Abstract
Airway epithelium ciliated cells play a central role in clearing the lung of inhaled pathogens and xenobiotics, and cilia length and coordinated beating are important for airway clearance. Based on in vivo studies showing that the airway epithelium of healthy smokers has shorter cilia than that of healthy nonsmokers, we investigated the mechanisms involved in cigarette smoke–mediated inhibition of ciliogenesis by assessing normal human airway basal cell differentiation in air–liquid interface (ALI) cultures in the presence of nontoxic concentrations of cigarette smoke extract (CSE). Measurements of cilia length from Day 28 ALI cultures demonstrated that CSE exposure was associated with shorter cilia (P < 0.05), reproducing the effect of cigarette smoking on cilia length observed in vivo. This phenotype correlated with a broad CSE-mediated suppression of genes involved in cilia-related transcriptional regulation, intraflagellar transport, cilia motility, structural integrity, and basal body development but not of control genes or epithelial barrier integrity. The CSE-mediated inhibition of cilia growth could be prevented by lentivirus-mediated overexpression of FOXJ1, the major cilia-related transcription factor, which led to partial reversal of expression of cilia-related genes suppressed by CSE. Together, the data suggest that components of cigarette smoke are responsible for a broad suppression of genes involved in cilia growth, but, by stimulating ciliogenesis with the transcription factor FOXJ1, it may be possible to maintain close to normal cilia length despite the stress of cigarette smoking.
Keywords: cigarette smoke, ciliogenesis
Clinical Relevance
We examined the effect of cigarette smoke on ciliogenesis in human airway epithelium in an in vitro setting to understand why cigarette smoking is associated with shortened cilia. This simple in vitro model can serve as a useful tool in understanding the human airway epithelium biology related to ciliogenesis and the response of differentiating basal cells to environmental stressors.
The ciliated cells of the mucociliary airway epithelium play a critical role in clearing the lung of inhaled pathogens, particulates, and xenobiotics (1). The motile cilia extend from the apical surface of the ciliated cells into the periciliary layer, with the cilia tips reaching the mucus gel layer of the airway lumen (2, 3). Mucus, secreted by the secretory cells of the airway epithelium, traps inhaled particles and is removed by cilia to cleanse the airways (2, 4). The coordinated beating and the length of the motile cilia are crucial for the mucociliary clearance process. The cilia motor proteins mediate coordinated and unidirectional beating, propelling the mucus gel layer cephalad (5, 6). If the cilia are shorter than the normal average of 6 to 7 μm (3, 7, 8), then it is logical to assume that the mucus gel layer cannot be propelled in a normal fashion, although a direct causative connection has not been established. When the cilia are defective in coordinated motility, length, or both, inhaled particles may remain in the respiratory tract. If toxic, as in the case of the cigarette smoke components, these particles can contribute to increased risk of developing airway epithelium lung diseases such as chronic obstructive pulmonary disease (COPD) and lung cancer (9, 10).
A variety of studies have assessed the mechanisms by which cigarette smoke inhibits ciliary beat frequency (11–17). For example, cigarette smoke extract (CSE)-induced oxidative stress and intracellular reactive oxygen species generation cause loss of the ciliated phenotype, suggesting that oxidative stress may play a major role in the CSE-induced effects on ciliogenesis (12). It is not understood, however, how cigarette smoke might alter the structure of cilia and particularly why smoking is associated with shorter cilia (8, 18–21). In this regard, we hypothesized that the effect of smoking on ciliogenesis occurs, at least in part, by suppression of cilia-related gene expression during the process of ciliated cell differentiation. The ciliated cells of the human airway epithelium are derived from basal cells, the stem/progenitor cell population that represents 10 to 15% of the airway epithelial cells (22). The airway epithelium is constantly renewed, estimated to turn over every 30 to 40 days, with the basal cells differentiating into ciliated and secretory cells (23, 24). To investigate the mechanisms involved in cigarette smoke–mediated suppression of human airway cilia development and growth, normal human airway basal cells were differentiated in air–liquid interface (ALI) cultures in the presence or absence of CSE. This in vitro model recapitulated the effect of cigarette smoking on shortening cilia length that is observed in vivo and permitted the assessment of CSE effect on the expression of genes relevant to ciliogenesis. The data demonstrate that CSE has a broad effect on inhibiting the expression of a variety of genes related to ciliogenesis and that overexpression of the cilia-related transcription factor FOXJ1 can prevent the CSE-mediated inhibition of cilia growth.
Materials and Methods
Cell Culture and Gene Expression Analysis
Nonsmoker basal cells (catalog no. CC2540S; Lonza, Walkersville, MD) were cultured and differentiated into mucociliary epithelium following standard procedures. For TaqMan PCR analysis, ALI cultures were washed with PBS and homogenized in TRIzol (Life Technologies, Carlsbad, CA), followed by RNA isolation, cDNA preparation, and real-time PCR analysis. Further details are provided in the online supplement.
Histology
The ALI cultures were washed once with PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 20 minutes at 23°C followed by three washes with PBS. After fixation, the ALI membranes were analyzed by top-stain immunofluorescence or sectioned and then analyzed by hematoxylin and eosin staining, immunofluorescence, or Alcian blue staining. Further details are provided in the online supplement.
Western Analysis
Cells were lysed directly in ALI cultures by adding into the transwell insert 100 μl of 1× NuPAGE LDS Sample Buffer (Life Technologies) diluted in radioimmunoprecipitation lysis buffer (Sigma, St. Louis, MO) containing Complete Protease Inhibitor Cocktail (Roche, Mannheim, Germany), Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL), and 50 mM dithiothreitol (Sigma) followed by standard procedures as described in the online supplement.
CSE Treatment of ALI Cultures
Cells were exposed to 0.1, 1, 3, and 6% CSE between Days 5 and 28 or between Days 28 and 42 of ALI culture from the basolateral side of the transwell inserts (see the online supplement for details regarding the CSE preparation protocol). Medium was changed every 2 to 3 days, and each time a fresh CSE aliquot was thawed and diluted accordingly. Cell viability was determined by using the Cytotoxicity Detection Kit (lactate dehydrogenase; Roche Applied Science, Indianapolis, IN) (further details are provided in the online supplement). Epithelial barrier integrity was determined by measuring the transepithelial electrical resistance (Ω × cm2) in Day 28 ALI cultures using the Millicell ERS-2 epithelial volt-ohm-meter (Millipore, Bedford, MA). To assess cilia length, Day 28 or Day 42 ALI cultures were washed once with PBS followed by dissociation from the transwell inserts by gently rubbing with a pipette tip and pipetting up/down in PBS. For each transwell insert, cells were resuspended in a total volume of 600 μl of PBS. The cell suspension (100 μl) was applied to a glass slide by cytocentrifugation (Cytospin 11; Shandon Instruments, Pittsburgh, PA), air dried, and stained with a Diff-Quik stain (Dade Behring, Deerfield, IL). Further details are provided in the online supplement.
Lentivirus-Mediated FOXJ1 Expression
Human FOXJ1 was constitutively overexpressed in ALI cultures under the CMV promoter via lentivirus-mediated transduction. The Lenti-FOXJ1 expressing lentiviral plasmid (catalog no. LV162690) and the Lenti-control lentiviral plasmid (catalog no. LV069) were from Applied Biological Materials Inc. (Richmond, British Columbia, Canada). Further details are provided in the online supplement (see Figure E4A in the online supplement for vector maps).
Results
ALI Cultures
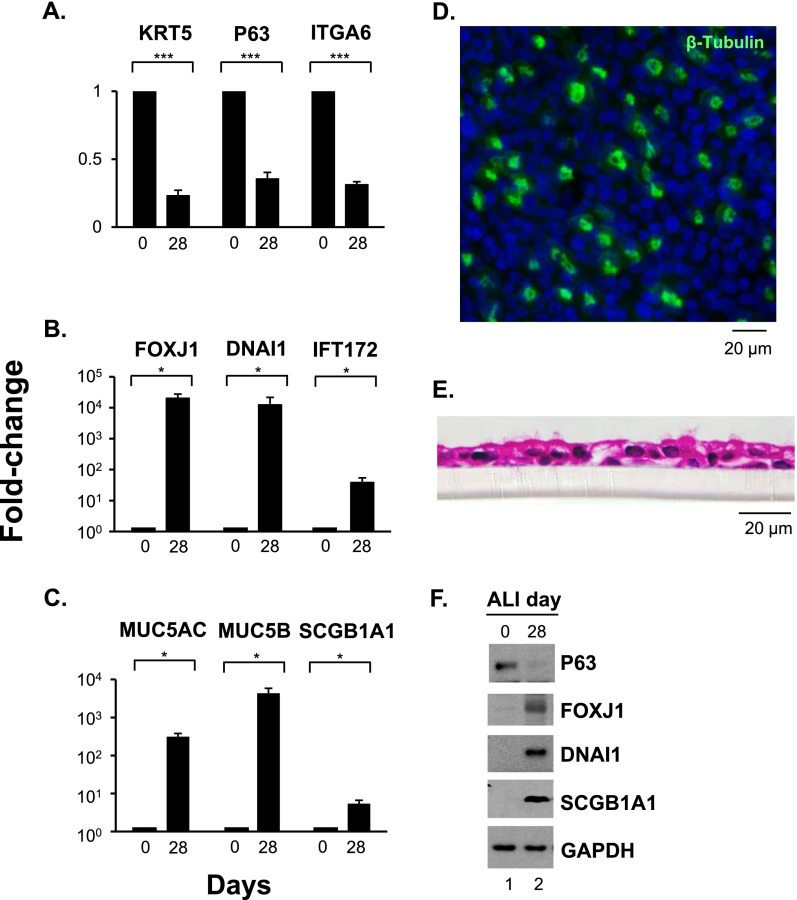
The basal cells differentiated in ALI cultures as expected. The gene expression of the basal cell–related markers KRT5, P63, and ITGA6 was significantly down-regulated (P < 0.001) on Day 28 of ALI cultures as compared with Day 0 (Figure 1A). In contrast, expression of the ciliated cell–related markers FOXJ1, DNAI1, and IFT172 (Figure 1B) and the secretory cell–related markers MUC5AC, MUC5B, and SCGB1A1 (Figure 1C) was significantly up-regulated (P < 0.05) on Day 28 compared with Day 0. β-Tubulin IV immunofluorescent staining verified that ciliated cells were present at Day 28 (Figure 1D). Hematoxylin and eosin staining of paraffin-embedded sections of Day 28 ALI cultures also confirmed that the basal cells differentiated into a multilayered ciliated epithelium (Figure 1E). Consistent with the differentiation of the basal cells to a mucociliary epithelium, Western analysis of cell protein lysates of Day 0 and Day 28 ALI cultures demonstrated that the level of P63 protein (basal cell) decreased, whereas the levels of FOXJ1 and DNAI1 (ciliated cell) and SCGB1A1 proteins (secretory cell) increased, on Day 28 of ALI cultures (Figure 1F).
Figure 1.
Differentiation of human airway basal cells in air–liquid interface (ALI) cultures. (A–C) TaqMan PCR analyses of differentiation-related genes from Day 0 and Day 28 ALI cultures. Basal cell genes (KRT5, P63, ITGA6) (A), ciliated cell genes (FOXJ1, DNAI1, IFT172) (B), and secretory cell genes (MUC5AC, MUC5B, SCGB1A1) (C). Averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student's t test. *P < 0.05; ***P < 0.001. (D) Immunofluorescent staining of ciliated cell marker β-tubulin IV (green) in Day 28 ALI cultures; cell nuclei stained with DAPI. Bar, 20 μm. (E) Hematoxylin and eosin staining of paraffin-embedded section of Day 28 ALI cultures. Bar, 20 μm. (F) Western analysis. Shown is expression of P63, FOXJ1, DNAI1, and SCGB1A1 proteins in whole cell lysates of Day 0 and Day 28 ALI cultures. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Data in D–F are representative of three independent experiments.
Overall Effects of CSE
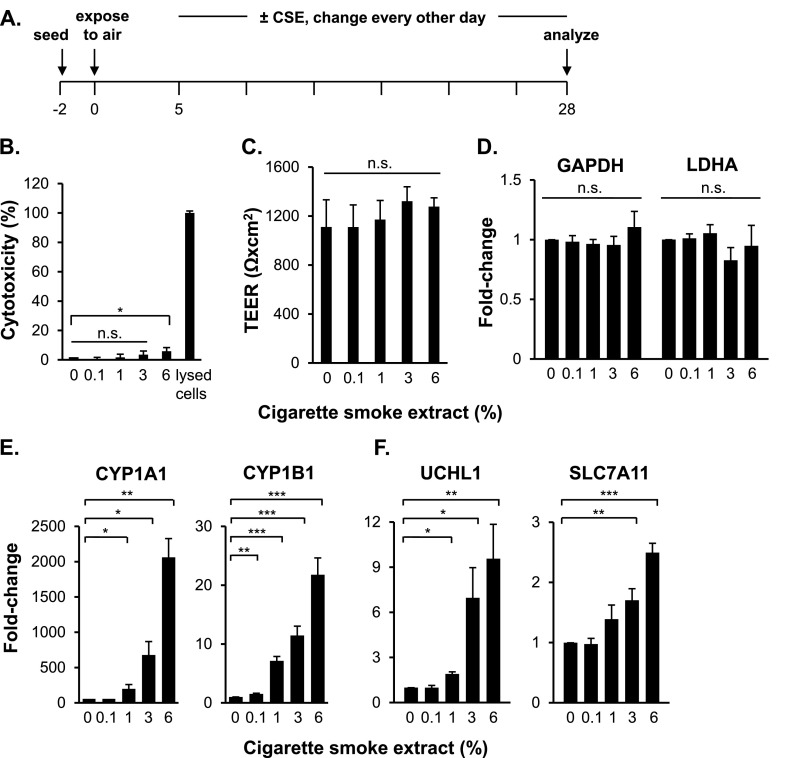
The basal cells were exposed to 0.1, 1, 3, and 6% CSE between Days 5 and 28 of ALI culture from the basolateral side of the transwell inserts (Figure 2A). The CSE concentrations used throughout the study had very low toxicity on the differentiating cells; the 0.1, 1, and 3% CSE treatments had no significant effect on cells, whereas 6% CSE increased cytotoxicity to 5.9% (P < 0.05) (Figure 2B). Measurements of the transepithelial electrical resistance showed that the CSE treatments had no effect on the barrier integrity of the differentiated epithelium (P > 0.05) (Figure 2C).
Figure 2.
Effect of cigarette smoke extract (CSE) on ALI cultures. (A) Experimental design. ALI cultures were exposed to 0, 0.1, 1, 3, and 6% CSE between Days 5 and 28 from the basolateral side of the transwell inserts. Medium was changed every 2 to 3 days, with fresh CSE added. (B) Viability. Assessment at Day 28 by lactate dehydrogenase release assay. (C) Barrier integrity. Shown is the effect of CSE on transepithelial electrical resistance (TEER) (expressed in Ω × cm2) in untreated and CSE-treated Day 28 ALI cultures. Data in B and C represent averages and standard errors of three independent experiments. P values were determined by two-tailed Student's t test. *P < 0.05. n.s., nonsignificant (P > 0.05). (D–F) TaqMan PCR analyses of housekeeping genes (GAPDH, lactate dehydrogenase A [LDHA]), oxidative stress genes (CYP1A1, CYP1B1), and smoking-induced genes (UCHL1, SLC7A11) in Day 28 ALI cultures. Averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001. n.s., nonsignificant (P > 0.05).
The expression of the housekeeping genes (GAPDH and LDHA) did not change in the presence of the CSE treatments (Figure 2D). In contrast, the expression of the oxidative stress-response genes (CYP1A1 and CYP1B1) was significantly up-regulated in the presence of the CSE treatments in a concentration-dependent manner (Figure 2E). Similarly, the expression of the smoking-induced genes (UCHL1 and SLC7A11), chosen because these genes are up-regulated by smoking in brushed samples of airway epithelium of smokers compared with nonsmokers (25), was significantly up-regulated in the presence of CSE in a concentration-dependent manner (Figure 2F).
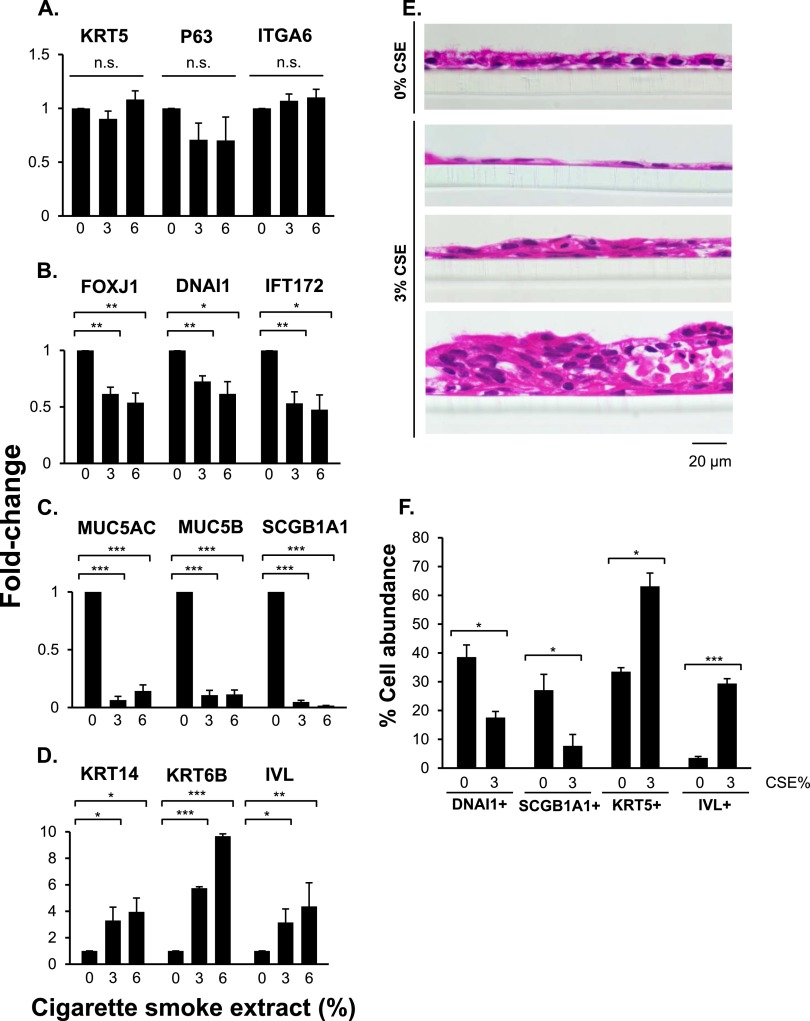
The expression of the basal cell genes (KRT5, P63, and ITGA6) did not change in the presence of CSE (P > 0.05 for all) (Figure 3A). However, CSE significantly down-regulated the expression of the ciliated cell–related genes, including FOXJ1, DNAI1, and IFT172 (Figure 3B), and secretory cell–related genes, including MUC5AC, MUC5B, and SCGB1A1 (Figure 3C). In contrast, the expression of the squamous cell–related genes, including KRT14, KRT6B, and IVL, was significantly up-regulated in the presence of CSE (Figure 3D).
Figure 3.
Effect of CSE on human airway basal cells differentiation in ALI cultures. Cells were differentiated in ALI cultures while being exposed to 0, 3, and 6% of CSE between Days 5 and 28. (A–D) TaqMan PCR analyses of differentiation-related genes at Day 28 of ALI cultures. Basal cell genes (KRT5, P63, ITGA6) (A), ciliated cell genes (FOXJ1, DNAI1, IFT172) (B), secretory cell genes (MUC5AC, MUC5B, SCGB1A1) (C), and squamous cell genes (KRT14, KRT6B, IVL) (D). Averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student's t test. *P < 0.05, **P < 0.01, and ***P < 0.001. (E) Hematoxylin and eosin staining of paraffin-embedded sections of Day 28 ALI cultures treated with 0 and 3% CSE. Bar, 20 μm. (F) Quantitative assessment of basal cells (KRT5+), ciliated cells (DNAI1+), secretory cells (SCGB1A1+), and squamous cells (IVL+) in ALI cultures. The percentage of basal, ciliated, secretory, and squamous cells was determined by counting the different epithelial cells, visualized by immunofluorescent or Alcian blue staining of ALI sections (representative images are shown in Figure E1) and dividing those values by the total number of cells, determined by counting the DAPI-stained nuclei in the same field. Shown are averages of means and standard errors of three independent experiments. P values were determined by two-tailed Student's t test. *P < 0.05; ***P < 0.001.
In addition to the gene expression analysis, we examined the effect of CSE on epithelium morphology and epithelial cell proportions. Hematoxylin and eosin staining of paraffin-embedded sections of Day 28 ALI cultures showed that exposure to CSE led to the development of regions with a flattened single-cell epithelial layer and regions with disordered thickened epithelium (Figure 3E). Cross-section measurements and quantification indicated that CSE exposure did not significantly change the average thickness of the epithelium throughout the transwell (P > 0.05) (see Table E2). However, in CSE-exposed ALI cultures there was a significant increase in the range distribution of epithelium thickness (Table E2). Day 28 ALI cross-section epithelial cell staining (Figures E1A–E1D) and quantification (Figure 3F) showed that CSE exposure reduced the percent abundance of ciliated cells (P < 0.05) and the percent abundance of secretory cells (P < 0.05). The percent abundance of Alcian blue–positive cells was very low (2.3%) and did not change significantly in the presence of CSE (not shown). In contrast, CSE treatment increased the percent abundance of basal cells (P < 0.05) and the percent abundance of squamous cells (P < 0.001). None of the cells in the Day 28 ALI cultures exposed to CSE were proliferating, as KI67 staining was negative (Figure E1E and see Figure E1F for KI67 antibody positive control in proliferating basal cells).
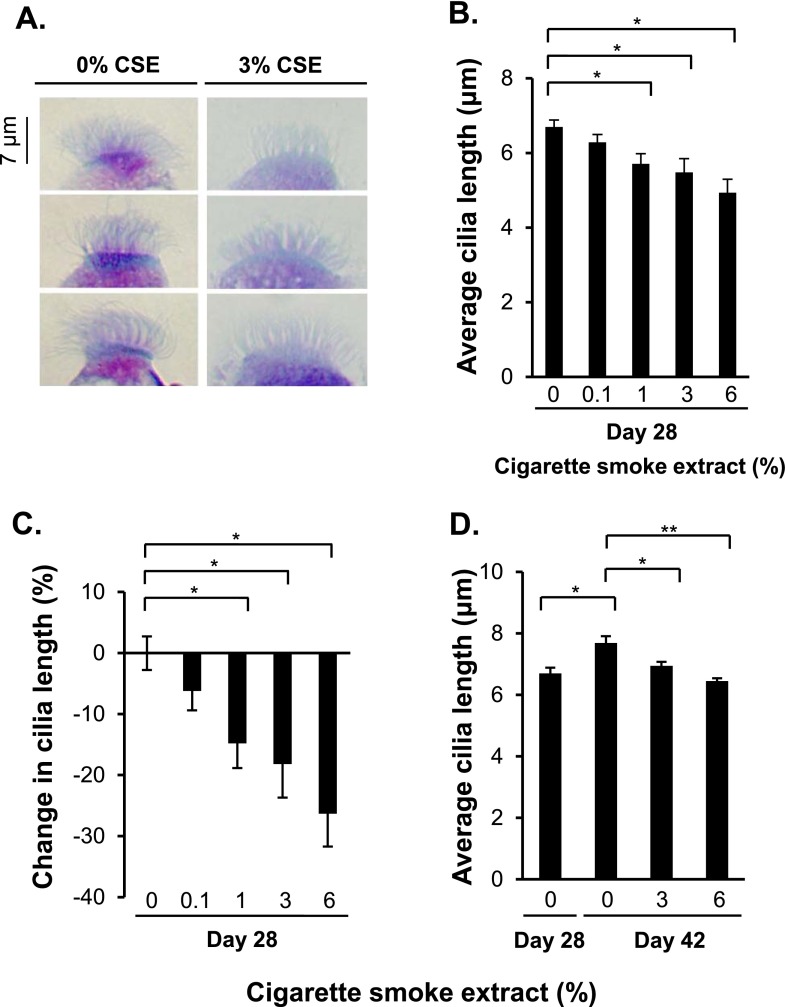
CSE Suppression of Cilia Length
Morphologic assessment of the ciliated cells from the 3 and 6% CSE-treated ALI cultures suggested that the ciliated cells had shorter cilia compared with the untreated ALI cultures (Figure 4A; 0 and 3% CSE treatments are shown for representative images of cilia). Quantitative analysis of cilia length showed that the untreated Day 28 ALI cultures had an average cilia length of 6.7 μm, whereas 1, 3, and 6% CSE treatments decreased cilia length to 5.7, 5.5, and 4.9 μm, respectively (P < 0.05 each in comparison to no CSE) (Figure 4B). The data reflected a significant decrease in the average cilia length (P < 0.05) (Figure 4C). Distributions of cilia length, represented as individual cilia measurements sorted by increasing length, demonstrate the global inhibition of cilia growth by CSE (Figures E2A–E2E; see Tables E3 and E4 for detailed statistical analysis of CSE-mediated inhibition of cilia growth).
Figure 4.
Suppression of ciliogenesis in cells differentiating in ALI cultures in the presence of CSE. Cells were differentiated in ALI cultures while being exposed to 0, 0.1, 1, 3, and 6% of CSE between Days 5 and 28. (A) Examples of the effect of CSE on cilia length. On Day 28 of ALI culture, suspensions of differentiated cells were applied to glass slides using a cytocentrifuge, air dried, and stained with Diff-Quik. Shown are representative images of cilia in 0 and 3% CSE-treated ALI cultures from three independent experiments. Bar, 7 μm. (B) Quantitative assessment of cilia length in untreated and CSE-treated Day 28 ALI cultures. Shown are means and standard errors of three independent experiments; in each experiment, 10 cilia on 10 different ciliated cells were measured (100 measurements in total per experiment) in 60× images of Diff-Quik–stained cytospins using ImageJ software. (C) Average percent decrease in cilia length. The average cilia length in untreated Day 28 ALI cultures was set to 100%, and the average cilia length in the CSE-treated Day 28 ALI cultures was normalized to the untreated values. (D) Effect of CSE on cilia length when ALI cultures continued beyond 28 days. Quantitative assessment of cilia length of untreated (Days 28 and 42) and CSE-treated cells (ALI cultures treated with 0, 3, and 6% of CSE between Days 28 and 42). Cilia lengths were determined using same methods as in B. In B–D, averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student’s t test. *P < 0.05 and **P < 0.01.
To validate the method for determining the average cilia length and to assess whether 100 cilia (10 cilia on each of 10 ciliated cells) were a representative sample, 500 cilia were measured (10 cilia on each of 50 ciliated cells; see Supplemental Methods). Distribution of cilia length from Day 28 ALI cultures treated with 0 or 3% CSE between Days 5 and 28 was constructed by creating histograms from 0.2-μm bins and then determining the frequency of cilia length for each bin (Figure E3). The distribution curves of cilia lengths were visually similar and not significantly different (all P > 0.05), suggesting that evaluating 100 cilia adequately represented the population of cilia length for each group. An average cilia length for each group was calculated using the 10-cell set (100 cilia), and this average was then compared with the average cilia length of the 50-cell set (500 cilia). This average cilia length was not significantly different (in untreated cultures: 6.53 μm for the 10-cell set and 6.59 μm for the 50-cell set; in 3% CSE cultures: 5.16 μm for the 10-cell set and 5.20 μm for the 50-cell set; all P > 0.05).
The effect of CSE treatment on cilia length in growing cilia was examined by allowing basal cells to differentiate for 28 days in ALI cultures and then continuously exposing the cells to 0, 3, and 6% of CSE between Days 28 and 42 of ALI. Quantitative analysis of cilia length showed that ciliated cells in the untreated Day 42 ALI cultures had longer cilia than Day 28 cultures (P < 0.05) (Figure 4D), whereas in the presence of 3 and 6% of CSE, this elongation of cilia was suppressed (Figure 4D).
CSE Suppression of the Cilia-Related Transcriptional Program
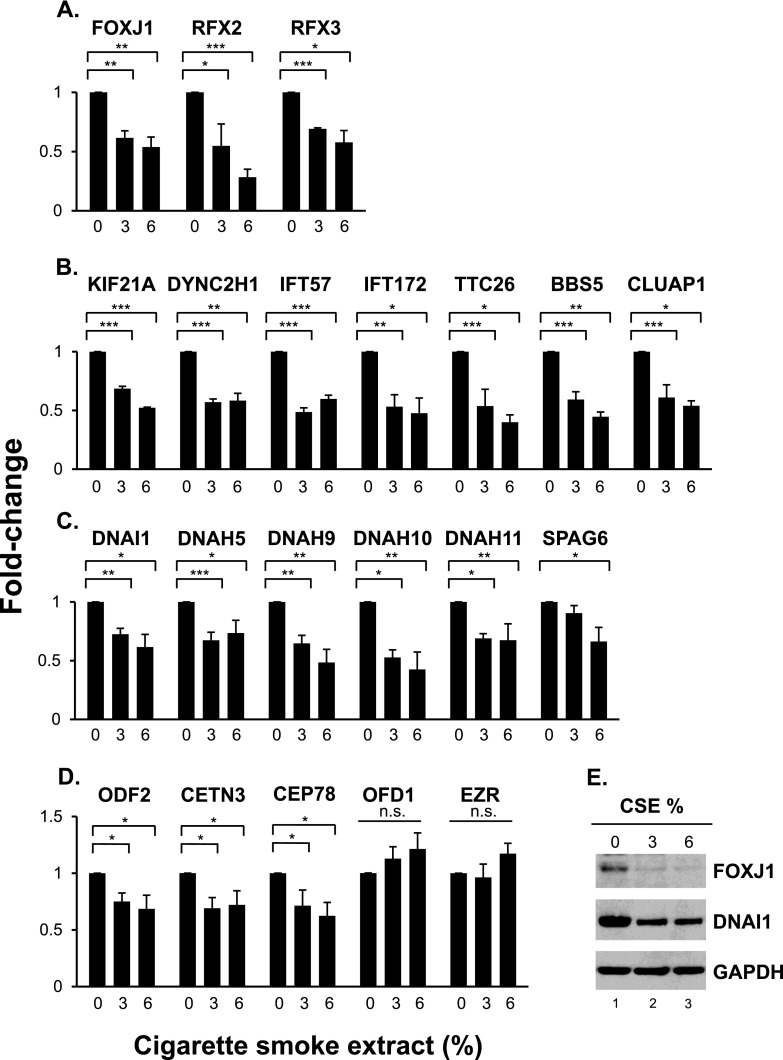
Four different categories of the ciliated cell–related genes were examined, including (1) cilia-related transcription factors FOXJ1 (forkhead box J1), RFX2 (regulatory factor X2), and RFX3 (regulatory factor X3); (2) cilia intraflagellar transport genes KIF21A (kinesin family member 21A), DYNC2H1 (dynein cytoplasmic 2 heavy chain 1), IFT57 (intraflagellar transport 57), IFT172 (intraflagellar transport 172), TTC26 (tetratricopeptide repeat domain 26), BBS5 (Bardet-Biedl syndrome 5), and CLUAP1 (clusterin associated protein 1); (3) cilia motility and structural integrity genes DNAI1 (dynein axonemal intermediate chain 1), DNAH5 (dynein axonemal heavy chain 5), DNAH9 (dynein axonemal heavy chain 9), DNAH10 (dynein axonemal heavy chain 10), DNAH11 (dynein axonemal heavy chain 11), and SPAG6 (sperm associated antigen 6); and (4) basal body development ODF2 (outer dense fiber of sperm tails 2), CETN3 (centrin EF-hand protein 3), CEP78 (centrosomal protein 78 kD), OFD1 (oral-facial-digital syndrome 1), and EZR (ezrin). The expression of the cilia-related transcription factor genes was significantly down-regulated in the presence of the CSE treatments, including FOXJ1, RFX2, and RFX3 (Figure 5A). Similarly, the expression of the cilia intraflagellar transport genes was significantly down-regulated in the presence of the CSE treatments, including KIF21A, DYNC2H1, IFT57, IFT172, TTC26, BBS5, and CLUAP1 (Figure 5B). The expression of the cilia motility and structural integrity genes was also significantly down-regulated in the presence of the CSE treatments, including DNAI1, DNAH5, DNAH9, DNAH10, DNAH11, and SPAG6 (Figure 5C). Likewise, the expression of the basal body development genes was significantly down-regulated in the presence of the CSE treatments, including ODF2, CETN3, and CEP78 (Figure 5D).
Figure 5.
CSE-mediated suppression of the cilia-related transcriptional program in differentiating ALI cultures. (A–D) TaqMan PCR analysis of cilia-related genes. Cilia-related transcription factors (FOXJ1, RFX2, RFX3) (A), intraflagellar transport (KIF21A, DYNC2H1, IFT57, IFT172, TTC26, BBS5, CLUAP1) (B), motility and structural integrity (DNAI1, DNAH5, DNAH9, DNAH10, DNAH11, SPAG6) (C), and basal body development (ODF2, CETN3, CEP78, OFD1, EZR) (D). Cells were differentiated in ALI cultures while being exposed to 0, 3, and 6% of CSE between Days 5 and 28. Averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001. n.s., nonsignificant (P > 0.05). (E) Western analysis of FOXJ1 and DNAI1 proteins in whole cell lysates of Day 28 ALI cultures treated with 0, 3, and 6% CSE. GAPDH was used as a loading control. Western analyses were representative of three independent experiments.
Consistent with the CSE-mediated suppression of mRNA levels, exposure of the differentiating basal cells to CSE down-regulated key ciliated cell–related proteins such as FOXJ1 and DNAI1. FOXJ1 is a well-characterized transcription factor involved in ciliated cell differentiation and has been suggested as a major regulator of the ciliated cell differentiation process (26–28), whereas DNAI1 (intermediate chain 1) is part of the outer dynein arm protein complexes, which, together with the inner dynein arm protein complexes, produces the force for ciliary axoneme bending (27, 29). Western analysis demonstrated that exposure to CSE between Days 5 and 28 of ALI culture resulted in lower levels of FOXJ1 and DNAI1 (Figure 5E).
FOXJ1 Expression Prevents CSE-Mediated Suppression of Cilia Growth
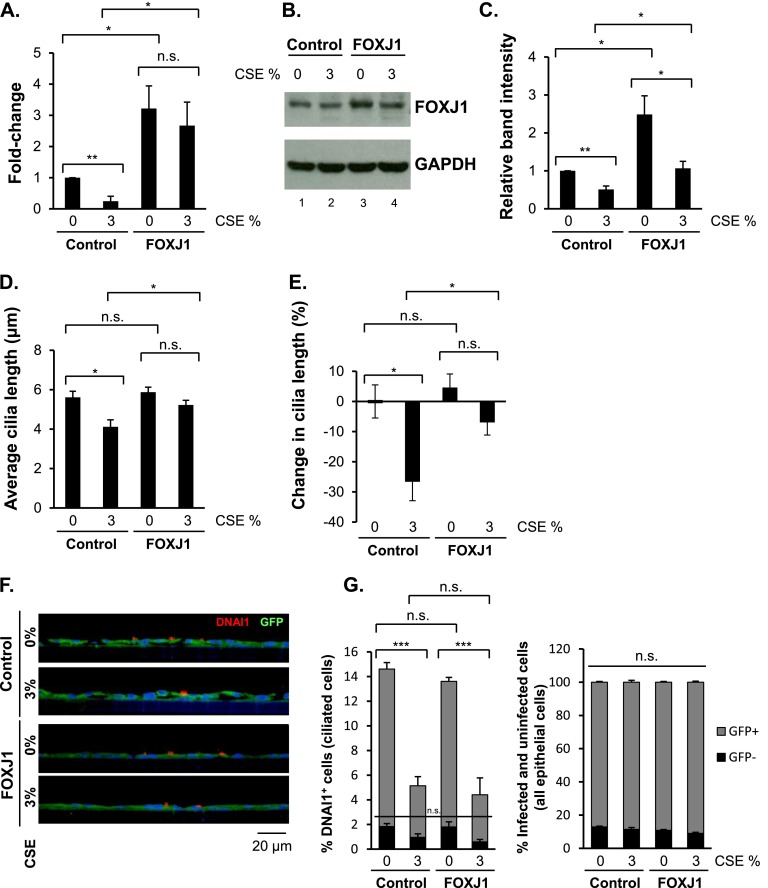
Because the FOXJ1 transcription factor is a major regulator of motile cilia growth, we hypothesized that the inhibitory effect of CSE on ciliogenesis might be circumvented by overexpression of FOXJ1 (26–28). Lenti-control and Lenti-FOXJ1–infected basal cells were differentiated in ALI cultures, and FOXJ1 gene expression was analyzed during the differentiation process. There was a higher induction in FOXJ1 expression in Lenti-FOXJ1–infected cells compared with the Lenti-control (Figure E4B). Lenti-control–infected and Lenti-FOXJ1–infected basal cells were differentiated in ALI cultures and were continuously treated with 3% CSE between Days 5 and 28 of culture. On Day 28 of ALI culture, the cells were analyzed for FOXJ1 gene expression. In Lenti-control–infected cultures, CSE treatment down-regulated FOXJ1 expression (P < 0.01) (Figure 6A), consistent with the uninfected ALI cultures that were treated with CSE (compare Figure 6A with Figure 5A). In Lenti-FOXJ1–infected ALI cultures, the expression of FOXJ1 was up-regulated by an average of 3.2-fold (P < 0.05) (Figure 6A), and in the presence of CSE, the FOXJ1 expression gene was up-regulated 2.7-fold (P < 0.05) (Figure 6A). In Lenti-control–infected cultures, the protein levels of FOXJ1 were decreased by CSE (Figures 6B [compare lanes 1 and 2] and 6C), correlating with the effect of CSE treatment in uninfected ALI cultures (Figure 5E). In Lenti-FOXJ1–infected cultures, there was a significant increase in the protein level of FOXJ1 (Figures 6B [compare lanes 1 and 3] and 6C). CSE treatment of Lenti-FOXJ1–infected cultures reduced the protein levels of FOXJ1 (Figures 6B [compare lanes 3 and 4] and 6C), but that level was still significantly higher in the Lenti-FOXJ1–infected cells than in the Lenti-control–infected cells (Figures 6B [compare lanes 2 and 4] and 6C). FOXJ1 overexpression did not affect the CSE-mediated induction of oxidative stress genes CYP1A1 and CYP1B1 or the smoking-induced genes UCHL1 and SLC7A11 (Figures E4E and E4F, respectively).
Figure 6.
Prevention of CSE-mediated suppression of cilia growth by FOXJ1 overexpression. Basal cells infected with Lenti-control or Lenti-FOXJ1 lentiviruses were differentiated in ALI cultures while being exposed to 0 and 3% CSE between Days 5 and 28. (A) TaqMan PCR analysis of FOXJ1 gene expression in Day 28 ALI cultures. Averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student’s t test. *P < 0.05. n.s., nonsignificant (P > 0.05). (B) Western analysis of FOXJ1 protein in whole cell lysates of Day 28 ALI cultures. GAPDH was used as a loading control. Representative Western analysis of three independent experiments is shown. (C) Quantification of relative band intensities of FOXJ1 protein in Western analysis from three independent experiments. FOXJ1 and GAPDH band intensities were quantified using ImageJ software followed by normalization of FOXJ1 to the GAPDH values. Averages and standard errors of three independent experiments are shown. P values were determined by two-tailed Student’s t test. *P < 0.05; **P < 0.01. (D) Quantitative assessment of cilia length in Day 28 ALI cultures. Shown are means and standard errors of three independent experiments, where in each experiment 10 cilia on 10 different ciliated cells were measured (100 measurements in total per experiment). Suspensions of differentiated cells were applied to glass slides using cytocentrifuge and were air dried. Slides were stained for GFP protein by immunohistochemistry, and cilia were measured in GFP-positive ciliated cells only. Cilia were measured in 60× images of stained cytospins using ImageJ software. (E) Average percent decrease in cilia length. The average cilia length in 0% CSE-treated Day 28 ALI cultures infected with Lenti-control was set to 100%, and the average cilia lengths in the subsequent samples were normalized to that value. (F) Representative immunofluorescence images of ciliated cell marker DNAI1 (red) and GFP (green) in Day 28 Lenti-control–infected and Lenti-FOXJ1–infected ALI cultures treated with 0 and 3% CSE. Cell nuclei were stained with DAPI. Bar, 20 μm. (G) Quantitative assessment of % DNAI1-positive, GFP-positive and DNAI1-positive, GFP-negative cells (infected and uninfected ciliated cells) and GFP-positive and GFP-negative cells (infected and uninfected epithelial cells) in Lenti-control–infected and Lenti-FOXJ1–infected ALI cultures treated with 0 and 3% CSE. For ciliated cell detection, DNAI1 antibody was used; for lentivirus infected cell detection, GFP antibody was used (representative images are shown in F). The percentage of specific cells for each condition was determined by counting the DNAI1-positive cells (ciliated cells) in ALI culture sections, visualized by immunofluorescence staining, and dividing those values by the total number of cells, determined by counting the DAPI-stained nuclei in the same field (in total, 500 cells were counted for each condition per experiment). Shown are averages of means and standard errors of three independent experiments. P values were determined by two-tailed Student's t test. ***P < 0.001. n.s., nonsignificant (P > 0.05).
To determine if FOXJ1 overexpression could prevent CSE-mediated inhibition of ciliogenesis, cilia lengths were measured in lentivirus-infected cells with or without continuous CSE treatment between Days 5 and 28 of culture. Before measuring cilia length, the Day 28 ALI cells were stained for green fluorescent protein (GFP) to distinguish infected cells from uninfected cells within the same ALI well (see Materials and Methods). Ciliated cells in the GFP-positive untreated Lenti-control–infected Day 28 ALI cultures had an average cilia length of 5.6 μm, whereas 3% CSE treatment decreased the cilia length to 4.1 μm (P < 0.05) (Figure 6D), consistent with the data from the uninfected ALI cultures exposed to CSE (Figure 4B). Ciliated cells in the GFP-positive untreated Lenti-FOXJ1–infected Day 28 ALI cultures had an average cilia length of 5.9 μm, with no significant change as compared with the GFP-positive untreated Lenti-control–infected cells (P > 0.05) (Figure 6D). Likewise, at an earlier time point during differentiation (Day 14), the average cilia length was similar in Lenti-control–infected and Lenti-FOXJ1–infected ciliated cells (P > 0.05) (Figure E4C). In the presence of CSE, in GFP-positive cells the average cilia length was significantly higher in Lenti-FOXJ1–infected cells (5.2 μm) than in Lenti-control–infected cells (4.1 μm; P < 0.05) (Figure 6D). In contrast, in GFP-negative (uninfected) ciliated cells within those same ALI cultures, CSE-mediated inhibition of cilia growth was observed in the Lenti-control and the Lenti-FOXJ1 groups (Figure E4D). In the presence of CSE, whereas there was a 26.6% decrease in cilia length in Lenti-control–infected cells, there was only a 6.9% decrease in cilia length in Lenti-FOXJ1–infected cells (P < 0.05) (Figure 6E,; see Tables E5 and E6 for detailed statistical analysis).
Lentivirus infection efficiency and whether FOXJ1 overexpression affected the number of ciliated cells in the absence and presence of CSE were determined by immunofluorescence staining of Day 28 ALI cultures with ciliated cell marker DNAI1 and infection marker GFP (Figure 6F) followed by quantification of the different cell types (Figure 6G). Counts of DNAI1-positive, GFP-positive cells revealed that treatment with CSE inhibited the percent abundance of ciliated cells in Lenti-control–infected and Lenti-FOXJ1–infected cultures (P < 0.001) (Figure 6G, left panel), whereas FOXJ1 overexpression neither increased the ciliated cell abundance nor prevented the CSE-mediated decrease in ciliated cell number (P > 0.05) (Figure 6G, left panel). Quantification of infection efficiency by counting the number of GFP-positive (infected) and GFP-negative (uninfected) cells showed that for all conditions the infection efficiency was close to 90% (Figure 6G, right panel).
Effect of FOXJ1 Overexpression on CSE-Mediated Suppression of the Cilia-Related Transcriptional Program
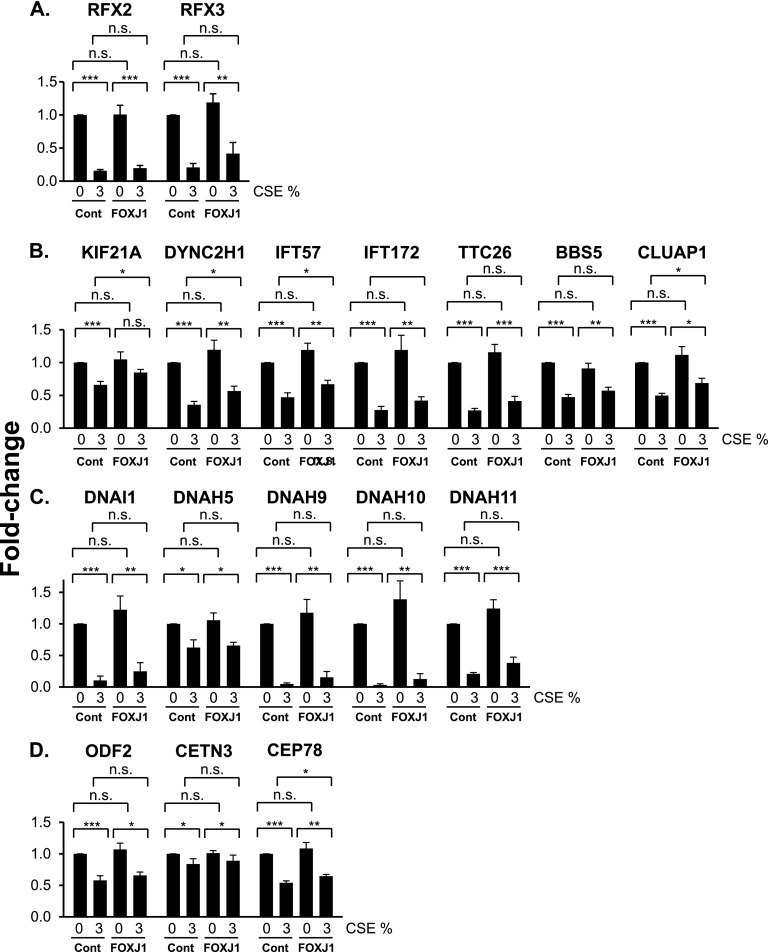
To determine if FOXJ1 overexpression could prevent the CSE-mediated suppression of the cilia-related transcriptional program, the expression of ciliated cell–related genes was analyzed in the Lenti-control–infected and Lenti-FOXJ1–infected cells with or without continuous CSE treatment between Days 5 and 28 of ALI culture, where those genes that were down-regulated by CSE (Figures 5A–5D) were analyzed. In Lenti-control–infected cells, CSE treatment inhibited the expression of all genes examined (Figures 7A–7D). None of the genes in the untreated samples were affected by FOXJ1 overexpression (Figures 7A–7D). FOXJ1 overexpression partially prevented the CSE-mediated inhibition of KIF21A, DYNC2H1, IFT57, CLUAP1, and CEP78 genes (Figures 7A–7D). The preventative effect of FOXJ1 overexpression on CSE-mediated inhibition of cilia-related genes was observed as a trend for the rest of the genes as well, although this finding was not significant when individual genes were analyzed (Figures 7A–7D). However, comparison of all the cilia-related genes as a group showed that FOXJ1 overexpression significantly up-regulated their expression (paired analysis of 3% CSE-treated Lenti-control and 3% CSE-treated Lenti-FOXJ1 samples; P < 0.001) (Figure E5).
Figure 7.
Effect of FOXJ1 overexpression on CSE-mediated suppression of cilia-related gene expression. Basal cells, infected with Lenti-control or Lenti-FOXJ1 lentiviruses, were differentiated in ALI cultures while being exposed to 0 and 3% CSE between Days 5 and 28. Shown is TaqMan PCR analysis of cilia-related genes. (A) Cilia-related transcription factors (RFX2, RFX3). (B) Intraflagellar transport (KIF21A, DYNC2H1, IFT57, IFT172, TTC26, BBS5, CLUAP1). (C) Motility and structural integrity (DNAI1, DNAH5, DNAH9, DNAH10, DNAH11). (D) Basal body development (ODF2, CETN3, CEP78). Averages and standard errors of five independent experiments are shown. P values were determined by two-tailed Student’s t test. *P < 0.05, **P < 0.01, and ***P < 0.001. n.s., nonsignificant (P > 0.05).
Discussion
The ciliated cells of the airway epithelium are important for clearing the lung airways of inhaled pathogens and xenobiotics (1). Based on the observations that the airways of healthy smokers have significantly shorter cilia when compared with the airways of healthy nonsmokers (8) and the transcriptional analysis of airway epithelium demonstrating that smoking is associated with suppressed expression of cilia-related genes (8), we hypothesized that components of cigarette smoke suppress cilia growth in the human airway epithelium. To test this hypothesis, we assessed the effect of nontoxic concentrations of CSE on the differentiation of normal human bronchial basal cells into a mucociliary epithelium in ALI cultures. This in vitro model of the effect of cigarette smoke on ciliogenesis recapitulated the observed effect of cigarette smoking on cilia length in vivo. The data demonstrated that, during basal cell differentiation into a mucociliary epithelium, there was a widespread suppression of ciliated cell–related gene expression, including genes involved in cilia-related transcriptional regulation, ciliary intraflagellar transport, cilia motility, structural integrity, and basal body development. This cigarette smoke–mediated inhibition of cilia growth during differentiation could be significantly reversed by overexpressing FOXJ1, a transcription factor that plays a central role in ciliogenesis (26–28). Of the four different categories of ciliated cell–related genes analyzed, FOXJ1 overexpression partially prevented the CSE-mediated down-regulation of the intraflagellar transport genes and a basal body development gene. In addition to inhibiting cilia growth, we observed that, in the presence of CSE, the differentiation of basal cells toward ciliated and secretory cells is altered, with abnormal differentiation toward the squamous cell phenotype. In cultures exposed to CSE, ciliated cells were less abundant, and in those ciliated cells that did appear in the presence of CSE, the cilia were shorter. This is in line with the observation that smokers exhibit a squamous cell metaplasia in the airway epithelium (30, 31).
Effect of Cigarette Smoke on Airway Ciliated Cells
The human airways are lined with a pseudostratified heterogeneous epithelium composed of four major cell types: ciliated, secretory, intermediate, and basal cells (23, 24). The airway epithelial ciliated cells are derived from basal cells, the stem/progenitor cells of the airway epithelium, which are capable of differentiating into secretory and ciliated cells (22). The ciliated cells account for 50 to 80% of the airway epithelial cells (1). Ciliated cell formation is a continuous process during the ongoing homeostasis and the repair of the airway epithelium (1). The motile cilia of the ciliated cells beat in coordinated fashion and move mucus across the airway lumen in a cephalad direction, mediating the removal of inhaled environmental contaminants from the airways (2–6). If this process is impaired, mucus accumulates in the airways, contributing to increased inflammation and infection, and, as in the case of cigarette smoke, the inhaled particles remaining in the respiratory tract can promote the development of COPD and lung cancer (9, 10).
Cigarette smoke has an inhibitory effect on at least four aspects of motile cilia relevant to their function in the airways, including beat frequency, beat coordination, ultrastructure, and length. Based on the mucociliary clearance models, all these properties of respiratory motile cilia play a role in the impaired process of mucus clearance observed in smokers (32–34). Numerous in vivo and in vitro studies have demonstrated that cigarette smoke can significantly suppress the beat frequency of the airway cilia. Although early studies of the effect of cigarette smoke on cilia beating were mainly descriptive (14–17), in more recent studies these observations have been quantified via digital high-speed video imaging, and involvement of the protein kinase A and protein kinase Cε pathways has been implicated (6, 12, 13). Using electron microscopy, cigarette smoke has also been shown to be associated with various ultrastructural abnormalities of the respiratory cilia, where different ciliary components are missing, including the outer and inner dynein arms, nexin links, radial spokes, central sheaths, and central and peripheral microtubules (21, 35–37). Such ultrastructural abnormalities may be associated with ineffective ciliary stroke motion and uncoordinated cilia beating (2, 38). In addition, we and others have shown in vivo evidence that cigarette smoking is associated with reduced cilia length in the human airway epithelium. In the respiratory airway epithelium obtained from lung autopsies of smokers and nonsmokers, it was observed that smokers had shorter cilia than nonsmokers (20). More recently, we measured cilia in the large airways of smokers and nonsmokers and found that smokers have significantly shorter cilia than nonsmokers (8). Shortened cilia have also been described in COPD (39, 40). Based on models of the airway periciliary and mucus gel layer structural characteristics, even a small decrease in the length of the cilium impairs mucus clearance (32, 34, 41, 42). Such a decrease in length would likely prevent the distal tip of the shortened cilium from reaching the mucus gel layer and would also result in reduced force of the forward stroke of the cilium, thus decreasing the overall mucus movement in the epithelium. In support of the concept that shortened cilia reduce mucus clearance, in vivo studies have demonstrated correlations between shortened cilia and impaired mucociliary clearance (43, 44). The motile cilia are shorter in the small airways as compared with the large airways (39, 40). Taken together with the hypothesis of reduced mucociliary clearance in the presence of shortened cilia, these findings indicate that the small airways likely accumulate greater amounts of cigarette smoke components than the large airways, and this may in part explain why COPD is associated with the small airways.
Disordered Ciliogenesis Related to Cigarette Smoking
To understand how cigarette smoking leads to shortened cilia in the respiratory airway epithelium, we exposed human bronchial basal cells to CSE during their differentiation into the mucociliary epithelium in ALI cultures and assessed the molecular changes that occurred in relation to the ciliogenesis process. Similar to the in vivo data we reported previously (8), the decrease in cilia length in the presence of CSE was 1.0 to 1.5 μm, which translates to approximately 20% decrease in the average cilia length. However, based on theoretical calculations, others have estimated that these “small” changes will potentially have a major impact on mucociliary clearance in the airways (32, 34, 41, 42). We observed that in the presence of CSE, there was widespread inhibition of ciliated cell–related gene expression. These data are consistent with the transcriptional analysis of the airway epithelium from smokers, demonstrating that smoking is associated with suppressed cilia-related gene expression (8, 45). All the major categories of the ciliated cell–related genes were inhibited when basal cells were exposed to CSE during the differentiation process, including cilia-related transcriptional regulators (FOXJ1, RFX2, and RFX3), ciliary intraflagellar transport (KIF21A, DYNC2H1, IFT57, IFT172, TTC26, BBS5, and CLUAP1), motility and structural integrity (DNAI1, DNAH5, DNAH9, DNAH10, DNAH11, and SPAG6), and basal body development (ODF2, CETN2, and CEP78). This suggests that cigarette smoke affects the expression of multiple genes central to the ciliogenesis process in the airway epithelium.
Airway epithelial basal cell differentiation into the ciliated cell lineage is under the control of the cilia-related transcription factors (26–28, 46, 47). Cilia growth is a highly dynamic process, where the expression of ciliary structural components and regulators of their assembly is specifically induced during the development of ciliated tissues. The underlying mechanisms controlling cilia growth are only partially defined. Genetic studies in different organisms have identified FOXJ1 and RFX family transcription factors as the key factors controlling the expression of cilia-related genes. Because the majority of cilia-related genes are believed to be under the transcriptional control of FOXJ1 and RFX (27), it is possible that the decreased expression of these transcription factors led to reduced expression of the examined cilia-related genes. The FOXJ1 transcription factor is the most well-characterized transcription factor and has been suggested as the major regulator of the ciliated cell differentiation process (26–28). Because CSE treatment suppressed the expression of FOXJ1 and has led to reduced cilia length in ALI cultures, we hypothesized that exogenous overexpression of FOXJ1 could prevent the CSE-mediated inhibition of cilia growth. Consistent with this hypothesis, we observed that FOXJ1 overexpression reversed the CSE-mediated inhibition of cilia growth. Furthermore, of the four different categories of ciliated cell–related genes analyzed, FOXJ1 overexpression partially prevented the CSE-mediated down-regulation of intraflagellar transport genes and a basal body development gene. It is an interesting question as to how CSE can reduce the expression of the cilia-related transcription factors. Recently, the Wnt/β-catenin signaling pathway has been shown to directly regulate FOXJ1 expression and ciliogenesis in zebrafish Kupffer’s vesicle (48). In turn, we have observed a down-regulation of the Wnt/β-catenin signaling pathway in the airway epithelium of healthy smokers and smokers with COPD (49). Therefore, it is possible that CSE decreased the expression of the cilia-related genes by targeting FOXJ1 via Wnt/β-catenin pathway inhibition. Overall, the cilia-related data suggest that several aspects of the ciliogenesis process are affected by CSE because the upstream components of the ciliogenesis process are inhibited; furthermore, the evidence for this finding is shown in the decreased expression of the intraflagellar transport, structural integrity, motility, and basal body development genes. Recently it has been shown that cilia length is affected by cigarette smoke exposure at the protein level via an autophagy-dependent pathway (50).
In addition to suppressing cilia-related transcriptional regulation and thus affecting the early stages of the ciliogenesis process, it is possible that cigarette smoke inhibits processes related to cilia growth, cilia length maintenance, and cilia function. According to the limited precursor model of cilia growth, cilia length can be limited by the quantities of the necessary precursor particles (51, 52). Thus, by reducing the expression of many cilia-related genes involved in this process, CSE treatment can lead to shortened cilia formation. Because the expression of the intraflagellar transport genes was widely suppressed by CSE treatment in ALI cultures, it is likely that the process by which the molecular motors transport and assemble ciliary building blocks at the distal ends of the cilia is impaired (53). Similarly, the reduced expression of the ciliary motility, structural integrity, and basal body development genes may explain the observations that cigarette smoking is associated with reduced cilia beat frequency, improper beat coordination, and ultrastructural abnormalities (8, 11–21, 35–37).
Acknowledgments
Acknowledgments
The authors thank B. Ferris and J. Heldrich for technical assistance, G. Wang for help with data interpretation, and N. Mohamed and D. N. McCarthy for help in preparing the manuscript.
Footnotes
This work was supported by National Institutes of Health grants R01 HL107882, P20 HL113443, P50 HL084936, UL1 TR000457, and UL1 RR024143 (A.B.), T32 HL094284 (A.B., R.G.C.), and K23 HL103837 (A.E.T.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0363OC on May 14, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lee RMKW, Forrest JB.Structure and function of ciliaCrystal RGeditor. The lung: scientific foundations2nd ed.. Philadelphia, PA: Lippincott-Raven Publishers; 1997459–478. [Google Scholar]
- 2.Wanner A, Salathé M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 3.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol. 2007;35:116–129. doi: 10.1080/01926230601060025. [DOI] [PubMed] [Google Scholar]
- 4.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1:451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 6.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 7.Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leopold PL, O’Mahony MJ, Lian XJ, Tilley AE, Harvey BG, Crystal RG. Smoking is associated with shortened airway cilia. PLoS ONE. 2009;4:e8157. doi: 10.1371/journal.pone.0008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J. 2007;29:438–445. doi: 10.1183/09031936.00124506. [DOI] [PubMed] [Google Scholar]
- 10.Brody JS, Spira A. State of the art: chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc. 2006;3:535–537. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 11.Yaghi A, Zaman A, Cox G, Dolovich MB. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respir Med. 2012;106:1139–1147. doi: 10.1016/j.rmed.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Milara J, Armengot M, Bañuls P, Tenor H, Beume R, Artigues E, Cortijo J. Roflumilast N-oxide, a PDE4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br J Pharmacol. 2012;166:2243–2262. doi: 10.1111/j.1476-5381.2012.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simet SM, Sisson JH, Pavlik JA, Devasure JM, Boyer C, Liu X, Kawasaki S, Sharp JG, Rennard SI, Wyatt TA. Long-term cigarette smoke exposure in a mouse model of ciliated epithelial cell function. Am J Respir Cell Mol Biol. 2010;43:635–640. doi: 10.1165/rcmb.2009-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vastag E, Matthys H, Köhler D, Gronbeck L, Daikeler G. Mucociliary clearance and airways obstruction in smokers, ex-smokers and normal subjects who never smoked. Eur J Respir Dis Suppl. 1985;139:93–100. [PubMed] [Google Scholar]
- 15.Goodman RM, Yergin BM, Landa JF, Golivanux MH, Sackner MA. Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers, and patients with chronic bronchitis. Am Rev Respir Dis. 1978;117:205–214. doi: 10.1164/arrd.1978.117.2.205. [DOI] [PubMed] [Google Scholar]
- 16.Lourenço RV, Klimek MF, Borowski CJ. Deposition and clearance of 2 micron particles in the tracheobronchial tree of normal subjects: smokers and nonsmokers. J Clin Invest. 1971;50:1411–1420. doi: 10.1172/JCI106624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camner P, Philipson K. Tracheobronchial clearance in smoking-discordant twins. Arch Environ Health. 1972;25:60–63. doi: 10.1080/00039896.1972.10666136. [DOI] [PubMed] [Google Scholar]
- 18.Tamashiro E, Xiong G, Anselmo-Lima WT, Kreindler JL, Palmer JN, Cohen NA. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009;23:117–122. doi: 10.2500/ajra.2009.23.3280. [DOI] [PubMed] [Google Scholar]
- 19.Nagai A, Thurlbeck WM. Scanning electron microscopic observations of emphysema in humans: a descriptive study. Am Rev Respir Dis. 1991;144:901–908. doi: 10.1164/ajrccm/144.4.901. [DOI] [PubMed] [Google Scholar]
- 20.Chang SC. Microscopic properties of whole mounts and sections of human bronchial epithelium of smokers and nonsmokers. Cancer. 1957;10:1246–1262. doi: 10.1002/1097-0142(195711/12)10:6<1246::aid-cncr2820100623>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Crémoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151:630–634. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 22.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 25.Harvey BG, Heguy A, Leopold PL, Carolan BJ, Ferris B, Crystal RG. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med (Berl) 2007;85:39–53. doi: 10.1007/s00109-006-0103-z. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- 27.Thomas J, Morlé L, Soulavie F, Laurençon A, Sagnol S, Durand B. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol Cell. 2010;102:499–513. doi: 10.1042/BC20100035. [DOI] [PubMed] [Google Scholar]
- 28.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2010;43:731–739. doi: 10.1165/rcmb.2009-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennarun G, Escudier E, Chapelin C, Bridoux AM, Cacheux V, Roger G, Clément A, Goossens M, Amselem S, Duriez B. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet. 1999;65:1508–1519. doi: 10.1086/302683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auerbach O, Stout AP, Hammond EC, Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med. 1961;265:253–267. doi: 10.1056/NEJM196108102650601. [DOI] [PubMed] [Google Scholar]
- 31.Wistuba II, Gazdar AF. Lung cancer preneoplasia. Annu Rev Pathol. 2006;1:331–348. doi: 10.1146/annurev.pathol.1.110304.100103. [DOI] [PubMed] [Google Scholar]
- 32.Sleigh MA, Blake JR, Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988;137:726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- 33.Yeates DB, Besseris GJ, Wong LB.Physicochemical properties of mucus and its propulsion Crystal RG.editor. The lung: scientific foundations2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1997487–503. [Google Scholar]
- 34.Fulford GR, Blake JR. Muco-ciliary transport in the lung. J Theor Biol. 1986;121:381–402. doi: 10.1016/s0022-5193(86)80098-4. [DOI] [PubMed] [Google Scholar]
- 35.Fox B, Bull TB, Oliver TN. The distribution and assessment of electron-microscopic abnormalities of human cilia. Eur J Respir Dis Suppl. 1983;127:11–18. [PubMed] [Google Scholar]
- 36.McDowell EM, Barrett LA, Harris CC, Trump BF. Abnormal cilia in human bronchial epithelium. Arch Pathol Lab Med. 1976;100:429–436. [PubMed] [Google Scholar]
- 37.Ailsby RL, Ghadially FN. Atypical cilia in human bronchial mucosa. J Pathol. 1973;109:75–78. doi: 10.1002/path.1711090108. [DOI] [PubMed] [Google Scholar]
- 38.Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- 39.Serafini SM, Michaelson ED. Length and distribution of cilia in human and canine airways. Bull Eur Physiopathol Respir. 1977;13:551–559. [PubMed] [Google Scholar]
- 40.Hessel J, Heldrich J, Fuller J, Staudt MR, Radisch S, Hollmann C, Harvey BG, Kaner RJ, Salit J, Yee-Levin J, et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS ONE. 2014;9:e85453. doi: 10.1371/journal.pone.0085453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DJ, Gaffney EA, Blake JR. Modelling mucociliary clearance. Respir Physiol Neurobiol. 2008;163:178–188. doi: 10.1016/j.resp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Rautiainen M, Nuutinen J, Collan Y. Short nasal respiratory cilia and impaired mucociliary function. Eur Arch Otorhinolaryngol. 1991;248:271–274. doi: 10.1007/BF00176753. [DOI] [PubMed] [Google Scholar]
- 44.Toskala E, Nuutinen J, Rautiainen M. Scanning electron microscopy findings of human respiratory cilia in chronic sinusitis and in recurrent respiratory infections. J Laryngol Otol. 1995;109:509–514. doi: 10.1017/s0022215100130580. [DOI] [PubMed] [Google Scholar]
- 45.Wang G, Wang R, Ferris B, Salit J, Strulovici-Barel Y, Hackett NR, Crystal RG. Smoking-mediated up-regulation of GAD67 expression in the human airway epithelium. Respir Res. 2010;11:150. doi: 10.1186/1465-9921-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beckers A, Alten L, Viebahn C, Andre P, Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc Natl Acad Sci USA. 2007;104:15765–15770. doi: 10.1073/pnas.0704344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Zein L, Ait-Lounis A, Morlé L, Thomas J, Chhin B, Spassky N, Reith W, Durand B. RFX3 governs growth and beating efficiency of motile cilia in mouse and controls the expression of genes involved in human ciliopathies. J Cell Sci. 2009;122:3180–3189. doi: 10.1242/jcs.048348. [DOI] [PubMed] [Google Scholar]
- 48.Caron A, Xu X, Lin X. Wnt/β-catenin signaling directly regulates Foxj1 expression and ciliogenesis in zebrafish Kupffer’s vesicle. Development. 2012;139:514–524. doi: 10.1242/dev.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang R, Ahmed J, Wang G, Hassan I, Strulovici-Barel Y, Hackett NR, Crystal RG. Down-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD. PLoS ONE. 2011;6:e14793. doi: 10.1371/journal.pone.0014793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lefebvre PA, Silflow CD, Wieben ED, Rosenbaum JL. Increased levels of mRNAs for tubulin and other flagellar proteins after amputation or shortening of Chlamydomonas flagella. Cell. 1980;20:469–477. doi: 10.1016/0092-8674(80)90633-9. [DOI] [PubMed] [Google Scholar]
- 52.Wemmer KA, Marshall WF. Flagellar length control in chlamydomonas: paradigm for organelle size regulation. Int Rev Cytol. 2007;260:175–212. doi: 10.1016/S0074-7696(06)60004-1. [DOI] [PubMed] [Google Scholar]
- 53.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]