Abstract
Background
Myofascial pain syndrome (MPS) in the shoulder is among the most prevalent pain problems in the middle-aged population worldwide. Evidence suggests that peripheral and central sensitization may play an important role in the development and maintenance of shoulder MPS. Given previous research supporting the potential efficacy of anodal transcranial direct current stimulation (tDCS) for modulating pain-related brain activity in individuals with refractory central pain, we hypothesized that anodal tDCS when applied over the primary motor cortex (M1) combined with standard treatment will be more effective for reducing pain in patients with MPS than standard treatment alone.
Method
Study participants were randomized to receive either (1) standard treatment with 5-consecutive days of 1 mA anodal tDCS over M1 for 20 min or (2) standard treatment plus sham tDCS. Measures of pain intensity, shoulder passive range of motion, analgesic medication use, and self-reported physical functioning were administered before treatment and again at post-treatment and 1-, 2-, 3-and 4-week follow-up.
Results
Thirty-one patients with MPS were enrolled. Participants assigned to the active tDCS condition reported significantly more pre- to post-treatment reductions in pain intensity that were maintained at 1-week post-treatment, and significant improvement in shoulder adduction PROM at 1-week follow-up than participants assigned to the sham tDCS condition.
Conclusion
5 consecutive days of anodal tDCS over M1 combined with standard treatment appears to reduce pain intensity, and may improve PROM, faster than standard treatment alone. Further tests of the efficacy and duration of effects of tDCS in the treatment of MPS are warranted.
Keywords: Transcranial direct current stimulation, Myofascial pain syndrome, Shoulder pain, Pain intensity, Physical functioning
Introduction
Myofascial pain syndrome (MPS) has been defined as pain referred from active myofascial trigger points (MTrPs) with associated dysfunction [1]. The pathophysiology of MTrPs is thought to be related to mechanical overload or mechanical trauma leading to increase fiber tension, which then result in taut bands, increased muscle tension and restricted range of motion [2]. In the absence of perpetuating factors, such as ongoing micro- and macro-trauma from over exertion, poor body mechanics and psychological stress, among others [3], acute MTrPs usually resolve [4]. However, in the presence of perpetuating factors or if inadequately managed, MTrPs are thought to persist and may propagate as secondary and satellite MTrPs, leading to progressively more severe and widespread chronic MPS [1,5–7].
However, the role that MTrPs play in the development and maintenance of MPS has not been confirmed, and a number of additional factors have been proposed as playing a causal role in MPS, including those related to peripheral sensitization [8], ischemic muscle spasm [9], neuromuscular dysfunction [10], motor end plate hyperactivity [11], and central sensitization [12,13]. Many of these factors have also been hypothesized as mechanisms of fibromyalgia [14]. Moreover, the various models of MPS are not necessarily mutually exclusive; it is possible–even likely–that MPS may be associated with or results from more than one of these purposed mechanisms [13]. The standard treatment of chronic MPS usually includes muscle stretching and physical therapy [1]. However, no treatment has been identified that successfully eliminated pain and disability in all patients with MPS [1,5–7]. There is therefore a need to develop and examine the efficacy of additional treatments for this disorder.
Transcranial direct current stimulation (tDCS) is a painless and safe method for focal brain stimulation [15]. tDCS is based on decade-old observations that neuronal firing can be modulated by low amplitude electrical direct current [16]. Even though the electrical current used in tDCS is low, it is large enough to decrease the threshold needed to generate an increase in action potentials in neurons immediately below the anodal (positive) electrode. Thus, it is thought to modulate cortical excitability by altering cell membrane potential. Although the precise mechanisms that underlie tDCS are not yet completely understood, the overall effect on the human cortex is reliable, such that anodal tDCS facilitates cortical activity and cathodal tDCS depresses cortical activity [17].
Recently, a number of studies have suggested that motor cortex stimulation with tDCS can decrease the intensity of chronic pain, at least for some pain conditions. For example, Fregni and colleagues randomly assigned individuals with pain associated with a spinal cord lesion to receive sham or active anodal tDCS over the left or right M1 (2 mA, 20 minutes) for 5 consecutive days [18]. They found that significantly greater pain intensity reductions in those who received active tDCS than in those who received sham tDCS. Maximal pain relief in this study was found at the end of one week of stimulation and remained significant three weeks later. A second study from this group showed that anodal tDCS over M1 also produced decreases in pain intensity in patients with fibromyalgia that were maintained for up to three weeks [19]. Finally, Silva and colleagues published a case report describing how anodal tDCS over M1 may induce analgesic effects in cancer pain [20]. The patient in this study had severe pain for several months and responded well to active, but not sham, anodal tDCS over M1. Other research suggests the possibility of anodal tDCS for treatment phantom limb pain [21] and migraine headache [22]. However, research findings do not universally support the efficacy of tDCS for reducing chronic pain [23]. As a group, these findings provide preliminary support for the possibility that anodal tDCS applied over M1 may be effective for treating some – but not all – types of chronic pain.
As mentioned previously, no research has yet examined the effects of tDCS for chronic MPS. However, given that chronic MPS may share some pathophysiology with other chronic pain conditions that could be amendable to neuromodulatory treatments – specifically, central sensitization [12,13] – it is reasonable to examine the potential effects of tDCS on MPS. We hypothesized that five days of tDCS stimulation would result in significantly more pre- to post-treatment decreases in pain intensity than five days of sham (placebo) tDCS stimulation, and that the differences in pain intensity would maintain for at least 4 weeks post-treatment. In addition to testing the primary hypothesis, and consistent with the call to assess the effects of pain treatments on more than just pain intensity [24], we also explored the effects of tDCS, relative to a sham condition, on measures of passive range of motion in the affected shoulder, analgesic medication use, and physical functioning.
METHODS AND MATERIALS
Participant recruitment and informed consent
Study participants were recruited via advertisement at the Physical Therapy clinic, Faculty of Associated Medical Science, Khon Kaen University, Khon Kaen, Thailand. The study procedures were described to any eligible patients who expressed an interest in participating in the study by clinic physicians. MPS diagnosis was confirmed by a physician. Study inclusion criteria included: (1) a diagnosis of chronic myofascial pain in the shoulder; (2) stable doses of analgesics for at least three months prior to study participation; (3) an average pain intensity over the past week score of 4 or greater on a 0–10 numerical rating scale; and (4) age between 18 and 65 years. The criteria used for a diagnosis of chronic myofascial pain was that described by Yunus [25]; and included: (1) pain of at least three months duration and (2) spontaneous upper back pain which had at least one trigger point in the serratus posterior superior, rhomboid, levator scapulae, supraspinatus, trapezius, infraspinatus, subscapularis, teres major, and teres minor muscle. Trigger points were defined as the presence of tender points within palpable taut bands of muscle in the areas that the patient identified as painful. Study exclusion criteria included: (1) previous diagnosis of shoulder pathology other than MPS that could contribute to pain, including subluxation, instability, fractures, frozen shoulder, infection, inflammation or degenerative changes such as osteoarthritis, bursitis, and tendinitis; (2) a diagnosis of neurological disease such as Alzheimers or Parkinsons; (3) severe psychiatric conditions or symptoms such as schizophrenia, major depression, or mania; (4) current pregnancy or lactation; (5) presence of a skull defect; and (6) current use of herbal remedies and other alternative therapies such as massage for pain.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Khon Kaen University (Identifier number: HE 542339). Written informed consent was obtained from all participants.
Experimental design
The current study was a randomized double-blind controlled trial performed over a total of 9 weeks consisting of (1) 4 weeks of baseline, (2) 5 days of treatment, and (3) 4 weeks of follow-up. Just before the treatment phase, study participants were randomized in a 1:1 ratio in blocks of four randomizations to receive either (1) standard care plus active tDCS stimulation or (2) standard care plus sham tDCS stimulation for one week. Participants were asked to continue their routine analgesic medication regimen throughout the duration of the 9-week trial.
Active and sham transcranial direct current stimulation
tDCS was applied via 0.9% NaCl-soaked pair of surface sponge electrodes (35 cm2) and delivered through battery-driven power supply. The constant current stimulator had a maximum output of 10 mA (Soterixmedical, Model 1224-B, New York, USA). The stimulation site over the M1, contralateral to the most painful side, located based on the international electroencephalography (EEG) 10/20 electrode placement system. The cathode (reference) electrode was placed over the supraorbital area contralateral to the anode electrode.
The tDCS device was designed to allow for masked (sham) stimulation. Specifically, the control switch was in front of the instrument, which was covered by an opaque adhesive during stimulation. The power indicator was on the front of the machine, which lit up during the time of stimulation both in active and sham stimulations. However, in sham stimulation, the current was discontinued after 30 seconds while the power indicator remained on [26].
Standard care
All study participants were also given the standard treatment for MPS provided in our clinical setting [1]. Specifically, after the trigger points were identified, patients were taught active stretching exercises. We followed Travell and Simons’ procedure [1] for this exercise program: slow-sustained stretching of the group of muscles throughout the available ROM. Stretching stops at the point of pain, and then moves slowly and gently just a little further with the goal of decreasing muscle tightness. Patients were asked to perform daily stretching activities of both shoulder throughout the study period, and received treatment at the hospital. Treatment also included ultrasound therapy for 5–10 min followed by the application of hot packs over the affected part for 20 min 3 times/week for 2 weeks. All participants also received the instructions to assume and maintain good posture [27].
Measures
Four outcome domains were assessed in this study: pain intensity, passive range of motion, analgesic medication use, and quality of life.
Average pain intensity
Pain intensity was the primary outcome variable, and was assessed by using a 0 – 10 numerical rating scale (NRS) with the endpoints “No pain” and “The most possible pain”. For the baseline (pre-treatment) assessment, participants were asked to provide daily ratings of average pain in the past 24 hours every day for 28 days during the baseline period on a daily diary. These 28 ratings were averaged into a single composite score of baseline average pain intensity. Immediately after treatment, participants were asked to rate their current pain on the same 0–10 scale. Finally, daily 24-hour recall ratings were administered for four weeks following treatment. 1-, 2-, 3-, and 4-week composite scores of average pain were computed as an average of the daily ratings for each epoch (i.e., the 1-week follow-up average pain intensity score = average of 7 daily ratings during the first week after treatment). 0 – 10 NRSs have a great deal of evidence supporting their reliability and validity as measures of pain intensity [28,32,53], and have been recommended as an outcome measure by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus group [28].
Passive range of motion
Shoulder passive range of motion (PROM) refers to the number of degrees of motion present in a shoulder joint. PROM of shoulder flexion/ extension, abduction/adduction, internal/external rotation were measured following the procedures described by Norkin and White [29], using a goniometer, by an experienced physical therapist (T.J.). PROM was assessed on the affected side at four weeks prior to treatment and again at post-treatment and 1-, 2-, 3-, and 4-weeks follow-up.
Analgesic medication use
Analgesic medication use was recorded on a medication diary as the total number of tablets of analgesics taken each week during the 4-week baseline period, the one week of treatment, and weekly at each follow-up assessment. All study participants were prescribed p.r.n. acetaminophen up to 1,000 mg every 6 hours for mild pain, and either p.r.n. ibuprofen 400 mg one tablet every 8 hours or p.r.n. diclofenac 25 mg one tablet every 12 hours, for moderate pain. These prescriptions were viewed as a component of standard treatment [30]. Participants were allowed to alter the amount of analgesic medication use as needed throughout the study, but were not prescribed any analgesics other than acetaminophen, ibuprofen, or diclofenac, and change in type or dose of analgesics used did not occur. Thus, the number of tablets taken represents a consistent measure of analgesic use over time for each participant.
Physical functioning
Physical functioning was assessed using the seven physical functioning items from the brief World Health Organization quality of life (WHOQOL), which has been translated and validated into Thai (WHOQOL-brief-Thai) [31]. With the WHOQOL, raw scores are transformed into a 0–100 metric. The items were administered just before treatment and again at 4-week follow-up
Data analysis
We first computed means and standard deviations of the demographic and outcome variables for descriptive purposes. Next, we compared the two treatment conditions (active tDCS versus sham tDCS) on all baseline outcome measures to ensure baseline equivalence using t-tests. Because dropouts could indicate either treatment failure or lack of improvement leading subjects to discontinue participation, we used intent-to-treat analyses, using last observation carried forward for imputing the endpoint scores for tests of the primary and exploratory hypotheses. Results are presented as means and SEM. Both the primary (related to pain intensity) and exploratory (related to passive range of motion, analgesic medication use, and physical functioning) hypotheses were tested using repeated measures analysis of variance (ANOVA) followed by LSD to help understand any significant effects found. To describe the clinically meaningfulness of any changes in pain, we computed the rates of participants in each condition who reported 30% and 50% reductions in pain intensity from pre- to post-treatment and from pre-treatment to 4-week follow-up [32]. For all analyses, p values of < 0.05 were considered statistically significant. Analyses were completed using Stata software, version 10.0 (StataCorp, College Station, TX).
Results
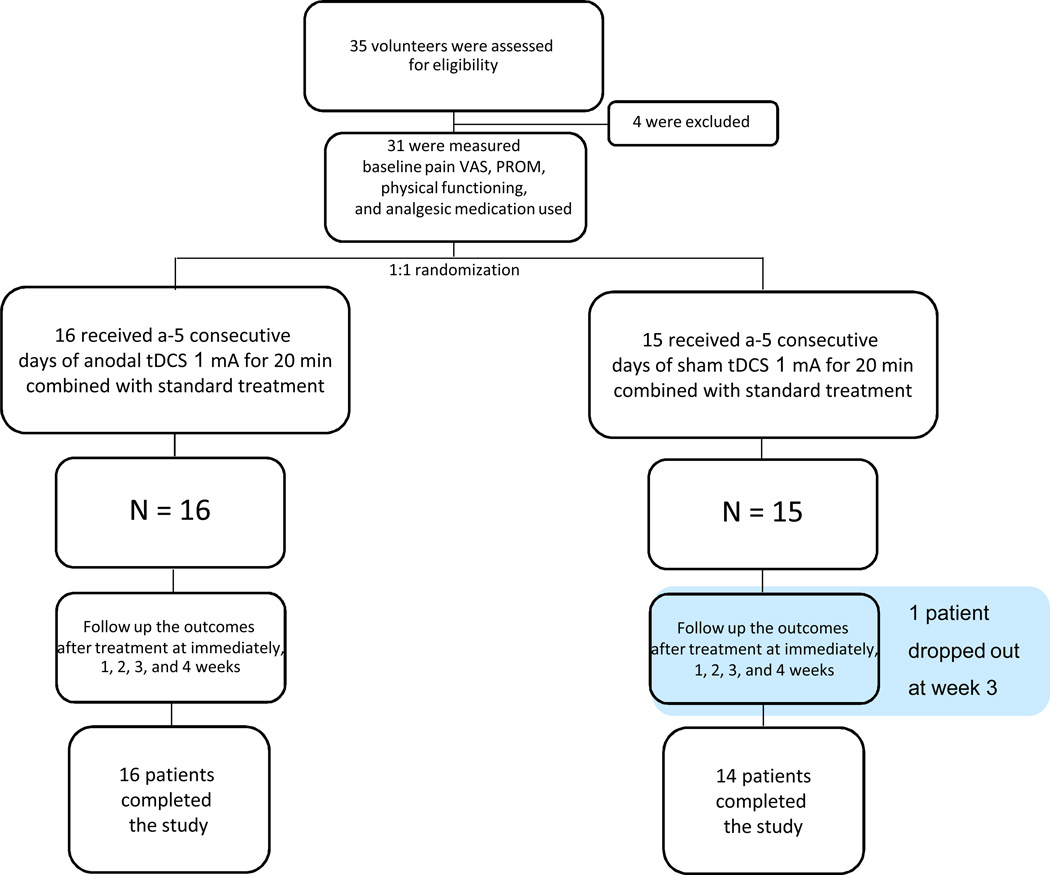
A total of 35 patients were recruited for participation in this study between December 2009 and April 2013. Four patients did not meet the inclusion criteria. Participant flow is shown in the Consort diagram in Figure 1. One of the participants in the sham group dropped out at week 4 for a personal reason unrelated to the study. Table 1 presents the descriptive variables for study participants
Figure 1.
Consort diagram of participant flow.
Table 1.
Study participant descriptive variables.
| Active tDCS with standard care |
Sham tDCS with standard care |
|
|---|---|---|
| No. of subject (female/male) | 16 (11/5) | 15 (11/4) |
| Age | ||
| Mean±SD | 49.94±8.25 | 45.93±10.24 |
| Range (years) | 38–60 | 29–60 |
| Site of the taut band | ||
| Right | 10 | 10 |
| Left | 6 | 5 |
| Number of trigger points | ||
| One trigger point | 12 | 13 |
| Two trigger points | 4 | 2 |
| Duration of pain episode (months) (mean ± SD) | 5.91±2.55 | 5.69±1.69 |
| Oral analgesic drugs | ||
| Acetaminophen | 13 | 12 |
| Ibuprofen | 2 | 1 |
| Diclofenac | 1 | 2 |
| Number of analgesic medications/ week (tablets) (mean±SD) | ||
| Acetaminophen | 1.27±0.67 | 1.17±0.49 |
| Ibuprofen | 0.38±0.18 | 0.50±0.00 |
| Diclofenac | 2.00±0.00 | 0.50±0.00 |
| Baseline pain intensity (VAS) (mean±SD) | 4.22±0.52 | 4.37±0.48 |
| Physical functioning (mean±SD) | 38.94±9.30 | 36.00±8.10 |
| Baseline shoulder flexion (degree) (mean±SD) | 168.94±9.31 | 168.47±8.88 |
| Baseline shoulder extension (degree) (mean±SD) | 58.50±8.61 | 59.00±7.45 |
| Baseline shoulder abduction (degree) (mean±SD) | 169.88±15.79 | 171.40±15.66 |
| Baseline shoulder internal rotation (degree) (mean±SD) | 70.38±11.27 | 72.13±10.27 |
| Baseline shoulder external rotation (degree) (mean±SD) | 95.13±7.94 | 95.47±8.11 |
Pain intensity
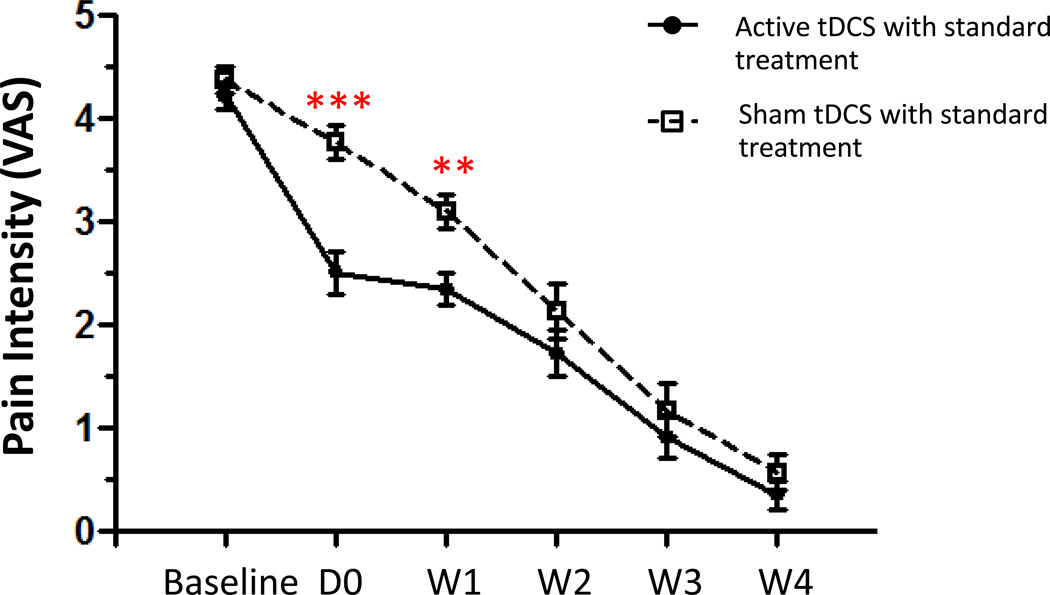
Repeated-measures ANOVA with group as a between-subjects factor and time as a within-subjects factor revealed a significant main effect for group (F (1, 29) = 6.479; p = 0.016), time (F (5, 29) = 214.93 ; p <0.001) and a significant Group × Time interaction (F (5, 29) = 4.946 ; p<0.001). Post-hoc analyses showed that participants in the active tDCS condition reported significantly lower pain intensity than participants in the sham tDCS condition at post-treatment (p < 0.001), and at 1 week follow-up (p = 0.002). However, no significant differences emerged between the two treatment conditions at 2-, 3-, and 4- week follow-up.
Meaningfulness of pain reduction
At post-treatment, none of the participants in either group reported a 50% or greater pre- to post-treatment reduction in pain. However, 12 (75%) of the participants in the tDCS group and none (0%) of the participants in the sham group reported a 30% or more reduction in pain intensity. This rate difference was statistically significant (χ2 (2) = 18.35, p < 0.001). At the 4-week follow-up point, all participants in both treatment conditions reported a 30% or greater reduction in pain intensity, relative to pre-treatment levels. However, at 4-weeks, 15 (94%) of the participants in the tDCS group reported a 50% or greater reduction in average pain intensity, while only 7 (47%) of the sham group participants reported these levels of pain reduction. This difference in responder rates was also statistically significant (χ2 (2) = 8.33, p = 0.004).
Passive range of motion
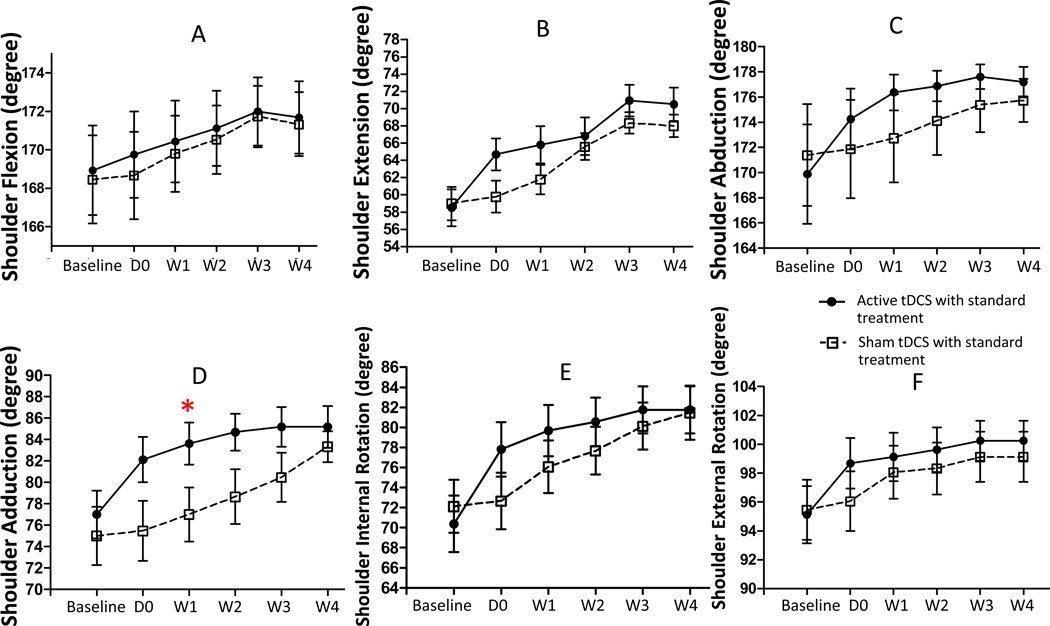
The repeated measures ANOVA using shoulder adduction PROM as a dependent variable revealed significant main effects for group (F (1, 29) = 5.11 ; p = 0.031) and time (F (5, 29) = 16.61; p < 0.001), and a significant Group X Time interaction (F (5, 29) = 6.72 ; p < 0.001). Post-hoc analysis showed that the shoulder adduction PROM was significantly greater in the tDCS group, relative to the sham tDCS group, at 1- week (p = 0.047). However, there was no statistically significant difference in shoulder adduction PROM at any other time point. Also, we found no statistically significant differences between the treatment conditions in PROM of shoulder flexion, shoulder extension, shoulder abduction, shoulder internal rotation, and shoulder external rotation. However, a review of the PROM scores across time (see Figure 3) indicated a trend of greater PROM across all domains for the tDCS group than the sham tDCS group.
Figure 3.
Effect of treatments on shoulder passive range of motion (PROM). Data are presented as mean of PROM at baseline and various time points after treatment: immediately (D0), 1, 2, 3, and 4 weeks after a 5-consecutive days tDCS. Vertical line represent SEM. *p=0.047.
Analgesic medication use
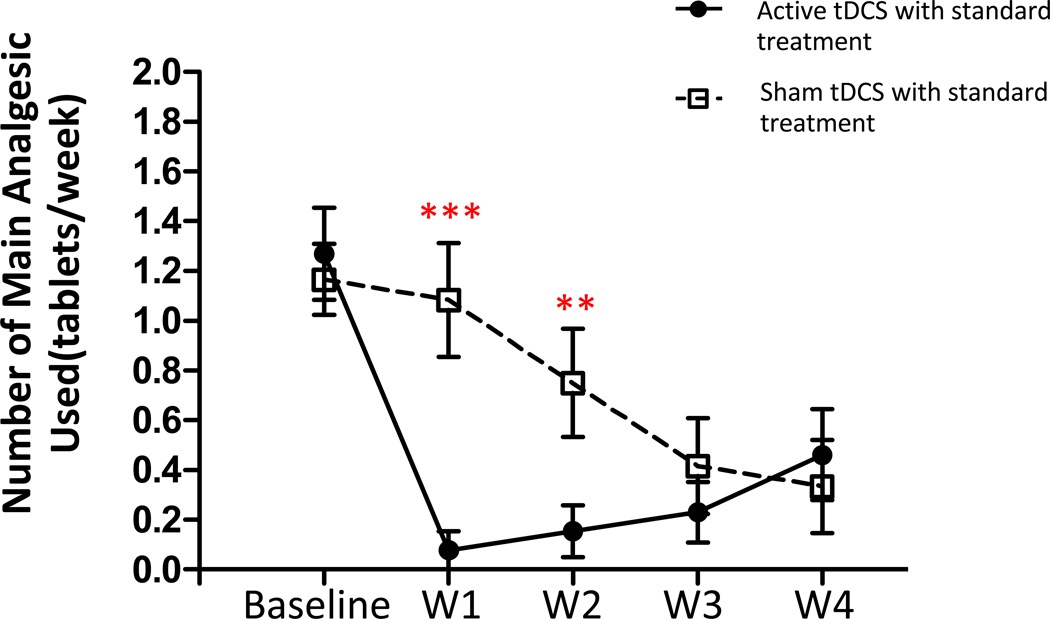
The majority of our patients (25/31) received acetaminophen for pain during the study period. We therefore compared use of acetaminophen between treatment groups. Repeated-measures ANOVA with group as a between-subjects factor and time as a within-subjects factor revealed a significant main effect on number of acetaminophen tablets taken for group (F (1, 23) = 65.80; p < 0.001), time (F (4, 23) = 11.75; p <0.001) and a significant Group X Time interaction (F (4, 23) = 5.32; p=0.001). Post-hoc analyses showed that participants in the active tDCS condition reported taking significantly fewer acetaminophen tablets than participants in the sham tDCS condition at 1 week (p < 0.001), and 2 week follow-up (p = 0.019). However, no significant differences in acetaminophen use were found between the two treatment conditions at 3 and 4 weeks follow-up (see Figure 4).
Figure 4.
Effect of treatment on number of primary analgesic (acetaminophen) tablets taken. Data are presented as mean of number of acetaminophen tablets taken at baseline and at 1-, 2-, 3-, and 4-week follow-up. Vertical lines represent SEM. ***p<0.001, **p=0.001.
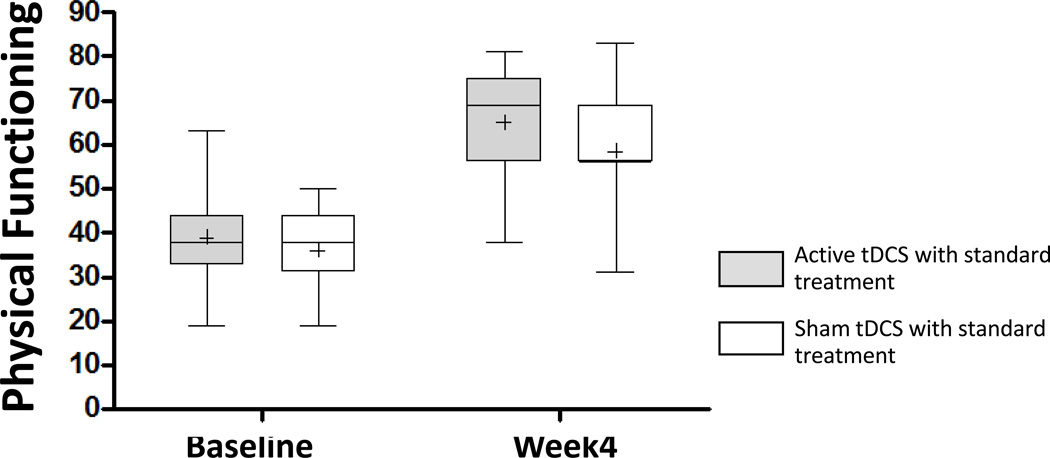
Physical functioning
The repeated-measures ANOVA using the physical domain of WHOQOL-brief-Thai as the dependent variable revealed significant main effects for group (F (1, 29) = 820.78; p < 0.001) and time (F (1, 29) = 153.15; p < 0.001), but no significant Group X Time interaction (F (1, 29) = 0.787; p =0.382). Despite the significant group main effect, post-hoc analyses revealed no statistically difference between groups at either time point (p = 0.180).
Adverse events
Two patients (13%) in active group developed a transient erythematous rash with no pruritus or pain under the reference electrode (anode), which resolved within 1 hour. No other adverse events in participants in the active or sham groups were reported by the participants or observed by the investigators.
Discussion
To the best of our knowledge, this is the first RCT examining the efficacy of anodal tDCS when combined with standard care in the treatment of patients with chronic MPS. The primary outcome revealed a significantly greater pre- to post-treatment decrease in pain intensity that maintained for one week among participants in the active tDCS condition, relative to those in the sham tDCS condition. We also found statistically significant between-group differences in the secondary outcome variables emerged for shoulder adduction PROM at 1 week post-treatment. These treatment effects on shoulder adduction PROM did not maintain at the 2- through 4-week assessments, although the pattern of findings for this any many of the other PROM measures were suggestive of better outcomes for the active tDCS group, relative to the sham tDCS group. In addition, we found significant decreases in the primary analgesic (acetaminophen) use from during the first 2 weeks following treatment in active tDCS group, relative to the sham tDCS group. No significant between-group differences emerged for physical functioning.
Because this is the first study evaluating anodal tDCS in patients with chronic MPS, a comparison with previous results of tDCS in this patient population is not possible. However, a number of previous studies have shown some promising beneficial effects of tDCS in patients with other chronic pain conditions. When benefits were found in those other studies, the analgesic effect occurred immediately after 5 days of anodal tDCS stimulation [18,19, 33–38]. Our results are consistent with these findings, as we also found statistically significant reductions in pain intensity immediately after 5 days of stimulation that maintained for one week. However, the significant between-group differences in pain intensity disappeared by the second week of follow-up. There are at least three possible reasons for the relatively short duration of effects. First, assuming that the treatment dose was ideal, it is possible that the longest duration of benefit that patients with shoulder MPS can expect with tDCS (without booster treatments) is one to two weeks. Second, the limited duration of effects might have occurred because we did not provide the best or most effective dose (frequency of treatment, duration of treatment sessions, current intensity) of tDCS. Further research is needed to determine if changes in treatment frequency, treatment duration, or current intensity impact the magnitude or duration of treatment benefits. Third, it is possible (as discussed in more detail below) that some or all of the benefits found in this trial could have been due to placebo effects. Although previous research has found effects of anodal tDCS on pain intensity [18–19,33–38] and brain metabolites [39–42], either or both of these effects could theoretically also occur with placebo treatment.
Statistically significant improvement does not necessarily translated to clinically meaningful improvements in pain. To more fully understand the clinical meaningfulness of the improvements observed in both treatment conditions, we computed the rates of 30% and 50% or more reduction in pain from pre-treatment to both post-treatment and 4-week follow-up [32]. We found that participants in the tDCS reported meaningful reductions in pain more often at both post-treatment and at 4-weeks follow-up than participants in the sham condition. This provides further support for the efficacy of tDCS, and that its benefits – at least in the short term – are clinically meaningful.
We also found more rapid improvements in PROM at one week after treatment and significantly lower analgesic used in participants who received active treatment than in participants in the sham tDCS condition at 1- and 2- week follow-up. These findings are consistent with the findings regarding pain intensity, supporting the short-term benefits of tDCS for MPS. However, no significant between-group differences emerged for physical functioning; participants in both treatment conditions reported significant (and similar) pre- to post-treatment improvements in physical functioning after treatment. The most likely explanation for the disconnect found between change in pain and change in functioning is the fact that pain intensity and physical functioning are different domains, and can influence each in complicated ways. Pain is one of many factors that can impact functioning. In order to have an impact on functioning, a treatment needs to either (1) effectively target functioning directly (such as the physical therapy treatment that both treatment groups in our study received) or (2) have a large enough impact on pain intensity for it to subsequently influence functioning. We speculate that although the effects of tDCS on pain intensity were statistically significant overall and clinically meaningful for many, the between-group differences in pain intensity that occurred were still not large enough to result in between-group differences in physical functioning.
The mechanisms of tDCS’s effects on pain intensity have not been confirmed. Some investigators have hypothesized that some individuals with chronic pain may have deficits in intracortical inhibition [35]. Because tDCS induces a weak, constant electric current that alters resting membrane potential, it increases overall firing activity in the cortical areas immediately below the anode electrode [43]. Thus, it is possible that tDCS may facilitate greater activity in brain areas involved in the inhibition of signals. Consistent with this possibility, neuroimaging research has shown that stimulation of the motor cortex with epidural electrodes changes activity in thalamic and subthalamic nuclei [44]. It is possible that thalamic nuclei activation which occurs following motor cortex stimulation could lead to changes in activity in other pain-related structures, such as the anterior cingulate, and the periaqueductal gray [45]. It is also possible that active tDCS increases the synaptic transmission modulation via the NMDA receptors [46].
On the other hand, a recent study suggests that the sham tDCS procedure commonly used in tDCS research – including the present study – might not be truly blind to patient participants [23]. Thus, it remains possible that the beneficial effects of active tDCS relative to sham tDCS may be due, at least in part, to placebo effects. Unfortunately, we did not test the efficacy of the blinding procedure in this study. Future research should test for the success of any blinding procedure used, and develop a better (i.e., more effectively blinding) sham tDCS procedure if other needed.
To the extent that the benefits obtained in this study with the tDCS plus standard care treatment are specific and not due to placebo or expectancy effects, and given the increase in shoulder adduction PROM that we observed, it is possible that in individuals with MPS, anodal tDCS might facilitate a relaxation of the MTrP taut band that could then respond more to stretching exercises used in standard MPS treatment [47]. Consistent with this possibility, our results are similar to those reported for the application of high-power ultrasound [48] or TENS plus stretching [49] over the MTrP within the upper trapezius, where an increase in cervical lateral flexion has been observed. In short, our findings suggest that anodal tDCS could be combined with standard care to help make standard care more comfortable for some patients, at least during the first 2 weeks of standard care. It may be particularly useful in patients for whom other options for pain relief are limited [50]. If improvements in range of motion contribute to an overall benefit for patients with MPS, our findings regarding the potential of tDCS for enhancing ROM warrant further exploration and testing.
An important limitation of the current study is the relatively small sample size. Thus, it may have been under-powered to detect the effects on PROM that appeared to emerge across a number of the PROM variables. Additional examination of tDCS’s impact on PROM in individuals with MPS, ideally in studies with larger sample sizes, is warranted. Second, we did not image brain activity before and after treatment, so we are not able to provide findings related to possible neurophysiological mechanisms of tDCS’s benefits.
Third, although we used standard procedures (i.e., the international 10–20 system) for electrode placement, we did not confirm that the electrode was directly over the motor cortex, using for example transcranial magnetic stimulation (TMS) to locate motor responses [51,52]. Moreover, given the size of the electrodes (35 cm2), tDCS procedures likely result in more generalized (hemi-cortical) stimulation than very specific stimulation. Thus, we cannot confirm that the M1 cortex (and only the M1 cortex) was stimulated in this study, and therefore whether M1 stimulation (versus other areas) explain or underlie the benefits found. Finally, as we have already mentioned, recent research (not available at the time that the current study was designed and conducted) suggests the possibility that the sham tDCS procedure used may not be effective as a truly blind sham procedure. Future studies should examine the efficacy of the blinding procedure used in study participants, and use an alternative sham procedure if needed to ensure successful blinding.
Nevertheless, despite the study’s limitations, to our knowledge this is the first study to demonstrate that anodal tDCS over the motor cortex when combined with standard treatment may have immediate beneficial effects on pain intensity, passive range of motion, and analgesic medication used in individuals with chronic shoulder pain. Further research is needed to examine these effects in larger samples of patients and to more closely examine the potential mechanisms of treatment using neuroimaging techniques.
Figure 2.
Effect of treatment conditions on pain intensity (average of VAS intensity ratings). Data are presented as mean of VAS at baseline and at post-treatment (D0) and at 1-, 2-, 3-, and 4-week follow-up. Vertical lines represent SEM. ***p<0.0001, **p<0.001.
Figure 5.
Effect of treatment on physical functioning (WHOQOL Physical Functioning scale). Lower lines of the boxes represent the lowest physical score, middle lines of the boxes represent median, plus symbol represent mean and upper lines of the boxes represent the highest physical domain QOL at baseline and 4 weeks after 5-consecutive days tDCS. Vertical lines represent SEM.
Acknowledgements
We thank Professor Michael A. Nitsche of Department of Clinical Neurophysiology George-August University; Assistant Professor Alexander Rotenberg of The Children Hospital Boston, Harvard University; for their guidance and valuable suggestions.
Funding
This research was supported in part by grant number R21 HD058049 from the National Institutes of Health, National Institute of Child Health and Human Development, National Center for Medical Rehabilitation Research. However, the contents of the article are solely the responsibility of the authors, and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no financial or personal conflicts of interest associated with this study.
Reference
- 1.Travell JG, Simons DG. Management of chronic myofascial pain syndrome. In: Travell JG, Simons DG, editors. Myofascial pain and dysfunction: the trigger point manual. Vol. 2. Baltimore: Lippincott Williams & Wilkins; 1999. pp. 541–551. [Google Scholar]
- 2.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Yap EC. Myofascial pain-an overview. Ann Acad Med Singapore. 2007;36(1):43–48. [PubMed] [Google Scholar]
- 4.Mense S, Simons DG, Russell IJ. Muscle pain understanding its nature, diagnosis, and treatment. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 205–288. [Google Scholar]
- 5.Wheeler AH. Myofascial pain disorders: theory to therapy. Drugs. 2004;64(1):45–62. doi: 10.2165/00003495-200464010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Roth RS, Horowitz K, Bachman JE. Chronic myofascial pain: knowledge of diagnosis and satisfaction with treatment. Arch Phys Med Rehabil. 1998;79(8):966–970. doi: 10.1016/s0003-9993(98)90096-x. [DOI] [PubMed] [Google Scholar]
- 7.De Andrés J, Cerda-Olmedo G, Valía JC, Monsalve V, Lopez-Alarcón, Minguez A. Use of botulinum toxin in the treatment of chronic myofascial pain. Clin J Pain. 2003;19(4):269–275. doi: 10.1097/00002508-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Wall PD, Melzack R. The textbook of pain. 3rd ed. Edinburgh: Churchill Livingstone; 1994. pp. 475–493. [Google Scholar]
- 9.Simons DG. Referred phenomena of myofascial trigger points. In: Vechiet L, Albe-Fessard D, Linbblom U, editors. New trends in referred pain and hyperalgesia. Amsterdam: Elsevier; 1993. p. 341. [Google Scholar]
- 10.Hubbard D, Berkoff G. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18:1803–1807. doi: 10.1097/00007632-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Simons DG. Clinical and etiological update of myofascial pain from trigger points. J Musculoskel Pain. 1996;4:93–121. [Google Scholar]
- 12.Quintner JL, Cohen ML. Referred pain of peripheral nerve origin: an alternative to the “myofascial pain” construct. Clin J Pain. 1994;10:243–251. doi: 10.1097/00002508-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Rivner MH. The neurophysiology of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5:432–444. doi: 10.1007/s11916-001-0054-6. [DOI] [PubMed] [Google Scholar]
- 14.Chandola HC, Chakraborty A. Fibromyalgia and myofascial pain syndrome-a dilemma. Indian J Anaesth. 2009;53(5):575–581. [PMC free article] [PubMed] [Google Scholar]
- 15.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- 17.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1–2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–3998. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 20.Silva G, Miksad R, Freedman SD, Pascual-Leone A, Jain S, Gomes DL, et al. Treatment of cancer pain with noninvasive brain stimulation. J Pain Symptom Manage. 2007;34:342–345. doi: 10.1016/j.jpainsymman.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. doi: 10.1016/j.pain.2013.03.040. In press. [DOI] [PubMed] [Google Scholar]
- 22.Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. 2012;52(8):1283–1295. doi: 10.1111/j.1526-4610.2012.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connell NE, Cossar J, Marston L, Wand BM, Bunce D, De Souza LH, et al. Transcranial direct current stimulation of the motor cortex in the treatment of chronic nonspecific low back pain: a randomized, double-blind exploratory study. Clin J Pain. 2013;29(1):26–34. doi: 10.1097/AJP.0b013e318247ec09. [DOI] [PubMed] [Google Scholar]
- 24.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Yunus MB. Fibromyalgia syndrome and myofascial pain syndrome: Clinical features, laboratory test, diagnosis, and pathophysiologic mechanisms. In: Rachlin ES, editor. Myofascial pain and fibromyalgia: trigger point management. St. Louis: Mosby; 1994. pp. 1–30. [Google Scholar]
- 26.Auvichayapat N, Rotenberg A, Gersner R, Ngodklang S, Tiamkao S, Tassaneeyakul W, Auvichayapat P. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 2013 Feb 9; doi: 10.1016/j.brs.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Hains G, Descarreaux M, Hains F. Chronic shoulder pain of myofascial origin: a randomized clinical trial using ischemic compression therapy. J Manipulative Physiol Ther. 2010;33:362–369. doi: 10.1016/j.jmpt.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149(2):177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Norkin CC, White DJ. Measurement of joint motion, a guide to Goniometry. 4th ed. Philadelphia: Davis Company; 2009. pp. 19–142. [Google Scholar]
- 30.Goadsby PJ, Raskin NH. Headache. In: Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, et al., editors. Harrison's principle of internal medicine. 17th ed. New York: McGraw- Hill; 2008. pp. 95–107. [Google Scholar]
- 31.Mahatnirunkul S, Tuntipivatanakul W, Pumpisanchai W. Comparison of the WHOQOL-100 and the WHQOL-BREF (26 items) Journal of Mental Health of Thailand. 1998;5:4–15. [Google Scholar]
- 32.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005 Jan;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Boggio PS, Amancio EJ, Correa CF, Cecilio S, Valasek C, Bajwa Z, et al. Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. Clin J Pain. 2009;25:691–695. doi: 10.1097/AJP.0b013e3181af1414. [DOI] [PubMed] [Google Scholar]
- 34.Mori F, Codeca C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain. 2009;11:436–442. doi: 10.1016/j.jpain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Antal A, Terney D, Kühnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010;39:890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010;133:2565–2577. doi: 10.1093/brain/awq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumru H, Soler D, Vidal J, Navarro X, Tormos JM, Pascual-Leone A, et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur J Pain. 2013;17:55–66. doi: 10.1002/j.1532-2149.2012.00167.x. [DOI] [PubMed] [Google Scholar]
- 38.Kuhnl S, Terney D, Paulus W, Antal A. The effect of daily sessions of anodal tDCS on chronic pain: Basic, Translational, and Clinical Research in Neuromodulation. Brain stimulation. 2008;1(3):281. [Google Scholar]
- 39.Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24(1):75–79. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 40.Pattany PM, Yezierski RP, Widerström-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23(6):901–905. [PMC free article] [PubMed] [Google Scholar]
- 41.Stanwell P, Siddall P, Keshava N, Cocuzzo D, Ramadan S, Lin A, et al. Neuro magnetic resonance spectroscopy using wavelet decomposition and statistical testing identifies biochemical changes in people with spinal cord injury and pain. Neuroimage. 2010;53(2):544–552. doi: 10.1016/j.neuroimage.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 42.Widerström-Noga E, Pattany PM, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, et al. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154(2):204–212. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Bonnefoi F, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg. 1997;68:141–148. doi: 10.1159/000099915. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83:259–273. doi: 10.1016/s0304-3959(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 46.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulationinduced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 47.Rodríguez-Fernández AL, Garrido-Santofimia V, Güeita-Rodríguez J, Fernández-de-Las-Peñas C. Effects of burst-type transcutaneous electrical nerve stimulation on cervical range of motion and latent myofascial trigger point pain sensitivity. Arch Phys Med Rehabil. 2011;92(9):1353–1358. doi: 10.1016/j.apmr.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Majlesi J, Ünalan MD. High-power pain threshold ultrasound technique in the treatment of active myofascial trigger points: a randomized, double-blind, case-control study. Arch Phys Med Rehabil. 2004;85:833–836. doi: 10.1016/j.apmr.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Kavcic NS, Lehman GH, McGill SM. Effect of modulated TENS on muscle activation, oxygenation, and pain: searching for a physiological mechanism. J Musculoskel Pain. 2005;13:19–30. [Google Scholar]
- 50.Bandy BD, Sanders B. Therapeutic Exercise for Physical Therapist Assistants Techniques for Intervention, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 64. [Google Scholar]
- 51.Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008 Jan 29;(1):82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- 53.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]