Abstract
Both glial fibrillary acidic protein (GFAP) and S100β are found in glial cells and are released into serum following a traumatic brain injury (TBI), however, the clinical utility of S100β as a biomarker has been questioned because of its release from bone. This study examined the ability of GFAP and S100β to detect intracranial lesions on computed tomography (CT) in trauma patients and also assessed biomarker performance in patients with fractures and extracranial injuries on head CT. This prospective cohort study enrolled a convenience sample of adult trauma patients at a Level I trauma center with and without mild or moderate traumatic brain injury (MMTBI). Serum samples were obtained within 4 h of injury. The primary outcome was the presence of traumatic intracranial lesions on CT scan. There were 397 general trauma patients enrolled: 209 (53%) had a MMTBI and 188 (47%) had trauma without MMTBI. Of the 262 patients with a head CT, 20 (8%) had intracranial lesions. There were 137 (35%) trauma patients who sustained extracranial fractures below the head to the torso and extremities. Levels of S100β were significantly higher in patients with fractures, compared with those without fractures (p<0.001) whether MMTBI was present or not. However, GFAP levels were not significantly affected by the presence of fractures (p>0.05). The area under the receiver operating characteristics curve (AUC) for predicting intracranial lesions on CT for GFAP was 0.84 (0.73–0.95) and for S100β was 0.78 (0.67–0.89). However, in the presence of extracranial fractures, the AUC for GFAP increased to 0.93 (0.86–1.00) and for S100β decreased to 0.75 (0.61–0.88). In a general trauma population, GFAP out-performed S100β in detecting intracranial CT lesions, particularly in the setting of extracranial fractures.
Key words: : computed tomography (CT), fractures, glial fibrillary acidic protein (GFAP), mild traumatic brain injury/concussion, S100β
Introduction
Research in the field of traumatic brain injury (TBI) biomarkers has increased exponentially over the last 20 years,1,2 with most of the publications on the topic occurring in the last 10 years.3 Accordingly, studies assessing biomarkers in TBI have looked at a number of potential markers that could lend diagnostic, prognostic, and therapeutic information. Despite the large number of published studies, there is still a lack of any U.S. Food and Drug Administration–approved biomarkers for clinical use in adults and children.2,3
Of the markers studied to date, S100β is the most well-studied biomarker in mild TBI for potentially screening patients for head computed tomography (CT). S100β is the major low-affinity calcium binding protein in astrocytes4 that helps to regulate intracellular levels of calcium, and is considered a marker of astrocyte injury or death. A number of studies have found significant correlations between elevated serum levels of S100β and CT abnormalities.5–7 It has been suggested that adding the measurement of S100β concentration to clinical decision tools for mild TBI patients could potentially reduce the number of CT scans by 30%.7 However, other investigators have failed to detect associations between S100β with CT abnormalities.8–11 Although S100β remains promising as an adjunctive marker, its utility in the setting of multiple trauma remains controversial because it is also elevated in trauma patients without head injuries,12–16 as it can also be found in non-neural cells such as adipocytes, chondrocytes, and melanocytes.17,18
In the 2009 American College of Emergency Physicians clinical policy for neuroimaging and decision-making in adult mild traumatic brain injury in the acute setting, S100β received a Level C recommendation.19 The recommendation suggested that in mild TBI patients without significant extracranial injuries and a serum S100β of level less than 0.1μg/L measured within 4 h of injury, consideration could be given to not performing a CT. At the time of these guidelines, glial fibrillary acidic protein (GFAP) had not yet been explored in the mild TBI population.
GFAP is a monomeric intermediate protein that was first isolated by Eng and colleagues in 1971 and found in astroglial skeleton in both white and gray brain matter.20 Current evidence indicates that serum GFAP might be a useful marker for severe traumatic brain injury.15,21–25 In a mild TBI population, Metting and colleagues demonstrated that serum GFAP was increased in patients with an abnormal CT after mild TBI, compared with those with a normal CT. They also found GFAP elevated in axonal injury on magnetic resonance imaging (MRI) in a subset of these patients at three months post-injury.26 Similarly, Papa and colleagues found serum GFAP was significantly higher in mild TBI patients with intracranial lesions on CT, compared with those without lesions and predicted patients who required neurosurgical intervention.27 In this same study, GFAP was detectable in serum in less than 1 h after a mild TBI and was able to distinguish mild TBI patients from other trauma patients (without head injury) who had orthopedic injuries or who were in motor vehicle crashes. These studies suggest that GFAP has a good specificity for brain injury acutely after injury.
This study examined the ability of both GFAP and S100β to detect intracranial lesions on CT in a general trauma population with and without traumatic brain injury, particularly in those with fractures and extracranial lesions on CT.
Methods
This prospective cohort study enrolled a convenience sample of adult trauma patients with and without suspected mild or moderate TBI (MMTBI; Glasgow Coma Scale [GCS] score of 9 to 15) presenting to the emergency department (ED) within 4 h of trauma. The study site was the ED of a Level I Trauma Center in Orlando Florida. This study was approved by the institutional review board and written informed consent was obtained from each patient and/or their legal authorized representative prior to enrollment.
Eligibility for suspected MMTBI was determined by the treating physician based on the history of blunt head trauma followed by either loss of consciousness, amnesia, or disorientation and presenting to the ED within 4 h of injury with a GCS of 9 to 15. Eligibility also was prospectively verified by the research team prior to enrollment. Head CT scans were performed at the discretion of the treating physician. Patients were excluded if they: 1) were younger than 18 years old; 2) had no history of trauma as their primary event (e.g., syncope or seizure); 3) had known dementia, chronic psychosis, or active central nervous system pathology; 4) were pregnant; 5) were incarcerated; or 6) had a systolic blood pressure of less than 100 mm Hg.
The non-TBI general trauma group included patients with a GCS score of 15 presenting to the ED with a traumatic mechanism of injury but without TBI. They experienced similar mechanisms of injury as the MMTBI group but all had a normal mental status since injury (as verified by the research team) and had no evidence of acute brain injury or hemodynamic instability. These patients were carefully screened to ensure they had no loss of consciousness, no amnesia, and no alteration in sensorium at any time after injury. The purpose of enrolling both TBI and general trauma patients was to simulate the real world setting in which TBI biomarkers would be used.
All initial patient assessments were made by board certified emergency medicine physicians trained by a formal 1-h session on evaluating patient eligibility. At the time of enrollment, the study team carefully reassessed every patient to ensure each patient met inclusion criteria and verified any exclusion. Blood samples were obtained from each TBI and trauma patient within 4 h of the reported time of injury. A single vial of approximately 5 mL of blood was collected and placed in clot tubes with a serum separator and allowed to clot at room temperature. The blood was centrifuged within 30 min and the serum was placed in bar-coded aliquot containers and stored in a freezer at −70°C until it was transported to a central laboratory (Banyan Biomarkers Inc.; Alachua, FL). There, the samples were analyzed in batches using sandwich enzyme-linked immunosorbent assays (ELISAs) to GFAP and S100β. After assessment and treatment in the ED patients were either discharged home or admitted to hospital based on severity of their injuries and patient management was not altered by the study.
Trauma patients underwent standard CT scan of the head according to the judgment of the treating physician. The CT scan ordering pattern at the participating Level I trauma centers is such that most patients with blunt head injury with subsequent symptoms have a head CT scan performed as part of usual care. Physicians also ordered CT scans of the head on the general trauma controls based on mechanism or clinical circumstances. CT examinations were interpreted by board-certified radiologists who recorded location, extent, and type of brain injury. Radiologists were blinded to the study protocol but had the usual clinical information. Lab personnel running the samples were blinded to the clinical data.
Outcome measures
The primary outcome measure was the presence of intracranial lesions on initial CT scan. Intracranial lesions on CT included any acute traumatic intracranial lesions visualized on CT scan. The secondary outcome measure included the performance of the biomarkers in the presence of extracranial fractures, including both 1) extracranial fractures on head CT (scalp or facial hematomas, facial fractures, and isolated skull fractures), and 2) extracranial fractures below the head including torso (chest, pelvis, and spine fractures) and extremity (limb) fractures.
Data analysis
Descriptive statistics with means and proportions were used to describe the data. For statistical analysis, biomarker levels were treated as continuous data, measured in ng/mL and expressed as medians with interquartile range. Data were assessed for equality of variance and distribution. Logarithmic transformations were conducted on non-normally distributed data. Group comparisons for different CT categories were performed using analysis of variance with multiple comparisons using Bonferoni's correction. Receiver operating characteristics (ROC) curves were created to explore the ability of the biomarkers to detect intracranial lesions on CT scan. Estimates of the area under these curves (AUC) were obtained (AUC=0.5 indicates no discrimination and an AUC=1.0 indicates a perfect diagnostic test). Classification performance was assessed by sensitivity, specificity, positive predictive value, and negative predictive value with 95% confidence intervals. GFAP and S100β cut-off points were selected based on the ROC curve to maximize the sensitivity and correctly identify as many patients with CT lesions as possible. All analyses were performed using the statistical software package PASW 17.0 (IBM Corporation®; Somers, NY).
Sample size calculations took into consideration earlier studies on GFAP and S100β.26,27 Additionally, preliminary data from our group on the performance of GFAP and S100β provided data to calculate a sample size for distinguishing TBI patients with a positive CT versus a negative CT in the presence of fractures. A sample of 10 patients from the positive CT group and 100 from the negative CT group achieves 80% power to detect a difference of 0.17 (estimated to be between 0.15 to 0.20) between a diagnostic test (GFAP) with an AUC of 0.90 and another diagnostic test (S100β) with an AUC of 0.73 (estimated to be between 0.70 to 0.75) using a two-sided z-test at a significance level of 0.05.
Biomarker analysis
Serum GFAP levels were measured in duplicate for each sample using a validated ELISA platform (Banyan Biomakers Inc.). The lower limit of quantification (LLOQ) for this assay is 0.030 ng/mL and upper limit of quantification is 50 ng/mL. The limit of detection (LOD) is 0.008 pg/mL. Serum S100β levels were measured in duplicate for each sample using an ELISA platform which is for research purposes only. The assay calibrators range from 0.016ng/mL to 2ng/mL. LOD of the S100β assay at 0.017 ng/mL and the LLOQ at 0.083 ng/mL. Any samples yielding a signal over the quantification or calibrator range were diluted and re-assayed.
Results
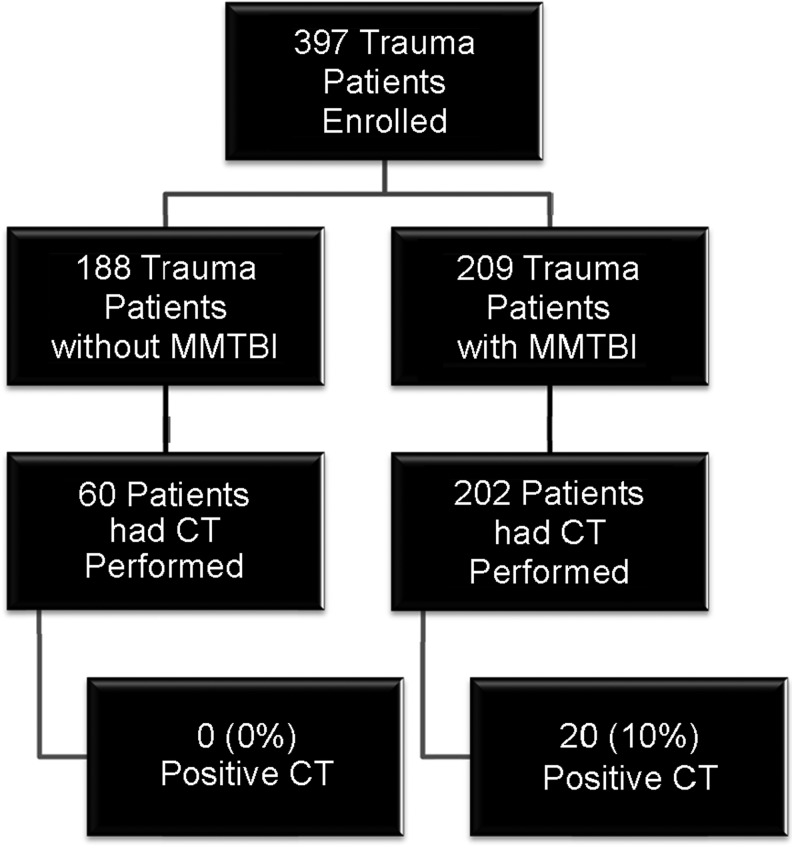
There were 397 general trauma patients enrolled, 209 (53%) had a MMTBI (206 with GCS 13–15, three with GCS 9–12) and 188 (47%) had trauma without MMTBI. The flow diagram in Figure 1 describes the distribution of enrolled patients. CT scan of the head was performed in 202 MMTBI patients and traumatic intracranial lesions on CT scan were evident in 20 (10%). Of these, 19 had a GCS 13–15 and one had a GCS 9–12. A CT scan also was performed in 60 trauma patients without TBI, all of which were negative. Therefore, there were a total of 262 CT scans performed. The median age of enrolled patients was 40 years (range 18–83) and 59% were male. The distribution of clinical characteristics in each group is presented in Table 1. There were no statistically significant differences in the age, gender, and race between trauma patients with and without MMTBI (Table 1). All patients had serum samples drawn within 4 h of injury, with the average time from injury to serum sample collection at 3.1 hours (95% CI 3.0–3.3) and SD 1.9 hours. The average time to serum collection for trauma patients without TBI was 3.2 h (95% CI 2.9–3.5) and for trauma patients with MMTBI it was 3.1 hours (95% CI 2.8–3.3). The temporal profile of GFAP and S100β all trauma patients within 4 hours post-injury is shown in Figure 2. Both GFAP and S100β demonstrated a rapid appearance in serum post-injury with levels detectible within an hour of injury. S100β rose quickly and appeared most elevated within the first 2 h; whereas elevations in GFAP remained steady over 4 h.
FIG. 1.
Flow diagram of enrolled patients. Flow diagram showing the number of enrolled trauma patients with and without mild or moderate traumatic brain injury.
Table 1.
Characteristics of Enrolled Patients
| Characteristics | Trauma patients with MMTBI N=209 | Trauma patients without MMTBI N=188 | Total N=397 | p Value |
|---|---|---|---|---|
| Mean age (years±SD) | 40 (±16) | 41 (±16) | 40 (±16) | 0.510 |
| Range | 18–76 | 18–83 | 18–83 | |
| Gender male(%) | 131 (63) | 103 (55) | 234 (59) | 0.126 |
| Race (%) | ||||
| Asian | 4 (2) | 1 (1) | 5 (1) | 0.131 |
| Black | 39 (19) | 49 (27) | 88 (23) | |
| Hispanic | 45 (22) | 42 (23) | 87 (22) | |
| Native American | 1 (1) | 3 (2) | 4 (1) | |
| White | 113 (55) | 88 (48) | 201 (51) | |
| Other | 7 (3) | 5 (3) | 12 (3) | |
| GCS score in ED (%) | ||||
| GCS 9–12 | 3 (2) | 0 (0) | 3 (1) | <0.001 |
| GCS 13 | 2 (1) | 0 (0) | 2 (1) | |
| GCS 14 | 27 (13) | 0 (0) | 27 (7) | |
| GCS 15 | 177 (85) | 188 (100) | 365 (92) | |
| Mechanism of Injury (%) | ||||
| Motor vehicle crash | 98 (47) | 112 (60) | 210 (53) | 0.001 |
| Fall | 44 (21) | 23 (12) | 67 (17) | |
| Motorcycle | 24 (12) | 12 (6) | 36 (9) | |
| Pedestrian struck | 10 (5) | 5 (3) | 15 (4) | |
| Bicycle | 12 (6) | 5 (3) | 17 (4) | |
| Assault | 9 (4) | 2 (1) | 11 (3) | |
| Sports | 2 (1) | 7 (4) | 9 (2) | |
| Other | 10 (5) | 22 (12) | 32 (8) | |
| Loss of consciousness (%) | 177 (85) | 0 (0) | 177 (45) | <0.001 |
| Amnesia (%) | 95 (46) | 0 (0) | 95 (24) | <0.001 |
| Admitted to hospital (%) | 77 (37) | 44 (23) | 121 (31) | 0.004 |
| Intoxicated (alcohol or drugs) (%) | 11 (5) | 1 (1) | 12 (3) | <0.001 |
| Fractures and CT lesions | ||||
| Intracranial lesions on head CT (%) | 20 (10) | 0 (0) | 20 (5) | 0.010 |
| All extracranial fractures (%) | 73 (35) | 76 (40) | 149 (38) | 0.299 |
| Extracranial fractures below head (%) | 64 (31) | 73 (39) | 137 (35) | 0.092 |
| Vertebral fractures | 15 (23) | 6 (8) | 21 (15) | |
| Upper extremity fractures | 26 (41) | 26 (36) | 57 (42) | |
| Lower extremity fractures | 12 (19) | 31 (43) | 38 (28) | |
| Fractures multiple locations | 11 (16) | 10 (14) | 21 (15) | |
Note: Due to rounding, percentages may not add up to 100
MMTBI, mild and moderate traumatic brain injury; SD, standard deviation; GCS, Glasgow Coma Scale; ED, emergency department; CT, computed tomography.
FIG. 2.
Temporal profile of S100β and glial fibrillary acidic protein (GFAP) in trauma patients within 4 h of injury. Bars represent median serum levels with interquartile ranges of S100β and GFAP (ng/mL) at different times post-injury. (n=1, 6, 38, 60, 89, 95, and 138, respectively).
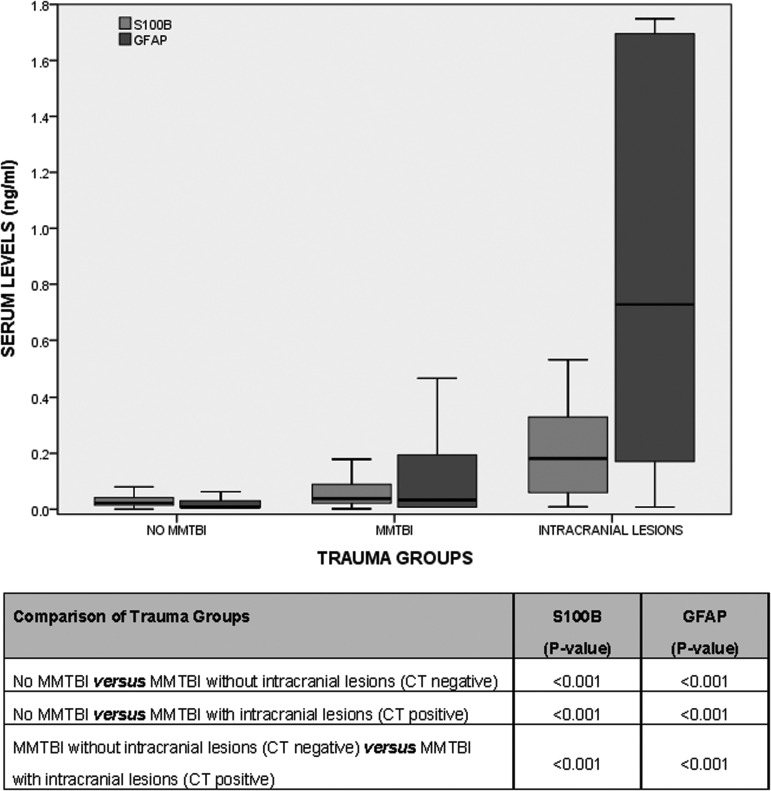
In Figure 3, levels of GFAP and S100β are compared in three groups of trauma patients: 1) no MMTBI, 2) MMTBI without intracranial lesions on CT, and 3) MMTBI with intracranial lesions on CT. There were statistically significant differences between each group for both GFAP (p<0.001) and S100β (p<0.001). When serum levels of GFAP and S100β were compared between patients with traumatic intracranial lesions on CT scan (CT positive) and those without CT lesions (CT negative), levels were significantly higher in those with lesions on CT scan for both biomarkers (p<0.001 for both). The area under the ROC curve (AUC) for discriminating between CT scan positive and CT scan negative intracranial lesions was 0.84 (95% CI 0.73–0.95) for GFAP and 0.78 (95% CI 0.67–0.89) for S100β (p=0.28; Fig. 4A). Additionally, when we isolated those patients with a GCS 14–15, the AUC for GFAP was 0.82 (95% CI 0.70–0.94) for S100β, 0.77 (95% CI 0.65–0.89; p=0.51; Fig. 4B).
FIG. 3.
Boxplot of levels of serum glial fibrillary acidic protein (GFAP) and S100β in general trauma patients divided into three groups: 1) No mild and moderate traumatic brain injury (MMTBI); 2) MMTBI (without intracranial lesions on CT); and 3) MMTBI (with intracranial lesions on CT). Bars represent median serum levels with interquartile ranges of S100β and GFAP (ng/mL) measured within 4 h of injury in trauma patients without MMTBI (n=188) versus trauma patients with MMTBI (n=189) versus trauma patients with intracranial lesions on CT (n=20). There were significant differences (p<0.001) between each of the three groups for both GFAP and S100β. Those with lesions on CT scan had the highest levels.
FIG. 4.
Receiver operating characteristics curves of glial fibrillary acidic protein (GFAP) versus S100β for detecting traumatic intracranial lesions on CT. (A) Performance of GFAP versus S100β measured within 4 h of injury in detecting intracranial lesions on CT in 262 trauma patients. (B) Performance of GFAP versus S100β measured within 4 h of injury in detecting intracranial lesions on CT in 257 trauma patients with GCS 14–15.
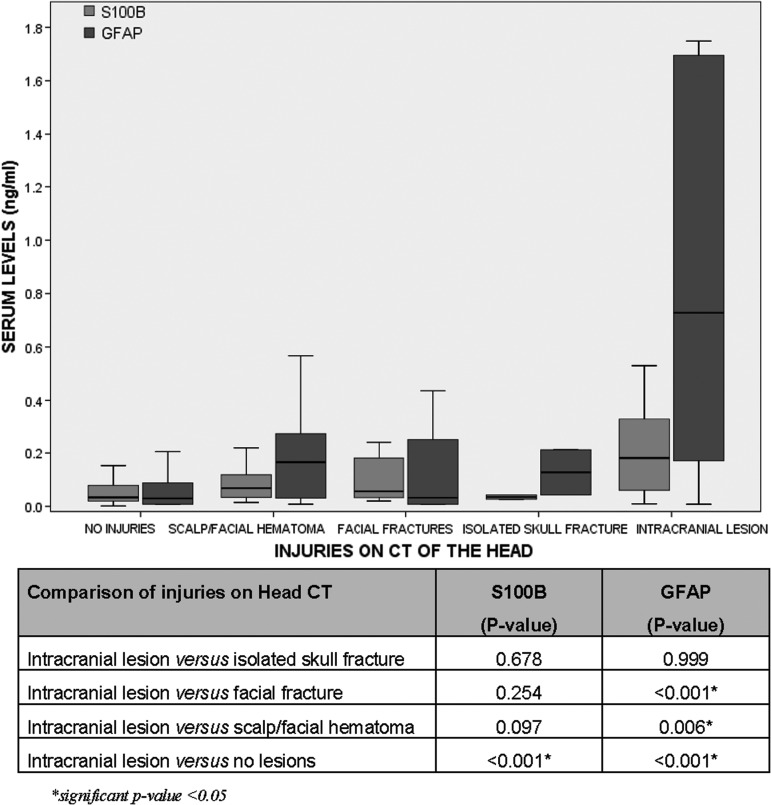
We also examined the performance of the biomarkers when extracranial lesions were present on head CT. We classified patients into five categories based on the injuries found on head CT. Among the 262 patients who had a head CT performed, 194 (74%) had no injuries on CT, 32 (12%) had scalp or facial hematomas, 14 (5%) had facial fractures, two (1%) had isolated skull fractures, and 20 (8%) had intracranial lesions. Levels of GFAP were significantly higher in those with intracranial lesions, compared with any of the extracranial lesions (scalp/facial hematoma and facial fractures). However, S100β was unable to discriminate between intracranial and extracranial lesions on CT (Fig. 5).
FIG. 5.
Comparison of levels of glial fibrillary acidic protein (GFAP) and S100β in various extracranial injuries (hematoma, facial fracture, isolated skull fracture) versus intracranial lesions found on head computed tomography (CT). Bars represent median serum levels with interquartile ranges of S100β and GFAP (ng/mL) measured within 4 h of injury in patients with extracranial versus intracranial lesions on head CT (n=194, 32, 14, 2, and 20, respectively). GFAP was able to discriminate between intracranial and extracranial lesions on CT. However, S100β was unable to discriminate between intracranial and extracranial lesions on CT.
There were 137 (35%) trauma patients who sustained extracranial fractures below the head to the torso and extremities. In trauma patients without MMTBI, levels of S100β were significantly higher in patients with fractures to the torso and extremities, compared with those without fractures (p<0.001). However, GFAP levels were not significantly affected by the presence of fractures (p=0.521; Fig. 6). When patients with MMTBI were included in the analysis, S100β was still significantly elevated (p<0.001) in those with fractures to the torso and extremities compared to those without fractures, whereas GFAP was not significantly different in those with and without fractures (p=0.104).
FIG. 6.
Boxplot of levels of serum glial fibrillary acidic protein (GFAP) and S100β in patients with fractures on the torso and extremities. Bars represent median serum levels with interquartile ranges of S100β and GFAP (ng/mL) measured within 4 h of injury. (A) Comparison of GFAP versus S100β levels in trauma patients without mild or moderate traumatic brain injury (MMTBI) who had fractures to the torso and extremities. Even without traumatic brain injury, S100B was significantly elevated (p<0.001) in those with fractures to the torso and extremities. There was, however, no significant elevation in GFAP (p=0.521). (B) Comparison of GFAP versus S100β levels in all trauma patients (with and without MMTBI) who had fractures to the torso and extremities. Again, S100β was significantly elevated (p<0.001) in those with fractures but GFAP was not (p=0.104).
Head CT was performed in 87/137 patients with extracranial fractures below the head and nine of the CT's showed intracranial lesions. In the presence of fractures, the AUC for GFAP increased to 0.93 (95% CI 0.86–1.00) and for S100β decreased to 0.75 (95% CI 0.61–0.88; p=0.003). Cutoff points for GFAP and S100β were derived from the ROC curves for detecting intracranial lesions on CT scan in the presence of fractures to maximize the sensitivity and correctly classify all CT positive lesions. Classification performance for detecting intracranial lesions on CT at a GFAP cutoff level of 0.067 ng/mL yielded a sensitivity of 100% (95% CI 63–100) and a specificity of 55% (95% CI 43–66; Table 2A). Classification performance for detecting intracranial lesions on CT at a S100β cutoff level of 0.020 ng/mL yielded a sensitivity of 100% (95% CI 63–100) and a specificity of 5% (95% CI 2–13; Table 2B).
Table 2A.
Classification Performance of Serum GFAP in Detecting Intracranial Lesions on CT in the Presence of Extracranial Fractures
| CT positive | CT negative | |||
|---|---|---|---|---|
| Sensitivity | 100% (63–100) | |||
| GFAP positive >0.067 ng/mL | 9 | 35 | Specificity | 55% (43–66) |
| GFAP negative ≤0.067 ng/mL | 0 | 43 | NPV | 100% (90–100) |
| PPV | 20% (10–36) |
Table 2B.
Classification Performance of Serum S100β in Detecting Intracranial Lesions on CT in the Presence of Extracranial Fractures
| CT positive | CT negative | |||
|---|---|---|---|---|
| Sensitivity | 100% (63–100) | |||
| S100β positive >0.020 ng/mL | 9 | 74 | Specificity | 5% (2–13) |
| S100β negative ≤0.020 ng/mL | 0 | 4 | NPV | 100% (40–100) |
| PPV | 11% (5–20) |
GFAP, glial fibrillary acidic protein; CT, computed tomography; NPV, negative predictive value; PPV, positive predictive value.
When all extracranial fractures (99/149) were taken together (head, torso, and extremities) the performance for detecting intracranial lesions on CT at a GFAP cutoff level of 0.067 ng/mL yielded a sensitivity of 100% (95% CI 63–100) and a specificity of 56% (95% CI 45–66). Classification performance for detecting intracranial lesions on CT at a S100β cutoff level of 0.020 ng/mL yielded a sensitivity of 100% (95% CI 63–100) and a specificity of 6% (95% CI 2–13).
Discussion
This prospective study compared the performance of GFAP and S100β in a cohort of general trauma patients presenting to a Level I trauma center in order to simulate the setting in which such biomarkers would likely be used clinically. In this population GFAP out-performed S100β in detecting traumatic intracranial lesions on CT, particularly in those with fractures and those with extracranial lesions on head CT. Both GFAP and S100β demonstrated a rapid appearance in serum post-injury with levels detectible within an hour of injury. The temporal profile of GFAP and S100β differed slightly. S100β rose quicker and peaked within the first 2 h, whereas GFAP rose more steadily over 4 h and tapered slightly at 4 h.
There are a number of studies that have compared serum GFAP with S100β in TBI in the last ten years. However, most of these have been in severe TBI patients.22,28–32 Recently, Metting and colleagues examined these two biomarkers in 94 cases of MTBI.26 All of these comparative studies have had sample sizes of less than 100 patients, making this study among the largest published studies to date to prospectively compare GFAP and S100β in a general trauma population with MMTBI.
Overall, GFAP had a greater AUC for detecting traumatic intracranial lesions on CT scan than S100β (0.84 vs. 0.78). This difference was more prominent when patients with fractures were examined, with the AUC falling to 0.75 for S100β and increasing for GFAP to 0.93. Both biomarkers achieved a high sensitivity for intracranial lesions (100%) but the specificity was markedly different. In the presence of fractures, GFAP had a specificity of 55%, whereas S100β had a specificity of 5%. Many patients with trauma have a combination of injuries and it is important that TBI biomarkers be brain-specific. Thus far, GFAP has shown to consistently be specific in MTBI patients. In a 2012 study, GFAP was able to distinguish MTBI patients from orthopedic controls and motor vehicle crash controls as well as in those MTBI patients with negative CT.27
The superior performance of GFAP over S100β in diagnosing TBI on neuroimaging has been shown in studies of severe TBI22,31,32 and more recently in a study of MTBI patients.26 Metting and colleagues found GFAP was increased in patients with an abnormal CT, compared with normal CT, acutely after injury. They also noted that GFAP was elevated in patients with axonal injury on MRI at three months post-injury. However, S100β did not perform as well and was not associated with findings on either CT or MRI.26 These findings support the superior performance of GFAP over S100β and is consistent with our results. However, there is a discrepancy in the performance of the S100β between our study and the Metting study. S100β was able to discriminate between CT positive and CT negative in our study but not in Metting's. This difference is likely due to the type of assay used.
Of note, we also examined the performance of the biomarkers when extracranial lesions were present on head CT without intracranial lesions. GFAP was not elevated significantly when only extracranial lesions such as scalp/facial hematomas or facial fractures were present on CT. GFAP levels were highest when intracranial lesions were present on CT. In contrast, S100β levels were not significantly different between those with either intracranial or extracranial lesions on head CT. This suggests GFAP has better discriminatory ability than S100β to separate extracranial from intracranial lesions. Taken together, these findings suggest that GFAP has better specificity for brain injury acutely than S100β.
The authors recognize that there are limitations to our study. This study addressed severity of injury in the acute care setting and did not describe long-term outcome in these patients. Outcome data will be assessed as these data become available in our ongoing studies. All patients presented to a single Level I trauma center in order to assess their performance in a multiple trauma setting. Since this study was at a single site, the generalizability to other centers might be questioned. However, the demographics of the MMTBI and trauma patients at this Level I trauma center is comparable to other trauma centers across the country.
Conclusion
In a general trauma population with and without MMTBI, GFAP out-performed S100β in detecting traumatic intracranial CT lesions, particularly in the setting of extracranial lesions on head CT and fractures to the torso and extremities. These results support the brain-specific nature of GFAP, compared with S100β. This study is among the largest published studies to date using a general trauma population, and the most cases of MTBI, to prospectively compare GFAP and S100β. These findings are undergoing further validation in a larger sample.
Author Disclosure Statement
This study was supported in part by Award Number R01NS057676 from the National Institute of Neurological Disorders and Stroke. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Drs. Papa, Brophy, and Demery are consultants of Banyan Biomarkers, Inc. but do not receive stocks or royalties from the company and will not benefit financially from this publication. Drs. Wang and Hayes own stock and receive royalties from Banyan Biomarkers Inc., and as such may benefit financially as a result of the outcomes of this research or work reported in this publication.
References
- 1.Kochanek P.M., Berger R.P., Bayr H., Wagner A.K., Jenkins L.W., and Clark R.S. (2008) Biomarkers of primary and evolving damage in traumatic and ischemic brain injury: diagnosis, prognosis, probing mechanisms, and therapeutic decision making Curr. Opin. Crit. Care 14, 135–141 [DOI] [PubMed] [Google Scholar]
- 2.Papa L. (2012) Exploring the role of biomarkers for the diagnosis and management of traumatic brain injury patients, in: Poteomics - Human Diseases and Protein Functions. Man T.K. and Flores R.J. (eds). In Tech Open Access Publisher [Google Scholar]
- 3.Papa L., Ramia M.M., Kelly J.M., Burks S.S., Pawlowicz A., and Berger R.P. (2013) Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma 30, 324–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong H., Liang W.L., and Wu X.R. (2000) [Pathophysiological alterations in cultured astrocytes exposed to hypoxia/reoxygenation]. Sheng Li Ke Xue Jin Zhan 31, 217–221 [PubMed] [Google Scholar]
- 5.Ingebrigtsen T., Romner B., Marup-Jensen S., Dons M., Lundqvist C., Bellner J., Alling C., and Borgesen S.E. (2000) The clinical value of serum S-100 protein measurements in minor head injury: a Scandinavian multicentre study. Brain Inj. 14, 1047–1055 [DOI] [PubMed] [Google Scholar]
- 6.Muller K., Townend W., Biasca N., Unden J., Waterloo K., Romner B., and Ingebrigtsen T. (2007) S100B serum level predicts computed tomography findings after minor head injury. J. Trauma 62, 1452–1456 [DOI] [PubMed] [Google Scholar]
- 7.Biberthaler P., Linsenmeier U., Pfeifer K.J., Kroetz M., Mussack T., Kanz K.G., Hoecherl E.F., Jonas F., Marzi I., Leucht P., Jochum M., and Mutschler W. (2006) Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock 25, 446–453 [DOI] [PubMed] [Google Scholar]
- 8.Phillips J.P., Jones H.M., Hitchcock R., Adama N., and Thompson R.J. (1980) Radioimmunoassay of serum creatine kinase BB as index of brain damage after head injury. BMJ 281, 777–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothoerl R.D., Woertgen C., Holzschuh M., Metz C., and Brawanski A. (1998) S-100 serum levels after minor and major head injury. J. Trauma 45, 765–767 [DOI] [PubMed] [Google Scholar]
- 10.Piazza O., Storti M.P., Cotena S., Stoppa F., Perrotta D., Esposito G., Pirozzi N., and Tufano R. (2007) S100B is not a reliable prognostic index in paediatric TBI. Pediatr. Neurosurg. 43, 258–264 [DOI] [PubMed] [Google Scholar]
- 11.Bechtel K, Frasure S, Marshall C, Dziura J, and Simpson C. (2009) Relationship of serum S100B levels and intracranial injury in children with closed head trauma. Pediatrics 124, e697–e704 [DOI] [PubMed] [Google Scholar]
- 12.Rothoerl R.D. and Woertgen C. (2001) High serum S100B levels for trauma patients without head injuries. Neurosurgery 49, 1490–1491 [DOI] [PubMed] [Google Scholar]
- 13.Romner B., Ingebrigtsen T. (2001) High serum S100B levels for trauma patients without head injuries. Neurosurgery 49, 1490. [DOI] [PubMed] [Google Scholar]
- 14.Anderson R.E., Hansson L.O., Nilsson O., Dijlai-Merzoug R., and Settergen G. (2001) High serum S100B levels for trauma patients without head injuries. Neurosurgery 49, 1272–1273 [DOI] [PubMed] [Google Scholar]
- 15.Pelinka L.E., Kroepfl A., Schmidhammer R., Krenn M., Buchinger W., Redl H., and Raabe A. (2004) Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J. Trauma 57, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 16.Unden J., Bellner J., Eneroth M., Alling C., Ingebrigtsen T., and Romner B. (2005) Raised serum S100B levels after acute bone fractures without cerebral injury. J. Trauma 58, 59–61 [DOI] [PubMed] [Google Scholar]
- 17.Zimmer D.B., Cornwall E.H., Landar A., and Song W. (1995) The S100 protein family: history, function, and expression. Brain Res. Bull. 37, 417–429 [DOI] [PubMed] [Google Scholar]
- 18.Olsson B., Zetterberg H., Hampel H., and Blennow K. (2011) Biomarker-based dissection of neurodegenerative diseases. Prog. Neurobiol. 95, 520–534 [DOI] [PubMed] [Google Scholar]
- 19.Jagoda A.S., Bazarian J.J., Bruns J.J., Jr., Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., and Whitson R.R. (2008) Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 20.Eng L.F., Vanderhaeghen J.J., Bignami A., and Gerstl B. (1971) An acidic protein isolated from fibrous astrocytes. Brain Res. 28, 351–354 [DOI] [PubMed] [Google Scholar]
- 21.Missler U., Wiesmann M., Wittmann G., Magerkurth O., and Hagenstrom H. (1999) Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin. Chem. 45, 138–141 [PubMed] [Google Scholar]
- 22.Pelinka L.E., Kroepfl A., Leixnering M., Buchinger W., Raabe A., and Redl H. (2004) GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma 21, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 23.van Geel W.J., de Reus H.P., Nijzing H., Verbeek M.M., Vos P.E., and Lamers K.J. (2002) Measurement of glial fibrillary acidic protein in blood: an analytical method. Clin. Chim. Acta 326, 151–154 [DOI] [PubMed] [Google Scholar]
- 24.Nylen K., Ost M., Csajbok L.Z., Nilsson I., Blennow K., Nellgard B., and Rosengren L. (2006) Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J. Neurol. Sci. 240, 85–91 [DOI] [PubMed] [Google Scholar]
- 25.Mondello S., Papa L., Buki A., Bullock R., Czeiter E., Tortella F., Wang K.K, and Hayes R.L. (2011) Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care 15:R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metting Z., Wilczak N., Rodiger L.A, Schaaf J.M., and van der Naalt J. (2012) GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 78, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 27.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012) Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bohmer A.E., Oses J.P., Schmidt A.P., Peron C.S., Krebs C.L., Oppitz P.P., D'Avila T.T., Souza D.O., Portela L.V., and Stefani M.A. (2011) Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 68, 1624–1630 [DOI] [PubMed] [Google Scholar]
- 29.Vos P.E., Jacobs B., Andriessen T.M., Lamers K.J., Borm G.F., Beems T., Edwards M., Rosmalen C.F., and Vissers J.L. (2010) GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 75, 1786–1793 [DOI] [PubMed] [Google Scholar]
- 30.Zurek J. and Fedora M. (2012) The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir. (Wien) 154, 93–103 [DOI] [PubMed] [Google Scholar]
- 31.Honda M., Tsuruta R., Kaneko T., Kasaoka S., Yagi T., Todani M., Fujita M., Izumi T., and Maekawa T. (2010) Serum glial fibrillary acidic protein is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. J. Trauma 69, 104–109 [DOI] [PubMed] [Google Scholar]
- 32.Vajtr D., Benada O., Linzer P., Samal F., Springer D., Strejc P., Beran M., Prusa R., and Zima T. (2013) Immunohistochemistry and serum values of S-100B, glial fibrillary acidic protein, and hyperphosphorylated neurofilaments in brain injuries. Soud Lek 57, 7–12 [PubMed] [Google Scholar]