Abstract
The goal for this study was to evaluate the effects of consumption of dried apple peel powder (DAPP) on joint function and range of motion (ROM). Additional in vitro and clinical testing was performed to suggest specific mechanisms of action. An open-label clinical pilot study involved 12 healthy people with moderate loss of joint ROM and associated chronic pain. The subjects consumed 4.25 g DAPP daily for 12 weeks, with evaluations at baseline, 2, 4, 8, and 12 weeks. ROM was evaluated at each visit using dual digital inclinometry. Pain scores were collected using Visual Analogue Scales. Blood draws enabled testing of serum antioxidant protective capacity using the cellular antioxidant protection (CAP-e) bioassay. Additional in vitro testing involved testing of cyclooxygenase-2 (COX-2) and lipoxygenase inhibition, cellular antioxidant protection by the CAP-e bioassay, and formation of reactive oxygen species (ROS) by polymorphonuclear (PMN) cells by flow cytometry. Twelve weeks of consumption of DAPP was associated with improved ROM. DAPP provided antioxidants that were available to enter into and protect cells from oxidative damage in vitro, and consumption of DAPP for 12 weeks was associated with a statistically significant improvement in serum antioxidant protective status. DAPP inhibited both COX-2 and lipoxygenase enzymes, and pretreatment of inflammatory PMN cells with DAPP before inflammatory stimulus resulted in reduced ROS formation. This suggests multifaceted anti-inflammatory properties of DAPP. Consumption of DAPP was associated with improved joint function and improved serum antioxidant protection status. The observed pain reduction may be associated with the improved antioxidant status and linked to the apple polyphenols' anti-inflammatory effects.
Key Words: : antinociceptive, antioxidant, anti-inflammatory, chronic pain, digital inclinometry
Introduction
Joint mobility affects normal activities of daily living as age-related wear and tear leads to significant structural, mechanical, and matrix changes.1 A progressive reduction in the ability of chondrocytes to maintain cartilage homeostasis contributes to overall loss of matrix tensile strength and stiffness that accompanies aging,2 resulting in a restriction of joint movement and loss of mobility.
As interest in natural, nutritional support for overall health has increased, several studies have shown that polyphenol flavonoids, such as anthocyanins, are capable of neuro-protective, anti-inflammatory, and analgesic functions. These naturally occurring compounds may contribute to primary prevention measures and provide an alternative to non-steroidal anti-inflammatory drugs used in the treatment of age-related decrease in joint function.
Contrary to acute inflammation, chronic inflammation results when the inflammatory response is incomplete, or the inflammatory initiators persist, rather than being controlled by the production of anti-inflammatory cytokines.3 While inflammation can be beneficial in that it recruits inflammatory cells to the affected tissue and stimulates changes in local blood vessels, prolonged inflammatory states have been linked to the development of rheumatoid arthritis and other inflammatory diseases, which reduce both joint range of motion (ROM) and activity levels.
Apples, and the apple peel specifically, have been associated with multiple health benefits through disease prevention and the maintenance of overall health. Apples have been shown to display antioxidant and antiproliferative activity, which may be responsible for protecting cellular components from oxidative damage as well as for inhibiting the growth of tumor cells.4 Evidence from epidemiological studies has shown that apples may play a significant role in reducing the risk of chronic diseases such as cancer, type II diabetes, cardiovascular disease, pulmonary disease, and asthma.5
Although apples contain an abundance of biologically active compounds, it is well known that the flesh and peel differ with regard to phenolic distribution. For example, although both contain compounds such as phloretin glycosides, phloridzin, and chlorogenic acid, the peel contains additional flavonoids that are not found within the flesh.6 In an evaluation of the nutritional quality of apple peels, the total phenolic and flavonoid contents, antioxidant activities, and inhibition of tumor cell growth were significantly higher in the peels than in flesh, regardless of the cultivar.6
The phytochemical profile of apples has been found to be affected by their cultivar, conditions of growth and maturation, as well as plant nutritive status and processing.7 In addition to the differences in phenolic and flavonoid contents between specific apple cultivars, changes in light exposure during the process of ripening may be significant in stimulating the production of specific phytochemicals. These variations in phytochemical concentration have been positively associated with total antioxidant activity.6 Although the duration of storage has not been shown to have significant effects on apple phenolics, the processing of apples for juice or other apple-related products has been shown to significantly reduce the concentration of phenolics and total antioxidant activity compared with fresh apples.5 In contrast, dried apples and their peel maintain a high level of polyphenolic antioxidants.6
Polyphenolic compounds found in fruits have been documented to display anti-inflammatory and antioxidant activity both in vitro and in vivo. Apple extracts have been observed to inhibit the expression of pro-inflammatory genes, inflammatory enzymes, and transcription factors,8 thereby modifying signal transduction pathways. Specifically, quercetin, a polyphenolic flavonoid found in fruits such as apples, red grapes, and blueberries, has been shown to have significant anti-inflammatory effects in human and animal models,9 suggesting a beneficial effect on chronic inflammatory diseases and overall joint health. In addition, a high intake of antioxidant-rich fruits in animal studies has been positively associated with higher bone mineral density, mass, thickness, enhanced bone formation, and suppression of bone resorption, which results in greater overall bone strength.10
It is well known that dietary polyphenolic antioxidants have been associated with improved joint function and anti-nociceptive effects in both animals and humans. Clinical evaluation of a beverage containing whole Acai (including the skin and pulp) showed improved joint ROM,11 as did a clinical study on a complex blend, including the mushroom-based amino-acid ergothioneine.12 In addition, anthocyanin-rich extracts from tart cherries have been shown to significantly reduce inflammation-induced thermal and mechanical hyperalgesia in rats.13 Therefore, due to their potential for reducing inflammation, improving mobility, and modifying pain perception, interest in a variety of fruits containing high levels of polyphenols has increased.
Dried apple peel powder (DAPP) is rich in polyphenolic antioxidants, and it has been demonstrated to inhibit cyclooxygenase-2 (COX-2), in addition to other potent mechanisms of anti-inflammatory action.14 This study was performed to explore effects in humans consuming DAPP over a period of 12 weeks. A detailed evaluation of joint function was performed, as well as an evaluation of antioxidant status and pain reduction.
Materials and Methods
Reagents
The following buffers and reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA): Histopaque 1077 and 1119, phosphate-buffered saline (PBS), RPMI-1640 culture medium, fetal calf serum, L-glutamine 200 mM, penicillin-streptomycin 100× solution, gallic acid, dimethylsulfate (DMSO), fibronectin, and bovine serum albumin. The precursor dye [5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester] (DCF-DA) was obtained from Molecular Probes (Eugene, OR, USA). 2,2′-Azobis (2-amidinopropane) dihydrochloride (AAPH) was obtained from Wako Chemical USA (Richmond, VA, USA); sodium azide (NaN3) was obtained from LabChem, Inc. (Pittsburgh, PA, USA). Leukotriene B4 was obtained from Cayman Chemical (Ann Arbor, MI, USA).
Dried apple peel powder
Leahy dried apple peel powder (Leahy DAPP™), commercially available as AppleActiv™ and AppleBoost™, was obtained from Leahy Orchards, Inc. (Quebec, Canada). For the in vitro testing, DAPP was prepared as follows: 500 mg DAPP was added to 5 mL saline, incubated on a rocker for 1 h, after which solids were removed by centrifugation followed by filtration through a sterile 0.22 μm cellulose acetate filter. The dose range chosen for the bioassays was based on an initial cellular viability assay, to establish the dose range to be used in cellular assays. For some tests, a low-molecular-weight (LMW) fraction was prepared by applying this sterile saline solution, containing the water-soluble portion of DAPP, to an ultrafiltration device and centrifuging according to the manufacturer's instructions to isolate compounds smaller than the 3 kDa cut-off for the ultrafiltration device. For the clinical pilot study, DAPP was encapsulated in veggie capsules. Participants consumed three capsules thrice daily, for a daily dose of 4.25 g, which is the upper daily dose recommended by the manufacturer.
Antioxidant capacity using the oxygen radical absorbance capacity assay
The antioxidant capacity was evaluated by a panel of chemical oxygen radical absorbance capacity (ORAC) tests, where each test measures quenching of specific oxidative reactions. Total ORAC provides a measure of the total antioxidant capacity against these five predominant reactive species: peroxyl radicals, hydroxyl radicals, peroxynitrite, super oxide anion, and singlet oxygen. Testing was performed at Brunswick Laboratories (Southborough, MA, USA).
Antioxidant capacity using the Folin–Ciocalteu assay
The antioxidant capacity was also tested using the Folin–Ciocalteu assay. The Folin–Ciocalteu's phenol reagent was added to serial dilutions of extract, thoroughly mixed, and incubated for 5 min. To start the chemical reaction, sodium carbonate was added to produce color. The microplate was incubated for 30 min at 37°C, and the optical absorbance at 765 nm was read in a colorimetric plate reader (BioTek PowerWave, Winooski, VT, USA).
COX-2 inhibition
The effects of DAPP on COX-2 was evaluated using the COX Inhibitor Screening Assay Kit (Cayman Chemical). Arachidonic acid was used as a substrate for human recombinant COX-2 enzyme. The assay measures PGF2α produced by SnCl2 reduction of COX-derived PGH2. The prostanoid product is quantified via enzyme immunoassay using a broadly specific antibody that binds to all major prostaglandin compounds.
Lipoxygenase inhibition
DAPP's effect on the enzymatic activity of lipoxygenase was tested using lipoxygenase Inhibitor Screening Assay Kit (Cayman Chemical). Purified soybean lipoxygenase was provided with the substrate arachidonic acid in the absence versus presence of DAPP. The hydroxyperoxides produced as a result of the lipoxygenase enzymatic reaction were measured in a colorimetric assay. Chromogen was used to stop enzyme catalysis and develop the reaction. The 96-well plate was read in a microplate reader (BioTek PowerWave) at 490–500 nm absorbance.
Purification of polymorphonuclear cells and erythrocytes
Healthy human volunteers between the ages of 18 and 65 years served as blood donors after written informed consent was obtained, as approved by the Sky Lakes Medical Center Institutional Review Board (FWA2603). Isolation of polymorphonuclear (PMN) cells was performed as previously described.15,16 The PMN cells were used for evaluation of anti-inflammatory activity in assays for production of reactive oxygen species (ROS) and migratory response to the inflammatory mediator leukotriene B4. The erythrocytes were stored at 4°C in aliquots for later use in the cellular antioxidant protection (CAP-e) bioassay.
CAP-e bioassay
The CAP-e assay was performed according to the method published by Honzel et al.,15 but using an accelerated and more sensitive microplate-based protocol, and the implementation of ex vivo storage of the erythrocytes to achieve improved interassay performance. Briefly, a suspension of erythrocytes was prepared for the CAP-e bioassay by adding 0.1 mL packed red blood cells to 10 mL physiological saline (pH 7.4) in a V-bottom 96-well microplate. Six wells served as negative controls (no induced oxidative damage), and six wells served as positive controls (maximum oxidative damage in the absence of any antioxidants). Gallic acid was used as a standard reference compound. For assessment of the cellular antioxidant protection provided by the DAPP in vitro, serial dilutions of the aqueous extract were tested in duplicate. For assessment of the changes in serum antioxidant protection capacity, serum samples were tested in quadruplicate. The cells were incubated for 20 min with aqueous extract or serum, to enable available antioxidants to penetrate into the cells. After this incubation, antioxidant compounds that were not absorbed into the erythrocyte cells were removed from the assay plate by two washes in physiological saline. Cell pellets were re-suspended and lysed in water to accelerate the final steps, and the precursor dye DCF-DA was added to the wells for 15 min. Subsequently, oxidative damage was induced by the addition of AAPH and incubation for 1 h. The oxidation leads to the transformation of the precursor dye to a green fluorescent marker, where the fluorescence intensity is a measure of the level of oxidative damage. The green fluorescence intensity was recorded at 488 nm using a Tecan Spectrafluor plate reader (Durham, NC, USA). Cellular antioxidant protection was calculated as the inhibition of oxidative damage reflected by the reduced fluorescence intensity in the wells where cells were pretreated with test products, compared with the baseline (negative controls) and maximum oxidative damage (positive controls).
Evaluation of ROS formation in PMN cells
PMN cells were used for the evaluation of ROS. They were incubated at 37°C, in 5% CO2 for 20 min as either untreated PMN cells or serial dilutions of DAPP aqueous extract treated PMN cells. The precursor dye DCF-DA was prepared by adding 0.18 mL DMSO to a 50 μg aliquot of DCF-DA (stock solution). By adding 0.01 mL stock to 10 mL PBS, the working solution was prepared. PBS was used to wash the PMN cells twice to remove any unabsorbed and unbound compounds. The DCF-DA working solution was used to resuspend the cells. They were incubated for 1 h at 37°C so the precursor dye could be absorbed into the PMN cells. All samples were then exposed to 167 mM H2O2 for 45 min to induce severe oxidative stress, except for the three negative controls. The peroxide was removed from the samples by washing twice in PBS, transferred to cold RPMI 1640 medium, and stored in the dark on ice. Flow cytometry (Becton Dickinson FACSCalibur, San Jose, CA, USA) was used to immediately analyze the DCF-DA fluorescence intensity. Data for controls and each dilution of the DAPP extract were collected in triplicate. Untreated, H2O2-treated, and extract-pretreated cells were compared using the mean fluorescence intensity of PMN cells.
Migratory response to the inflammatory mediator leukotriene B4
The effects of DAPP on inflammatory cell behavior was tested using a transwell migration assay. Each control and treatment was performed in quadruplicate following the experimental model. Serial dilutions of DAPP aqueous were incubated with cells. The plate was allowed to sit for 30 min with 50 μg/mL fibronection coating the top compartments of Millipore transwell (3.0 μm pore size) migration plates. Culture medium containing leukotriene B4 (12 nM) was placed in the bottom chamber at a volume of 150 μL in the transwell migration plate. Aspiration of the fibronectin from the top wells was performed before plating of cells. The top chambers were plated with 50 μL of cells (1×106/mL), then lowered into the bottom plate, and allowed to incubate for 4 h at 37°C. The top chambers were removed after the incubation. CyQuant® was used to stain the relative number of cells that had migrated to the bottom chambers. The Tecan Spectrafluor plate reader (Durham, NC, USA) was used to measure the fluorescence intensity. Experiments were repeated thrice using PMN cells from three different healthy donors.
Clinical pilot study
A 12-week clinical pilot study was performed, following an open-label design. Twelve study participants were screened and enrolled after written informed consent, as approved by Sky Lakes Medical Center Institutional Review Board (FWA 2603). Screening involved an interview to ensure that subjects met the study inclusion/exclusion criteria, and a prestudy ROM assessment was performed for two purposes: (1) to ensure that a given study participant had reduced ROM in specific joints, (2) to accommodate a learning curve for the detailed ROM assessment, so that data collection would not be affected at later visits. Inclusion criteria were as follows: Subjects of either gender, 45–75 years of age, eating a balanced Western diet, with more than 6 months of chronic pain in well-defined area(s). Exclusion criteria were recent trauma that would affect ROM and pain scoring, and recent changes in diet, supplements, or medication that could potentially affect joint health and pain scores. Daily consumption of over-the-counter pain medications as well as supplements that may be beneficial to joint health was not an exclusion criterion; however, subjects were instructed to maintain this constant during the study.
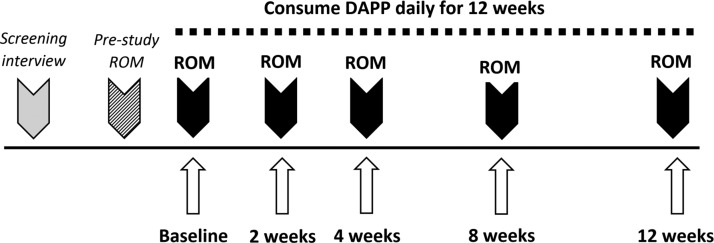
The study subjects consumed 4.25 g (nine capsules) of DAPP daily for 12 weeks, spread over three daily doses of three capsules. Study participants were instructed to keep diet and lifestyle constant during the study, and were allowed to consume over-the-counter pain medication if needed. Compliance was evaluated during each visit, including product consumption, diet and lifestyle, and medications. The study participants were monitored at baseline and after 2, 4, 8, and 12 weeks, where questionnaires, ROM assessment, and blood draws were performed (Fig. 1). Serum samples were banked at −80°C during the clinical phase of the study, and used for the testing of serum antioxidant protection capacity in the CAP-e bioassay after the clinical phase was completed.
FIG. 1.
Diagram showing the study design for the 12-week open-label study. Range of motion (ROM) along the vertical weight-bearing column (neck to knees) and shoulders was performed using dual digital inclinometry. Additional data involved questionnaire-based data collection pertaining to pain levels in areas of primary and secondary pain areas identified at study start. Blood draws for serum testing was performed at baseline, 2, 4, 8, and 12 weeks.
ROM assessment using dual digital inclinometry
The evaluation of ROM was conducted using a detailed protocol where the ROM of the entire vertical weight-bearing axis of the body was studied from the neck to the knees, as well as the shoulders. The rationale behind this detailed assessment is that often a person's primary complaint (e.g., left knee) will lead to a compensated posture and ROM of other anatomical areas such as the lower back, as the person strives to alleviate pressure on the affected joint. This complete assessment (15 distinct ROM measurements) optimizes our ability to show significant changes as a result of consumption of a nutritional product. The ROM assessment was active, that is, the study participants performed movements and the extent of movement was recorded. This method is very reliable, as the software provides voice prompts for whether three readings gave a good average, and will prompt approximately six readings, if needed, for each ROM measurement. A screening ROM exam was performed before study entry to ensure the subject was used to the examination procedure so subsequent readings were more accurate and not affected by learning the instructions and expected motions. All ROM assessments were performed by a single examiner (D.M.A.), using wireless dual digital inclinometry (JTECH Tracker Freedom; JTECH Medical, Salt Lake City, UT, USA).
Pain assessment
Pain is inherently a subjective parameter, as pain perception is affected by many physiological, emotional, and stress-related factors. A person's self-reported pain under different conditions (at rest versus at use when performing physical activities) is considered the most reliable tool.17 Scoring in a research setting is optimally performed using neutral scoring tools, such as an unmarked 100 mm Visual Analogue Scale (VAS).18 For this study, each person's anatomical areas of primary and secondary joint stiffness and reduced function were identified before the study start. At each visit, pain levels for both the primary and secondary areas were scored for “pain at rest” and “pain at use,” using VAS. General pain at the time of the visit was also tracked. The VAS were 100 mm without increment marks, where one end was labeled “no pain,” and the other end was labeled “intense pain.” The score was measured on the scale in millimeters and scored in percentage.
Statistical analysis
For the in vitro testing, average and standard deviation for each data set was calculated using Microsoft Excel. Statistical analysis of in vitro data was performed using the two-tailed, dependent t-test. For the evaluation of ROM and pain scores, a comparison of arithmetic means was performed using Student's t-test, using Microsoft Excel (Microsoft, Redmond, WA, USA). Statistical significance of changes from baseline to later assessments was evaluated by “within-subject” analysis performed using the two-tailed paired t-test. Statistical significance was indicated if P<.05, and a high level of significance was indicated if P<.01.
Results
Clinical pilot study
The 12-week open-label pilot study involved 12 people of both genders with well-defined reduced joint function in several anatomical locations. These locations were identified at study start as the primary and secondary complaints (Table 1).
Table 1.
Demographics and Areas of Reduced Range of Motion at Study Start
| Anatomical area with limited ROM at study start | ||||
|---|---|---|---|---|
| Vol no. | Gender | Age | Primary | Secondary |
| V01 | F | 72 | Hands | Right knee |
| V02 | F | 48 | Knees | Fingertips |
| V03 | M | 73 | Shoulder | Neck |
| V04 | F | 62 | Feet | Hips |
| V05 | M | 61 | Hands | Achilles tendon |
| V06 | M | 56 | Neck/shoulders | Knees |
| V07 | F | 61 | Right shoulder | Hips |
| V08 | M | 56 | Left knee | Right hand |
| V09 | M | 68 | Right ankle | Back |
| V10 | F | 63 | Lower back/hip | Knees |
| V11 | M | 61 | Lower back | Upper back spine area |
| V12 | F | 56 | Neck | Back |
ROM, range of motion.
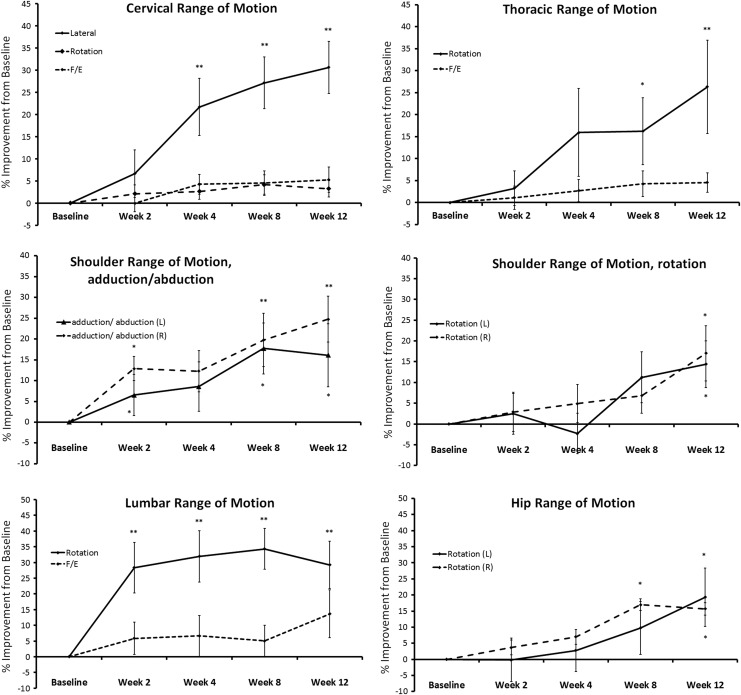
Improved ROM was seen rapidly for some joints, and more slow improvements were seen for other areas (Fig. 2). The lumbar area and shoulders showed rapid improvements, where some ROM measures were significantly increased already at 2 weeks, when compared with baseline ROM for each motion (P<.05). Particularly, the right shoulder adduction/abduction reached a high level of significance when compared with baseline at 8 and 12 weeks (P<.01). Both the cervical lateral ROM and thoracic ROM showed improvements at 4 weeks, where the improvement in cervical lateral motion was statistically significant compared with baseline ROM (P<.05). Cervical lateral ROM, thoracic and lumbar rotation, hip ROM, as well as all ROM associated with both shoulders were significantly improved after 12 weeks of DAPP consumption when compared with baseline ROM (P<.05). The improvements in cervical rotation, thoracic and lumbar rotation also reached a high level of significance (P<.01), with the lumbar rotation already showing significance after 2 weeks of consumption.
FIG. 2.
Changes in joint ROM during 12 weeks of consumption of dried apple peel powder (DAPP). The ROM was evaluated along the vertical weight-bearing column (neck to knees) as well as shoulders, using dual digital inclinometry. Rapid improvements were seen in lumbar and shoulder ROM, where some types of ROM improvements were statistically significant already at 2 weeks, when compared with baseline ROM (P<.05). The cervical lateral ROM and thoracic ROM showed improvements at 4 weeks, where the cervical lateral ROM was significantly improved compared with baseline ROM (P<.05). Cervical lateral ROM, thoracic and lumbar rotation, hip ROM, as well as all ROM associated with the left and right shoulders were significantly improved after 12 weeks of DAPP consumption when compared with baseline ROM (P<.05). The level of significance, as calculated by the paired t-test (“within-subject” analysis), is indicated by * if P<.05 and by ** if P<.01.
For eight of the study participants, the anatomical area of primary pain was in a joint where ROM was being measured during the study. For seven of these eight people, the anatomical area of primary pain showed improved ROM, and for six of these people the improvement was robust.
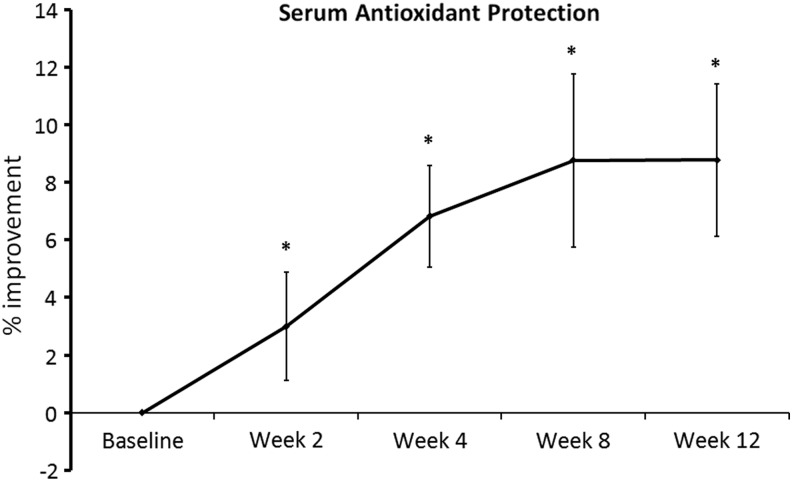
Improved antioxidant status was measured after 2 weeks of DAPP consumption, and the serum antioxidant protection status continued to improve throughout the initial 8 weeks of the study. After the 8 weeks, a plateau was seen, and the serum antioxidant protection status at week 12 was similar to week 8, and significantly above baseline (P<.05) (Fig. 3).
FIG. 3.
Changes in serum antioxidant protection during 12 weeks of consumption of DAPP. Serum samples collected during the study were tested in a modified cellular antioxidant protection (CAP-e) bioassay, where the antioxidant protection data reflect whether serum contained antioxidants of a chemical composition that are available to enter into and protect cells from oxidative stress. An improvement in serum antioxidant protection status was statistically significant already after 2 weeks of consumption, and continued to increase until 8 weeks, after which a plateau was seen. The improvement from baseline remained statistically significant at all time points (*P<.05).
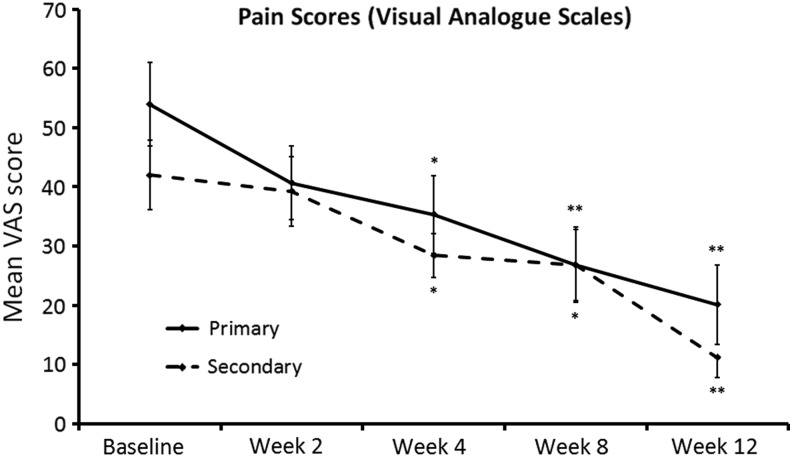
Reduction of chronic pain associated with each person's primary and secondary complaint areas was reported already at the first return visit at 2 weeks, and reached statistical significance at 4 weeks for both the primary and secondary complaint areas (P<.05). The pain reduction continued throughout the 12 week study and reached a high level of statistical significance at study exit (P<.01) (Fig. 4).
FIG. 4.
Pain scores during 12 weeks of consumption of DAPP. Joint pain was evaluated at baseline and at all the next visits using Visual Analogue Scales. Pain reduction was observed already after 2 weeks of DAPP consumption, and it was statistically significant after 4 weeks of consumption (*P<.05). The improvement continued throughout the 12 week study, at which time the improvement from baseline was highly significant (**P<.01).
Antioxidant capacity of DAPP
The antioxidant capacity of DAPP was evaluated by the measurement of the ORAC assay (Table 2). The antioxidant capacity of DAPP was primarily seen in the hydrophilic compounds, with a small contribution from lipophilic antioxidant compounds.
Table 2.
Antioxidant Capacity of Dried Apple Peel Powder
| ORAChydro | μm TE/g | 327 |
| ORAClipo | μm TE/g | 3 |
| ORACtotal | μm TE/g | 330 |
| H-ORAC | μm CAE/g | 57 |
| N-ORAC | μm TE2/g | 23 |
| SOD | kU SOD eq/g | 1.8 |
ORAC, oxygen radical absorbance capacity; TE, trolox equivalent; CAE, caffeic acid equivalent; SOD, superoxide dismutase.
Cellular antioxidant protection by DAPP
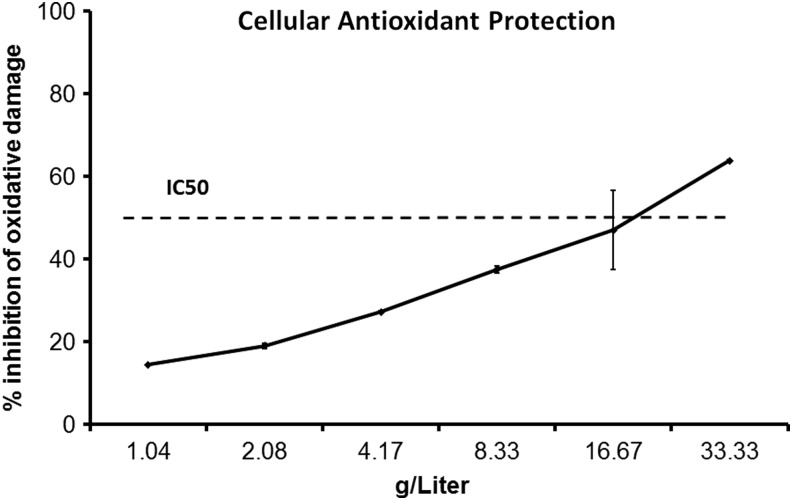
Given the high level of antioxidant capacity in the water-soluble fraction of DAPP, an aqueous extract of DAPP was tested for its ability to provide cellular antioxidant protection in the CAP-e bioassay. A clear dose response was seen, where measureable cellular protection from oxidative stress was provided by DAPP starting at a dose of 1 g/L (Fig. 5).
FIG. 5.
Cellular antioxidant protection provided by DAPP was measured using the CAP-e bioassay. Human erythrocytes were treated with serial doses of DAPP before being exposed to oxidative stress. The fluorescence intensity of a reporter dye reflected the level of intracellular oxidative damage. The inhibition of intracellular damage is shown as the average+standard deviation of duplicate data points, in reference to negative and positive controls, each of which are performed in hexaplicate. The protective effects provided by antioxidants in DAPP, able to enter into and protect the living cells, were dose dependent, with an IC50 around 20 mg/mL.
Reduced production of ROS by PMN cells in the presence of DAPP
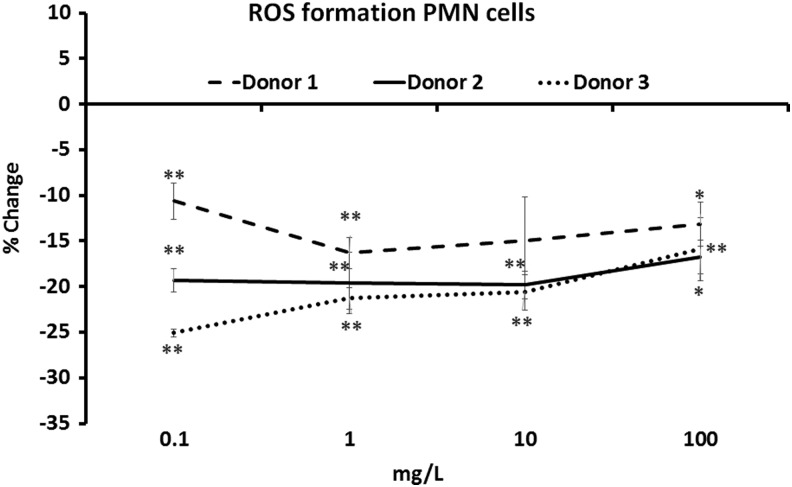
The PMN cell type comprises ∼70% of all circulating white blood cells in humans. The cell type is inflammatory in nature, and has the capacity to, on an inflammatory stimulus, very rapidly produce robust levels of free radicals, including ROS. Many polyphenol-rich natural products significantly reduce the production of ROS.15,17,18 This is not only a matter of antioxidant protection but also likely involves signaling events, programming the PMN cell to a less inflammatory behavior.19 The PMN cell type is subject to individual variation between blood donors, depending on the donor's nutritional and inflammatory status; therefore, we present data on the cellular ROS production after DAPP treatment from three healthy donors (Fig. 6). The treatment of PMN cells with DAPP before triggering an inflammatory response resulted in much reduced production of ROS in cell cultures from all three donors, across a wide dose range of DAPP. The reduction was statistically significant compared with untreated cells at all doses of DAPP tested (P<.05).
FIG. 6.
Changes in intracellular production of reactive oxygen species (ROS) were evaluated using an in vitro bioassay with inflammatory polymorphonuclear (PMN) cells from three healthy donors. Pretreatment of PMN cels with DAPP before an inflammatory insult was introduced resulted in reduction of the levels of ROS in cells from all three donors, across the dose range of 0.0001–0.1 mg/mL DAPP (P<.05). The level of significance, as calculated by the independent two-tailed t-test, is indicated by * if P<.05 and by ** if P<.01.
Inhibition of inflammatory cell migration in response to leukotriene B4
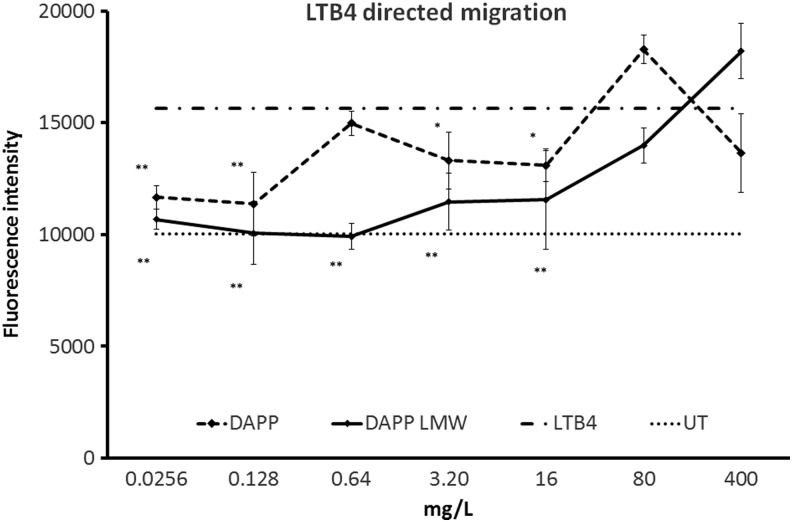
The recruitment of inflammatory cells into tissue involves the process of chemotaxis where the inflammatory cells sense a chemotactic compound and initiate directional migration toward it. A bioassay using transwell migration plates was used to test the effect of DAPP on this cellular behavior. The inflammatory chemotactic compound leukotriene B4 was added to cell culture medium in the bottom chambers, and cells pre-treated with various doses of DAPP were added to the top chambers of the transwell plates. The separating barrier between the two chambers had 3 μm pores to enable directional cellular transmigration. Cells pretreated with DAPP showed a mild reduction in migratory behavior. Importantly, the LMW fraction of DAPP showed a stronger inhibition of inflammatory migration across a wider dose range (down to the low dose of 25 μg/L). The inhibition by DAPP was predominantly associated with the <3 kDa LMW fraction, where the inhibition was highly significant when compared with cells exposed to leukotriene B4 in the absence of DAPP LMW, across a dose range of 0.025–3.2 mg/L (P<.01) (Fig. 7).
FIG. 7.
Cellular migration in response to the inflammatory chemo-attractant leukotriene B4 (LTB4) was evaluated using PMN cells in vitro. Pretreatment of PMN cells with DAPP before cells were placed within range of the chemotactic compound LTB4 in transwell migration chambers resulted in a reduction in migratory behavior of the cells. The<3 kDa low-molecular-weight fraction of DAPP (DAPP LMW) showed high potency at very low doses where cellular migration in response to LTB4 was reduced to baseline levels (UT: untreated cells). Asterisks indicate doses where the results were statistically signficant when compared with the positive control (LTB4) where cells were exposed to LTB4 in the absence of DAPP or DAPP LMW (P<.05). The level of significance was calculated by the independent two-tailed t-test and is indicated by * if P<.05 and by ** if P<.01.
Inhibition of inflammatory enzymes COX-2 and lipoxygenase by DAPP
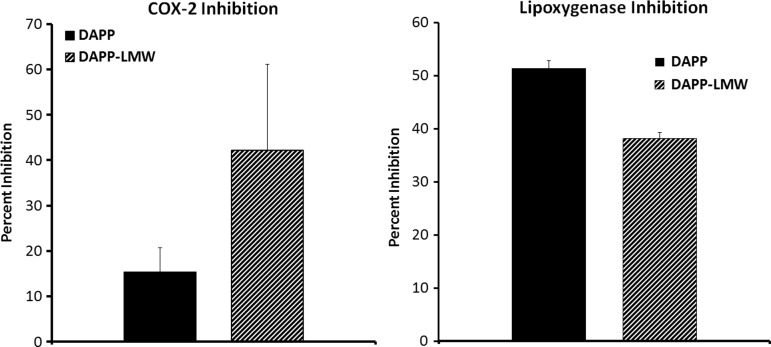
DAPP was tested for its ability to inhibit two enzymes involved in inflammatory processes: COX-2 and lipoxygenase, comparing a crude water extract of DAPP with an LMW fraction (DAPP LMW). Both COX-2 and lipoxygenase enzymes were inhibited by DAPP at a dose of 10 g/L (Fig. 8). Data from the comparison of the crude and LMW fractions suggest that several synergistic compounds act on both enzymes, where the LMW fraction provided better inhibition of COX-2 than lipoxygenase when compared with the crude water extract, suggesting that compounds below 3 kDa are involved in the inhibition of COX-2. The LMW fraction showed less inhibition than crude DAPP, but still provided robust inhibition of lipoxygenase enzyme activity, suggesting that lipoxygenase inhibitors are present in both the high- and LMW fractions.
FIG. 8.
DAPP was tested for its ability to inhibit two enzymes involved in inflammatory processes: Cyclooxygenase-2 (COX-2) and lipoxygenase. The tests compared a crude water extract of DAPP to a<3 kDa DAPP LMW. The crude extract showed inhibition of both enzymes at a dose of 10 mg/mL. The<3 kDa LMW fraction provided a proportionally better inhibition of COX-2 than lipoxygenase when compared with the crude water extract, suggesting that compounds below 3 kDa are involved in the inhibition of COX-2.
Discussion
The importance of maintaining joint mobility with aging is a major factor for remaining physically active and independent of caregivers. The resulting cost savings pertaining to overall physical and mental health, social functioning, and reduced costs for assisted living are undeniable. The interest in nutritional products that help maintain healthy joint function is on a rise, and was an incentive for the study we are reporting here.
The daily consumption of a DAPP in an older population with some joint mobility limitations resulted in rapid and sustained improvements in joint function. This improvement was not limited to each person's identified problem areas; rather, a general improvement in the function of many joints was seen. Over the course of the study, the general upper and lower back ROM improved, with significant improvements in thoracic, lumbar, and hip rotation (P<.05). Furthermore, even though only three study participants complained about reduced function of shoulders and neck, cervical lateral motion (the bending of the head forward and backward), as well as shoulder adduction/abduction (arching of the shoulder/arm from above the head to down across the body) showed significant improvements when compared with baseline, with some improvements reaching statistical significance already after 2 weeks. The highly significant improvement of ROM for the right shoulder at 8 and 12 weeks (P<.01), given a predominantly right-handed population, is noteworthy in light of a general tendency to shoulder and neck tension due to poor posture during work-related tasks.
The improvement in joint ROM (Fig. 2) was parallel to an improvement in serum antioxidant protection status (Fig. 3), as measured by significant improvements in serum CAP-e results already at 2 weeks. The importance of the test results using the CAP-e bioassay is that the improvement is not solely a reflection of an improved level of serum antioxidants, but it is also associated with an improved ability of serum to provide antioxidants of a composition that are able to enter into and protect live cells from free radical damage.
The changes in ROM and antioxidant status were also paralleled by reduced pain scores. The reduced pain levels were seen for both primary and secondary pain areas, and they were preceded by the improved antioxidant status, suggesting an association between antioxidant uptake and subsequent pain reduction. Improved antioxidant status reached statistical significance above baseline already at 2 weeks, whereas the reduced pain scores reached significance after 4 weeks (P<.05), and reached a high level of significance after 8 weeks (P<.01).
In terms of understanding possible underlying mechanisms of action of DAPP that may help put the clinical data in perspective, several data from in vitro testing point to antioxidant properties, as well as to some anti-inflammatory activity. The cellular antioxidant protection by DAPP seen in serum samples from people consuming DAPP was mirrored when DAPP was added to cells in culture using the CAP-e bioassay, verifying that DAPP contains antioxidants of a chemical composition that are capable of entering into living cells and protecting these cells from free radical damage both in vitro and in vivo. In parallel to that activity, DAPP triggered a reduction in free radical formation by PMN cells. DAPP pretreatment also resulted in a reduced migratory activity of cells in response to the inflammatory chemokine leukotriene B4. DAPP was shown to contain compounds that are able to inhibit the enzymatic activity of both COX-2 and lipoxygenase enzymes. The LMW fraction, containing water-soluble compounds smaller than 3 kDa, showed the strongest inhibition of cellular migration, and also strongly contributed to COX-2 inhibition, pointing to the presence of small molecules with the ability to quench inflammatory responses, and due to the COX-2 inhibition, likely contribute to pain reduction. Taken together, these data point to multifaceted mechanisms of action that may help explain the observed improvements in joint function, antioxidant status, and pain reduction in older people consuming DAPP.
In conclusion, the improvements in joint function associated with consumption of DAPP were not limited to an isolated joint problem. The general improvement seen in this exploratory pilot study suggests that a general improvement in antioxidant status may have led to improvements in joint function in this population. In future studies, the ROM assessments should include an assessment before study start, to accommodate the learning aspect of performing the motions, such that an improvement at later visits is not simply associated with an increased familiarity with the testing method. Further studies are warranted, and should include a placebo-controlled dose study to evaluate at which daily dose improvements in joint function and antioxidant status can be detected, and should incorporate tracking of diet and exercise, as well as frequency of additional adjunct therapies. Future studies may also include assessment of joint health support in younger populations, including athletes.
Acknowledgments
The study was conducted at NIS Labs, an independent contract research laboratory specializing in natural products research. The Leahy DAPP™ product was provided by Leahy Orchards, Inc (Franklin Centre, Quebec, Canada). The study was sponsored by Michael Leahy, CEO Leahy Orchards, Inc.
Author Disclosure Statement
All authors are associated with NIS Labs and have no competing financial interest in the subject matter.
References
- 1.Horton WE, Jr, Bennion P, Yang L: Cellular, molecular, and matrix changes in cartilage during aging and osteoarthritis. J Musculoskelet Neuronal Interact 2006;6:379–381 [PubMed] [Google Scholar]
- 2.Martin JA, Buckwalter JA: Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 2002;3:257–264 [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z: Inflammation and cancer. Nature 2002;420:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyson DA: A comprehensive review of apples and apple components and their relationship to human health. Adv Nutr 2011;2:408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer J, Liu RH: Apple phytochemicals and their health benefits. Nutr J 2004;3:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe K, Wu X, Liu RH: Antioxidant activity of apple peels. J Agric Food Chem 2003;51:609–614 [DOI] [PubMed] [Google Scholar]
- 7.Van der Sluis A, Dekker M, de Jager A, Jongen W: Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. J Agric Food Chem 2001;49:3606–3613 [DOI] [PubMed] [Google Scholar]
- 8.Jung M, Triebel S, Anke T, Richling E, Erkel G: Influence of apple polyphenols on inflammatory gene expression. Mol Nutr Food Res 2009;53:1263–1280 [DOI] [PubMed] [Google Scholar]
- 9.Heeba GH, Mahmoud ME, El Hanafy AA: Anti-inflammatory potential of curcumin and quercetin in rats: role of oxidative stress, heme oxygenase-1 and TNF-α. Toxicol Ind Health 2012;30:551–560 [DOI] [PubMed] [Google Scholar]
- 10.Shen CL, von Bergen V, Chyu MC, Jenkins MR, Mo H, Chen CH, Kwun IS: Fruits and dietary phytochemicals in bone protection. Nutr Res 2012;32:897–910 [DOI] [PubMed] [Google Scholar]
- 11.Jensen GS, Ager DM, Redman KA, Mitzner MA, Benson KF, Schauss AG: Pain reduction and improvement in range of motion after daily consumption of an acai (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J Med Food 2011;14:702–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson KF, Ager DM, Landes B, Aruoma OI, Jensen GS: Improvement of joint range of motion (ROM) and reduction of chronic pain after consumption of an ergothioneine-containing nutritional supplement. Prev Med 2012;54Suppl:S83–S89 [DOI] [PubMed] [Google Scholar]
- 13.Tall JM, Seeram NP, Zhao C, Nair MG, Meyer RA, Raja SN: Tart cherry anthocyanins suppress inflammation-induced pain behaviour in rat. Behav Brain Res 2004;153:181–188 [DOI] [PubMed] [Google Scholar]
- 14.Denis MC, Furtos A, Dudonné S, Montoudis A, Garofalo C, Desjardins Y, Delvin E, Levy E: Apple peel polyphenols and their beneficial actions on oxidative stress and inflammation. PLoS One 2013;8:e53725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Honzel D, Carter SG, Redman KA, Schauss AG, Endres JR, Jensen GS: Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J Agric Food Chem 2008;56:8319–8325 [DOI] [PubMed] [Google Scholar]
- 16.Jensen GS, Redman KA, Benson KF, Carter SG, Mitzner MA, Reeves S, Robinson L: Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: results of a placebo-controlled double-blinded crossover pilot study. J Med Food 2011;14:1002–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frampton CL, Hughes-Webb P: The measurement of pain. Clin Oncol (R Coll Radiol) 2011;23:381–386 [DOI] [PubMed] [Google Scholar]
- 18.Phan NQ, Blome C, Fritz F, et al. : Assessment of pruritus intensity: prospective study on validity and reliability of the Visual Analogue Scale, Numerical Rating Scale and Verbal Rating Scale in 471 patients with chronic pruritis. Acta Derm Venereol 2012;92:502–507 [DOI] [PubMed] [Google Scholar]
- 19.Kang J, Li Z, Wu T, Jensen GS, Schauss AG, Wu X: Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). J Agric Food Chem 2010;122:610–617 [Google Scholar]