 to 0·71) | 0·29 (−0·19 to 0·77) | 0·23 (−0·25 to 0·71) | |
| Hip | 57·30 (4·00) | 57·36 (4·11) | 0·07 (−0·48 to 0·61) | 0·33 (−0·14 to 0·81) | 0·26 (−0·22 to 0·73) | |
| Upper leg | 31·11 (2·91) | 31·21 (2·91) | 0·10 (−0·30 to 0·49) | 0·26 (−0·09 to 0·61) | 0·21 (−0·14 to 0·56) | |
| Mid–upper arm | 15·94 (1·40) | 15·99 (1·38) | 0·04 (−0·15 to 0·23) | 0·11 (−0·06 to 0·27) | 0·10 (−0·07 to 0·26) | |
| Renal dimension (cm) | ||||||
| Right length | 7·90 (0·55) | 7·89 (0·57) | −0·01 (−0·08 to 0·07) | 0·00 (−0·07 to 0·07) | −0·01 (−0·08 to 0·06) | |
| Right anteroposterior distance | 2·98 (0·26) | 3·00 (0·28) | 0·02 (−0·01 to 0·06) | 0·02 (−0·01 to 0·06) | 0·02 (−0·02 to 0·06) | |
| Left length | 8·25 (0·57) | 8·22 (0·58) | −0·03 (−0·11 to 0·05) | −0·03 (−0·10 to 0·05) | −0·05 (−0·12 to 0·03) | |
| Left anteroposterior distance | 3·29 (0·32) | 3·30 (0·32) | 0·02 (−0·03 to 0·06) | 0·02 (−0·03 to 0·06) | 0·01 (−0·03 to 0·06) | |
| Blood pressure (mm Hg) | ||||||
| Systolic | 98·06 (7·14) | 98·08 (8·10) | 0·02 (−1·02 to 1·05) | −0·06 (−1·10 to 0·98) | −0·20 (−1·23 to 0·83) | |
| Diastolic | 61·16 (7·36) | 61·29 (8·27) | 0·13 (−0·93 to 1·19) | 0·19 (−0·87 to 1·25) | 0·15 (−0·92 to 1·22) | |
Data are mean (SD) unless otherwise stated.
Multivariable regression models included variables describing air pollution, dietary diversity, food security, maternal education and height, household asset score, and residence, by use of robust standard errors. Age and sex were included if not intrinsic to Z score.
Multivariable regression models tended to increase the effect size, but the results did not reach statistical significance. The only outcome that did was head circumference (table 3). Table 4 shows stratification by sex; we recorded no differences and no evidence of interaction (p=0·24).
Table 4.
Child anthropometry by allocation group and child sex at 8·5 years
| Control group | Intervention group | Unadjusted difference (95% CI) | Multivariable regression*(95% CI) | Multivariable regression restricted to children without major or chronic illness (95%CI) | |
|---|---|---|---|---|---|
| Weight (kg) | |||||
| Girls | 19·55 (2·96) | 19·71 (3·15) | 0·16 (−0·44 to 0·75) | 0·51 (−0·03 to 1·04) | 0·51 (−0·03 to 1·06) |
| Boys | 20·53 (3·57) | 20·53 (3·49) | −0·00 (−0·67 to 0·66) | 0·101 (−0·43 to 0·63) | 0·01 (−0·51 to 0·53) |
| Height, standing (cm) | |||||
| Girls | 120·40 (5·92) | 119·98 (5·93) | −0·42 (−1·57 to 0·74) | 0·33 (−0·65 to 1·32) | 0·44 (−0·51 to 1·39) |
| Boys | 121·05 (5·89) | 121·38 (6·12) | 0·33 (−0·80 to 1·47) | 0·36 (−0·55 to 1·27) | 0·14 (−0·78 to 1·06) |
| Weight-for-age Z score | |||||
| Girls | −2·14 (0·92) | −2·02 (1·02) | 0·12 (−0·07 to 0·31) | 0·17 (−0·01 to 0·35) | 0·17 (−0·01 to 0·35) |
| Boys | −2·02 (1·09) | −2·04 (1·09) | −0·02 (−0·23 to 0·18) | 0·03 (−0·15 to 0·21) | −0·02 (−0·19 to 0·16) |
| Height-for-age Z score | |||||
| Girls | −1·55 (0·91) | −1·53 (0·93) | 0·02 (−0·16 to 0·20) | 0·06 (−0·11 to 0·23) | 0·08 (−0·08 to 0·24) |
| Boys | −1·47 (0·93) | −1·44 (0·98) | 0·03 (−0·15 to 0·21) | 0·06 (−0·10 to 0·22) | 0·02 (−0·14 to 0·18) |
| BMI-for-age Z score | |||||
| Girls | −1·73 (0·87) | −1·58 (0·97) | 0·15 (−0·03 to 0·33) | 0·18 (0·00 to 0·36) | 0·16 (−0·02 to 0·34) |
| Boys | −1·61 (1·04) | −1·67 (0·99) | −0·06 (−0·25 to 0·13) | −0·02 (−0·20 to 0·16) | −0·05 (−0·23 to 0·13) |
Data are mean (SD) unless otherwise stated.
Multivariable regression models included variables describing air pollution, dietary diversity, food security, maternal education and height, household asset score and residence, by use of robust standard errors. Age was included if not intrinsic to Z score.
We noted no difference between allocation groups in direct effects of antenatal multiple micronutrient supplementation, not mediated by birth-size, on Z score: 0·04 (95% CI –0·08 to 0·17) for weight-for-age, 0·06 (–0·06 to 0·17) for height-for-age, and 0·05 (–0·08 to 0·18) for BMI-for-age. The appendix shows conditional relative growth results. Generally, there was a tendency for positive effect sizes up to 2·5 years and negative effect sizes up to 8·5 years on both weight-for-age and height-for-age, but these did not reach statistical significance.
Overall, the population was food secure and children had a diverse diet. In the intervention group, 9% of households were food insecure and in the control group 8%. For households in which there was some food insecurity in terms of access, the median HFIAS score was six of 27 in the control group and eight of 27 in the intervention group. The median dietary diversity score was nine of 12 during 7 days in both intervention and control groups. The mean air pollution 24 h time-weighted average (particle mass <4 μm) was 167·2 μg/L (SD 25·2) in the control group and 168·5 μg/L (26·6) in the intervention group.
Discussion
At follow-up at 8·5 years, we recorded no differences in anthropometric outcomes (with or without adjustment for birth size), conditional relative growth, or blood pressure between groups whose mothers were allocated antenatally to either multiple micronutrient or iron and folic acid supplements. Only head circumference differed in multivariable analysis, to a similar degree as at 2·5 years, but we are cautious about interpreting this finding as one outcome among many.
We can be confident of the findings because TEM and TEM% values were below consensual norms (TEM <3 mm for height and <2 mm for body circumferences and TEM% <1% [<5% for skinfold thicknesses]).44, 45 Because of balanced allocation, the primary analysis was unadjusted. We developed adjusted models from a conceptual diagram with uncertain assumptions because of the complexity of childhood growth as an outcome. We used causal diagram analysis to make these assumptions explicit, and attempted to adjust for important variables, without overadjustment. The references used to calculate anthropometric Z scores differed from those used for children younger than 5 years in the trial and previous follow-up.39 This might have led to inconsistencies, but the WHO reference ranges for older children are based on statistical extrapolation from the younger ranges and were designed for compatibility.
We assume that the previous follow-up findings showed a true difference between intervention and control groups in early life (the trial was of high quality and the results in keeping with similar trials). Our interpretation of the findings is that either the differences between groups had disappeared by mid-childhood or that they were not manifest in our indicators. Five other trials of antenatal UNIMMAP supplements have followed up cohorts into childhood (panel). Three groups have assessed child growth,47, 48, 49, 50 three have done cognitive tests,56, 57, 58 and one has assessed mortality.46 Overall, results of follow-up studies have not shown a lasting difference in anthropometry in children born to mothers taking antenatal multiple micronutrient supplements. In Burkina Faso, Roberfroid and colleagues49 recorded increased length-for-age and weight-for-age after 1 year, but the difference had disappeared by 2·5 years. In China, Wang and colleagues50 found no difference in wasting, stunting, and underweight at 30 months of age. In Bangladesh, Khan and colleagues47, 48 reported no difference in weight or body composition at 54 months of age, but reported lower linear growth in children born to mothers in the iron and folic acid control group.
Panel. Research in context.
Systematic review
We did a systematic review of follow-up studies of antenatal multiple micronutrient supplementation in low-income and middle-income countries. We searched for randomised controlled trials, with no date restrictions, published in English in Medline, Embase, and PsychINFO with the search terms “micronutrient”, “multiple micronutrient”, or “UNIMMAP” and “pregnancy”, “antenatal”, or “prenatal”. This search yielded 136 records from which we identified 12 relevant trials of multiple micronutrients. We found ten follow-up studies from these trials. One study looked only at mortality and found no difference in overall mortality up to 2 years of age.46 Three trials had follow-up studies investigating long-term anthropometry and cardiovascular outcomes. 47, 48, 49, 50, 51 Additionally, two trials of similar antenatal multiple micronutrient combinations to the UNICEF, WHO, United Nations University international multiple micronutrient preparation (UNIMMAP) were followed up for anthropometry and cardiovascular outcomes.52, 53, 54, 55 Overall, results of follow-up studies showed no difference in anthropometry in children born to mothers who took multiple micronutrients antenatally compared with those born to mothers who took iron and folic acid. In Bangladesh,51 use of multiple micronutrients was associated with a small increase in diastolic blood pressure at 4·5 years of age 0·87 mm Hg (95% CI 0·18–1·56) compared with a control sample that received 30 mg iron and 400 μg folate. There was no difference in systolic blood pressure. In the follow-up from Sarlahi, Nepal, there was no difference in any of the cardiovascular outcomes measured.54
Interpretation
Overall, findings of studies have shown an increase in birthweight but no consistent lasting effects on anthropometry or cardiovascular outcomes. Our study had the greatest increase in birthweight and, by contrast with the others, was the only one to show to show an increase in weight at 2 years combined with a lower systolic blood pressure. Although our results do not rule out future improvements, combined with those from other trials, they do not suggest a long-term benefit on anthropometry and cardiovascular outcomes.
If the effects of antenatal micronutrient supplementation are transient, they might have been vitiated by childhood in a challenging environment. What this means mechanistically is unclear. Periconceptual and intrauterine development, followed by a time in early childhood of unknown duration, provide an opportunity for epigenetic pattern setting, most commonly via methylation at the CpG dinucleotide site.3 Presumably, either methylation is reversible or any changes happened too late to modify growth trajectory if epigenetic mechanisms caused the initial differences.
The second possibility is that long-term effects were present but undetected. Multivariable models showed a difference in mean weight of 295 g, similar to the difference at 2·5 years of age, but a much smaller proportion of the weight of a child aged 8 years. To be able to detect a difference of this magnitude would require a sample roughly ten times as large, and we are unable to say whether or not it was true. A true difference of 300 g would have been equivalent to roughly 1·5% of the cohort mean weight. This amount of difference could have clinical implications, especially in a population such as ours in which many children were undernourished. Perhaps anthropometric differences will emerge at subsequent follow-up, and the present ages of cohorts across all the trials are insufficient to see an effect. Children have low growth rates between the age of 7 and 9 years and the groups might diverge again in adolescence. There is some evidence for this divergence from a study in baboons, in which an effect of changing weaning diet was manifest in females only in adolescence.59
If weight is too crude a marker of long-term change, body composition can be more useful. However, neither bioelectrical impedance analysis nor skinfold thickness showed a difference between allocation groups, which suggests no global difference in either lean or fat mass or distribution.
We recorded an increased effect of antenatal micronutrient supplementation at birth in girls, and a differential effect might be biologically plausible (eg, through sex-specific DNA methylation),60, 61, 62 but we found no interaction of child sex and anthropometric indices at follow-up. Other trials have been inconclusive. Friis and colleagues63 reported a marginally significant interaction with length, Stewart and colleagues52 recorded a non-significant difference in height-for-age Z scores in which boys were taller than girls, and Roberfroid and coworkers found no interaction.64
Malnutrition was high. Estimates for the Terai from the 2011 Nepal Demographic and Health Survey suggest that 37% of children under 5 were stunted, 11% wasted, and 30% underweight.65 We found a 23% higher estimate of low BMI as an index of underweight. The fact that our children were older raises concerns about further deterioration in nutritional status beyond the age of 5 years. Although growth failure is greatest in early life, there seems to be some scope for catch-up in height.
Weight-for-age and levels of obesity in terms of BMI were low, but fat percentage was relatively high. When we applied reference equations for UK children,66 the children in our cohort had high fat mass (Z score for fat mass −1·66 and −1·92 for lean mass). This finding is in keeping with the so-called thin–fat phenotype common in the region.67, 68 The effect was determined by relatively high fat mass in boys, with no obvious difference in girls. We noted no difference between the trial groups in morbidity rates. On the basis of parental recall, the data were potentially prone to bias and we were unable to corroborate them reliably with medical records. However, we would expect recall bias to have been similar in both trial groups. The 2·5 mm Hg difference in blood pressure seen at 2·5 years was not sustained at 8·5 years, and there was no difference in kidney dimensions.69
Generalisation of the findings might be restricted by non-representative recruitment to the original trial or differential loss to follow-up. Women who chose to participate might have differed systematically from those who did not. Although we adjusted internally for socioeconomic status, rural participants might have been from more affluent groups who could afford to travel to the urban hospital for antenatal care.
So far, meta-analyses have not resulted in a recommendation on the basis of benefits other than increased birthweight.16, 17, 18, 19, 20 Our supplements began at a minimum 12 weeks' gestation and we await the results of trials investigating periconceptional supplementation. Neither transgenerational nor current environmental influences are likely to be redressed over the course of two trimesters of micronutrient supplementation, but the birthweight advantage might have longer-term health effects. For example, the slower growth of children born in the intervention group compared with children in the control group might be associated with physiological differences that might affect later non-communicable disease.43 Further research will undoubtedly add to our understanding of gene–environment interactions, but needs to be augmented by cohort follow-up.
Acknowledgments
Acknowledgments
We would like to thank the families who kindly took part in the study; study team members Gagan Dev Chaube, Sonali Jha, Bhim Prasad Shrestha, Maharudrakumar Thakur, Chandra Maya Thapa, Durna Thapa, Rupesh Yadav, and Sushil Yadav who collected the data; and Andrea Rehman and Rhian Daniel who advised on the statistical analysis. This study was funded by the Wellcome Trust (grant number 092121/Z/10/Z).
Contributors
The study was designed by DD and DO, with technical advice from JCKW and JGA. DD oversaw the study and SSC coordinated the data collection. DSM, NMS, and AC provided overall supervision and management of the field site and staff. DD analysed the data and wrote the first draft. All authors were involved in data interpretation, and read and criticised drafts of the manuscript.
Declaration of interests
JCKW received two bio-electrical impedance analysers and £15 000 from Tanita UK for separate research in 2006. The company had no involvement in the current study. We declare no further competing interests.
Supplementary Material
References
- 1.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 2.Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140:437–445. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 3.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 4.Fall CH, Yajnik CS, Rao S, Davies AA, Brown N, Farrant HJ. Micronutrients and fetal growth. J Nutr. 2003;133(suppl 2):1747S–1756S. doi: 10.1093/jn/133.5.1747S. [DOI] [PubMed] [Google Scholar]
- 5.Costello AM, Osrin D. Micronutrient status during pregnancy and outcomes for newborn infants in developing countries. J Nutr. 2003;133(suppl 2):1757S–1764S. doi: 10.1093/jn/133.5.1757S. [DOI] [PubMed] [Google Scholar]
- 6.UNICEF/WHO/UNU . Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. United Nations Children's Fund; New York: 1999. [Google Scholar]
- 7.Osrin D, Vaidya A, Shrestha Y. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 8.Bhutta ZA, Qadir M. Addressing maternal nutrition and risks of birth asphyxia in developing countries. Arch Pediatr Adolesc Med. 2009;163:671–672. doi: 10.1001/archpediatrics.2009.97. [DOI] [PubMed] [Google Scholar]
- 9.Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr. 2005;59:1081–1089. doi: 10.1038/sj.ejcn.1602215. [DOI] [PubMed] [Google Scholar]
- 10.Persson LA, Arifeen S, Ekström EC, Rasmussen KM, Frongillo EA, Yunus M, the MINIMat Study Team Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. JAMA. 2012;307:2050–2059. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 11.Roberfroid D, Huybregts L, Lanou H, the MISAME Study Group Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88:1330–1340. doi: 10.3945/ajcn.2008.26296. [DOI] [PubMed] [Google Scholar]
- 12.Shankar AH, Jahari AB, Sebayang SK, the Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371:215–227. doi: 10.1016/S0140-6736(08)60133-6. [DOI] [PubMed] [Google Scholar]
- 13.Sunawang UB, Utomo B, Hidayat A, Kusharisupeni. Subarkah Preventing low birthweight through maternal multiple micronutrient supplementation: a cluster-randomized, controlled trial in Indramayu, West Java. Food Nutr Bull. 2009;30(suppl):S488–S495. doi: 10.1177/15648265090304S403. [DOI] [PubMed] [Google Scholar]
- 14.Zagré NM, Desplats G, Adou P, Mamadoultaibou A, Aguayo VM. Prenatal multiple micronutrient supplementation has greater impact on birthweight than supplementation with iron and folic acid: a cluster-randomized, double-blind, controlled programmatic study in rural Niger. Food Nutr Bull. 2007;28:317–327. doi: 10.1177/156482650702800308. [DOI] [PubMed] [Google Scholar]
- 15.Zeng L, Dibley MJ, Cheng Y. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fall CH, Fisher DJ, Osmond C, Margetts BM, the Maternal Micronutrient Supplementation Study Group Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. 2009;30(suppl):S533–S546. doi: 10.1177/15648265090304S408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah PS, Ohlsson A, the Knowledge Synthesis Group on Determinants of Low Birth Weight and Preterm Births Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta-analysis. CMAJ. 2009;180:E99–108. doi: 10.1503/cmaj.081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai K, Spiegelman D, Shankar AH, Fawzi WW. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: meta-analysis and meta-regression. Bull World Health Organ. 2011;89:402–411B. doi: 10.2471/BLT.10.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan U, Grant FK, Goldenberg T, Bui V, Imdad A, Bhutta ZA. Effect of multiple micronutrient supplementation on pregnancy and infant outcomes: a systematic review. Paediatr Perinat Epidemiol. 2012;26(suppl 1):153–167. doi: 10.1111/j.1365-3016.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- 20.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD004905.pub3. CD004905. [DOI] [PubMed] [Google Scholar]
- 21.Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children's weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371:492–499. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Central Intelligence Agency World Factbook. 2013. http://www.cia.gov/library/publications/the-world-factbook (accessed Jan 22, 2012).
- 23.Klugman J. Human Development Report 2011: Sustainability and equity: a better future for all. United Nations Development Programme. 2011. http://hdr.undp.org/sites/default/files/reports/271/hdr_2011_en_complete.pdf (accessed Oct 10, 2014).
- 24.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v.3) USAID Food and Nutrition Technical Assistance Project, Academy for Educational Development; Washington, DC: 2007. [Google Scholar]
- 25.Swindale A, Bilinsky P. Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide. USAID, Food and Nutrition Technical Assistance Project, Academy for Educational Development; Washington, DC: 2006. [Google Scholar]
- 26.Shrestha BP, Bhandari B, Manandhar DS, Osrin D, Costello A, Saville N. Community interventions to reduce child mortality in Dhanusha, Nepal: study protocol for a cluster randomized controlled trial. Trials. 2011;12:136. doi: 10.1186/1745-6215-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. [Google Scholar]
- 28.WHO Multicentre Growth Reference Study Group Reliability of anthropometric measurements in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl. 2006;450:38–46. doi: 10.1111/j.1651-2227.2006.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 29.Wells JC, Williams JE, Chomtho S. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr. 2010;91:610–618. doi: 10.3945/ajcn.2009.28428. [DOI] [PubMed] [Google Scholar]
- 30.Great Ormond Street Hospital for Children Clinical guideline Blood Pressure Monitoring. 2010. http://www.gosh.nhs.uk/clinical_information/clinical_guidelines/ (accessed May 16, 2011).
- 31.Bruce NG, Dherani MK, Das JK. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 2013;13(suppl 3):S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health and Safety Executive Methods for the determination of hazardous substances. Section 14/3 general methods for sampling and gravimetric analysis of respirable and inhalable dust. Health and Safety Executive. 2000. http://www.hse.gov.uk/pubns/mdhs/ (accessed Oct 10, 2014).
- 33.Devakumar D, Semple S, Osrin D. Biomass fuel use and the exposure of children to particulate air pollution in southern Nepal. Environ Int. 2014;66:79–87. doi: 10.1016/j.envint.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 35.Dahlberg G. Statistical methods for medical and biological students. George Allen and Unwin; London: 1940. [Google Scholar]
- 36.Mueller WH, Martorell R. In: Anthropometric standardization: reference manual. Lohman TG, Roche AF, Martorell R, editors. Human Kinetics Books; Champaign, IL: 1988. Reliability and accuracy of measurements; pp. 83–86. [Google Scholar]
- 37.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–177. doi: 10.1017/s0007114599001348. [DOI] [PubMed] [Google Scholar]
- 38.Norton K, Olds T. Anthropometrica. University of New South Wales Press; Sydney: 1996. [Google Scholar]
- 39.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel RM, Kenward MG, Cousens SN, De Stavola BL. Using causal diagrams to guide analysis in missing data problems. Stat Methods Med Res. 2012;21:243–256. doi: 10.1177/0962280210394469. [DOI] [PubMed] [Google Scholar]
- 41.Stock JH, Watson MW. Introduction to econometrics. 2nd edn. Pearson Addison Wesley; Boston: 2007. [Google Scholar]
- 42.WHO Multi-Centre Growth Reference Study Group . WHO Child growth standards: growth velocity based on weight, length and head circumference: Methods and development. World Health Organization; Geneva: 2009. [Google Scholar]
- 43.Adair LS, Fall CH, Osmond C, the COHORTS group Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sicotte M, Ledoux M, Zunzunegui MV, Ag Aboubacrine S, Nguyen VK, the ATARAO group Reliability of anthropometric measures in a longitudinal cohort of patients initiating ART in West Africa. BMC Med Res Methodol. 2010;10:102. doi: 10.1186/1471-2288-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Australian Sport Commission . In: Gore CJ, editor. Human Kinetics; Champaign, IL: 2000. Physiological tests for elite athletes. [Google Scholar]
- 46.Andersen GS, Friis H, Michaelsen KF. Effects of maternal micronutrient supplementation on fetal loss and under-2-years child mortality: long-term follow-up of a randomised controlled trial from Guinea-Bissau. Afr J Reprod Health. 2010;14:17–26. [PubMed] [Google Scholar]
- 47.Khan AI, Kabir I, Ekström EC. Effects of prenatal food and micronutrient supplementation on child growth from birth to 54 months of age: a randomized trial in Bangladesh. Nutr J. 2011;10:134. doi: 10.1186/1475-2891-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan AI, Kabir I, Hawkesworth S. Early invitation to food and/or multiple micronutrient supplementation in pregnancy does not affect body composition in offspring at 54 months: follow-up of the MINIMat randomised trial, Bangladesh. Matern Child Nutr. 2012 doi: 10.1111/mcn.12021. published online Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberfroid D, Huybregts L, Lanou H, the MISAME study group Impact of prenatal multiple micronutrients on survival and growth during infancy: a randomized controlled trial. Am J Clin Nutr. 2012;95:916–924. doi: 10.3945/ajcn.111.029033. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Yan H, Zeng L, Cheng Y, Wang D, Li Q. No effect of maternal micronutrient supplementation on early childhood growth in rural western China: 30 month follow-up evaluation of a double blind, cluster randomized controlled trial. Eur J Clin Nutr. 2012;66:261–268. doi: 10.1038/ejcn.2011.190. [DOI] [PubMed] [Google Scholar]
- 51.Hawkesworth S, Wagatsuma Y, Kahn AI. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. J Nutr. 2013;143:728–734. doi: 10.3945/jn.112.168518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart CP, Christian P, LeClerq SC, West KP, Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90:132–140. doi: 10.3945/ajcn.2008.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakrishnan U, Neufeld LM, Flores R, Rivera J, Martorell R. Multiple micronutrient supplementation during early childhood increases child size at 2 y of age only among high compliers. Am J Clin Nutr. 2009;89:1125–1131. doi: 10.3945/ajcn.2008.26874. [DOI] [PubMed] [Google Scholar]
- 54.Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139:1575–1581. doi: 10.3945/jn.109.106666. [DOI] [PubMed] [Google Scholar]
- 55.Christian P, Murray-Kolb LE, Khatry SK. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010;304:2716–2723. doi: 10.1001/jama.2010.1861. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Yan H, Zeng L. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics. 2009;123:e685–e692. doi: 10.1542/peds.2008-3007. [DOI] [PubMed] [Google Scholar]
- 57.Tofail F, Persson LA, El Arifeen S. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the Maternal and Infant Nutrition Interventions, Matlab (MINIMat) study. Am J Clin Nutr. 2008;87:704–711. doi: 10.1093/ajcn/87.3.704. [DOI] [PubMed] [Google Scholar]
- 58.Prado EL, Alcock KJ, Muadz H, Ullman MT, Shankar AH, the SUMMIT Study Group Maternal multiple micronutrient supplements and child cognition: a randomized trial in Indonesia. Pediatrics. 2012;130:e536–e546. doi: 10.1542/peds.2012-0412. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DS, Bertrand HA, McMahan CA, McGill HC, Jr, Carey KD, Masoro EJ. Preweaning food intake influences the adiposity of young adult baboons. J Clin Invest. 1986;78:899–905. doi: 10.1172/JCI112678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabory A, Ferry L, Fajardy I. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS One. 2012;7:e47986. doi: 10.1371/journal.pone.0047986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlin J, George R, Reyes TM. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One. 2013;8:e63549. doi: 10.1371/journal.pone.0063549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen PY, Ganguly A, Rubbi L. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics. 2013;45:565–576. doi: 10.1152/physiolgenomics.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friis H, Gomo E, Nyazema N. Effect of multimicronutrient supplementation on gestational length and birth size: a randomized, placebo-controlled, double-blind effectiveness trial in Zimbabwe. Am J Clin Nutr. 2004;80:178–184. doi: 10.1093/ajcn/80.1.178. [DOI] [PubMed] [Google Scholar]
- 64.Roberfroid D, Huybregts L, Lanou H. Prenatal micronutrient supplements cumulatively increase fetal growth. J Nutr. 2012;142:548–554. doi: 10.3945/jn.111.148015. [DOI] [PubMed] [Google Scholar]
- 65.Population Division Ministry of Health and Population. New ERA, Measure DHS, USAID 2011 Nepal Demographic and Health Survey Preliminary Report. 2011. http://dhsprogram.com/pubs/pdf/FR257/FR257%5B13April2012%5D.pdf (accessed Oct 10, 2014).
- 66.Wells JC, Williams JE, Chomtho S. Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am J Clin Nutr. 2012;96:1316–1326. doi: 10.3945/ajcn.112.036970. [DOI] [PubMed] [Google Scholar]
- 67.Yajnik CS, Fall CH, Coyaji KJ. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 68.Joglekar CV, Fall CH, Deshpande VU. Newborn size, infant and childhood growth, and body composition and cardiovascular disease risk factors at the age of 6 years: the Pune Maternal Nutrition Study. Int J Obes (Lond) 2007;31:1534–1544. doi: 10.1038/sj.ijo.0803679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1:335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.