Abstract
In this study, the authors evaluate the biological effects of irradiation of hepatocellular carcinoma cells by internal exposure with 125I-labeled 5-iodo-2′-deoxyuridine (125I-UdR)-chitosan drug loading nanoparticles (125I-UdR-CS-DLN). The authors observed that accumulation of nanoparticles was significantly (p<0.05) higher in hepatocellular carcinoma cells HepG2 than normal liver cells HL-7702 after treated with 125I-UdR-CS-DLN for 30 minutes. Survival of HepG2 cells was significantly lower at 125I-UdR-CS-DLN doses higher than 37 kBq/mL (more significant in the G1 phase and G2/M phase) than the HL-7702 cells. In addition, 125I-UdR-CS-DLN induced a higher level of DNA double-strand breaks than 125I-UdR, and HepG2 cells exhibited a lower level of DNA repair when compared with HL-7702 cells. In vivo animal experiments, TUNEL staining, after targeted treatment, showed that 125I-UdR-CS-DLN induced significant cell apoptosis in rabbit hepatocellular tumors in situ than 125I-UdR infusion at the same dose. In conclusion, hepatocellular carcinoma cells were significantly irradiated with 125I-UdR-CS-DLN compared with 125I-UdR, and 125I-UdR-CS-DLN irradiation enhanced DNA damage, induced liver cancer cell apoptosis, and prevented DNA damage repair. However, evaluating the extent of damage and organ sparing in vivo should also be considered.
Key words: : biological effects, 125I-UdR-CS-DLN, liver cancer, nanoparticles, passive targeting
Introduction
The 125I-labeled 5-iodo-2′-deoxyuridine (125I-UdR) is a radiotherapy drug that is highly lethal for tumor cells.1 Chitosan nanoparticles (CS-NP) may be used as a 125I-UdR carrier to protect the drug from premature degradation and achieve tumor-targeted delivery and intracellular sustained release, thereby enhancing the tumor-killing effect with a lower drug dose.2 However, this novel approach has not yet been validated on a global level. Based on the results of the previous studies, the authors prepared 125I-UdR-CS-drug loading nanoparticles (125I-UdR-CS-DLN) with an average particle size of 175.8 nm through process optimization. This carrier had high biocompatibility, loading capacity, and encapsulation efficiency, and its release characteristics met the requirements of long-acting formulations, since it showed significant sustained release.3 After hepatic arterial infusion, 125I-UdR-CS-DLN satisfactorily achieved passive targeting of the hepatocellular carcinoma with an intratumoral slow-release time of up to 48 hours. Furthermore, previous studies showed that3 the use of 125I-UdR-CS-DLN significantly suppressed the inhibition of 125I-UdR on bone marrow hematopoietic cells and alleviated toxic effects of 125I-UdR to the gastrointestinal mucosa.
This study included a series of in vivo and in vitro experiments to simulate the environment of drug action in tumor cells and assessed the effect of 125I-UdR-CS-DLN on hepatocellular carcinoma proliferation. This study also evaluated the biological effects of 125I-UdR-CS-DLN irradiation on hepatocellular carcinoma cells.
Materials and Methods
Experimental material
125I-UdR-CS-DLN and fluorescein isothiocyanate-labeled chitosan nanoparticles
125I-UdR-CS-DLN and fluorescein isothiocyanate-labeled chitosan nanoparticles (FITC-CS-NP) were prepared in advance at the laboratory. 125I-UdR was dissolved in 100 mL solution of chitosan [0.1 g chitosan (3 kDa), acetate buffer (0.1 M sodium acetate/0.1 M acetic acid), pH 5.5], then tripolyphosphate (TPP, 0.5 M, 10 mL) was added dropwise under stirring at room temperature for 30 minutes to form chitosan NPs according to a previously reported procedure.4–6
Chemical reagents
Chitosan was procured from Zhejiang Golden-Shell Biochemical Co. Ltd. After deacetylation and radiation degradation, the product had a molecular weight of 3 kDa and a deacetylation degree of 93.062%±2.384%.
The TUNEL staining kit was procured from Roche. Trypan blue, FITC, sodium lauroyl sarcosine, dimethyl sulfoxide (DMSO), and agarose were procured from Shanghai Sangon Biological Engineering Technology and Service Co., Ltd. Neutral lysates and electrophoresis gel were prepared according to the instructions provided in the product inserts. Other chemical reagents used in this study were procured from the Sinopharm Chemical Reagent Co., Ltd.
Cell culture
The hepatocellular carcinoma cell line (HepG2) and normal human liver cell line (HL-7702), were purchased from the Cell Resources Center of Shanghai Institutes for Biological Sciences. Cells were adherently cultured in a 5% CO2-containing incubator at 37°C. After 2–5 days, cells in the logarithmic phase were trypsinized (0.25%) for passaging or future treatment.
Animal model
Male New Zealand white rabbits (n=20, weighted 4.1±0.5 kg) were provided by the Laboratory Animal Center of Soochow University. Rabbit-derived VX2 tumor tissue was gifted by Dr. Yonghai Jin and Dr. Dayong Zhou. The development of the rabbit VX2 liver tumor model was as per the methods proposed by Lee et al.7 The animal treatment protocol used in this study was approved by the Institutional Animal Care and Use Committee of Soochow University (Suzhou, China).
Instruments and equipment
Instruments and equipment used in this study included a radioactivity meter (CAPINTEC CRC-15R), a laser scanning confocal microscope (Leica SP2), a color ultrasound (GE, Logiq Book XP), a digital subtraction angiography instrument (Siemens AXIOM ARTIS), and a single-photon emission computed tomography (SPECT) system (PHILIPS FORTE). Other materials like a 3F microcatheter, 4F catheter sheath, 4F RH catheter (Terumo), and 18G lumbar puncture needle (Hakko) were also included.
Experimental methods
Intracellular distribution of FITC-CS-NP
A sterilized coverslip (1×1 cm) was placed in the bottom of each well in a six-well plate, and logarithmically growing HepG2 and HL-7702 cells were seeded at a concentration of 1×106 cells per well. Each cell line (i.e., HepG2 and HL-7702) had three repeats. Cells were cultured for adherent growth (5% CO2, 37°C, 24 hours) until the confluence reached 60%–80% and were then cultured in 50 μg/mL FITC-CS-NP-containing serum-free medium for 30 minutes in a dark environment. Subsequently, the coverslip was removed from the well, and cells were washed thrice with ice-cold phosphate-buffered saline (PBS), fixed by 4% paraformaldehyde for 3 minutes, and washed again thrice with PBS. Thereafter, cells were mounted on a slide with 40% glycerol and observed under a confocal microscope for observing the intracellular distribution of fluorescence.
Biological effects of 125I-UdR-CS-DLN irradiation
Measuring cell survival with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
Logarithmically growing HepG2 and HL-7702 cells were inoculated in a 96-well plate at a concentration of 1×104 cells per well. Cells were cultured (5% CO2, 37°C, 24 hours) up to 60%–70% confluence and then cultured in 100 μL serum-free medium containing differently concentrated 125I-UdR-CS-DLN (radioactivity set at 1, 10, 37, 100, 200, and 500 kBq, respectively). Positive control cells were cultured in the 125I-UdR-containing medium with the same radioactivity, while equal volumes of cell-free medium were used as blank controls. After incubation for 30 minutes, cells were washed thrice with a serum-free medium, and cultured in a serum-containing medium. At 24 and 48 hours postculturing, the MTT solution (5 mg/mL, 20 μL) was added, followed by 4 hours of incubation. At the end of the incubation period, the supernatant was aspirated carefully, and cells were washed thrice with PBS. Then, cells were treated with 100 μL of DMSO and placed on a shaker for 15 minutes. Cell survival was determined by detecting the absorbance at 490 nm using an enzyme-linked immunosorbent plate reader.8
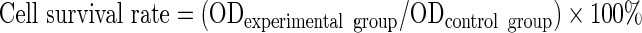 |
Cell cycle analysis
HepG2 and HL-7702 cells in the logarithmic phase were inoculated in a sterile Petri dish (diameter 6 cm) at a concentration of 5×105 cells per dish. Each cell (i.e., HepG2 and HL-7702) had three repeats. Cells were cultured (5% CO2, 37°C, 24 hours) until 60%–70% confluence and added to the 125I-UdR-CS-DLN-free and serum-free medium, 125I-UdR-CS-DLN-containing (37 kBq/mL) serum-free medium, and 125I-UdR-containing serum-free medium with the same radioactivity, respectively. After 30 minutes of incubation, cells were washed with the serum-free medium, again cultured in the serum-containing medium for another 48 hours, and then washed twice with precooled PBS (4°C) before overnight ethanol fixation (70%, ice cold). Thereafter, cells were washed and resuspended in 2.5 mL PBS solution and placed in an ice bath after treating with 5 mL precooled ethanol (70%, 4°C). After 30 minutes, cells were pipetted and passed through a 300-mesh nylon filter, centrifuged at 800 g for 5 minutes, and incubated with 100 μL RNase (5 mg/mL) at 37°C for 30 minutes. The reaction was terminated in the ice bath, and staining of the cells was done with 100 μg/mL propidium iodide dye for 30 minutes in a dark environment. Cell division was analyzed by a flow cytometry, in which the PI fluorescence was excited by argon ions with an excitation wavelength at 488 nm, and the red fluorescence at 620 nm was filtered and collected for data analysis. Data processing was done using the Multicycle (Beckman Coulter) software. The relative proportion of cells in G1, S, and G2 phases was determined, cell cycle was observed, and proliferation index (PI) was calculated.9,10
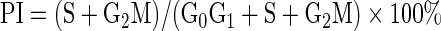 |
Single-cell gel electrophoresis
Five milliliters of logarithmically growing HepG2 and HL-7702 cells (1×105 cells/mL) was inoculated in a sterile Petri dish (diameter 6 cm). Each group had three repeats. Cells were cultured (5% CO2, 37°C, 24 hours) until 60%–70% confluence and, respectively, added with the 125I-UdR-CS-DLN-free and serum-free medium, 125I-UdR-CS-DLN-containing (37 kBq/mL) serum-free medium, and 125I-UdR-containing serum-free medium with the same radioactivity. After 30 minutes of culturing, cells were washed with the serum-free medium and cultured in the serum-containing medium. Single-cell suspension was prepared at 0.5, 2, and 24 hours postculturing, and single-cell gel electrophoresis was carried out after cell counting. Thereafter, the gel was stained with the PI dye (5 μg/mL) for 20 minutes and observed under a fluorescence microscopy after bleaching. For each group, 200 cells were randomly shot, and the rate of tailing cells was calculated. The comet images of randomly selected cells (n=30) were analyzed by the software CASP (CASPLab), and the data were presented as median values.
Verifying the passive targeting ability of 125I-UdR-CS-DLN in vivo
Hepatic arterial infusion and SPECT imaging
Rabbits with in situ hepatocellular carcinoma (n=15) were randomly assigned into the experimental group (n=6), the control group (n=6), and the sham operation group (n=3). Rabbits were fed with 1% perchlorate aqueous solution, after fasting for 24 hours, to enclose the thyroid gland and anesthetized with 3% pentobarbital (1 mL/kg) after 6 hours of water deprivation.
The femoral artery was catheterized using a 3F microcatheter and the hepatic artery was superselected for infusion of 125I-UdR-CS-DLN at a radioactivity of 72 MBq. Thereafter, the femoral arteries were ligated and the wounds were sutured; they were then subsequently fed with conventional diet and intramuscularly injected with antibiotics to prevent infection. Rabbits in the control and sham operation groups were infused with 125I-UdR and PBS, respectively.
Three rabbits were randomly selected from each group for SPECT imaging at 24 hours postirradiation. Images were acquired using a 256×256 matrix low-energy high-resolution collimator and the region of interest was plotted to calculate the ratio between tumor tissue and nontumor tissue (T/NT). At 48 hours postirradiation, rabbits' tumor tissues were sampled for TUNEL staining.
TUNEL staining of tumor tissues
Tumor tissues were sampled from both the experimental and control groups and fixed in 10% neutral buffered formalin for 4 hours. After paraffin embedding, sections were obtained, and TUNEL staining was carried out according to the instructions provided in the product inserts. Thereafter, tissue sections were restained with hematoxylin, dehydrated, mounted, and observed under a microscope.
Statistical analysis
Data were analyzed using the software SPSS (SPSS, Inc.) and the significance was tested by the t-test. Statistical significance was defined at p<0.05.
Results
Intracellular distribution of FITC-CS-NP
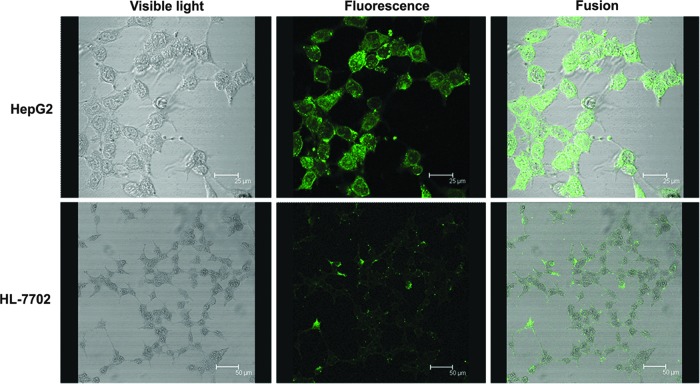
Under the confocal microscope, FITC-CS-NP directly exhibited the tumor-targeting ability of CS-NP. Figure 1 shows the distribution of FITC-CS-NP in HepG2 and HL-7702 cells at 30 minutes postirradiation. As shown in the Figure 1, the HL-7702 cell surface shows slightly higher fluorescence intensity, whereas the cytoplasm exhibits significantly lower fluorescence intensity, when compared with HepG2 cells. It was observed that HepG2 cells exhibited a significantly increased fluorescence in the cytoplasm, with gradually decreasing intensity radiating from the outside to the center. In addition, the HepG2 nucleus had weak fluorescent accumulation, and several cells showed a highly intensive and uniform fluorescent signal. These results indicated that the quantity of CS-NPs that entered hepatoma cells significantly (p<0.05) exceeded the quantity that entered normal liver cells.
FIG. 1.
Fluorescence distribution after fluorescein isothiocyanate-labeled chitosan nanoparticle (FITC-CS-NP) treatment in HepG2 and HL-7702 cells under a confocal microscope.
The biological effects of FITC-CS-NP, FITC-CS-NP irradiation
Cell survival rate
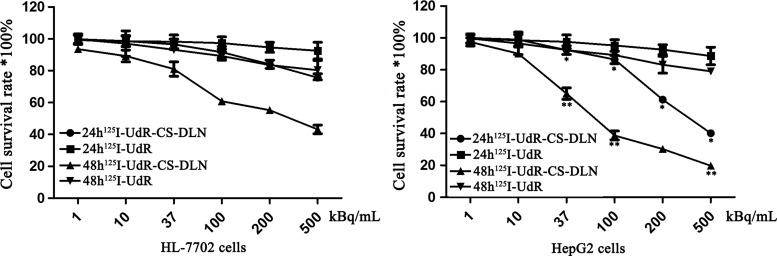
The results showed that HL-7702 and HepG2 cells treated with low-dose 125I-UdR-CS-DLN (1–10 kBq/mL) showed no significant difference (p>0.05) in the cell survival rate at 24 and 48 hours postirradiation. However, cells treated with high-dose 125I-UdR-CS-DLN (37–500 kBq/mL) showed a significantly decreased (p<0.01) survival rate at 48 hours post-treatment, when compared with that at 24 hours (Fig. 2). These results suggest that high-dose 125I-UdR-CS-DLN irradiation was more lethal for both normal and tumor cells. Further analysis showed that compared to HL-7702 cells (75.69±2.36, 43.17±2.69), HepG2 cells displayed a significantly (40.12±0.99, p<0.05) and a more significantly decreased (19.78±1.39, p<0.01) survival rate at 24 and 48 hours after irradiation, respectively. This indicated that high-dose 125I-UdR-CS-DLN had an enhanced lethal effect on hepatic tumor cells, resulting in an earlier damage in HepG2 cells.
FIG. 2.
Effect of 125I-labeled 5-iodo-2′-deoxyuridine-chitosan drug loading nanoparticles (125I-UdR-CS-DLN) on HL-7702 and HepG2 cell survival. HL-7702 and HepG2 cells treated with different doses of 125I-UdR or 125I-UdR-CS-DLN for 24 and 48 hours, and the cell survival rate was assessed by the MTT assay (*p<0.05, **p<0.01).
Cell cycle analysis
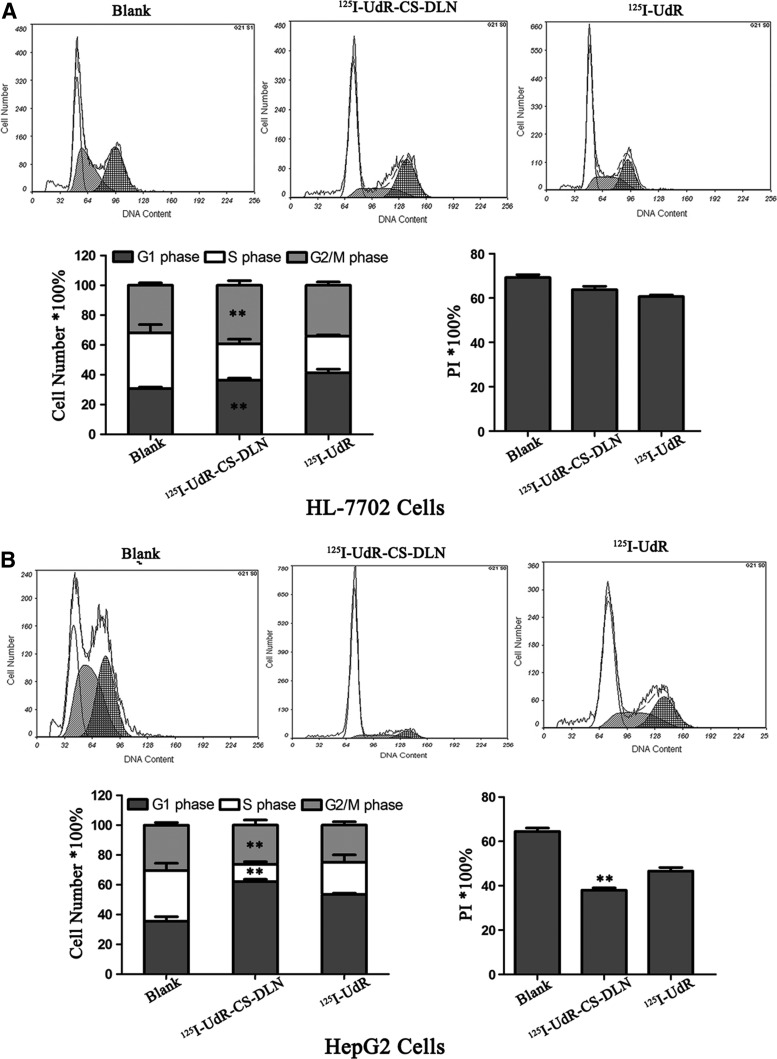
Flow cytometry showed that 125I-UdR-CS-DLN (37 kBq/mL) and 125I-UdR irradiation significantly (p<0.01) decreased the proportion of cells in S phase, whereas increased the proportion of cells in G1 phase in both HL-7702 and HepG2 cell lines at 48 hours postculturing (Fig. 3), as compared to blank. However, HL-7702 cells exhibited an increased ratio between G2 and M phase cells, whereas HepG2 cells showed a decline in this ratio. This finding suggested that both 125I-UdR-CS-DLN and 125I-UdR could destroy proliferating cells, since the synthesis of DNA during S phase requires high dNTP uptake, which facilitates the incorporation, enhances the toxicity of 125I-UdR and, therefore, arrests proliferating cells in G1 phase. 125I-UdR-CS-DLN exhibited a higher intracellular concentration and a longer release time, and thus it showed a more significant killing ability to DNA-synthesizing G2/M HepG2 cells.
FIG. 3.
Effect of 125I-UdR-CS-DLN on HL-7702 and HepG2 cell cycle. HL-7702 and HepG2 cells treated with 125I-UdR or 125I-UdR-CS-DLN (37 kBq/mL) for 48 hours, Cell division was analyzed by a flow cytometry (**p<0.01). (A) HL-7702 cells. (B) HepG2 cells.
Degree of DNA damage
The results of single-cell gel electrophoresis are presented in Table 1 and Figure 4. The degree of DNA double-strand breaks was determined by software CASP. The results showed that when compared with 125I-UdR-treated HepG2 and HL-7702 cells, cells treated with 125I-UdR-CS-DLN showed a significantly increased level of DNA damage at 2 hours postirradiation, as the 125I-UdR-CS-DLN significantly increased the comet tail length, the rate of cell tailing, and the proportion of DNA migration (p<0.05). Since the quantity of 125I-UdR-CS-DLN in HL-7702 cells was significantly lower than that in HepG2 cells, 125I-UdR-CS-DLN only induced mild DNA damage in HL-7702 cells, but still, the extent of DNA damage was higher than that in HL-7702 cells treated with 125I-UdR. At 24 hours postirradiation, cells treated with 125I-UdR-CS-DLN still exhibited significantly increased DNA tail length, tail distance, rate of cell tailing, and proportion of DNA that migrated to the comet tail (p<0.05). These results showed that the ability of DNA damage repair in 125I-UdR-CS-DLN-treated cells was significantly lower than that in 125I-UdR-treated cells, and also that the ability of DNA damage repair in 125I-UdR-CS-DLN-treated HepG2 cells was significantly lower than that in HL-7702 cells.
Table 1.
DNA Damage at 24 Hours Postirradiation
| Drugs | Cells | Dose (kBq/mL) | Tail length median (μm) | Olive tail distance | Comet tail DNA (%) | Rate of cell tailing (%) |
|---|---|---|---|---|---|---|
| 125I-UdR | HL-7702 | 0 | 5 | 0.51±0.10 | 1.37±0.01 | 3 |
| 37 | 3 | 0.66±0.26 | 0.93±0.18 | 20 | ||
| HepG2 | 0 | 5 | 0.49±0.23 | 1.27±0.94 | 4 | |
| 37 | 3 | 0.70±0.32 | 0.95±0.45 | 22 | ||
| 125I-UdR-CS-DLN | HL-7702 | 0 | 5 | 0.52±0.38 | 1.37±0.01 | 3 |
| 37 | 3 | 2.07±1.34a | 7.08±5.40a | 28a | ||
| HepG2 | 0 | 5 | 0.50±0.25 | 1.36±0.49 | 4 | |
| 37 | 17a,b | 8.37±5.51a,b | 24.23±10.2a,b | 56a,b |
p<0.05 when compared with the same cell lines treated by 125I-UdR.
p<0.05 when compared with HL-7702 cells treated by the same drugs.
I-UdR, 125I-labeled 5-iodo-2′-deoxyuridine; 125I-UdR-CS-DLN, 125I-labeled 5-iodo-2′-deoxyuridine-chitosan drug loading nanoparticles; HepG2, hepatocellular carcinoma cell line; HL-7702, normal human liver cell line.
FIG. 4.
Comet tailing phenomenon at 24 hours postirradiation.
The proapoptotic effects of hepatic arterial infusion of 125I-UdR-CS-DLN on in situ liver cancer
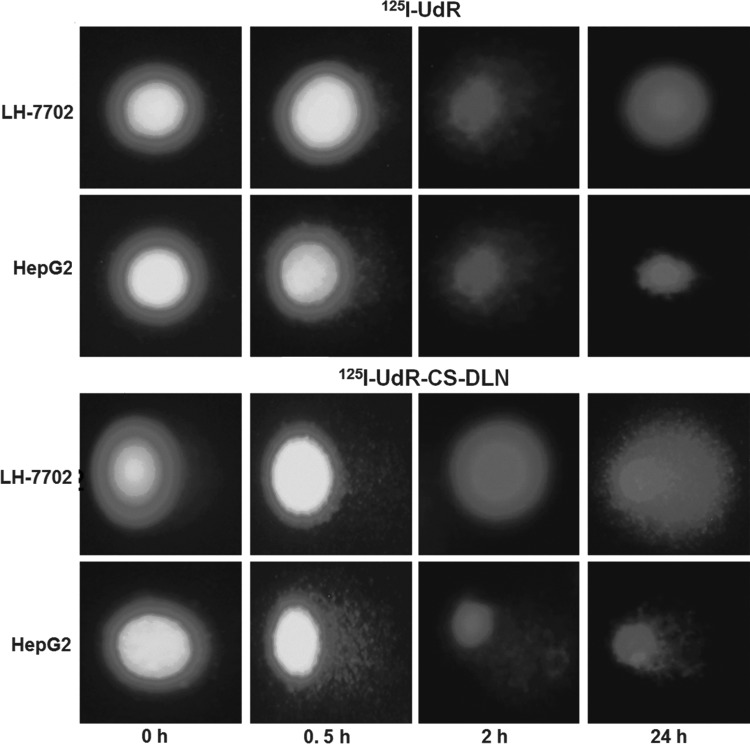
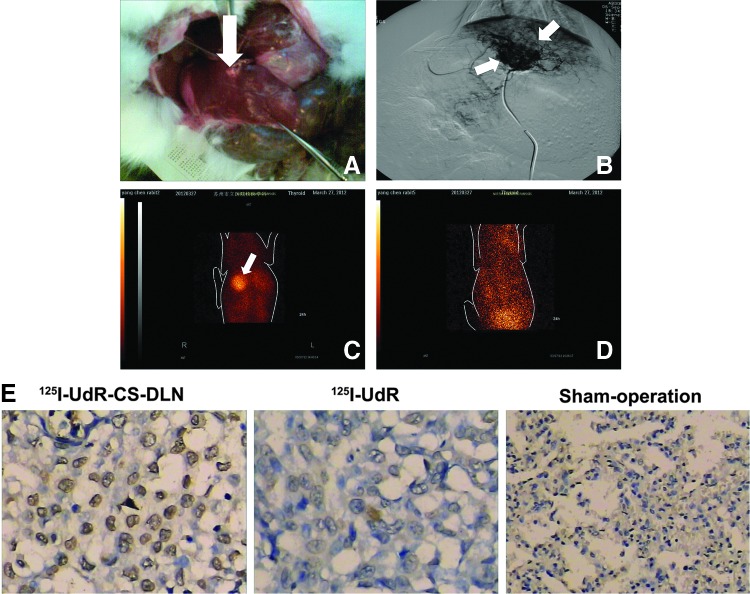
After successful establishment of the rabbit liver VX2 tumor model, 15 rabbits with an average lesion size of 9.2±1.3 mm in their left liver lobe were selected for future treatment (Fig. 5A). Under the guidance of CT, rabbits' hepatic artery was superselected through the femoral artery and infused with 125I-UdR-CS-DLN (72 MBq) using a Seldinger microcatheter. As shown in Figure 5B, the Seldinger microcatheter superselected the left hepatic artery successfully, and the drug release was controlled primarily in and around the tumor tissues. Since the tumor tissue had rich neovascularization, chaotic angiogenesis, and poor blood flow, the highly concentrated drugs were accumulated in tumors.
FIG. 5.
Gross pathology—digital subtraction angiography and single-photon emission computed tomography (SPECT) imaging of rabbit liver VX2 tumor model. (A) Left liver lobe with 9.2±1.3 mm average lesion size. (B) Left hepatic artery superselected by Seldinger microcatheter. (C) SPECT imaging at 24 hours postirradiation. (D) 125I-UdR-treated rabbits with weak radioactivity. (E) TUNEL staining of rabbit liver VX2 tumor at 48 hours postinfusion of 125I-UdR-CS-DLN, 125I-UdR, and sham operation group. White arrows indicate location and size of the tumor.
SPECT imaging at 24 hours postirradiation suggested that in 125I-UdR-CS-DLN-treated rabbits, the radioactivity in tumor tissues was significantly higher than paratumor tissues, the T/NT ratio reached 4.49±1.22, and the image of the tumor was very clear (Fig. 5C). However, the 125I-UdR-treated rabbits showed weak radioactivity in the bladder, and the image of an intact tumor tissue was difficult to obtain (Fig. 5D). This indicated that 125I-UdR was scavenged rapidly after infusion, but since the 125I-UdR-CS-DLN has a slow-release characteristic, it can be retained in tumor tissues for up to 24 hours.
TUNEL staining showed that at 48 hours postirradiation, 125I-UdR-CS-DLN significantly induced tumor cell apoptosis in rabbits with in situ hepatic cancer. However, rabbits treated with 125I-UdR showed negative TUNEL staining, and the difference was nonsignificant when compared with the sham operation group (Fig. 5E).
Discussion
Drug-carrying nanoparticles could achieve targeted drug delivery, protect the drug from early degradation, increase the amount of drug entering the cells, and enhance the killing efficacy of drugs on tumor cells.2,11 This study also showed that the amount of 125I-UdR-CS-DLN entering tumor cells was significantly greater than that entering normal cells, and that 125I-UdR-CS-DLN could be retained for a longer time in the cytoplasm. Due to the slow release characteristic of 125I-UdR-CS-DLN,7 it also exerts a damaging effect, which is different from previous studies.
Previous studies on the biological function of 125I-UdR mainly focused on its effect at 24 hours post-treatment or even longer.12–14 In practical application, however, the in vivo half-life of 125I-UdR was only 5 minutes, thus single administration of 125I-UdR could not maintain such a long duration of action. In this study, the authors controlled the time of drug–cell interaction within 30 minutes to simulate the environment of drug action in vivo and evaluated and validated the radiation damaging effects of 125I-UdR-CS-DLN in tumor cells as well as in situ tumor models.
Since the drug–cell interaction time was limited to 30 minutes, only a small amount of 125I-UdR could enter the cells, but for 125I-UdR-CS-DLN, it could rapidly enter and accumulate in the cell cytoplasm, thereby exerting a significantly enhanced radiation damaging effect on tumor cells. It should be noted that the lethality of high-dose 125I-UdR-CS-DLN on normal liver cells was higher compared with 125I-UdR; this may be because the study used immortalized normal liver cells with a higher capacity of proliferation and endocytosis compared with normal liver cells. This means that the high 125I-UdR-CS-DLN uptake and great damage exhibited by cells in this study could not totally reflect the actual extent of radiation damaging effects in vivo. Thus, evaluating the damage and reducing the toxicity of chemotherapy drugs on normal cells in vivo should be taken into consideration in future studies.
The 125I-UdR-CS-DLN-treated cells showed significantly lower ability of DNA damage repair when compared with cells treated with 125I-UdR. This may be due to the slow release of 125I-UdR-CS-DLN, which prolonged the energy deposition of 125I auger electron on DNA strand and led to increased time for DNA radiation damage. In addition, the damage repair capacity of 125I-UdR-CS-DLN-treated HepG2 cells was significantly lower than HL-7702 cells, since the incorporation of 125I-UdR mainly depends on the speed of DNA replication and hepatic tumor cells exhibits exuberant proliferation, short replication time, and active DNA synthesis.
In conclusion, the amount of 125I-UdR-CS-DLN entering liver cancer cells by internal exposures significantly exceeded that of 125I-UdR, and 125I-UdR-CS-DLN irradiation enhanced DNA damage, induced liver cancer cell apoptosis, and prevented DNA damage repair.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 31270897, 30870585), the Graduate Innovation Foundation of Medical College of Soochow University, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosure Statement
No potential conflicts of interest were disclosed.
References
- 1.Ma XD, Larry ED, Jerry RW. Local delivery of polymeric 125I-IUdR to experimental human malignant gliomas. Chin J Neurosurg Dis Res 2004;4:293 [Google Scholar]
- 2.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol 2008;26:57. [DOI] [PubMed] [Google Scholar]
- 3.Yang C, Liu F, Song M, et al. Preparation and in vitro evaluation of chitosan nanoparticles containing 5-[125I] Iodo-2′-deoxyuridine. Clin J Radiol Med Prot 2010;30:650 [Google Scholar]
- 4.Tanima B, Susmita M, Ajay KS, et al. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int J Pharm 2002;243:93. [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Yin R, Tong Z, et al. Synthesis and characterization of water-soluble glucosyloxyethyl acrylate modified chitosan. Int J Biol Macromol 2011;48:753. [DOI] [PubMed] [Google Scholar]
- 6.Nasti A, Zaki NM, Leonardis P, et al. Chitosan/TPP and chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm Res 2009;26:1918. [DOI] [PubMed] [Google Scholar]
- 7.Lee KH, Liapi E, Buijs M, et al. Considerations for implantation site of VX2 carcinoma into rabbit liver. J Vasc Interv Radiol 2009;20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.homas C, Martin J, Devic C, et al. Impact of dose-rate on the low-dose hyper-radiosensitivity and induced radioresistance (HRS/IRR) response. Int J Radiat Biol 2013;89:813. [DOI] [PubMed] [Google Scholar]
- 9.Arienti C, Zoli W, Pignatta S, et al. Efficacy of different sequences of radio- and chemotherapy in experimental models of human melanoma. J Cell Physiol 2014;229:1548. [DOI] [PubMed] [Google Scholar]
- 10.Rezáčová M, Rudolfová G, Tichý A, et al. Accumulation of DNA damage and cell death after fractionated irradiation. Radiat Res 2011;175:708. [DOI] [PubMed] [Google Scholar]
- 11.Power S, Slattery MM, Lee MJ. Nanotechnology and its relationship to interventional radiology. Part II: Drug delivery, thermotherapy, and vascular intervention. Cardiovasc Intervent Radiol 2011;34:676. [DOI] [PubMed] [Google Scholar]
- 12.Elmroth K, Stenerlow B. DNA-incorporated 125I induces more than one double-strand break per decay in mammalian cells. Radiat Res 2005;163:369. [DOI] [PubMed] [Google Scholar]
- 13.Pomplun E, Sutmann G. Is coulomb explosion a damaging mechanism for (125)IUdR? Int J Radiat Biol 2004;80:855. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, He Y, Shen Y. Killing effect of 125I-UdR on human pancreatic cancer cell line Bax-PC. Chin J Nucl Med 2004;24:136 [Google Scholar]