Abstract
Spergularia marina Griseb. (SM) is a halophyte that grows in mud flats. The aerial portions of SM have been eaten as vegetables and traditionally used to prevent chronic diseases in Korea. However, there has been no scientific report that demonstrates the pharmacological effects of SM. Glucagon-like peptide-1 (GLP-1) is important for the maintenance of glucose and energy homeostasis through acting as a signal in peripheral and neural systems. To discover a functional food for regulating glucose and energy homeostasis, we evaluated the effect of an aqueous ethanolic extract (AEE) of SM on GLP-1 release from enteroendocrine NCI-H716 cells. In addition, we explored the Takeda G-protein-coupled receptor 5 (TGR5) agonist activity of AEE-SM in Chinese hamster ovary (CHO)-K1 cells transiently transfected with human TGR5. As a result, treatment of NCI-H716 cells with AEE-SM increased GLP-1 secretion and intracellular Ca2+ and cyclic AMP (cAMP) levels in a dose-dependent manner. Transfection of NCI-H716 cells with TGR5-specific small interference RNA inhibited AEE-SM-induced GLP-1 secretion and the increase in Ca2+ and cAMP levels. Moreover, AEE-SM showed that the TGR5 agonist activity in CHO-K1 cells transiently transfected with TGR5. The results suggest that AEE-SM might be a candidate for a functional food to regulate glucose and energy homeostasis.
Key Words: : bile acid receptor, diabetes, enteroendocrine cells, glucagon-like peptide-1, obesity, Spergularia marina
Introduction
Spergularia (Caryophyllaceae) is a cosmopolitan genus distributed from temperate to subtropical climate zones of all continents.1,2 Pharmacological studies of Spergularia purpurea have revealed to be active as a diuretic,3 hyocholesterolemic,4 hypoglycemic,5,6 and hypotensive7 agent in rats. In addition, Spergularia rubra has been shown to be antidiabetic, anticholinesterase, and antioxidant in vitro.1
Spergularia marina Griseb. (SM) is a halophyte that grows in mud flats. The aerial portions of SM have been eaten as vegetables and traditionally used to prevent chronic diseases in Korea.8 However, there has been no scientific report that demonstrates the nutritional or pharmacological effects of SM.
Glucagon-like peptide-1 (GLP-1) is important for the maintenance of glucose and energy homeostasis through acting as a signal in the peripheral and neural systems. GLP-1 release is believed to reflect the direct sensing of luminal nutrients and non-nutrients by the apical processes of enteroendocrine L cells, which are specialized epithelial cells in the gut.9 Activation of G-protein-coupled receptors (GPCRs), such as Takeda G-protein-coupled receptor 5 (TGR5, also known as the bile acid receptor, GPR131, M-BAR, or GPBAR1),10 regulates GLP-1 release from L cells. TGR5 employs Gαs as a G protein. Activation of Gαs stimulates adenylate cyclase, resulting in an increase in the concentration of cyclic AMP (cAMP) within the cells, which is linked to an increase in the intracellular ATP/ADP ratio and a subsequent rise in intracellular Ca2+ mobilization.11
NCI-H716 cells are used as a model for human enteroendocrine L cells. These cells secrete GLP-1 through the activation of GPCRs, including TGR5.10 To characterize SM as a potential functional food for regulating glucose and energy homeostasis, we evaluated the effect of SM on GLP-1 release from NCI-H716 cells and explored the mechanisms underlying SM-induced GLP-1 secretion.
Materials and Methods
Plant material and preparation of plant extract
The aerial portions of SM were obtained from Sanchaewon Co. (Whasun, Korea) and identified by professor Hocheol Kim (Kyung Hee University, Korea). The voucher specimen (No. 72) was deposited at the No. 3-4-S Laboratory of the Division of Metabolism and Functionality Research at Korea Food Research Institute. SM was washed with distilled water, freeze-dried (yield 8.51%, w/w), and powdered. The SM powder was twice extracted with 50% (v/v) ethanol for 2 h at 25°C. After filtration and lyophilization, the aqueous ethanolic extract of SM (AEE-SM) was stored at −70°C until assayed. The yield of AEE-SM from freeze-dried powder was 35% (w/w). AEE-SM was dissolved in DMSO. The concentrations of DMSO in the cell-based assays were below 0.01% (v/v).
HPLC-based fingerprinting analysis
Fingerprints were produced by screening at 200–650 nm on a photodiode array detector (PDA). HPLC was performed on the JASCO HPLC system (Tokyo, Japan), consisting of a PU-980 Intelligent HPLC pump, an LG-2080-20 Ternary gradient unit, a DG-2080-53 Degasser, and a PDA detector. The column was a Waters XTerra RP18 5 μm column (150×4.6 mm, 5 μm particle size; Waters Corporation, Milford, MA, USA). The mobile phase consisted of pH 2.5 with trifluoroacetic acid (A) and acetonitrile (B). The gradient elutions were 0–5 min, 90% A; 5–10 min, 80% A; 10–20 min, 50% A; 20–25 min, 80% A; and 25–30 min, 90% A at a flow rate of 0.5 mL/min. The column temperature was maintained at 30°C. AEE-SM was dissolved in 20% acetonitrile at 10 mg/mL, and 20 μL was injected.
Determination of total polyphenol content
The total polyphenol content in the extract was estimated by the modified Folin–Ciocalteu method.12 The extract (0.067 mg/mL) was incubated with 0.067 N Folin–Ciocalteu reagent and 0.03 g/mL Na2CO3 at 25°C for 60 min. Absorbance was then measured at 750 nm using the Victor3 multilabel plate reader (PerkinElmer, Waltham, MA, USA). A calibration curve was generated for standard tannic acid, and the content was expressed as mg of tannic acid equivalents per g extract or per g aerial portion of SM.
Determination of total flavonoids
The total flavonoid content was estimated using the AlCl3 colorimetric method.12 The extract (0.40 mg/mL) was incubated with 0.01 g/mL AlCl3 at 25°C for 10 min. Absorbance was then measured at 340 nm using the Victor3 multilabel plate reader. Quantification was expressed by reporting the absorbance in the calibration graph of quercetin, which was used as a flavonoid standard. The results were expressed as mg of quercetin equivalents per g extract or per g aerial portion of SM.
Cell culture and viability assay
All the experiments using NCI-H716 and Chinese hamster ovary (CHO)-K1 cells were performed, as described previously.13 Culture and endocrine differentiation of NCI-H716 cells (the American Type Culture Collection [ATCC], Manassas, VA, USA) were performed using previously published methods.14 CHO-K1 cells (ATCC) were maintained in Ham's F-12K (Invitrogen, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum at 5% CO2. Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich Chemical Co., St. Louis, MO, USA) assay.15 The cell density in a microplate and the incubation time for endocrine differentiation before the MTT assay were the same as in the GLP-1 secretion and Ca2+ and cAMP measurement in NCI-H716 cells. The cell density in a microplate and the incubation time with TGR5 transfection before the MTT assay were the same as in cAMP measurement in TGR5-transfected CHO-K1 cells. AEE-SM was incubated for 1 h at 0, 10, 50, 100, 250, 500, and 1000 μg/mL on NCI-H716 cells and on CHO-K1 cells.
GLP-1 secretion from NCI-H716 cells
The cells were plated at 5.0×104 cells per well in 96-well microplates coated with Matrigel and incubated for 48 h. On the day of the experiment, cells were incubated with the Krebs-Ringer bicarbonate buffer (128.8 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 5 mM NaHCO3, 10 mM HEPES, and 0.2% bovine serum albumin, pH 7.4) containing AEE-SM (0, 100, 250, and 500 μg/mL) and a positive control (lithocholic acid [LCA]). After incubating at 37°C for 1 h, the supernatants were collected and GLP-1 was measured by a GLP-1 active ELISA kit (Millipore, Billerica, MA, USA). The concentrations of GLP-1 in the supernatants were calculated by the supplied GLP-1 standard, and the values were expressed as folds of the untreated control.
Measurement of intracellular Ca2+ levels in NCI-H716 cells
The cells were plated at 5×104 cells per well in Matrigel-coated 96-well clear-bottom plates (Corning, Inc., Bedford, MA, USA) and incubated for 48 h at 37°C. Cells were incubated with a calcium indicator dye from the FLIPR Calcium 5 Assay Kit (Molecular Devices, Sunnyvale, CA, USA) for 1 h at 37°C. Subsequently, the measurements were made using a FlexStation 3 (Molecular Devices). Fluorescence changes (i.e., excitation at 485 nm and emission at 525 nm with a cutoff at 515 nm) were monitored at 2-sec intervals. A 150-μL aliquot of the assay buffer supplemented with 4×test agents was added at 20 sec, and scanning was continued for an additional 100 sec. The response of each well was determined as ΔRFU (relative fluorescent units), calculated as (maximum fluorescent value)−(minimum fluorescent value).
Measurement of intracellular cAMP levels in NCI-H716 cells and in TGR5-transfected CHO-K1 cells
After NCI-H716 cells were plated at 5.0×104 cells per well in Matrigel-coated 96-well plates and incubated for 48 h, the cells were incubated with AEE-SM for 1 h and then intracellular cAMP levels were measured. The CHO-K1 cells were transfected with TGR5 plasmid by electroporation, plated at 1.0×104 cells per well in 96-well plates, and incubated for 72 h before cAMP measurements. Intracellular cAMP accumulation was measured by the HTRF-cAMP dynamic kit (Cisbio International, Paris, France).
Small interference RNA transfection in NCI-H716 cells
The validated small interference RNA (siRNA) targeted for TGR5 (Dharmacon RNA Technology, Lafayette, CO, USA) was as follows: ON-TARGETplus SMARTpool siGPBAR1 (G-protein coupled bile acid receptor 1, TGR5) human NM_170699 sequence. ONTARGETplus SMARTpool nontargeting pool was used as negative control to distinguish sequence-specific silencing from nonspecific effects. Electroporation by the Neon transfection system (Invitrogen) was used to transfer siRNA. Gene knockdown was verified by real-time PCR 48 h after the transfection. The NCI-H716 cells were transfected with siRNA and differentiated 48 h before functional assays were performed.
Gene expression profiling in NCI-H716 cells
Total RNA was extracted from cells using RNeasy Plus mini kits (Qiagen, Valencia, CA, USA). cDNA was synthesized by the Transcriptor first-strand cDNA synthesis kit from Roche (Mannheim, Germany). Real-time quantitative PCR (RT-PCR) was performed on a Roche LC480 (Roche Diagnostics, Penzberg, Germany) by LightCycler TaqMan Master (Roche). After denaturing at 94°C for 10 min, the amplification was obtained by 50 cycles of 94°C for 10 sec, 59°C for 10 sec, and 72°C for 10 sec each. For normalized data, the ratio between TGR5 gene and reference gene (GAPDH) expression was calculated by using the LC480 software (Roche). The sequences of the forward primer and reverse primer for target genes were 5′-CAGCAACTCCCTGACACTCA-3′ and 5′-GGCGTCATCTT GGTCCTG-3′ for TGR5 and 5′-AGCCACATCGCTCAGACAC-3′ and 5′-GCCCAATACGACCAAATCC-3′ for internal control GAPDH. The mRNA levels were presented as relative mRNA copy numbers compared with the internal control of GAPDH expression level.
TGR5 transfection in CHO-K1 cells
Human TGR5 cDNA cloned to the pCMV6-XL5 expression vector was purchased from Origene Technologies, Inc. (Rockville, MD, USA) and amplified in chemically component cells by the heat shock method. CHO-K1 cells were transfected with the TGR5 expression plasmid using electroporation by the Neon transfection system and seeded at 1.0×104 cells per well in 96-well half-plates. After incubation for 72 h, the medium was replaced by a serum-free medium, and then the cells were treated for 1 h with test agents. Subsequently, intracellular cAMP accumulation was measured by the HTRF-cAMP dynamic kit (Cisbio International).
Statistical analyses
Experiments were performed in quadruplicate and replicated thrice. All values are expressed as mean and standard error of the mean. One-way analysis of variance followed by Tukey's test and paired t-test was used for statistical analyses (IBM SPSS statistic 20 software).
Results
HPLC fingerprint and total polyphenol and flavonoid contents
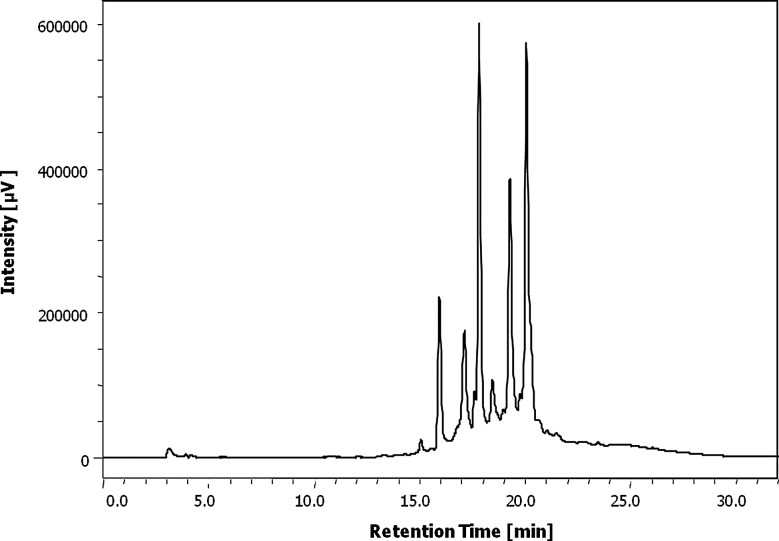
UV absorption maxima of AEE-SM were screened at 200–650 nm by HPLC on PDA. AEE-SM showed a UV absorption maximum at 330 nm and HPLC fingerprint of AEE-SM at 330 nm, as shown in Figure 1. Polyphenols and flavonoids have strong UV absorptions at 325 and 360 nm, respectively.16 Therefore, the total polyphenol and flavonoid contents of AEE-SM were determined.
FIG. 1.
HPLC fingerprint of the aqueous ethanolic extract of Spergularia marina (AEE-SM). Shown as peaks detected at 330 nm.
A linear calibration curve of tannic acid, a standard for polyphenol, was constructed in the range 20–1000 μg/mL with an r2 value of 0.999. Also, a linear calibration curve of quercetin, a standard for flavonoids, was constructed in the range 200–1000 μg/mL with an r2 value of 0.996. The total polyphenol and flavonoid contents of AEE-SM were 67.10±6.18 mg tannic acid equivalents and 54.81±1.36 mg quercetin equivalents per g extract, respectively (Table 1).
Table 1.
Total Phenolic and Flavonoid Content in Spergularia marina
| Per g extract DW | Per g aerial portion of SM DW | Per g aerial portion of SM FW | |
|---|---|---|---|
| Total phenolic content | 67.10±6.18 | 23.96±0.88 | 2.04±0.07 |
| Total flavonoid content | 54.81±1.36 | 19.16±0.48 | 1.63±0.04 |
Total phenolic content expressed as milligram tannic acid equivalent per gram. Total flavonoid content expressed as milligram quercetin equivalent per gram.
Extract, 50% ethanolic extract; DW, dry weight; FW, fresh weight; SM, Spergularia marina Griseb.
Effect of AEE-SM on the viability of NCI-H716 cells and TGR5-transfected CHO-K1 cells
We determined the cytotoxicity of AEE-SM in NCI-H716 cells and TRG5-transfected CHO-K1 cells. AEE-SM up to 1000 μg/mL was applied to NCI-H716 cells and to TGR5-transfected CHO-K1 cells for 1 h. AEE-SM at concentrations from 10 to 500 μg/mL did not affect the viability of NCI-H716 cells (data not shown). AEE-SM from 10 to 100 μg/mL did not affect the viability of TGR5-transfected CHO-K1 cells (data not shown). Based on the cytotoxicity results and preliminary experiments on AEE-SM-induced TGR5 activation and GLP-1 release, NCI-H716 cells were treated with 100, 250, and 500 μg/mL AEE-SM, whereas TGR5-transfected CHO-K1 cells were treated with 10, 50, and 100 μg/mL AEE-SM.
Effect of AEE-SM on GLP-1 secretion and intracellular Ca2+ and cAMP levels
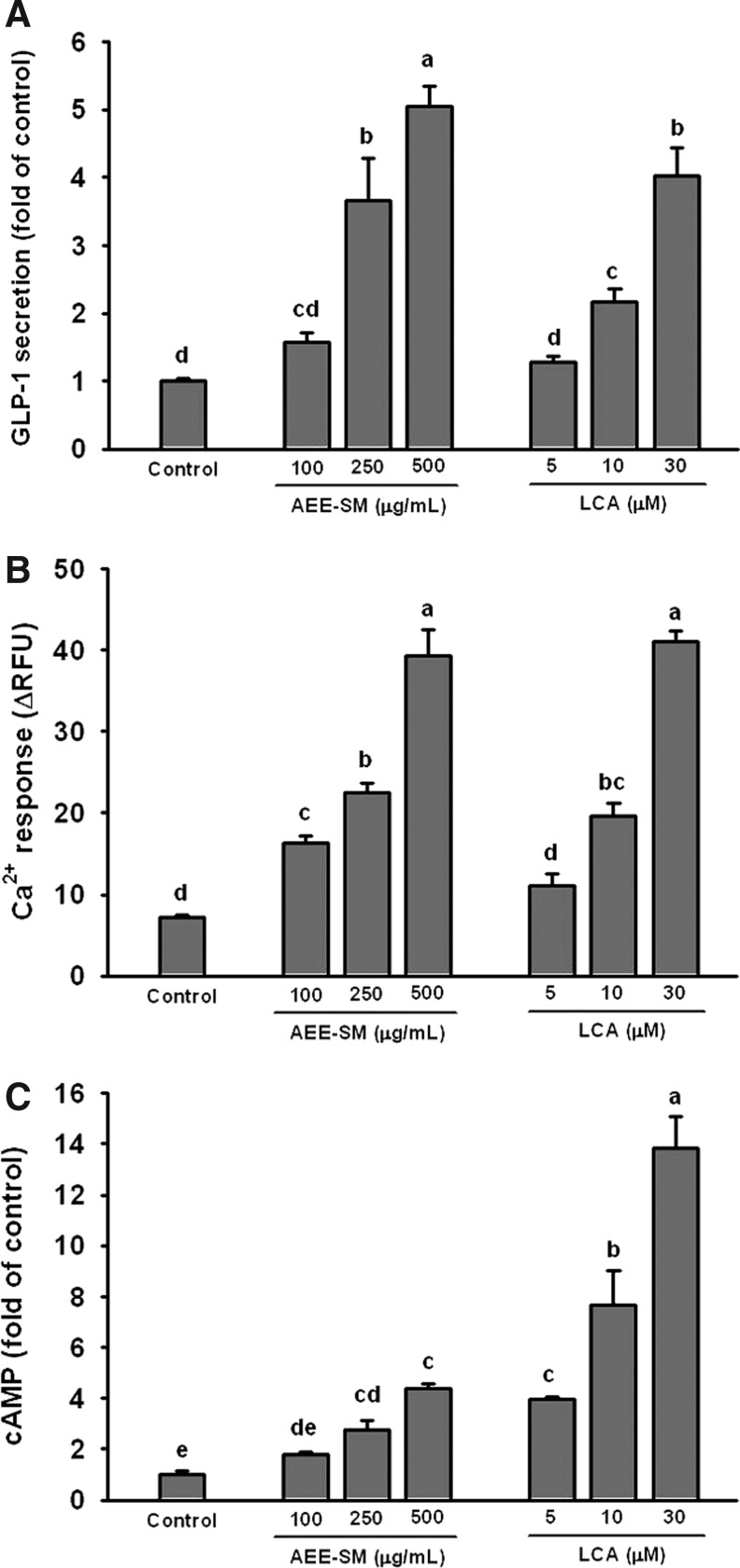
Treatment of NCI-H716 cells with AEE-SM at 100, 250, and 500 μg/mL significantly stimulated GLP-1 secretion in a concentration-dependent manner (Fig. 2A). The half-maximal effective concentration (EC50) of AEE-SM for GLP-1 release was 117 μg/mL when the response to 30 μM (11.29 μg/mL) LCA (a positive control) was regarded to be the maximum response.
FIG. 2.
(A) Glucagon-like peptide-1 (GLP-1) secretion from NCI-H716 cells in response to AEE-SM. (B) Increases in intracellular Ca2+ concentrations in response to AEE-SM in the cells. (C) Increases in intracellular cyclic AMP (cAMP) levels in response to AEE-SM in the cells. All values are mean±standard error of the mean (SEM) of 12 wells. Values not sharing a common letter are significantly (P<.05) different by analysis of variance (ANOVA) and Tukey's test.
To investigate whether AEE-SM affected intracellular Ca2+ levels, cells were loaded with the Ca2+-indicator dye from the FLIPR Calcium 5 Assay Kit, incubated, and fluorescence monitored during the application of AEE-SM. Administration of 100, 250, and 500 μg/mL AEE-SM significantly increased fluorescence compared to basal levels (Fig. 2B). The EC50 of AEE-SM for increasing Ca2+ levels was 199 μg/mL when the response to 30 μM LCA was regarded to be the maximum response.
We also examined whether AEE-SM induced an increase in intracellular cAMP levels. Treatment of NCI-H716 cells with 100, 250, and 500 μg/mL AEE-SM significantly increased intracellular cAMP levels in a dose-dependent manner (Fig. 2C). The EC50 of AEE-SM for increasing cAMP levels was 875 μg/mL when the response to 30 μM LCA was regarded to be the maximum response.
Effect of transfection of siRNA targeting TGR5 on AEE-SM-induced secretion of GLP-1 and increase in intracellular Ca2+ and cAMP levels
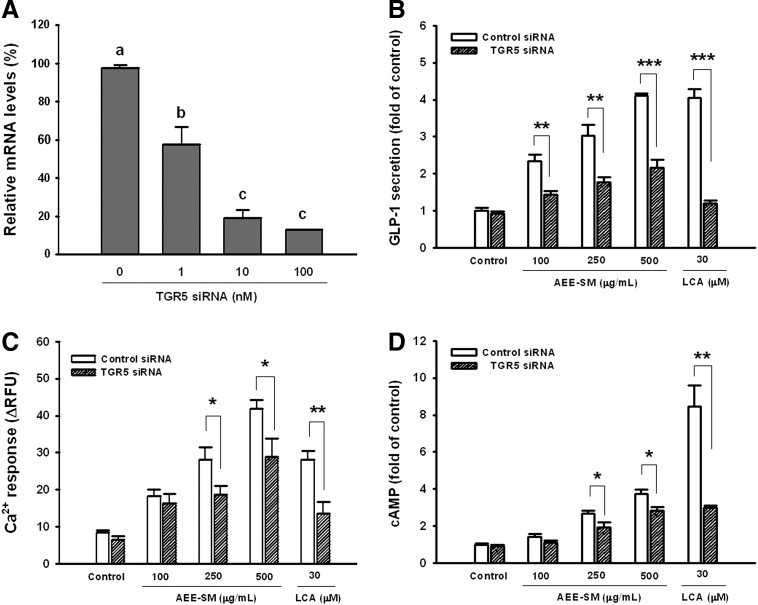
To determine the involvement of TGR5 in AEE-SM-induced GLP-1 release from NCI-H716 cells, we inhibited the expression of TGR5 mRNA by siRNA transfection and determined the effects of this on AEE-SM-induced GLP-1 secretion and intracellular Ca2+ increase. We confirmed significant knockdown of TGR5 mRNA expression 48 h after transfection (Fig. 3A). Relative TGR5 mRNA levels in siRNA knockdown cells were reduced by 39.8% at 1 nM, 78.3% at 10 nM, and 84.6% at 100 nM siRNA compared with the control. We therefore used 10 nM siRNA for subsequent experiments to determine the involvement of TGR5 in AEE-SM-induced GLP-1 release. When cells were transfected with 10 nM siRNA targeting TGR5, GLP-1 secretion induced by 30 μM LCA was significantly inhibited by 91.7% (Fig. 3B), the Ca2+ increase by 30 μM LCA was inhibited by 63.7% (Fig. 3C), and the cAMP increase by 30 μM LCA was inhibited by 72.4% (Fig. 3D). AEE-SM-induced GLP-1 secretion significantly decreased by 55.0% at 100 μg/mL, 57.5% at 250 μg/mL, and 60.0% at 500 μg/mL AEE-SM when the expression of TGR5 mRNA was inhibited by siRNA (Fig. 3B). The AEE-SM-induced Ca2+ response was significantly decreased by 36.8% at 250 μg/mL and 32.6% at 500 μg/mL AEE-SM in TGR5 knockdown cells (Fig. 3C). AEE-SM-induced cAMP increase significantly decreased by 40.0% at 250 μg/mL and 30.5% at 500 μg/mL AEE-SM in TGR5 knockdown cells (Fig. 3D).
FIG. 3.
Inhibition of Takeda G-protein-coupled receptor 5 (TGR5) mRNA expression by small interference RNA (siRNA), and effect on AEE-SM-induced GLP-1 release and intracellular Ca2+ and cAMP increases from NCI-H716 cells. Transfections using nontargeting siRNA were used as controls. (A) TGR5 mRNA assessed by real-time quantitative PCR 48 h posttransfection. (B) Effect of TGR5 knockdown on AEE-SM-induced GLP-1 release. (C) Effect of TGR5 knockdown on AEE-SM-induced increases in intracellular Ca2+ levels. (D) Effect of TGR5 knockdown on AEE-SM-induced increases in intracellular cAMP levels. The data are mean±SEM (n=12); values not sharing a common letter are significantly different (P<.05 by ANOVA and Tukey's test). *P<.05, **P<.01, ***P<.001 versus control siRNA.
Effect of AEE-SM on TGR5 activation in TGR5-transfected CHO-K1 cells
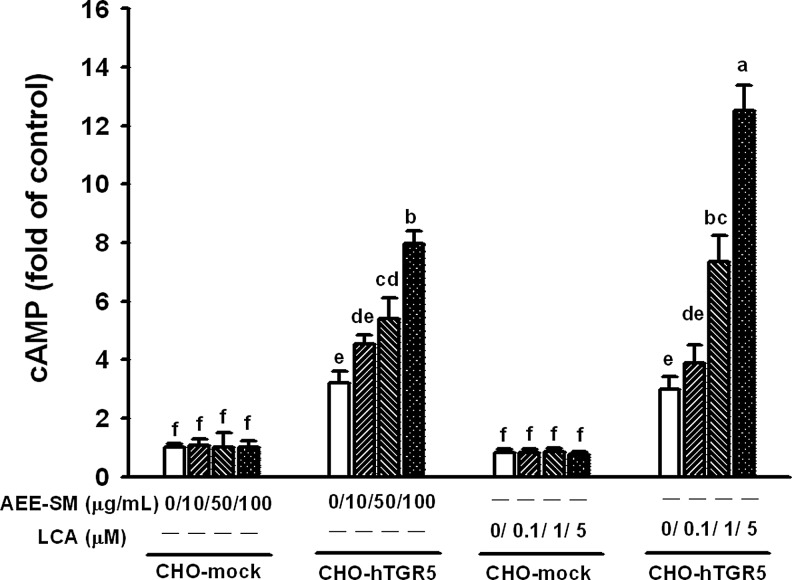
We next examined intracellular cAMP levels in CHO-K1 cells expressing human TGR5. The expression vector for human TGR5 cDNA was transfected into CHO-K1 cells, and the effects of AEE-SM on intracellular cAMP levels were measured. LCA (a known agonist of TGR5) at 0.1, 1, and 5 μM induced a significant increase in intracellular cAMP in cells transfected with TGR5, but not in cells transfected with an empty vector (Fig. 4). AEE-SM at 10, 50, and 100 μM induced a significant increase in intracellular cAMP in cells transfected with TGR5, and the EC50 of AEE-SM for increasing cAMP levels in TGR5-transfected cells was 63.4 μg/mL when the response to 5 μM (1.88 μg/mL) LCA was regarded to be the maximum.
FIG. 4.
Effect of AEE-SM on intracellular cAMP levels in Chinese hamster ovary (CHO)-K1 cells transiently transfected with human TGR5 expression plasmid or mock (without TGR5 plasmid). Data are mean±SEM (n=12). Values not sharing a common letter are significantly different (P<.05 by ANOVA and Tukey's test).
Dissussion
In the present study, we report for the first time that AEE-SM can elicit GLP-1 secretion from enteroendocrine NCI-H716 cells. AEE-SM-induced GLP-1 secretion was dependent on TGR5 activation. In addition, we demonstrated that AEE-SM is a natural agonist for human TGR5.
We also observed that the stimulatory effect of AEE-SM on GLP-1 may be, at least, associated with elevations in intracellular Ca2+ and cAMP levels in NCI-H716 cells, which is supported by GLP-1 secretion and Ca2+ and cAMP responses after AEE-SM stimulation. On the other hand, AEE-SM might induce GLP-1 secretion not only through the mechanism by which LCA induces GLP-1 but also through other mechanisms by which LCA does not share in GLP-1 secretion, which is supported by the observation that AEE-SM showed a lower response in cAMP levels than the response in Ca2+ levels and GLP-1 secretion compared with LCA.
When GLP-1 secretion is involved in the activation of GPCRs coupled to G-proteins in the Gαs family, GLP-1 secretion is coupled to increases in both intracellular Ca2+ and cAMP levels in NCI-H716 cells. AEE-SM significantly increased both Ca2+ and cAMP levels in cells. These results raised the possibility that AEE-SM-induced GLP-1 secretion is involved with activation of GPCRs coupled to Gαs.
TGR5 is a Gαs-coupled receptor. TGR5 stimulates GLP-1 release through coupling to Gαs, which elevates intracellular Ca2+ and cAMP levels in NCI-H716 cells.11 Transfection of cells with TGR5-specific siRNA significantly inhibited AEE-SM-induced GLP-1 secretion and the increase in Ca2+ and cAMP levels (Fig. 3). These results indicate that TGR5 mediates the AEE-SM-stimulated GLP-1 release and the increase in Ca2+ and cAMP levels in NCI-H716 cells.
To further evaluate the human TGR5 agonist activity of AEE-SM, we employed CHO-K1 cells transiently transfected with human TGR5. Our data demonstrated that AEE-SM significantly activates TGR5 (Fig. 4). Activation of TGR5 by AEE-SM was specific, because AEE-SM had no effects on control cells that did not overexpress TGR5 (Fig. 4).
NCI-H716 cells are a useful in vitro tool to study GLP-1 secretion from the human intestine. AEE-SM induced GLP-1 release through a TGR5-dependent pathway, although other signaling pathways may also be involved. In this study, we examined the involvement of TGR5 in AEE-SM-induced GLP-1 release, because TGR5 has been shown to be expressed in NCI-H716 cells and involved in GLP-1 release. However, other GPCRs coupled to Gαs besides TGR5 may be expressed and involved in GLP-1 release by NCI-H716 cells. However, GPR119, which is a GPCR coupled to Gαs and activated by oleoylethanolamide (a food-associated lipid metabolite), has been reported to induce GLP-1 release in enteroendocrine L cells9; the expression or involvement of GPR119 in GLP-1 release in NCI-H716 cells has not yet been studied.
GLP-1 is secreted by L-cells in response to digestive products of carbohydrates, fats, and proteins through diverse lists of GPCRs. Digestive products of carbohydrates lead to GLP-1 release, in part, through activation of sweet taste receptors. Free fatty acids derived from fats lead to GLP-1 release through activation of free fatty acid receptors. The mechanisms of protein-triggered GLP-1 release remain unclear. Ingestion of carbohydrates, fats, and proteins supply calories to the body.11 However, AEE-SM with the GLP-1-releasing activity might maintain glucose and energy homeostasis, while providing low intake of calories.
GLP-1 plays an essential role in the maintenance of normal glucose homeostasis by its peripheral and neural actions, including stimulation of glucose-dependent insulin secretion, proinsulin gene expression, and β-cell proliferative and antiapoptotic pathways.10 We showed that AEE-SM stimulates GLP-1 secretion, suggesting that AEE-SM might have regulatory effects on glucose homeostasis. Moreover, GLP-1 regulates energy homeostasis by its neural actions that include inhibiting glucagon release, gastric emptying, food intake, and appetite.17 AEE-SM appears to regulate energy homeostasis through GLP-1 secretion. In addition, we found that AEE-SM is a specific agonist for human TGR5. TGR5 is ubiquitously expressed in the small intestine, placenta, spleen, lungs, liver, stomach, adipose tissue, skeletal muscle, kidney, and pancreas. TGR5 activation results in a range of beneficial metabolic effects that include the maintenance of glucose homeostasis and insulin sensitivity, resistance to weight gain and hepatic steatosis, and preservation of the liver and pancreatic function.10 Therefore, AEE-SM is likely to have multiple pharmacological functions in the body.
In conclusion, we demonstrated that AEE-SM stimulates GLP-1 secretion in NCI-H716 cells and that the secretion involves TGR5 activation. Also, we showed that AEE-SM is an agonist for TGR5. The findings suggest that AEE-SM might have efficacy as a functional food with multiple functions, including improving glucose and energy homeostasis.
Acknowledgments
This work was supported by the Korea Food Research Institute (the grant number E0131203) and the National Research Foundation of Korea (grant number 2010-0024475).
Author Disclosure Statement
The authors have declared no conflicts of interest.
References
- 1.Vinholes J, Grosso C, Andrade PB, et al. : In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem 2011;129:454–462 [DOI] [PubMed] [Google Scholar]
- 2.Hartman RL, Rabeler RK: Spergularia. In: Flora of North America, Vol. 5 (Flora of North America Editorial Committee, ed.). Oxford University Press, New York, 2005, pp. 16–23 [Google Scholar]
- 3.Jouad H, Lacaille-Dubois MA, Eddouks M: Chronic diuretic effect of the water extract of Spergularia purpurea in normal rats. J Ethnopharmacol 2001;75:219–223 [DOI] [PubMed] [Google Scholar]
- 4.Jouad H, Lemhadri A, Maghrani M, Zeggwagh NA, Eddouks M: Cholesterol-lowering activity of the aqueous extract of Spergularia purpurea in normal and recent-onset diabetic rats. J Ethnopharmacol 2003;87:43–49 [DOI] [PubMed] [Google Scholar]
- 5.Jouad H, Eddouks M, Lacaille-Dubois MA, Lyoussi B: Hypoglycemic effect of Spergularia purpurea in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol 2000;71:169–177 [DOI] [PubMed] [Google Scholar]
- 6.Eddouks M, Jouad H, Maghrani M, Lemhadri A, Burcelin R: Inhibition of endogenous glucose production accounts for hypoglycemic effect of Spergularia purpurea in streptozotocin mice. Phytomed 2003;10:594–599 [DOI] [PubMed] [Google Scholar]
- 7.Jouad H, Lacaille-Dubois MA, Lyoussi B, Eddouks M: Effects of the flavonoids extracted from Spergularia purpurea Pers. on arterial blood pressure and renal function in normal and hypertensive rats. J Ethnopharmacol 2001;76:159–163 [DOI] [PubMed] [Google Scholar]
- 8.Heo BG, Park Yj, Park YS, et al. : Distribution status, physicochemical composition, and physiological activity of Spergularia marina cultivated. Korean J Community Liv Sci 2009;20:181–191 [Google Scholar]
- 9.Reimann F, Habib AM, Tolhurst G, Parker HE, et al. : Glucose sensing in L cells: a primary cell study. Cell Metab 2008;8:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas C, Gioiello A, Noriega L, Strehle A, et al. : TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009;10:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiman F, Tolhurst G, Gribble FM: G-protein-coupled receptors in intestinal chemosensation. Cell Metab 2012;4:421–431 [DOI] [PubMed] [Google Scholar]
- 12.Jimoh FO, Adedapo AA, Afolayan AJ: Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food Chem 2010;48:964–971 [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Park M, Lee YM, Rhyu MR, Kim HY: Ginsenoside metabolite compound K stimulates glucagon-like peptide-1 secretion in NCI-H716 cells via bile acid receptor activation. Arch Pharm Res 2014;37:1193–1200 [DOI] [PubMed] [Google Scholar]
- 14.Reimer RA, Darimont C, Gremlich S, Nicolas-Metral V, Ruegg UT, Mace K: A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. J Endocrinol 2001;142:4522–4528 [DOI] [PubMed] [Google Scholar]
- 15.Di Matteo MA, Loweth AC, Thomas S, Mabley JG, Morgan NG, Thorpe JR, Green IC: Superoxide, nitric oxide, peroxynitrite and cytokine combinations all cause functional impairment and morphological changes in rat islets of langerhans and insulin-secreting cell lines but dictate cell death by different mechanism. Appotosis 1997;2:164–177 [DOI] [PubMed] [Google Scholar]
- 16.An H, Wang H, Lan Y, Hashi Y, Chen S: Simultaneous qualitative and quantitative analysis of phenolic acids and flavonoids for the quality control of Apocynum venetum L. leaves of HPLC-DAD-ESI-IT-TOF-MS and HPLC-DAD. J Pharm Biomed Anal 2013;85:295–304 [DOI] [PubMed] [Google Scholar]
- 17.Lim GE, Brubaker PL: Glucagon-like peptide 1 secretion by the L-cell: the view from within. Diabetes 2006;55:S70–S77 [Google Scholar]