Abstract
Malignant mesothelioma (MM), lung cancers, and asbestosis are hyperproliferative diseases associated with exposures to asbestos. All have a poor prognosis; thus, the need to develop novel and effective therapies is urgent. Vandetanib (Van) (ZD6474, ZACTIMA) is a tyrosine kinase inhibitor that has shown equivocal results in clinical trials for advanced non-small cell lung cancer. However, tyrosine kinase inhibitors alone have shown no significant clinical activity in phase II trials of patients with unresectable MM. Using epithelioid (HMESO) and sarcomatoid (H2373) human MM lines, the efficacy of tumor cell killing and signaling pathways modulated by Van with and without doxorubicin (Dox) was examined. Van alone reduced total cell numbers in HMESO MM and synergistically increased the toxicity of Dox in HMESO and H2373 cells. Most importantly, we identified two novel cell survival/resistance pathways, ERK5 and cyclic AMP response element binding protein (CREB), that were inhibited by Van and Dox. After silencing of either ERK5 or CREB, significant decreases in cell numbers in the Dox-resistant sarcomatoid H2373 line were observed. Results suggest that a plethora of cell signaling pathways associated with cell survival are induced by Dox but inhibited by the addition of Van in MM. Data from our study support the combined efficacy of Van and Dox as a novel approach in the treatment of MM that is further enhanced by blocking ERK5 or CREB signaling cascades.
Keywords: tyrosine kinases, extracellular signal-regulated kinases, vandetanib, doxorubicin, malignant mesothelioma
Clinical Relevance
Malignant mesothelioma is a tumor with no effective therapeutic strategies. This report reveals two new signaling pathways blocked by a kinase inhibitor and suggests a trimodality approach to treatment.
Asbestos fibers increase cell proliferation by activated receptor tyrosine kinases in progenitor cells of malignant mesothelioma (MM), lung cancers, and asbestosis (1, 2). The incidence of MM increased in the United States from the late 1950s until the beginning of this century, where it has remained stable, with 2,500 to 3,000 cases reported annually (3). Most patients with MM survive less than 12 to 18 months after initial diagnosis (4). The majority of patients with MM are diagnosed at an advanced stage, thus reducing the effectiveness of therapies and making them poor candidates for resection. Therefore, more effective therapeutic strategies for MM are urgently needed.
Numerous preclinical and clinical studies using tyrosine kinase inhibitors and other molecular targeted therapies have shown great promise in the treatment of various malignant tumors (5). These therapies are designed to inhibit key signaling pathways involved in tumor growth and metastasis as well as chemoresistance. One of the important targets in a variety of tumors is the epidermal growth factor receptor (EGFR), a tyrosine kinase receptor belonging to the ErbB family and expressed in a large number of solid tumors (6). EGFR phosphorylation induces subsequent activation of several downstream intracellular targets, including mitogen-activated protein kinases (MAPK) and other tumor-promoting pathways. EGFR activation of MAPK also has been linked to chemoresistance because it is abolished in drug-resistant cells when compared with wild-type tumor cells (7).
Extracellular signal-regulated kinases (ERKs) are under intense scrutiny as potential molecular targets of EGFR phosphorylation because of their association with a number of neuropathies, cancers, and nonmalignant lung diseases. In addition, they mediate a number of fundamental cell processes, including injury, apoptosis, survival, differentiation, cytoskeletal dynamics, and responses to oncogenes and growth factors. These studies suggest that ERK1 and ERK2 compete for upstream mitogen-activated protein kinases (MEK1/2), and a multiplicity of substrates have been identified (8, 9).
Increased ERK1 and ERK2 phosphorylation occurs in MMs (10) and a variety of other cancers, and their activation has been identified as a major survival pathway in several tumor types. Asbestos is also known to activate ERKs through various pathways (2, 4). Recent studies from our lab also show that chemotherapeutic drugs such as doxorubicin (Dox) can activate ERKs (11). Moreover, inhibition of various ERKs can attenuate MM tumor growth in SCID mice (11–13).
ERK5 or Big MAPK, a distinctly different member of the ERK family (14), is strongly activated by EGF and by other receptor tyrosine kinases and is required for cell proliferation and cell cycle progression (15, 16). In human MMs, ERK5 is constitutively activated and further elevated by Dox, and ERK5 silencing attenuates invasion of MMs in vitro and reduction of MM growth (12). Moreover, crocidolite asbestos fibers, the most potent asbestos type in the causation of MM, cause protracted activation of ERK1/2 and ERK5 via EGFR-dependent versus independent pathways in rodent mesothelial and lung epithelial cells (1, 17). We have also demonstrated that hepatocyte growth factor/scatter factor mediates proliferation of human MMs through a phosphatidylinositol 3-kinase (PI3K)/mitogen extracellular signal-regulated kinase 5 (MEK5)/fos-related antigen 1 pathway (18).
The AKT/mammalian target of rapamycin (mTOR) pathway also is frequently activated in MM (19, 20), and inhibition of this pathway retards cell growth and increases sensitivity to conventional chemotherapeutic agents such as cisplatin (21, 22). The ability of AKT to interfere with apoptosis may be central to its ability to favor tumor growth (23, 24). In some studies, antiapoptotic/promalignant status is attributed to a major AKT downstream target, mTOR, suggesting that blockade of mTOR could be an effective anti-cancer strategy; however, blockade of mTOR can enhance AKT activity by feedback mechanisms downstream of mTOR, inducing undesirable compensatory resistance mechanisms (25, 26).
cAMP response element binding protein (CREB) has been classically studied in the physiology of nerve or contractile cells and most recently in some cancers (27–29). Signaling cascades responsible for CREB activation by extracellular stimuli include protein kinase A (PKA), protein kinase C (PKC), Ca2+/calmodulin-dependent kinase (CaM kinases), p90 ribosomal S6 kinase, and ERK1 and ERK2 (30). We first demonstrated that crocidolite asbestos causes CREB activation in human mesothelial cells via EGFR and PKA-dependent pathways (31). Moreover, human MM cell lines and human MM tissue arrays showed high endogenous activation of CREB1 that was further increased by Dox (31).
Because vandetanib (Van) (ZD6474, ZACTIMA) is a novel, orally active agent that inhibits the tyrosine kinase activity of vascular endothelial growth factor receptor-2 (VEGFR-2) and EGFR (32) and has shown significant antitumor activity in various xenograft models of human cancer including MM (32), we hypothesized that several of the multiple signaling pathways observed in MMs could be targeted by this drug. Moreover, we tested the hypothesis that Van might act synergistically with conventional chemotherapeutic drugs in killing of MM cells. We selected Dox for our studies because this DNA intercalating agent is the most successful drug of choice to treat MMs in single-agent studies (33, 34) and is used to treat MM and a number of other neoplasms in combination with other chemotherapeutic agents (35, 36). In studies described here, we performed dose–response toxicity studies with Van and Dox alone and in combination on two well-characterized MM cell lines that are known to be sensitive (HMESO) or resistant (H2373) to Dox (37). We then examined, using Western blot analysis, levels of phosphorylated and total EGFR, ERK1, ERK2, ERK5, AKT, and CREB under these identical circumstances. We show two new (ERK5, CREB) survival pathways activated by Dox in MM cells that are inhibited by coadministration of Van, correlating with decreases in cell viability. We also demonstrate, using RNA interference, that blocking either pathway in combination with Dox or Dox/Van treatment in the chemoresistant H2373 sarcomatoid MM line further increases cell killing. These studies suggest a trimodal approach to therapy of aggressive MMs.
Materials and Methods
MM Lines and Reagents
Epithelioid HMESO cells were characterized by Reale and colleagues (38). Sarcomatoid H2373 MM cells were provided by Dr. Harvey I. Pass (NYU Langone Medical Center, New York, NY). Cells were confirmed as mesothelial using a panel of specific antibodies and were tested periodically for mycoplasma. Cells were maintained in vitro as described previously (12). Van was obtained from LC Laboratories (Woburn, MA) and reconstituted to 25 mM in DMSO. Dox was obtained from Sigma (St. Louis, MO) and reconstituted to a 50 μM stock concentration in water. Solvent controls received DMSO alone. Cells were grown to 80 to 90% confluence and maintained in medium including 0.5% FBS for 24 hours before the addition of agents. Cell viability was studied using Van alone (5 μM), Dox alone (25 μM for HMESO and 100 μM for chemoresistant H2373 cells (37), and Van pretreatment 1 hour before Dox. Both cell lines were evaluated in dose-response studies for Dox toxicity that were used here (37). Twenty-five μM Dox is approximately equal to Dox concentrations in peritoneal fluids (average, 18.4 μM) after intracavitary Dox chemotherapy in patients with mesotheliomas (39). Five to 50 μM Van were administered for 24 hours before trypsinization and counting of cells using a hemocytometer. Images from viability studies were captured using an Olympus IX70 inverted light microscope (Olympus America, Lake Success, NY) with an attached Q Imaging Retiga 2000R digital CCD camera (Advanced Imaging Concepts, Inc., Princeton, NJ).
Western Blots
To measure the activation (phosphorylation) of ERK1, ERK2, ERK5, AKT, CREB, and EGFR after exposure to Van, Dox, and the combination, Western blot analyses were performed as previously described using rabbit polyclonal antibodies specific to total (diluted 1:1,000) and phosphorylated (diluted 1:500) ERK1, ERK2, ERK5, AKT, CREB, and EGFR (Cell Signaling Technology, Danvers, MA) (11). β-Actin (mouse 1:2,000 dilution) was used as a loading control (Abcam, Cambridge, MA). Protein bands were quantified with Quantity One software (Bio-Rad, Hercules, CA). Phosphorylated levels were normalized to total protein levels of each signaling protein.
Transfection and Characterization of Short Hairpin RNA Expressing Cell Lines ERK5 and Short Hairpin RNA Expressing Cell Lines CREB H2373 MM Lines
Using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), confluent H2373 cells were transfected with ERK5, CREB, or scrambled control Sure Silencing Plasmids (four sh constructs per gene per cell line) from SA Biosciences (Valencia, CA). After selection for 14 days in G418-containing medium, clones were screened for inhibition of ERK5 mRNA and CREB mRNA levels as compared with scrambled control (shCon)-transfected clones by using real-time quantitative PCR. Two maximally inhibited clones from short hairpin RNA expressing cell lines (sh) ERK5 and shCREB-transfected H2373 cells were processed by limited dilution to obtain stable cell lines in which ERK5 (12) and CREB (A. Shukla, unpublished observations) were inhibited by more than 70% in comparison to shCon clones, as determined by real-time quantitative PCR and Western blot analyses.
Statistical Analyses
Data were evaluated by one-way ANOVA using the Student-Neuman-Keul’s procedure for adjustment of multiple pairwise comparisons or an unpaired Student’s t test where indicated. All experiments were repeated in duplicate or triplicate.
Results
Synergistic Toxic Effects of Van and Dox on HMESO and H2373 Cells
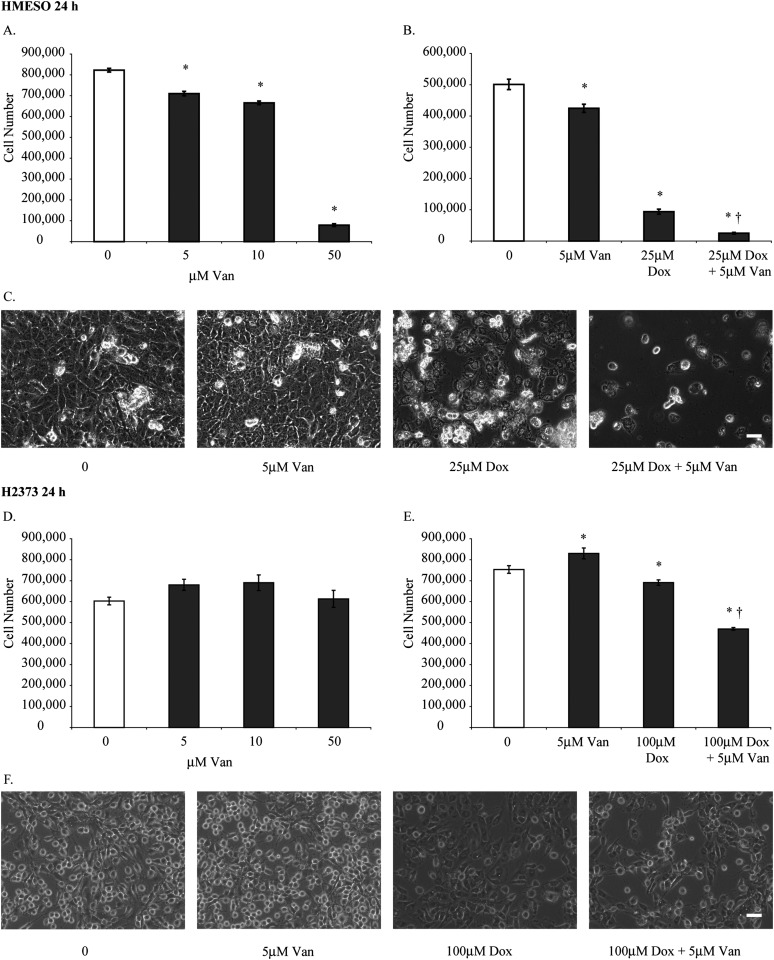
After treating HMESO cells with concentrations of 5 to 50 μM Van, total cell numbers were significantly decreased at all concentrations compared with untreated controls (Figure 1A). The lowest dose of Van (5 μM) was chosen for further experiments with Dox (25 μM) (37), a dose causing a 5-fold decrease in cell numbers, to demonstrate synergy (Figure 1B). The augmentation of toxicity by both agents was also demonstrated dramatically in phase contrast micrographs (Figure 1C). Van alone had no significant effects on the cell viability of H2373 cells in initial dose-response studies (i.e., 5, 10, and 50 μM) (Figure 1D). However, subsequent experiments showed a small but significant increase in cell viability at the lowest dose of Van and a significant decrease in total cell numbers when Dox (100 μM) was added (Figure 1E). These data are in line with the observation that this sarcomatoid line is the most resistant to Dox as established in a panel of primarily epithelioid MMs (37). In both cell lines, a combination of Dox preceded by 5 μM Van synergistically decreased cell viability (P < 0.05) compared with cells exposed to Dox alone (Figures 1B and 1E). Microscopy images correlated with the extent of quantitative cell death in both cell lines (Figures 1C and 1F).
Figure 1.
A combination of doxorubicin (Dox) and vandetanib (Van) is more cytotoxic than either agent alone. (A, D) Two different malignant mesothelioma (MM) cell lines (HMESO and H2373) were treated with 5, 10, and 50 μM Van for 24 hours, and total cell numbers were determined. Results (using both the colorimetric MTS Cell Proliferation Assay and counting of adherent, viable cells at 24 h) of dose-response studies using Dox at a range of concentration from 0.1 to 100 μM concentrations have been previously reported for the HMESO and H2373 (also referred to as PPM Mill) mesothelioma cell lines (11, 37). Based on these results, concentrations of Dox (25 μM for HMESO and 100 μM for the more resistant H2373 line) were selected for studies here. A comparable minimally toxic concentration of Van (5 μM) was used in both lines to demonstrate synergistic effects with Dox based on dose-response data. After exposure to Dox, we used the Apostain assay and LDH assay to show that necrosis and apoptosis occur in a number of MM lines in studies cited above. (B, E) Viability of HMESO and H2373 cells was assessed after treatment with 5 μM Van, 25 μM Dox for HMESO, 100 μM Dox for H2373, or a combination of Dox and Van (25 μM Dox + 5 μM Van for HMESO and 100 μM Dox + 5 μM Van for H2373) for 24 hours. (C, F) Inverted phase microscopy images correlate with total cell numbers in HMESO and H2373 cells after treatment for 24 hours (scale bar = 50 μm). *P ≤ 0.05 as compared with control (0); †P ≤ 0.05 as compared with Dox alone (n = 3 per group/experiment).
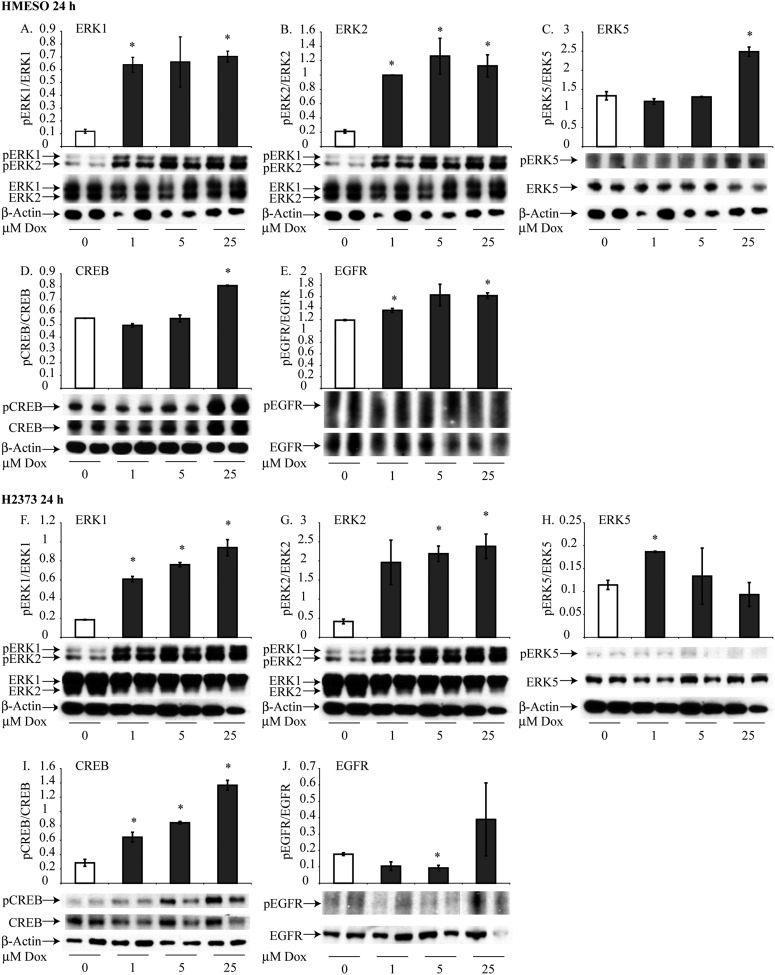
Dox Causes Activation (Phosphorylation) of Multiple Protein Kinases in HMESO and H2373 Cells
HMESO and H2373 cells were treated with 1, 5, and 25 μM Dox for 8 hours (see Figure E1 in the online supplement) or for 24 hours (Figure 2) to determine the activation of ERK1, ERK2, ERK5, CREB, and EGFR by Western blot analyses. Treatment with Dox induced significant increases in phosphorylation of ERK1, ERK2, ERK5, and CREB in a dose-dependent fashion in both cell lines (Figures 2A–2D, 2F–2I). At 8 hours, pEGFR was reduced significantly at 5 and 25 μM Dox in HMESO cells but increased at these concentrations in H2373 cells. At 24 hours, however, phosphorylation of EGFR by Dox was significantly decreased in both cell types (Figures 2E and 2J).
Figure 2.
Dox activates cell signaling proteins and survival factors in HMESO and H2373 cells. Western blot analysis was performed using specific antibodies for each cell signaling protein. Activation of extracellular signal-regulated kinase (ERK)1, ERK2, ERK5, and cyclic AMP response element binding protein (CREB) was determined after treatment with 1, 5, and 25 μM Dox for 24 hours in HMESO cells (A–E) and in H2373 cells (F–J). Data are expressed as the ratio of phosphorylated to unphosphorylated levels of proteins. *P ≤ 0.05 as compared with control (0) (n = 2 per group/experiment). pCREB, phosphorylated CREB; pERK, phosphorylated ERK.
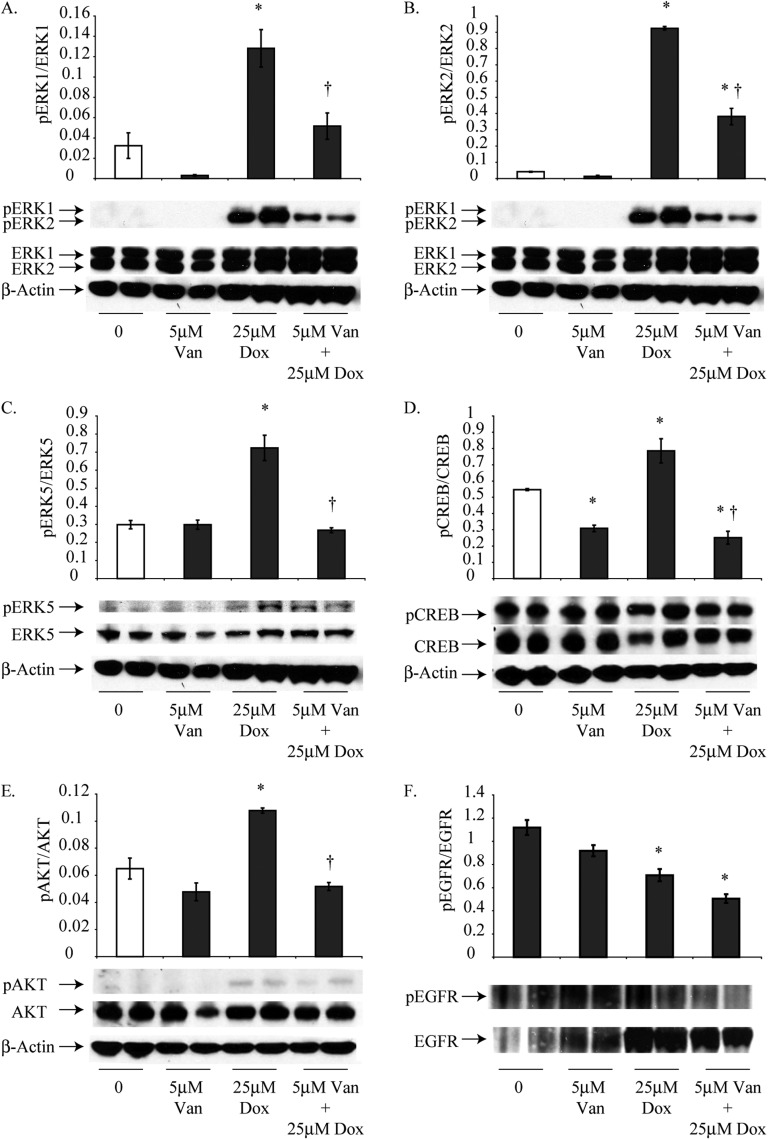
Van Pretreatment Attenuates Dox-Induced Phosphorylation of EGFR, ERK1, ERK2, ERK5, AKT, and CREB
Van alone (5 μM) did not cause significant changes in the phosphorylation of ERK1, ERK2, ERK5, AKT, or EGFR in HMESO cells at 24 hours, although significant decreases in the activation of pCREB/CREB were observed (Figure 3). Treatment with 25 μM Dox alone significantly increased the phosphorylation of ERK1, ERK2, ERK5, CREB, and AKT but decreased phosphorylation of EGFR (Figure 3).
Figure 3.
Van pretreatment attenuates Dox-induced activation of cell survival pathways in epithelioid HMESO cells at 24 hours. Activation of ERK1, ERK2, ERK5, CREB, and AKT were assessed by Western blot analysis after treatment with 5 μM Van, 25 μM Dox, and the combination for 24 hours. (A–E) Combination treatment significantly reduced these survival signals relative to Dox induction levels. (F) Dox (25 μM) alone and in combination with 5 μM Van decreased activation of EGFR. *P ≤ 0.05 as compared with control (0), †P ≤ 0.05 as compared to Dox alone (n = 2 per group/experiment in duplicate).
At 8 hours, the combination of Dox with Van significantly decreased the phosphorylation of ERK1 and ERK2 contrary to increases observed with Dox alone (25 μM) in HMESO cells (Figure E2). No significant differences in the phosphorylation of ERK5, AKT, and CREB occurred with Dox or Van in combination as compared with Dox alone (Figure E2). However, combination therapy reduced phosphorylated ERK5 by Van (5 μM) at 8 hours. At 24 hours, a combination of Dox and Van significantly decreased the phosphorylation of ERK1, ERK2, ERK5, CREB, and AKT relative to HMESO cells treated with 25 μM Dox alone (Figure 3).
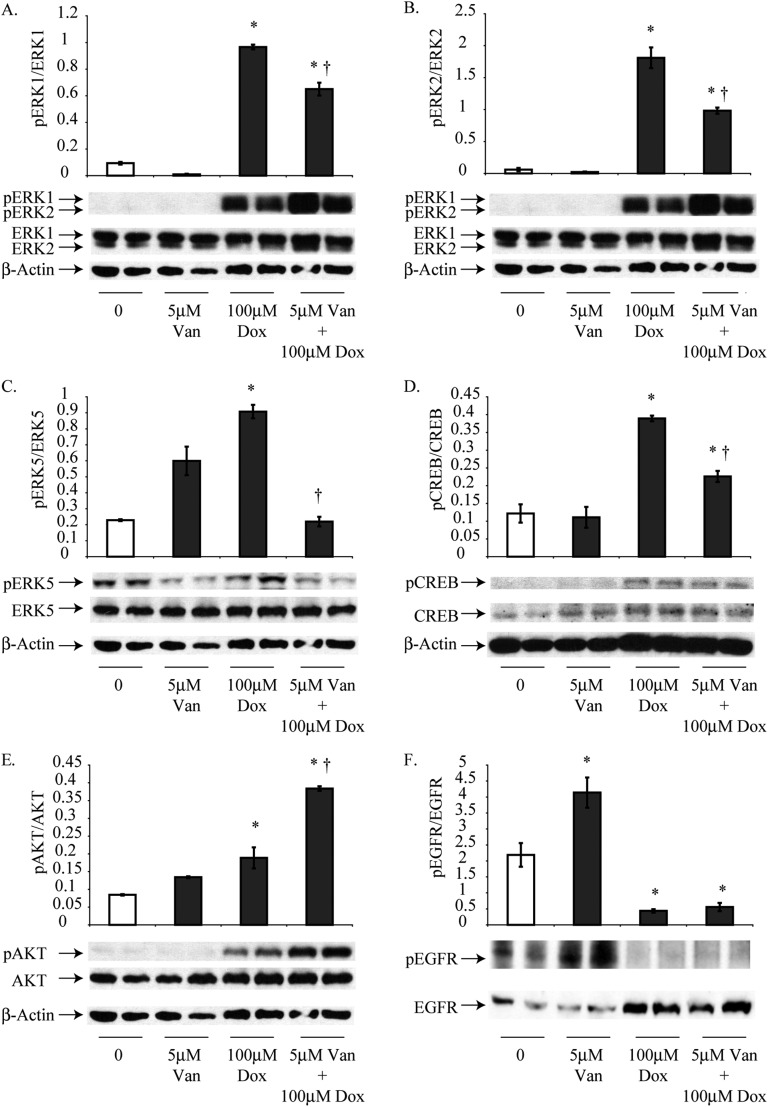
In H2373 cells, treatment for 24 hours with Dox alone (100 μM) significantly increased the phosphorylation of ERK1, ERK2, ERK5, CREB, and AKT, whereas EGFR phosphorylation was significantly decreased relative to untreated H2373 cells (Figure 4). Treatment with 5 μM Van alone did not cause any significant differences in phosphorylation of EGFR, but significant decreases in the phosphorylation of all downstream signaling pathway proteins were observed in combination groups when compared with cells exposed to Dox alone. In contrast, AKT showed an increase in phosphorylation under these conditions (Figure 4).
Figure 4.
Van pretreatment inhibits Dox-induced activation of cell signaling and survival cascades in sarcomatoid H2373 cells at 24 hours. (A–D) In H2373 cells, activation of ERK1, ERK2, ERK5, and CREB was assessed by Western blot analysis after treatment with 100 μM Dox for 24 hours. (E) A combination of 5 μM Van and 100 μM Dox for 24 hours increased the activation of AKT compared with 100 μM Dox treatment. (F) Treatment with 100 μM Dox alone or Dox and Van together significantly decreased levels of pEGFR (F). *P ≤ 0.05 as compared with control (0); †P ≤ 0.05 as compared with Dox alone (n = 2 per group/experiment in duplicate).
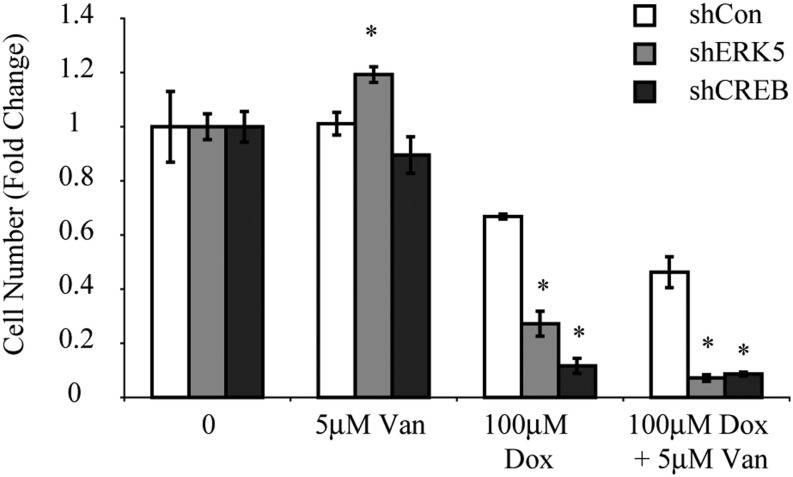
ERK5 and CREB Silencing Enhance MM Cell Toxicity
To further understand the role of ERK5 and CREB in MM cell toxicity in chemoresistant cells, sarcomatoid H2373 cell lines silenced for ERK5 or CREB expression (shERK5 or shCREB) were created and compared with a nontargeted control (shCon) line. Although a modest but significant increase in viability was observed in the shERK5 line exposed to Van alone, both shERK5 and shCREB cell lines showed significant decreases in cell survival (Figure 5). This observation suggests that these pathways are involved in cell survival and chemoresistance.
Figure 5.
Silencing ERK5 or CREB shows enhanced cell toxicity in H2373 MMs. A significant decrease in cell numbers after treatment with 100 μM Dox or a combination of 100 μM Dox and 5 μM Van was observed compared with shCon values. *P ≤ 0.05 as compared with control (0) (n = 3 per group/experiment). sh, short hairpin RNA expressing cell lines.
Discussion
Our study reveals that toxic (as in HMESO and H2373 cells) and nontoxic (as in H2373 cells) concentrations of Dox cause increases in phosphorylation of ERK1, ERK2, ERK5, AKT, and CREB, indicating several survival pathways that are up-regulated by this commonly used chemotherapeutic agent. The combination of Van and Dox did not affect EGFR phosphorylation synergistically in HMESO cells compared with Dox alone but decreased the phosphorylation of all downstream survival pathways (excluding AKT in resistant H2373 cells) in comparison to cells treated with Dox alone (summarized in Table 1). In line with our results, a search of the literature reveals that Van activated AKT and induced cancer “side populations” of cells with multiresistance capabilities in a salivary gland tumor cell line (40).
Table 1.
Significant Effects of Vandetanib and Doxorubicin Alone and in Combination on the Phosphorylation of Different Cell Signaling Pathways as Observed by Western Blot Analyses (24 h)
| Cells | Treatment | pERK1 | pERK2 | pERK5* | pCREB* | pAKT | pEGFR |
|---|---|---|---|---|---|---|---|
| HMESO | Van 5 μM | ↓† | |||||
| Dox 25 μM | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | |
| Van 5 μM + Dox 25 μM‡ | ↓ | ↓ | ↓ | ↓ | ↓ | ||
| H2373 | Van 5 μM | ||||||
| Dox 100 μM | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | |
| Van 5 μM + Dox 100 μM | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ |
Definition of abbreviations: Dox, doxorubicin; pAKT, phosphorylated AKT; pCREB, phosphorylated cyclic AMP response element binding protein; pEGFR, phosphorylated epidermal growth factor receptor; pERK, phosphorylated extracellular signal-regulated kinase; Van, andetanib.
pERK5 and pCREB present novel findings for this study.
Arrows represent P < 0.05 increases (up arrows) or decreases (down arrows).
All comparisons with Van + Dox are with the Dox alone group. Comparisons involving individual agents are with the control group.
This study revealed two new pathways, ERK5 and CREB, that augment cell survival and are inhibited by Dox and Van in combination. The former pathway is particularly relevant because synthetic ERK inhibitors are being used in clinical trials in a number of cancers (41) and because an ERK5-specific inhibitor has recently been developed (42). Previously published research demonstrated that Van was an antitumor agent that blocks two pathways, EGFR and VEGFR-2 signaling (32). However, it was also reported that VEGFR-2 was not found in MM cells affected by Van (43, 44). Therefore, we hypothesized that Van likely exerts its effects by inhibiting other survival and chemoresistance pathways. We demonstrate for the first time the effect of Van on the signaling/transcription factor pathways, ERK5 and CREB, and show that Van modulates these and other critical survival pathways in response to Dox, which can be linked to their synergistic effects on MM cell toxicity.
EGFR activation is known to be a key driver of cell proliferation in all asbestos-related diseases, and EGFR is present in the majority of MM cells (45). Although Van significantly decreases EGFR phosphorylation in some studies (43, 44, 46), the use of Van alone did not in this study. One possible problem in interpreting the data is that Van also up-regulates the EGFR ligands TGF-α and EGF in tumor tissues (46).
The AKT signaling pathway is frequently activated in MM cells, and inhibition of this pathway hinders cell growth and increases sensitivity to the chemotherapeutic agent cisplatin (22). In this study, the use of Dox with Van significantly decreased the activation of AKT by Dox alone in epithelioid HMESO cells. On the contrary, in the H2373 cell line, the combination of Van and Dox increased AKT activation. Sarcomatoid H2373 cells are also known to be resistant to Dox (37). Because the activation of AKT was further increased with both Van and Dox, perhaps the greater resistance of H2373 cells to chemotherapy is linked to this pathway because patients with sarcomatoid MM exhibit high endogenous levels of AKT (22).
In addition to other functional changes in neoplasia, ERK activation is linked to protection of cells from drug-induced cell death (47, 48). Our previous work confirms that increased activation of ERK1 and ERK2 after the addition of Dox is linked to attempted cell survival of MMs (11, 13). Dox and Van significantly decreased the activation of ERK1 and ERK2 observed in response to Dox alone in HMESO and H2373 cells, suggesting more effective drug-induced MM cell death after using these agents in combination.
Our results suggest the following scenario of events. A main mechanism by which Dox inhibits cancer growth is intercalation with DNA, thereby leading to DNA damage and/or inhibition of cell replication. However, as a consequence of Dox toxicity, MM cells up-regulate prosurvival signals such as ERK1, ERK2, ERK5, CREB, and AKT, events coinciding with changes in activation of EGFR that may be MM type specific. This observation suggests that there is an EGFR-independent pathway by which ERK1, ERK2, ERK5, CREB, and AKT are activated in the presence of Dox. In MM cells receiving a combination of Van and Dox, levels of EGFR activation are not further reduced in HMESO cells, but levels of other downstream effector molecules (with the exception of AKT in sarcomatoid H2373 cells) are brought back to (or below) baseline levels. A search of the literature reveals no reports on Dox-induced effects on phosphorylation of EGFR in MMs or other tumors.
In conclusion, the present study reveals that Van is capable of interrupting several different cell survival signaling pathways that are stimulated by Dox, including two novel, previously unreported pathways, ERK5 and CREB. Inhibition of these pathways correlates with an increased MM cell sensitivity to Dox and Dox/Van toxicity in a trimodal combination therapy approach that may be merited in chemoresistant MMs as well as other hyperproliferating cells in lung cancers and asbestosis. This approach is bolstered by the encouraging results of preclinical studies using Van, carboplatin, and pemetrexed in MM cells (43) and Van and DNA damaging agents in leukemia cell lines (49). A very recent report on a randomized phase 2 study for patients with advanced non-small cell lung cancer evaluated the effects of a combination of docetxel, carboplatin, and Van followed by maintenance therapy with Van or placebo (50). This study suggests a role for Van in amelioration of further tumor growth and progression-free survival.
Acknowledgments
Acknowledgments
The authors thank Dr. Harvey I. Pass and Dr. Joseph R. Testa for providing MM cell lines and Jennifer L. Díaz for manuscript editing.
Footnotes
This research was supported by grants from the Mesothelioma Applied Research Foundation (A.S., B.T.M.) and by training grant T32 ES007122 from the National Institute of Environmental Health Sciences (B.T.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0373TR on June 18, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zanella CL, Posada J, Tritton TR, Mossman BT. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996;56:5334–5338. [PubMed] [Google Scholar]
- 2.Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J Toxicol Environ Health B Crit Rev. 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009;20:935–944. doi: 10.1007/s10552-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 4.Mossman BT, Shukla A, Heintz NH, Verschraegen CF, Thomas A, Hassan R. New insights into understanding the mechanisms, pathogenesis, and management of malignant mesotheliomas. Am J Pathol. 2013;182:1065–1077. doi: 10.1016/j.ajpath.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bicknell R. The realisation of targeted antitumour therapy. Br J Cancer. 2005;92:S2–S5. doi: 10.1038/sj.bjc.6602602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 7.Mattingly RR, Milstein ML, Mirkin BL. Down-regulation of growth factor-stimulated MAP kinase signaling in cytotoxic drug-resistant human neuroblastoma cells. Cell Signal. 2001;13:499–505. doi: 10.1016/s0898-6568(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 8.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 9.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 10.de Melo M, Gerbase MW, Curran J, Pache JC. Phosphorylated extracellular signal-regulated kinases are significantly increased in malignant mesothelioma. J Histochem Cytochem. 2006;54:855–861. doi: 10.1369/jhc.5A6807.2006. [DOI] [PubMed] [Google Scholar]
- 11.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Pass HI, Carbone M, Testa JR, Mossman BT. Blocking of ERK1 and ERK2 sensitizes human mesothelioma cells to doxorubicin. Mol Cancer. 2010;9:314. doi: 10.1186/1476-4598-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla A, Miller JM, Cason C, Sayan M, MacPherson MB, Beuschel SL, Hillegass J, Vacek PM, Pass HI, Mossman BT. Extracellular signal-regulated kinase 5: a potential therapeutic target for malignant mesotheliomas. Clin Cancer Res. 2013;19:2071–2083. doi: 10.1158/1078-0432.CCR-12-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla A, Hillegass JM, MacPherson MB, Beuschel SL, Vacek PM, Butnor KJ, Pass HI, Carbone M, Testa JR, Heintz NH, et al. ERK2 is essential for the growth of human epithelioid malignant mesotheliomas. Int J Cancer. 2011;129:1075–1086. doi: 10.1002/ijc.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 15.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD. Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature. 1998;395:713–716. doi: 10.1038/27234. [DOI] [PubMed] [Google Scholar]
- 17.Scapoli L, Ramos-Nino ME, Martinelli M, Mossman BT. Src-dependent ERK5 and Src/EGFR-dependent ERK1/2 activation is required for cell proliferation by asbestos. Oncogene. 2004;23:805–813. doi: 10.1038/sj.onc.1207163. [DOI] [PubMed] [Google Scholar]
- 18.Ramos-Nino ME, Blumen SR, Sabo-Attwood T, Pass H, Carbone M, Testa JR, Altomare DA, Mossman BT. HGF mediates cell proliferation of human mesothelioma cells through a PI3K/MEK5/Fra-1 pathway. Am J Respir Cell Mol Biol. 2008;38:209–217. doi: 10.1165/rcmb.2007-0206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cedrés S, Montero MA, Martinez P, Martinez A, Rodríguez-Freixinós V, Torrejon D, Gabaldon A, Salcedo M, Ramon Y Cajal S, Felip E. Exploratory analysis of activation of PTEN-PI3K pathway and downstream proteins in malignant pleural mesothelioma (MPM) Lung Cancer. 2012;77:192–198. doi: 10.1016/j.lungcan.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Varghese S, Chen Z, Bartlett DL, Pingpank JF, Libutti SK, Steinberg SM, Wunderlich J, Alexander HR., Jr Activation of the phosphoinositide-3-kinase and mammalian target of rapamycin signaling pathways are associated with shortened survival in patients with malignant peritoneal mesothelioma. Cancer. 2011;117:361–371. doi: 10.1002/cncr.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman ML, Esposito JM, Yeap BY, Sugarbaker DJ. Combined treatment with cisplatin and sirolimus to enhance cell death in human mesothelioma. J Thorac Cardiovasc Surg. 2010;139:1233–1240. doi: 10.1016/j.jtcvs.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Altomare DA, You H, Xiao GH, Ramos-Nino ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB, Testa JR. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene. 2005;24:6080–6089. doi: 10.1038/sj.onc.1208744. [DOI] [PubMed] [Google Scholar]
- 23.Barbone D, Yang TM, Morgan JR, Gaudino G, Broaddus VC. Mammalian target of rapamycin contributes to the acquired apoptotic resistance of human mesothelioma multicellular spheroids. J Biol Chem. 2008;283:13021–13030. doi: 10.1074/jbc.M709698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KU, Wilson SM, Abayasiriwardana KS, Collins R, Fjellbirkeland L, Xu Z, Jablons DM, Nishimura SL, Broaddus VC. A novel in vitro model of human mesothelioma for studying tumor biology and apoptotic resistance. Am J Respir Cell Mol Biol. 2005;33:541–548. doi: 10.1165/rcmb.2004-0355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 26.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal S, Kim SW, Ryu SH, Chung WC, Koo JS. Growth suppression of lung cancer cells by targeting cyclic AMP response element-binding protein. Cancer Res. 2008;68:981–988. doi: 10.1158/0008-5472.CAN-06-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhabra A, Fernando H, Watkins G, Mansel RE, Jiang WG. Expression of transcription factor CREB1 in human breast cancer and its correlation with prognosis. Oncol Rep. 2007;18:953–958. [PubMed] [Google Scholar]
- 29.Shankar DB, Cheng JC, Kinjo K, Federman N, Moore TB, Gill A, Rao NP, Landaw EM, Sakamoto KM. The role of CREB as a proto-oncogene in hematopoiesis and in acute myeloid leukemia. Cancer Cell. 2005;7:351–362. doi: 10.1016/j.ccr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 31.Shukla A, Bosenberg MW, MacPherson MB, Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR, Mossman BT. Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis. Am J Pathol. 2009;175:2197–2206. doi: 10.2353/ajpath.2009.090400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciardiello F, Caputo R, Damiano V, Caputo R, Troiani T, Vitagliano D, Carlomagno F, Veneziani BM, Fontanini G, Bianco AR, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546–1556. [PubMed] [Google Scholar]
- 33.Bowman RV, Manning LS, Davis MR, Robinson BW. Chemosensitivity and cytokine sensitivity of malignant mesothelioma. Cancer Chemother Pharmacol. 1991;28:420–426. doi: 10.1007/BF00685817. [DOI] [PubMed] [Google Scholar]
- 34.Isobe H, Wellham L, Sauerteig A, Sridhar KS, Ramachandran C, Krishan A. Doxorubicin retention and chemoresistance in human mesothelioma cell lines. Int J Cancer. 1994;57:581–585. doi: 10.1002/ijc.2910570423. [DOI] [PubMed] [Google Scholar]
- 35.Saxena A, Chua TC. Results of systemic pemetrexed-based combination chemotherapy versus cytoreductive surgery and hyperthermic intraperitoneal cisplatin and doxorubicin on survival in malignant peritoneal mesothelioma. Lung Cancer. 2009;66:269–270. doi: 10.1016/j.lungcan.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Scherpereel A, Berghmans T, Lafitte JJ, Colinet B, Richez M, Bonduelle Y, Meert AP, Dhalluin X, Leclercq N, Paesmans M, et al. European Lung Cancer Working Party (ELCWP) Valproate-doxorubicin: promising therapy for progressing mesothelioma. A phase II study. Eur Respir J. 2011;37:129–135. doi: 10.1183/09031936.00037310. [DOI] [PubMed] [Google Scholar]
- 37.Hillegass JM, Blumen SR, Cheng K, MacPherson MB, Alexeeva V, Lathrop SA, Beuschel SL, Steinbacher JL, Butnor KJ, Ramos-Niño ME, et al. Increased efficacy of doxorubicin delivered in multifunctional microparticles for mesothelioma therapy. Int J Cancer. 2011;129:233–244. doi: 10.1002/ijc.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reale FR, Griffin TW, Compton JM, Graham S, Townes PL, Bogden A. Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res. 1987;47:3199–3205. [PubMed] [Google Scholar]
- 39.Van der Speeten K, Stuart OA, Mahteme H, Sugarbaker PH. A pharmacologic analysis of intraoperative intracavitary cancer chemotherapy with doxorubicin. Cancer Chemother Pharmacol. 2009;63:799–805. doi: 10.1007/s00280-008-0800-0. [DOI] [PubMed] [Google Scholar]
- 40.Fujishiro Y, Tonogi M, Ochiai H, Matsuzaka K, Yamane GY, Azuma T. The receptor tyrosine kinase inhibitor vandetanib activates Akt and increases side population in a salivary gland tumor cell line (A253) Int J Oncol. 2012;41:362–368. doi: 10.3892/ijo.2012.1434. [DOI] [PubMed] [Google Scholar]
- 41.Morris EJ, Jha S, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–750. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 42.Yang Q, Lee JD. Targeting the BMK1 MAP kinase pathway in cancer therapy. Clin Cancer Res. 2011;17:3527–3532. doi: 10.1158/1078-0432.CCR-10-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giovannetti E, Zucali PA, Assaraf YG, Leon LG, Smid K, Alecci C, Giancola F, Destro A, Gianoncelli L, Lorenzi E, et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br J Cancer. 2011;105:1542–1553. doi: 10.1038/bjc.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nutt JE, O’Toole K, Gonzalez D, Lunec J. Growth inhibition by tyrosine kinase inhibitors in mesothelioma cell lines. Eur J Cancer. 2009;45:1684–1691. doi: 10.1016/j.ejca.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Jänne PA, Taffaro ML, Salgia R, Johnson BE. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res. 2002;62:5242–5247. [PubMed] [Google Scholar]
- 46.Inoue K, Torimura T, Nakamura T, Iwamoto H, Masuda H, Abe M, Hashimoto O, Koga H, Ueno T, Yano H, et al. Vandetanib, an inhibitor of VEGF receptor-2 and EGF receptor, suppresses tumor development and improves prognosis of liver cancer in mice. Clin Cancer Res. 2012;18:3924–3933. doi: 10.1158/1078-0432.CCR-11-2041. [DOI] [PubMed] [Google Scholar]
- 47.Seidman R, Gitelman I, Sagi O, Horwitz SB, Wolfson M. The role of ERK 1/2 and p38 MAP-kinase pathways in taxol-induced apoptosis in human ovarian carcinoma cells. Exp Cell Res. 2001;268:84–92. doi: 10.1006/excr.2001.5262. [DOI] [PubMed] [Google Scholar]
- 48.Sheridan C, Brumatti G, Martin SJ. Oncogenic B-RafV600E inhibits apoptosis and promotes ERK-dependent inactivation of Bad and Bim. J Biol Chem. 2008;283:22128–22135. doi: 10.1074/jbc.M800271200. [DOI] [PubMed] [Google Scholar]
- 49.Macy ME, DeRyckere D, Gore L. Vandetanib mediates anti-leukemia activity by multiple mechanisms and interacts synergistically with DNA damaging agents. Invest New Drugs. 2012;30:468–479. doi: 10.1007/s10637-010-9572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aisner J, Manola JB, Dakhil SR, Stella PJ, Sovak MA, Schiller JH. Vandetanib plus chemotherapy for induction followed by vandetanib or placebo as maintenance for patients with advanced non-small-cell lung cancer: a randomized phase 2 PrECOG study (PrE0501) J Thorac Oncol. 2013;8:1075–1083. doi: 10.1097/JTO.0b013e3182937317. [DOI] [PubMed] [Google Scholar]