Abstract
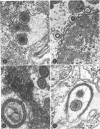
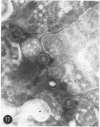
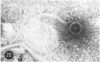
Ultrastructural observations on a new viral isolate, designated iguana virus, indicate that it is consistently present in intranuclear inclusions, possesses a ± 115-nm nucleocapsid, ranges from 165 to 300 nm in diameter in the enveloped form, and exhibits cubic symmetry (probably 162 capsomeres). It is concluded that it is a herpes-type virus, the morphological evidence being in agreement with and supporting the biological and physical characteristics presented by Clark and Karzon. Several fine-structural features, among them the encapsidization of small 35-nm hexagonal bodies and the viral membrane envelope enclosure of cell-derived moieties are presented and discussed.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Chandra S. Undulating tubules associated with endoplasmic reticulum in pathologic tissues. Lab Invest. 1968 Apr;18(4):422–428. [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Iguana virus, a herpes-like virus isolated from cultured cells of a lizard, Iguana iguana. Infect Immun. 1972 Apr;5(4):559–569. doi: 10.1128/iai.5.4.559-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Karzon D. T. Terrapene heart (TH-1), a continuous cell line from the heart of the box turtle Terrapene carolina. Exp Cell Res. 1967 Nov;48(2):263–268. doi: 10.1016/0014-4827(67)90351-5. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., HENLE G., ACHONG B. G., BARR Y. M. MORPHOLOGICAL AND BIOLOGICAL STUDIES ON A VIRUS IN CULTURED LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Exp Med. 1965 May 1;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Kondratick J., Ablashi D. V., Armstrong G. R., Dalton A. J. Ultrastructural and biological properties of a cytomegalovirus rescued from a human paraganglioma. Cancer Res. 1971 May;31(5):542–549. [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNGER P. D. THE ISOLATION AND MORPHOLOGY OF THE LUCK'E FROG KIDNEY TUMOR VIRUS. Virology. 1964 Oct;24:138–145. doi: 10.1016/0042-6822(64)90096-0. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazerian K., Burmester B. R. Electron microscopy of a herpes virus associated with the agent of Marek's disease in cell culture. Cancer Res. 1968 Dec;28(12):2454–2462. [PubMed] [Google Scholar]
- Nazerian K., Solomon J. J., Witter R. L., Burmester B. R. Studies on the etiology of Marek's disease. II. Finding of a herpesvirus in cell culture. Proc Soc Exp Biol Med. 1968 Jan;127(1):177–182. doi: 10.3181/00379727-127-32650. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. R., Jenson A. B., Phillips C. A., Melnick J. L. Herpes simplex virus hepatitis in mice: an electron-microscopic study. Exp Mol Pathol. 1968 Feb;8(1):34–48. doi: 10.1016/0014-4800(68)90004-x. [DOI] [PubMed] [Google Scholar]
- Shipkey F. H., Erlandson R. A., Bailey R. B., Babcock V. I., Southam C. M. Virus biographies. II. Growth of herpes simplex virus in tissue culture. Exp Mol Pathol. 1967 Feb;6(1):39–67. doi: 10.1016/0014-4800(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Toplin I., Mizell M., Sottong P., Monroe J. Zonal centrifuge applied to the purification of Herpesvirus in the Lucké frog kidney tumor. Appl Microbiol. 1971 Jan;21(1):132–139. doi: 10.1128/am.21.1.132-139.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzman B. G., Saito H., Kasac M. Tubular arrays in the endoplasmic reticulum in human tumor cells. Lab Invest. 1971 Jun;24(6):492–498. [PubMed] [Google Scholar]
- Winocour E. Further studies on the incorporation of cell DNA into polyoma-related particles. Virology. 1968 Apr;34(4):571–582. doi: 10.1016/0042-6822(68)90078-0. [DOI] [PubMed] [Google Scholar]
- Witter R. L., Nazerian K., Purchase H. G., Burgoyne G. H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am J Vet Res. 1970 Mar;31(3):525–538. [PubMed] [Google Scholar]
- Zambernard J., Mizell M. Viral particles of the frog renal adenocarcinoma: causative agent or passenger virus? I. Fine structure of primary tumors and subsequent intraocular transplants. Ann N Y Acad Sci. 1965 Aug 10;126(1):127–145. doi: 10.1111/j.1749-6632.1965.tb14272.x. [DOI] [PubMed] [Google Scholar]