Abstract
Secreted phosphoprotein 1 (Spp1) is located within quantitative trait loci associated with lung function that was previously identified by contrasting C3H/HeJ and JF1/Msf mouse strains that have extremely divergent lung function. JF1/Msf mice with diminished lung function had reduced lung SPP1 transcript and protein during the peak stage of alveologenesis (postnatal day [P]14–P28) as compared with C3H/HeJ mice. In addition to a previously identified genetic variant that altered runt-related transcription factor 2 (RUNX2) binding in the Spp1 promoter, we identified another promoter variant in a putative RUNX2 binding site that increased the DNA protein binding. SPP1 induced dose-dependent mouse lung epithelial-15 cell proliferation. Spp1(−/−) mice have decreased specific total lung capacity/body weight, higher specific compliance, and increased mean airspace chord length (Lm) compared with Spp1(+/+) mice. Microarray analysis revealed enriched gene ontogeny categories, with numerous genes associated with lung development and/or respiratory disease. Insulin-like growth factor 1, Hedgehog-interacting protein, wingless-related mouse mammary tumor virus integration site 5A, and NOTCH1 transcripts decreased in the lung of P14 Spp1(−/−) mice as determined by quantitative RT-PCR analysis. SPP1 promotes pneumocyte growth, and mice lacking SPP1 have smaller, more compliant lungs with enlarged airspace (i.e., increased Lm). Microarray analysis suggests a dysregulation of key lung developmental transcripts in gene-targeted Spp1(−/−) mice, particularly during the peak phase of alveologenesis. In addition to its known roles in lung disease, this study supports SPP1 as a determinant of lung development in mice.
Keywords: osteopontin, chronic obstructive pulmonary disease, asthma, emphysema, pulmonary fibrosis
Clinical Relevance
Inappropriate lung development is a risk factor for lower basal pulmonary function as well as defective repair and remodeling processes after lung injury, thereby predisposing individuals to asthma, pulmonary fibrosis, and chronic obstructive pulmonary diseases (COPD). This study examines the role of secreted phosphoprotein 1 (SPP1), a protein previously associated with pulmonary fibrosis and COPD, in lung development in mice. A mouse strain with decreased lung function has decreased lung SPP1 during postnatal alveologenesis. Mice have a genetic variant in the Spp1 promoter that is near a similar transcription factor binding site that is variant in humans. Spp1-deficent mice have smaller alveoli and decreased lung function.
Chronic lung diseases are leading causes of death worldwide (1). Impaired lung development is associated with lower basal pulmonary function and with defective repair and remodeling processes after lung injury, thereby predisposing individuals to chronic lung disease (2–7). Recently, the molecular pathways of lung development have been described (8, 9), and genes associated with lung function have been identified by genome-wide association studies (GWAS) (10–14). However, genetic variants in significant loci explained only a modest portion of the variance for FEV1/FVC (15, 16). Thus, much of the heritability remains unexplained by individual variants identified in GWAS, which is common with complex phenotypes (17, 18). In addition, the functional consequence of these genes and the downstream effectors of lung function have not been fully explored.
To further address the genetics of lung development and function, we used a diverse panel of inbred mice (19), a model organism with an extensive genetic architecture. We previously identified several quantitative trait loci (QTL) for lung function in mice by contrasting two strains (C3H/HeJ versus JF1/Msf) with extremely divergent pulmonary function (e.g., total lung capacity [TLC] of C3H/HeJ = 1,443 ± 30 μl and JF1/Msf = 874 ± 17 μl) (19, 20). We had previously identified candidate genes located in QTL on various regions of mouse chromosome 5, including superoxide dismutase 3, extracellular (Sod3) (21, 22), and c-Kit oncogene (Kit) (23), as determinants of dead space volume and lung compliance (CL), respectively. In children, we found that SOD3 single-nucleotide polymorphisms (SNPs) were associated with decreased FEV1 and maximal expiratory flow at 25% volume (22). In adults, SOD3 SNPs have been associated with lower lung function (24, 25) and increased risk of developing chronic obstructive pulmonary disease (COPD) (26). These findings support the rapid identification of candidate genes in mice that can later be tested in human populations.
In this study we sought to determine whether secreted phosphoprotein 1 (Spp1, a.k.a. osteopontin) is a functional candidate gene for lung development in mice. Spp1 is located within another QTL associated with lung function on mouse chromosome 5 bounded by markers D5Mit20 to D5Mit403 (97.8–106.2 Mbp) (20, 21), which is syntenic to human chromosome 4 (81.8–90.2 Mbp). SPP1 has been associated with chronic lung diseases, including pulmonary fibrosis (27) and COPD (28). An approximately 44 kD glycosylated phosphoprotein, SPP1, is commonly found in adhesive bone matrix protein. It is also recognized as a key cytokine involved in immune cell recruitment and type-1 (Th1) cytokine expression at sites of inflammation (29, 30) and as a mediator of tissue repair and remodeling (31, 32). Past studies on SPP1 focused mainly on its association with bone metabolism, inflammation, and cancer; however, the role of SPP1 in lung development is unknown.
In this study we examined lung SPP1 expression in mice and found strain-specific differences during development. Previously, Shen and Christakos (33) reported that the mouse Spp1 promoter contained functional runt-related transcription factor 2 (RUNX2) (−136 to −130 bp from the transcription start site) and vitamin D response element (−757 to −743) binding sites that cooperatively regulate transcriptional activation by 1,25-dihydroxyvitamin D3 [1,25(OH) D3]. In cells transfected with hes family bHLH transcription factor 1 (a.k.a. hairy and enhancer of split 1 [HES1]), basal and 1,25(OH) D3-induced SPP1 transcripts increased, indicating involvement of the NOTCH1 pathway. Subsequently, Sowa and colleagues (34) PCR amplified and sequenced the mouse Spp1 promoter in the C3H/HeJ and compared this sequence with the promoter of the reference C57BL/6J strain. One variant, a 13-bp insertion (rs234069704) at position −130 (5′-TTTTTTTTTTTTA-3′), was located at the 3′ end of the RUNX2 binding site. This insertion increased transcriptional responsiveness to RUNX2 in the C3H/HeJ promoter as compared with that of the C57BL/6J promoter. Based on these studies, we further examined Spp1 promoter polymorphisms in mice.
Materials and Methods
Detailed methods are provided in the online supplement. Briefly, studies were approved by the Bavarian Animal Research Authority and by the IACUC of the University of Pittsburgh. Mice (C3H/HeJ, JF1/Msf, Spp1(−/−) [B6.129S6(Cg)-Spp1tm1Blh/J], and Spp1(+/+) [C57BL/6J]) were purchased from Jackson Laboratory (Bar Harbor, ME). Quantitative RT-PCR (qRT-PCR) was used to determine lung SPP1 transcripts using the 2−ΔΔCT method normalized to actin, β (ACTB) as described previously (21). ELISA was used to determine lung SPP1 protein levels. Mouse lung epithelial (MLE)-15 cells are an immortalized cell line obtained from transgenic mice containing the simian virus 40 large T antigen under the transcriptional control of the human surfactant protein C promoter (35, 36). To measure the effect of SPP1 on cell proliferation, subconfluent MLE15 cells were serum deprived for 24 hours, 0 (control) or 1 to 4 μg/ml SPP1 was added to the culture medium, and growth was assessed at 48 hours using an alamarBlue (Life Technologies, Grand Island, NY) cell viability assay. To assess the effects of SNPs on the binding of nuclear protein, PCR amplification of the Spp1 promoter region was performed using genomic DNA from C3H/HeJ and JF1/Msf mice and sequenced in forward and reverse directions (Sequiserve, Vaterstetten, Germany). Two of the identified SNPs were used for an electrophoretic mobility shift assay (EMSA) performed using nuclear protein extracts from MLE15 cells. Double-stranded 25-mer oligonucleotides were prepared by annealing complementary synthetic oligonucleotides corresponding to the Spp1 promoter region containing G/T rs264140167 or A/G rs47003578 alleles. Lung function was measured in 27 strains of inbred mice (females, 13–17 wk; n = 252) and Spp1(−/−) and strain-, sex-, and age-matched control Spp1(+/+) mice as described (19, 20, 37). To assess lung morphology, mean airspace chord length (Lm) was measured from images to estimate the alveolar size of Spp1(−/−) mice and compared with strain-, sex-, and age-matched Spp1(+/+) as described (22). For immnunohistochemical localization, Spp1(+/+) lung sections were stained using a goat anti-mouse SPP1 antibody (AF-808; R&D Systems, Inc., Pittsburgh, PA) and biotinylated horse anti-goat secondary antibody (1:200 dilution) (Vector Laboratories, Inc., Burlingame, CA). Lung transcript levels were measured by microarray (Whole Mouse Genome Kit 4 × 44K; Agilent Technologies, Santa Clara, CA) comparing postnatal day (P)14 Spp1(−/−) with P14 Spp1(+/+) and P28 Spp1(−/−) with P28 Spp1(+/+) mice. P14 and P28 were chosen based on reduced SPP1 transcript expression pattern in JF1/Msf lungs compared with C3H/HeJ during peak phase of alveologenesis (P14) and completion of alveologenesis (P28). In addition, insulin-like growth factor 1 (IGF1), wingless-related mouse mammary tumor virus integration site 5A (WNT5A), Hedgehog-interacting protein (HHIP), notch 1 (NOTCH1), CD44 antigen (CD44) transcripts were assessed by qRT-PCR using lung RNA from P14 or P28 Spp1(−/−) or from P14 or P28 Spp1(+/+) mice. Data are presented as mean values of n observations ± the standard error (SE). Group comparisons were performed using ANOVA and all pairwise comparisons procedure (Holm-Sidak method) (Plot 11.0 software; Sigma). Significant differences in transcript levels for the microarray data were analyzed using ANOVA (Partek Genomics Suite; Partek, St. Louis, MO).
Results
Lung SPP1 Transcript and Protein Expression
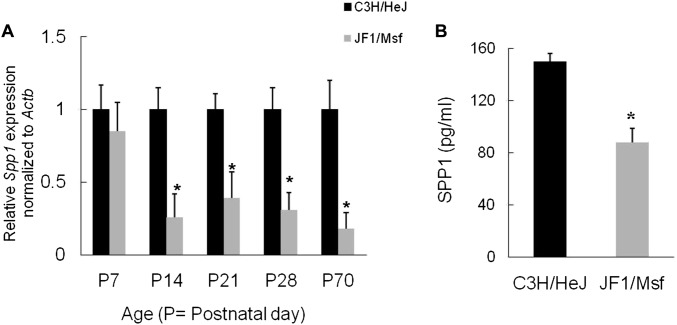
Lung SPP1 transcripts decreased in JF1/Msf mice as compared with C3H/HeJ mice during various stages of postnatal lung development between P14 and P70 (Figure 1A). At P7, lung SPP1 transcripts in JF1/Msf mice were not significantly different than in C3H/HeJ mice. However, from P14 onward, lung SPP1 transcripts decreased in JF1/Msf as compared with C3H/HeJ mice. At P28, lung SPP1 protein decreased in JF1/Msf as compared with C3H/HeJ mice (Figure 1B).
Figure 1.
Lung secreted phosphoprotein 1 (SPP1) transcript and protein decreased in JF1/Msf mice as compared with C3H/HeJ mice. (A) During postnatal lung development (i.e., postnatal day [P]7–P70), lung SPP1 transcript decreased in JF1/Msf mice as compared with C3H/HeJ mice. Previously we determined that JF1/Msf mice have diminished lung function as compared with C3H/HeJ mice (20, 21). Reduced transcripts (∼ 4-fold) were first noted at P14, which is the peak stage of alveologenesis. (B) Lung SPP1 protein decreased 1.7-fold in P28 JF1/Msf mice as compared with P28 C3H/HeJ mice. Values are mean ± SE (n = 5 mice/strain). Statistical significance (*P < 0.05) was determined by ANOVA and by the all pairwise comparisons procedure (Holm-Sidak method).
Spp1 Promoter Analysis
The mouse Spp1 promoter contained a functional RUNX2 binding site (−136 to −130) (33). A 13-bp insertion (rs234069704) at position −130 (5′-TTTTTTTTTTTTA-3′) was located at the 3′ end of this binding site that increases transcriptional responsiveness to RUNX2 in the C3H/HeJ promoter as compared with that of the C57BL/6J promoter (34). To further analyze the mouse Spp1 promoter, approximately 700-bp fragments 5′ from the transcription start site of the mouse Spp1 gene were PCR amplified using the JF1/Msf or C3H/HeJ DNA as a template and sequenced. These sequences were aligned to the reference sequence (obtained from C57BL/6J) (see Figure E1 in the online supplement), and six genetic variants (four single SNPs and two insertions) were identified that differed between JF1/Msf and C3H/HeJ mice (see Table E1). Like the reference C57BL/6J, the JF1/Msf promoter lacks the 13-bp insertion rs234069704. The variation in sequence was then analyzed using Matinspector (38) to identify which of the other four genetic variants could alter putative transcriptional binding sites. Two of the identified SNPs at position −158 (rs264140167) and −198 (rs47003578) could alter putative binding sites.
Sequence information of these two SNPs was used to generate 25-mer biotinylated oligonucleotide probes for EMSA of nuclear protein extract prepared from MLE-15 cells. SNP rs264140167 (−158 nucleotides from the transcription start site) in the Spp1 promoter region alters the nuclear protein–target DNA binding capacity. The 25-mer probes (−144 to −168 bp) containing C3H/HeJ T allele in the middle of the biotinylated oligonucleotide increased the DNA protein binding (i.e., the C3H/HeJ T allele formed slow migrating complexes and enhanced the intensity of a faster migrating complex compared with the JF1/Msf G allele) (Figure 2). The C3H/HeJ T allele forms an additional putative RUNX2 binding site not present in the JF1/Msf G allele. No difference in protein binding was noted in the EMSA when probes were generated from the rs47003578 SNP (JF1/Msf G allele versus C3H/HeJ A allele), which is located −198 nucleotides from the transcription start site (Figure E2).
Figure 2.
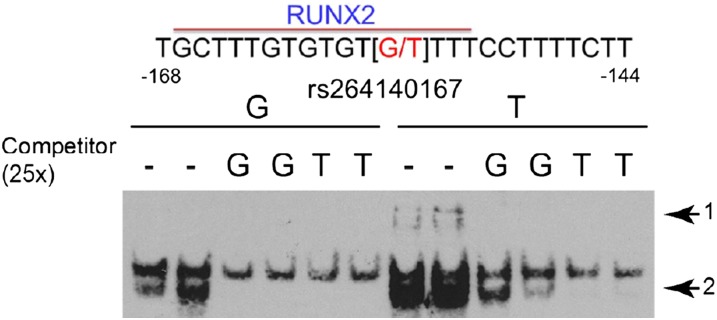
Genetic variant in secreted phosphoprotein 1 (Spp1) promoter alters nuclear protein binding capacity. Electrophoretic mobility shift assay of nuclear protein extract prepared from mouse lung epithelial cells (MLE15) and 25-mer probes (−144 to −168 bp from the start site). The single nucleotide polymorphism rs264140167 (−158 nucleotides from the transcription start site) in the Spp1 promoter region was used to generate 25-mer probes containing the C3H/HeJ T allele or the JF1/Msf G allele in the middle of the biotinylated oligonucleotide. The C3H/HeJ T allele increased the DNA protein binding to form slow migrating complexes (arrow 1) and enhanced the intensity of a faster migrating complex (arrow 2) compared with the JF1/Msf G allele. The C3H/HeJ T allele forms an additional putative runt-related transcription factor 2 (RUNX2) binding site not present in the JF1/Msf G allele.
We examined the lung functions of 36 inbred strains of mice to determine the possible functional consequence of rs47003578 SNP (JF1/Msf G allele versus C3H/HeJ A allele). This includes nine strains that had been previously phenotyped (19, 20) and 27 additional mouse strains (Table 1). Mice with the JF1/Msf allele had decreased TLC (G allele = 1,144 ± 13 versus T allele = 1,205 ± 25 μl; n = 345 mice), decreased specific TLC/body weight (G allele = 54 ± 1 versus T allele = 57 ± 1 μl/g; n = 369 mice), and increased specific compliance (CL/TLC) (G allele = 58 ± 1 versus T allele = 54 ± 1 μl/cm H2O/ml; n = 354 mice) (n = 7–15 mice/strain, 12–14 wk). These differences are ∼ 11, 17, and 21%, respectively, of the phenotypic difference we have previously observed in the extremely divergent mouse strains (19, 20). Body weight (G allele = 22.5 ± 0.4 versus T allele = 22.6 ± 0.6 g; n = 382 mice) and other lung function measurements (e.g., dead space volume) were not statistically different between genotypes.
Table 1.
Lung Function Values of 27 Inbred Mouse Strains (female; N = 252 mice)
| Strain | Mice Phenotyped/Strain | Age* (wk) | BW* (g) | BW SE | TLC* (μl) | TLC SE | TLC/BW* (μl/g) | TLC/BW SE | sCL* (ml) | sCL SE | VD* (μl) | VD SE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AKR/J | 8 | 16.0 | 30.6 | 1.2 | 1,176 | 32 | 40.9 | 1.5 | 49.8 | 2.7 | 231 | 4 |
| BALB/cJ | 10 | 13.9 | 22.7 | 0.8 | 1,277 | 17 | 60.2 | 2.5 | 63.9 | 3.0 | 233 | 2 |
| BPL/1J | 10 | 14.2 | 16.9 | 0.4 | 1,046 | 16 | 66.5 | 1.7 | 72.4 | 3.2 | 230 | 2 |
| BTBR T+tf/J | 8 | 13.9 | 31.9 | 0.6 | 1,409 | 26 | 46.5 | 1.6 | 58.2 | 1.7 | 251 | 3 |
| BUB/BnJ | 10 | 14.1 | 25.6 | 0.6 | 1,097 | 38 | 45.1 | 1.2 | 58.0 | 3.2 | 225 | 2 |
| C3HeB/FeJ | 10 | 14.4 | 25.7 | 0.9 | 1,480 | 33 | 61.6 | 2.3 | 72.9 | 4.0 | 232 | 3 |
| C57BL/10J | 10 | 14.3 | 20.7 | 0.4 | 1,034 | 11 | 52.7 | 1.0 | 51.2 | 0.7 | 233 | 4 |
| C57BLKS/J | 10 | 14.3 | 21.7 | 0.6 | 1,131 | 24 | 54.8 | 1.2 | 55.6 | 2.7 | 233 | 5 |
| C57BR/cdJ | 9 | 13.9 | 24.0 | 1.1 | 1,116 | 28 | 49.1 | 2.2 | 54.4 | 3.0 | 221 | 4 |
| C57L/J | 9 | 15.8 | 22.7 | 0.3 | 1,209 | 26 | 55.8 | 0.9 | 55.0 | 1.3 | 244 | 2 |
| C58/J | 10 | 14.7 | 19.8 | 0.5 | 1,002 | 20 | 53.7 | 0.9 | 53.2 | 1.1 | 215 | 2 |
| CBA/J | 10 | 15.3 | 28.6 | 0.5 | 1,177 | 17 | 43.4 | 0.9 | 59.8 | 3.4 | 242 | 1 |
| DBA/1J | 8 | 16.4 | 20.9 | 0.5 | 971 | 14 | 49.2 | 1.4 | 52.7 | 2.1 | 231 | 5 |
| DBA/2J | 8 | 15.4 | 24.2 | 0.8 | 1,054 | 17 | 45.8 | 1.7 | 58.0 | 1.3 | 232 | 3 |
| KK/HlJ | 7 | 13.9 | 35.0 | 1.3 | 1,304 | 29 | 39.6 | 1.5 | 47.0 | 1.9 | 246 | 2 |
| LP/J | 8 | 14.3 | 18.9 | 0.5 | 1,074 | 29 | 59.6 | 1.7 | 61.6 | 2.9 | 241 | 4 |
| MRL/MpJ | 10 | 13.9 | 35.5 | 1.3 | 1,519 | 42 | 45.0 | 1.1 | 56.3 | 3.7 | 251 | 3 |
| NOD/ShiLtJ | 8 | 15.1 | 21.9 | 0.6 | 1,012 | 25 | 48.8 | 1.6 | 60.7 | 2.4 | 222 | 2 |
| NON/ShiLtJ | 8 | 14.3 | 31.3 | 0.9 | 1,426 | 26 | 48.4 | 1.6 | 75.1 | 3.2 | 255 | 2 |
| NZL/LtJ | 9 | 14.4 | 36.3 | 1.9 | 1,409 | 34 | 41.9 | 2.2 | 59.6 | 2.5 | 230 | 2 |
| NZW/LacJ | 8 | 14.3 | 27.4 | 0.8 | 1,218 | 26 | 46.8 | 0.7 | 75.9 | 5.5 | 250 | 4 |
| PL/J | 10 | 14.1 | 20.5 | 0.7 | 956 | 13 | 49.7 | 1.2 | 49.0 | 1.2 | 210 | 3 |
| PWD/PhJ | 14 | 15.2 | 15.8 | 0.3 | 967 | 21 | 64.8 | 1.5 | 33.4 | 1.2 | 202 | 5 |
| RIIIS/J | 10 | 14.2 | 17.8 | 0.4 | 995 | 25 | 59.2 | 2.0 | 55.1 | 3.3 | 221 | 3 |
| SJL/J | 10 | 13.9 | 20.2 | 0.5 | 857 | 11 | 44.6 | 1.2 | 48.5 | 2.1 | 198 | 5 |
| SM/J | 10 | 15.4 | 14.1 | 0.3 | 881 | 25 | 62.6 | 1.6 | 47.1 | 2.3 | 222 | 7 |
| WSB/EiJ | 10 | 14.9 | 14.3 | 0.3 | 744 | 28 | 53.8 | 1.9 | 40.8 | 1.7 | 201 | 4 |
Definition of abbreviations: BW, body weight; sCL, specific static compliance of the lung [CL/TLC in ml (μl/cm H2O/ml TLC)]; SE, standard error; TLC, total lung capacity; TLC/BW, specific total lung capacity; VD, dead space volume.
Values are means.
SPP1 Induces Mouse Pneumocyte Growth
Considering that lung SPP1 transcripts and protein decreased in JF1/Msf mice during the peak stage of alveologenesis, we investigated whether SPP1 protein could stimulate the growth of MLE cells. MLE-15 cell proliferation increased 48 hours after treatment with 2 and 4 μg/ml SPP1 (Figure E3).
Lung Function of Spp1(−/−) Mice
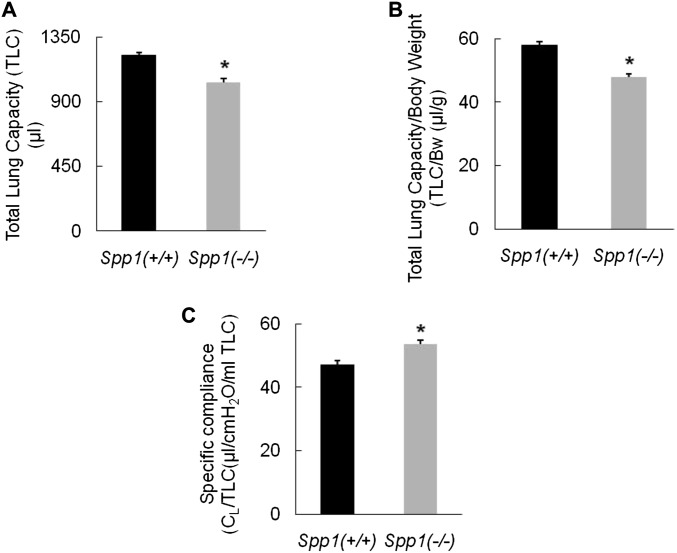
Analysis of lung function revealed that Spp1(−/−) had decreased TLC (Spp1(−/−) = 1,034 ± 25 versus Spp1(+/+) = 1,220 ± 22 μl), decreased specific TLC/body weight (Spp1(−/−) = 48 ± 1 versus Spp1(+/+) = 58 ± 1 μl/g), and increased specific CL (Spp1(−/−) = 54 ± 1 versus Spp1(+/+) = 47 ± 1 μl/cm H2O/ml) (n = 8 mice/strain, 12–14 wk) (Figure 3). These differences are approximately 33, 38, and 16%, respectively, of the phenotypic difference we have previously observed in the extremely divergent mouse strains (20). Other lung function measurements (including dead space volume and diffusion capacity/TLC) and BW (Spp1(−/−) = 21.5 ± 0.9 versus Spp1(+/+) = 21.0 ± 0.8 g) were not statistically different between Spp1(−/−) and Spp1(+/+) mice.
Figure 3.
Lung function measurements of secreted phosphoprotein 1–deficient [Spp1(−/−)] mice compared with strain-matched control [Spp1(+/+)] mice. (A) Spp1(−/−) mice have 15% decreased total lung capacity (TLC) [Spp1(−/−) = 1,034 ± 25 versus Spp1(+/+) = 1,220 ± 22 μl]. (B) Spp1(−/−) mice have 17% decreased specific TLC (TLC/body weight) (Spp1(−/−) = 48 ± 1 versus Spp1(+/+) = 58 ± 1 μl/g). (C) Spp1(−/−) mice have 14% increased specific compliance (sCL) compared with Spp1(+/+) mice (Spp1(−/−) = 54 ± 1 versus Spp1(+/+) = 47 ± 1 μl/cm H2O/ml). Values are mean ± SE (n = 8 mice/strain; age = 12–14 wk). Statistical significance (*P < 0.001) was determined by ANOVA and by all pairwise comparisons procedure (Holm-Sidak method).
Lung Morphometry and SPP1 Immunohistochemical Location
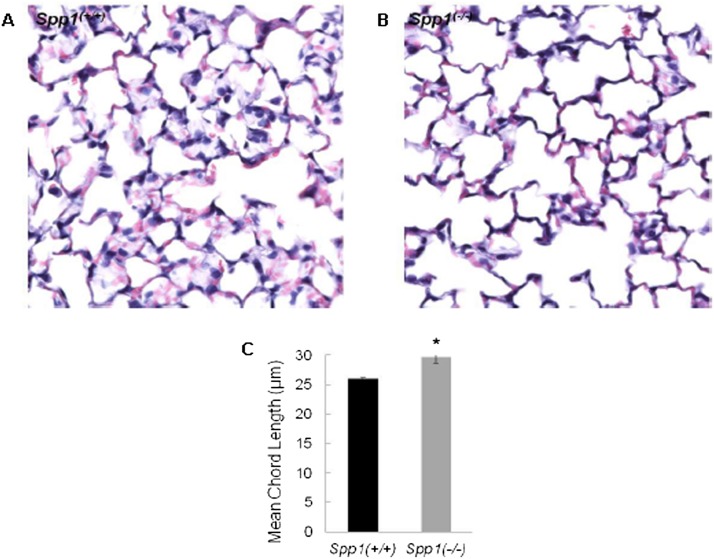
Increased Lm indicates decreased alveolar surface area. The mean chord length in Spp1(−/−) increased as compared with Spp1(+/+) mice (Figure 4). This was detected in P28 mice when lung development is just completed and is indicative of impaired alveologenesis. Immunohistological analysis localized SPP1 protein to the bronchial epithelium, alveolar macrophage, and weakly to the alveolar type II cell in adult Spp1(+/+) mice (Figure E4).
Figure 4.
Comparison of alveolar air space size between Spp1(−/−) and strain-matched control [Spp1(+/+)] mice at the age of 4 weeks when alveolarization is completed. (A) Spp1(+/+); (B) Spp1(−/−). Lung morphometric analysis revealed 14% increased mean chord length in Spp1(−/−) mice (Lm: 29.7 ± 0.3 μm) compared with Spp1(+/+) mice (Lm: 26.0 ± 0.3 μm) (C). Values are mean ± SE (n = 5 mice/strain). Statistical significance (*P < 0.05) was determined by ANOVA and by all pairwise comparisons procedure (Holm-Sidak method).
Transcriptomic analysis
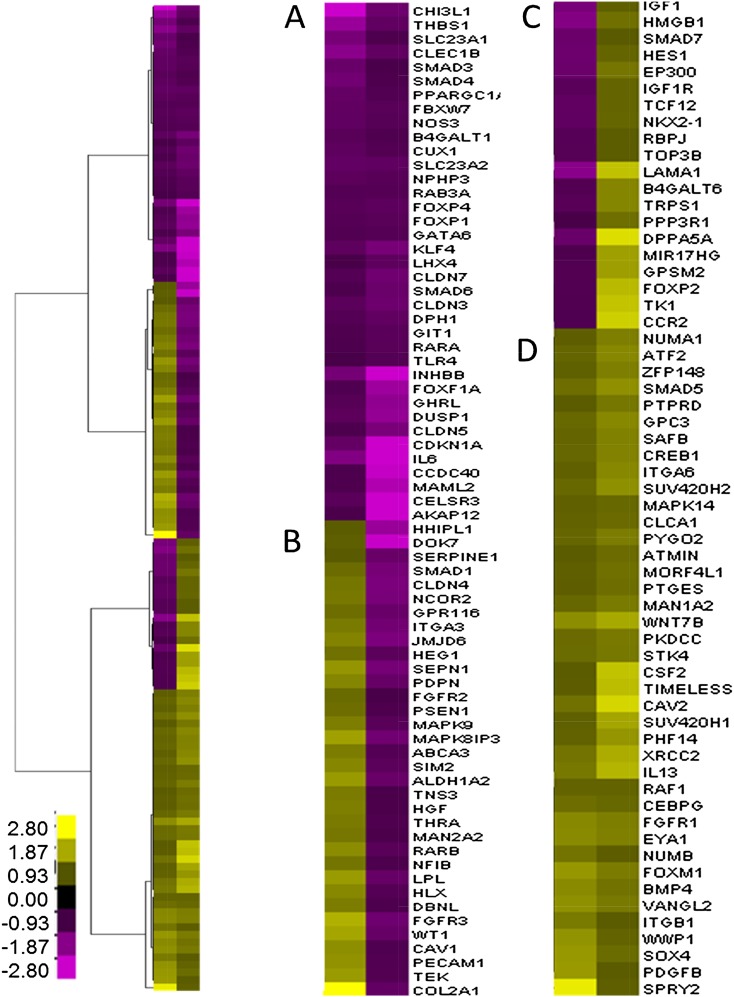
To assess the lung transcriptomic profile during P14 (peak alveologenesis phase) and P28 (completion of alveologenesis and lung development), microarray analysis was performed with mRNA isolated from Spp1(−/−) and Spp1(+/+) mouse lung. P14 and P28 were chosen based on reduced SPP1 transcript expression pattern in JF1/Msf lungs compared with C3H/HeJ. Initially, we examined the transcripts that were increased or decreased at P14 or P28 (n = 7,384). These transcripts were significantly enriched in genes associated with gene onotogy category GO:0030324 lung development (n = 132 significant of 387 in the category; P = 1.0E-08) (Figure 5). These 132 transcripts grouped into four clusters including transcripts that decreased at P14 and P28, increased at P14 and decreased at P28, decreased at P14 and increased at P28, or increased at P14 and P28. These clusters were analyzed for enrichment in transcripts associated with canonical pathways using Ingenuity Pathway Analysis. The top pathway for each cluster included retinoic acid receptor activation (P = 1.7E-06), aryl hydrocarbon receptor signaling (P = 1.4E-04), notch signaling (P = 7.8E-04), bone morphogenetic protein signaling pathway (P = 1.8E-0.8), and fibroblast growth factor (FGF) signaling (P = 1.5E-0.6), respectively.
Figure 5.
Hierarchical clustering of altered transcripts associated with lung development in Spp1(−/−) compared with strain-matched control [Spp1(+/+)] mice at P14 or P28. Transcripts were significantly enriched in genes associated with lung development (n = 132 significant of 387 in the category; P = 1E-08). Four major clusters were identified: decreased at P14 and P28 (A), increased at P14 and decreased at P28 (B), decreased at P14 and increased at P28 (C), or increased at P14 and P28 (D). Significant difference in transcript levels for the microarray data was analyzed using ANOVA (P < 0.05). Fold change color scale is adjacent to the heat map. Values are means (n = 6–14 mice/strain), with yellow denoting increased and purple denoting decreased in Spp1(−/−) compared with strain-matched control (Spp1(+/+)) mice. Each row represents a gene, and each column represents mean difference for P14 or P28.
Transcripts ≥ 1.5-fold increased or ≤ 1.5-fold decreased in Spp1(−/−) lung as compared with Spp1(+/+) were analyzed for enriched pathways using Database for Annotation, Visualization, and Integrated Discovery (DAVID) (39, 40). The enriched categories of Gene Ontogeny (GO) molecular function, GO biological process, GO cell component, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were determined. Ten transcripts with the greatest difference between strains at age P14 or P28 in each of these GO/KEGG categories are listed in Tables 2–5.
Table 2.
Increased Lung Transcripts in Secreted Phosphoprotein 1–Deficient Mice at Postnatal Day 14
| Gene Symbol | Entrez Gene ID | Fold Change | P Value | Description | Enrichment Score |
|---|---|---|---|---|---|
| Molecular function: GO:0004713 protein tyrosine kinase activity |
1.8 | ||||
| Dyrk1a | 13548 | 2.24 | 0.002 | dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 1a | |
| Ltk | 17005 | 2.11 | 0.028 | leukocyte tyrosine kinase | |
| Fgfr3 | 14184 | 1.95 | <0.001 | fibroblast growth factor receptor 3 | |
| Map2k6 | 26399 | 1.82 | 0.005 | mitogen-activated protein kinase kinase 6 | |
| Cdc7 | 12545 | 1.74 | 0.034 | cell division cycle 7 (Saccharomyces cerevisiae) | |
| Tyk2 | 54721 | 1.64 | <0.001 | tyrosine kinase 2 | |
| Blk | 12143 | 1.59 | 0.029 | B lymphoid kinase | |
| Epha5 | 13839 | 1.59 | <0.001 | Eph receptor A5 | |
| Ntrk2 | 18212 | 1.55 | 0.002 | neurotrophic tyrosine kinase, receptor, type 2 | |
| Ephb3 | 13845 | 1.51 | <0.001 | Eph receptor B3 | |
| Biological process: GO:0048514 blood vessel morphogenesis |
2.3 | ||||
| Hif1a | 15251 | 2.14 | 0.005 | hypoxia inducible factor 1, alpha subunit | |
| Tbx20 | 57246 | 2.09 | 0.001 | T-box 20 | |
| Wt1 | 22431 | 1.83 | 0.029 | Wilms tumor 1 homolog | |
| Hmox1 | 15368 | 1.69 | 0.001 | heme oxygenase (decycling) 1 | |
| Foxm1 | 14235 | 1.67 | 0.027 | forkhead box M1 | |
| Cav1 | 12389 | 1.58 | 0.018 | caveolin 1, caveolae protein | |
| Ovol2 | 107586 | 1.57 | <0.001 | ovo-like 2 (Drosophila) | |
| Ntrk2 | 18212 | 1.55 | 0.002 | neurotrophic tyrosine kinase, receptor, type 2 | |
| Dll4 | 54485 | 1.55 | 0.001 | delta-like 4 (Drosophila) | |
| Ccbe1 | 320924 | 1.51 | 0.014 | collagen and calcium binding EGF domains 1 | |
| Tnni3 | 21954 | 1.50 | 0.045 | troponin I, cardiac 3 | |
| Cellular component: GO:0042995 cell projection |
.8 | ||||
| Itln1 | 16429 | 4.32 | 0.018 | intelectin 1 (galactofuranose binding) | |
| Prph | 19132 | 2.12 | 0.027 | peripherin | |
| Exph5 | 320051 | 2.03 | 0.005 | exophilin 5 | |
| Myl7 | 17898 | 1.86 | 0.015 | myosin, light polypeptide 7, regulatory | |
| Dynlt1c | 1E+08 | 1.80 | <0.001 | dynein light chain Tctex-type 1C | |
| Ctnnd1 | 12388 | 1.79 | 0.020 | catenin (cadherin associated protein), delta 1 | |
| Dpysl5 | 65254 | 1.71 | 0.001 | dihydropyrimidinase-like 5 | |
| C2cd3 | 277939 | 1.67 | 0.050 | C2 calcium-dependent domain containing 3 | |
| Cyth3 | 19159 | 1.67 | 0.006 | cytohesin 3 | |
| Tesc | 57816 | 1.64 | 0.024 | tescalcin | |
| KEGG pathway: mmu05414: dilated cardiomyopathy |
1.9 | ||||
| Grik2 | 14806 | 2.68 | 0.001 | glutamate receptor, ionotropic, kainate 2 (beta 2) | |
| Myh6 | 17888 | 2.22 | 0.017 | myosin, heavy polypeptide 6, cardiac muscle, alpha | |
| Mybpc3 | 17868 | 2.09 | 0.004 | myosin binding protein C, cardiac | |
| Atp1a2 | 98660 | 1.88 | 0.006 | ATPase, Na+/K+ transporting, alpha 2 polypeptide | |
| Adrb1 | 11554 | 1.83 | <0.001 | adrenergic receptor, beta 1 | |
| Tnnc1 | 21924 | 1.80 | 0.035 | troponin C, cardiac/slow skeletal | |
| Adrb3 | 11556 | 1.72 | <0.001 | adrenergic receptor, beta 3 | |
| Actg1 | 11465 | 1.70 | 0.003 | actin, gamma, cytoplasmic 1 | |
| Atp2a2 | 11938 | 1.66 | 0.004 | ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2 | |
| Tnni3 | 21954 | 1.50 | 0.045 | troponin I, cardiac 3 | |
Definition of abbreviations: GO, gene ontogeny; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Table 5.
Decreased Lung Transcripts in Secreted Phosphoprotein 1–Deficient Mice at Postnatal Day 28
| Gene Symbol | Entrez Gene ID | Fold Change | P Value | Description | Enrichment Score |
|---|---|---|---|---|---|
| Molecular function: GO:0004672 protein kinase activity |
2.4 | ||||
| Taok2 | 381921 | −3.12 | 0.001 | TAO kinase 2 | |
| Phkg1 | 18682 | −3.03 | 0.003 | phosphorylase kinase gamma 1 | |
| Tnk1 | 83813 | −2.70 | 0.015 | tyrosine kinase, non-receptor, 1 | |
| Csnk1e | 27373 | −2.64 | 0.002 | casein kinase 1, epsilon | |
| Mapk6 | 50772 | −2.49 | 0.006 | mitogen-activated protein kinase 6 | |
| Plk3 | 12795 | −2.19 | <0.001 | polo-like kinase 3 (Drosophila) | |
| Sgk2 | 27219 | −2.16 | 0.025 | serum/glucocorticoid regulated kinase 2 | |
| Irak2 | 108960 | −2.15 | 0.025 | interleukin-1 receptor-associated kinase 2 | |
| Mtor | 56717 | −2.14 | 0.005 | mechanistic target of rapamycin (serine/threonine kinase) | |
| Trib1 | 211770 | −2.10 | <0.001 | tribbles homolog 1 (Drosophila) | |
| Biological process: GO:0007243 protein kinase cascade |
2.4 | ||||
| Gna13 | 14674 | −3.06 | <0.001 | guanine nucleotide binding protein, alpha 13 | |
| Osm | 18413 | −2.82 | 0.005 | oncostatin M | |
| Tlr6 | 21899 | −2.62 | 0.007 | Toll-like receptor 6 | |
| Muc20 | 224116 | −2.18 | 0.048 | mucin 20 | |
| Irak2 | 108960 | −2.15 | 0.025 | interleukin-1 receptor-associated kinase 2 | |
| Ghrl | 58991 | −2.00 | 0.005 | ghrelin | |
| Edn1 | 13614 | −1.99 | 0.012 | endothelin 1 | |
| Pxn | 19303 | −1.71 | 0.005 | paxillin | |
| Smad1 | 17125 | −1.65 | 0.045 | MAD homolog 1 (Drosophila) | |
| Dapk3 | 13144 | −1.62 | 0.045 | death-associated protein kinase 3 | |
| Fgfr3 | 14184 | −1.53 | 0.033 | fibroblast growth factor receptor 3 | |
| Cellular component: GO:0005911 cell–cell junction |
2.5 | ||||
| Myl2 | 17906 | −8.71 | 0.027 | myosin, light polypeptide 2, regulatory, cardiac, slow | |
| Nrap | 18175 | −3.29 | 0.003 | nebulin-related anchoring protein | |
| Myh7 | 140781 | −2.96 | 0.002 | myosin, heavy polypeptide 7, cardiac muscle, beta | |
| Ppp2ca | 19052 | −2.60 | <0.001 | protein phosphatase 2 (formerly 2A), catalytic subunit, alpha isoform | |
| Csnk2a2 | 13000 | −2.40 | 0.003 | casein kinase 2, alpha prime polypeptide | |
| Shroom3 | 27428 | −2.27 | 0.016 | shroom family member 3 | |
| Pcp4 | 18546 | −2.10 | 0.006 | Purkinje cell protein 4 | |
| Prkcz | 18762 | −2.07 | 0.005 | protein kinase C, zeta | |
| Ppp2r2b | 72930 | −2.05 | 0.039 | protein phosphatase 2 (formerly 2A), regulatory subunit B (PR 52), beta isoform | |
| Cldn23 | 71908 | −1.88 | 0.023 | claudin 23 | |
| KEGG pathway: mmu04530: tight junction |
1.9 | ||||
| Exoc4 | 20336 | −1.86 | 0.032 | exocyst complex component 4 | |
| Gja4 | 14612 | −1.81 | 0.001 | gap junction protein, alpha 4 | |
| Pard6b | 58220 | −1.77 | 0.002 | par-6 (partitioning defective 6) homolog beta (Caenorhabditis elegans) | |
| Myh9 | 17886 | −1.77 | 0.002 | myosin, heavy polypeptide 9, nonmuscle | |
| Cldn4 | 12740 | −1.70 | 0.039 | claudin 4 | |
| Ahnak | 66395 | −1.70 | 0.020 | AHNAK nucleoprotein (desmoyokin) | |
| Cldn5 | 12741 | −1.69 | 0.006 | claudin 5 | |
| Llgl2 | 217325 | −1.58 | 0.017 | lethal giant larvae homolog 2 (Drosophila) | |
| Cldn7 | 53624 | −1.55 | 0.016 | claudin 7 | |
| Cldn3 | 12739 | −1.51 | 0.004 | claudin 3 | |
The GO/KEGG categories at P14 containing increased transcripts (n = 738 transcripts with unique Entrez Gene ID) in Spp1(−/−) mouse lung were protein tyrosine kinase activity, blood vessel morphogenesis, and cell projection (Table 2). Several genes or gene products in these pathways have been associated with abnormal lung development or lung disease (e.g., asthma, or COPD). Noteworthy transcripts in these categories/pathways included FGF receptor 3 (FGFR3) (41); hypoxia inducible factor 1, α subunit (HIF1A) (42); intelectin 1 (ITLN1) (43, 44); and heme oxygenase 1 (HMOX1) (45, 46).
The GO/KEGG categories at P14 containing decreased transcripts (n = 388) in Spp1(−/−) mouse lung were peptidase activity, response to wounding, extracellular space, and cytokine–cytokine receptor interaction (Table 3). Noteworthy transcripts in these categories/pathways included matrix metalloproteinase 25 (MMP25; aka MT-MMP6) (47), thrombospondin 1 (THBS1) (48, 49), Toll-like receptor 1 (TLR1) (50, 51), TLR5 (52), chitinase 3-like 1 (CHIL3L1) (53, 54), and IL 12b (IL12B) (55, 56).
Table 3.
Decreased Lung Transcripts in Secreted Phosphoprotein 1–Deficient Mice at Postnatal Day 14
| Gene Symbol | Entrez Gene ID | Fold Change | P Value | Description | Enrichment Score |
|---|---|---|---|---|---|
| Molecular function GO:0008233 peptidase activity |
2.4 | ||||
| Adamts4 | 240913 | −5.8 | 0.005 | a disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 4 | |
| Adam28 | 13522 | −2.5 | 0.007 | a disintegrin and metallopeptidase domain 28 | |
| Agbl3 | 76223 | −2.5 | 0.001 | ATP/GTP binding protein-like 3 | |
| Mmel1 | 27390 | −2.3 | 0.026 | membrane metallo-endopeptidase-like 1 | |
| Adamdec1 | 58860 | −2.2 | 0.044 | ADAM-like, decysin 1 | |
| Qpct | 70536 | −1.8 | 0.042 | glutaminyl-peptide cyclotransferase (glutaminyl cyclase) | |
| Acr | 11434 | −1.7 | 0.037 | acrosin prepropeptide | |
| Ecel1 | 13599 | −1.7 | 0.025 | endothelin converting enzyme-like 1 | |
| Mmp25 | 240047 | −1.6 | 0.004 | matrix metallopeptidase 25 | |
| Usp36 | 72344 | −1.5 | 0.003 | ubiquitin specific peptidase 36 | |
| Biological process GO:0009611 response to wounding |
1.5 | ||||
| Gp9 | 54368 | −2.4 | 0.012 | glycoprotein 9 (platelet) | |
| Thbs1 | 21825 | −2.1 | 0.010 | thrombospondin 1 | |
| Tnfrsf1b | 21938 | −2.1 | <0.001 | tumor necrosis factor receptor superfamily, member 1b | |
| Tlr1 | 21897 | −2.0 | 0.033 | Toll-like receptor 1 | |
| P2ry12 | 70839 | −1.9 | 0.003 | purinergic receptor P2Y, G-protein coupled 12 | |
| Pf4 | 56744 | −1.7 | <0.001 | platelet factor 4 | |
| Tlr5 | 53791 | −1.6 | 0.048 | Toll-like receptor 5 | |
| Hps5 | 246694 | −1.6 | 0.032 | Hermansky-Pudlak syndrome 5 homolog (human) | |
| Treml1 | 71326 | −1.6 | 0.017 | triggering receptor expressed on myeloid cells-like 1 | |
| Ccl19 | 24047 | −1.5 | 0.029 | chemokine (C-C motif) ligand 19 | |
| Cellular component GO:0005615 extracellular space |
2.8 | ||||
| Afm | 280662 | −4.6 | 0.001 | afamin | |
| Enpp1 | 18605 | −2.9 | 0.045 | ectonucleotide pyrophosphatase/phosphodiesterase 1 | |
| Chi3l1 | 12654 | −2.8 | 0.024 | chitinase 3-like 1 | |
| Cilp | 214425 | −2.8 | 0.005 | cartilage intermediate layer protein, nucleotide pyrophosphohydrolase | |
| Spon2 | 100689 | −2.7 | 0.016 | spondin 2, extracellular matrix protein | |
| Apoc2 | 11813 | −2.7 | 0.025 | apolipoprotein C-II | |
| Adam28 | 13522 | −2.5 | 0.007 | a disintegrin and metallopeptidase domain 28 | |
| Gdf3 | 14562 | −2.5 | 0.029 | growth differentiation factor 3 | |
| Mmp8 | 17394 | −2.4 | 0.034 | matrix metallopeptidase 8 | |
| Grp | 225642 | −2.3 | 0.004 | gastrin releasing peptide | |
| KEGG pathway mmu04060: cytokine-cytokine receptor interaction |
1.8 | ||||
| Ccr8 | 12776 | −2.8 | 0.014 | chemokine (C-C motif) receptor 8 | |
| Ccl7 | 20306 | −2.4 | 0.047 | chemokine (C-C motif) ligand 7 | |
| Cxcr2 | 12765 | −2.3 | 0.010 | chemokine (C-X-C motif) receptor 2 | |
| Tnfrsf13c | 72049 | −2.3 | 0.038 | tumor necrosis factor receptor superfamily, member 13c | |
| Tnfrsf9 | 21942 | −2.1 | 0.039 | tumor necrosis factor receptor superfamily, member 9 | |
| Ccl12 | 20293 | −2.1 | 0.041 | chemokine (C-C motif) ligand 12 | |
| Il12b | 16160 | −2.0 | <0.001 | interleukin 12b | |
| Mpl | 17480 | −1.7 | 0.012 | myeloproliferative leukemia virus oncogene | |
| Pf4 | 56744 | −1.7 | >0.001 | platelet factor 4 | |
| Inhbb | 16324 | −1.6 | 0.004 | inhibin beta-B | |
The GO/KEGG categories at P28 containing increased transcripts (n = 1,436) in Spp1(−/−) mouse lung were zinc ion binding, regulation of transcription, microtubule cytoskeleton, and cell cycle (Table 4). Noteworthy transcripts in these categories/pathways included forkhead box P2 (FOXP2) (57), midline 1 (MID1) (58), metal response element binding transcription factor 1 (MTF1) (59), SRY-box containing gene 9 (SOX9) (60), SOX5 (61), nuclear factor I/A (NFIA) (62), and sperm-associated antigen 17 (SPAG17) (63).
Table 4.
Increased Lung Transcripts in Secreted Phosphoprotein 1 Deficient Mice at Postnatal Day 28
| Gene Symbol | Entrez Gene ID | Fold Change | P Value | Description | Enrichment Score: |
|---|---|---|---|---|---|
| Molecular function: GO:0008270 zinc ion binding |
9.2 | ||||
| Rag1 | 19373 | 11.07 | 0.013 | recombination activating gene 1 | |
| Zfp14 | 243906 | 4.13 | 0.001 | zinc finger protein 14 | |
| Trim59 | 66949 | 3.38 | <0.001 | tripartite motif-containing 59 | |
| Birc5 | 11799 | 2.82 | 0.016 | baculoviral IAP repeat-containing 5 | |
| Snai2 | 20583 | 2.46 | 0.033 | snail homolog 2 (Drosophila) | |
| Foxp2 | 114142 | 2.06 | 0.024 | forkhead box P2 | |
| Mid1 | 17318 | 1.97 | 0.034 | midline 1 | |
| Naip6 | 17952 | 1.94 | 0.013 | NLR family, apoptosis inhibitory protein 6 | |
| Rfwd2 | 26374 | 1.77 | 0.004 | ring finger and WD repeat domain 2 | |
| Mtf1 | 17764 | 1.72 | <0.001 | metal response element binding transcription factor 1 | |
| Biological process: GO:0045449 regulation of transcription |
14.3 | ||||
| Dnmt3a | 13435 | 2.80 | 0.010 | DNA methyltransferase 3A | |
| Runx1 | 12394 | 2.67 | 0.008 | runt related transcription factor 1 | |
| Sox9 | 20682 | 2.52 | 0.005 | SRY-box containing gene 9 | |
| Ccna2 | 12428 | 2.39 | 0.044 | cyclin A2 | |
| Sox5 | 20678 | 2.16 | 0.011 | SRY-box containing gene 5 | |
| Myb | 17863 | 2.15 | 0.009 | myeloblastosis oncogene | |
| Egr3 | 13655 | 2.02 | 0.042 | early growth response 3 | |
| Maf | 17132 | 1.98 | 0.028 | avian musculoaponeurotic fibrosarcoma (v-maf) AS42 oncogene homolog | |
| Nfia | 18027 | 1.80 | 0.011 | nuclear factor I/A | |
| E2f2 | 242705 | 1.74 | 0.017 | E2F transcription factor 2 | |
| Cellular component: GO:0015630 microtubule cytoskeleton |
7.7 | ||||
| Tube1 | 71924 | 3.63 | 0.001 | epsilon-tubulin 1 | |
| Aurka | 20878 | 3.46 | <0.001 | aurora kinase A | |
| Kifc1 | 1E+08 | 2.66 | 0.012 | kinesin family member C1 | |
| Spag17 | 74362 | 2.36 | 0.005 | sperm associated antigen 17 | |
| Tubd1 | 56427 | 2.22 | 0.001 | tubulin, delta 1 | |
| Cep55 | 74107 | 2.01 | <0.001 | centrosomal protein 55 | |
| Haus8 | 76478 | 1.95 | 0.006 | 4HAUS augmin-like complex, subunit 8 | |
| Rpgrip1l | 244585 | 1.83 | 0.021 | Rpgrip1-like | |
| Ttll7 | 70892 | 1.78 | 0.041 | tubulin tyrosine ligase-like family, member 7 | |
| C2cd3 | 277939 | 1.72 | 0.016 | C2 calcium-dependent domain containing 3 | |
| KEGG pathway: mmu04110 cell cycle |
2.9 | ||||
| Cdk1 | 12534 | 2.92 | 0.036 | cyclin-dependent kinase 1 | |
| Bub1 | 12235 | 2.89 | 0.001 | budding uninhibited by benzimidazoles 1 homolog (Saccharomyces cerevisiae) | |
| Skp2 | 27401 | 2.70 | 0.002 | S-phase kinase-associated protein 2 (p45) | |
| Ccnb1 | 268697 | 2.47 | 0.004 | cyclin B1 | |
| Ccna2 | 12428 | 2.39 | 0.044 | cyclin A2 | |
| Chek2 | 50883 | 2.25 | 0.043 | CHK2 checkpoint homolog (Schizosaccharomyces pombe) | |
| Cdc20 | 107995 | 2.25 | 0.006 | cell division cycle 20 homolog (S. cerevisiae) | |
| Plk1 | 18817 | 2.10 | 0.008 | polo-like kinase 1 (Drosophila) | |
| Cdc25c | 12532 | 2.09 | 0.038 | cell division cycle 25 homolog C (S. pombe) | |
| Anapc10 | 68999 | 1.81 | 0.011 | anaphase promoting complex subunit 10 | |
The GO/KEGG categories at P28 containing decreased transcripts (n = 1,161) in Spp1(−/−) mouse lung were protein kinase activity, protein kinase cascade, cell–cell junction, and tight junction (Table 5). Noteworthy transcripts in these categories/pathways included casein kinase 1, epsilon (CSNK1E) (64), mitogen-activated protein kinase 6 (MAPK6; a.k.a. ERK3) (65), mechanistic target of rapamycin (serine/threonine kinase) (MTOR) (66), oncostatin M (OSM) (67, 68), TLR6 (69), mucin 20 (MUC20) (70, 71), MAD homolog 1 (SMAD1) (72, 73), FGFR3, protein phosphatase 2 (formerly 2A), catalytic subunit, α isoform (PPP2CA) (74, 75), claudin 3 (CLDN3), CLDN4, CLDN5, CLDN7 (76–78), and lethal giant larvae homolog 2 (Drosphila) (LLGL2) (79, 80).
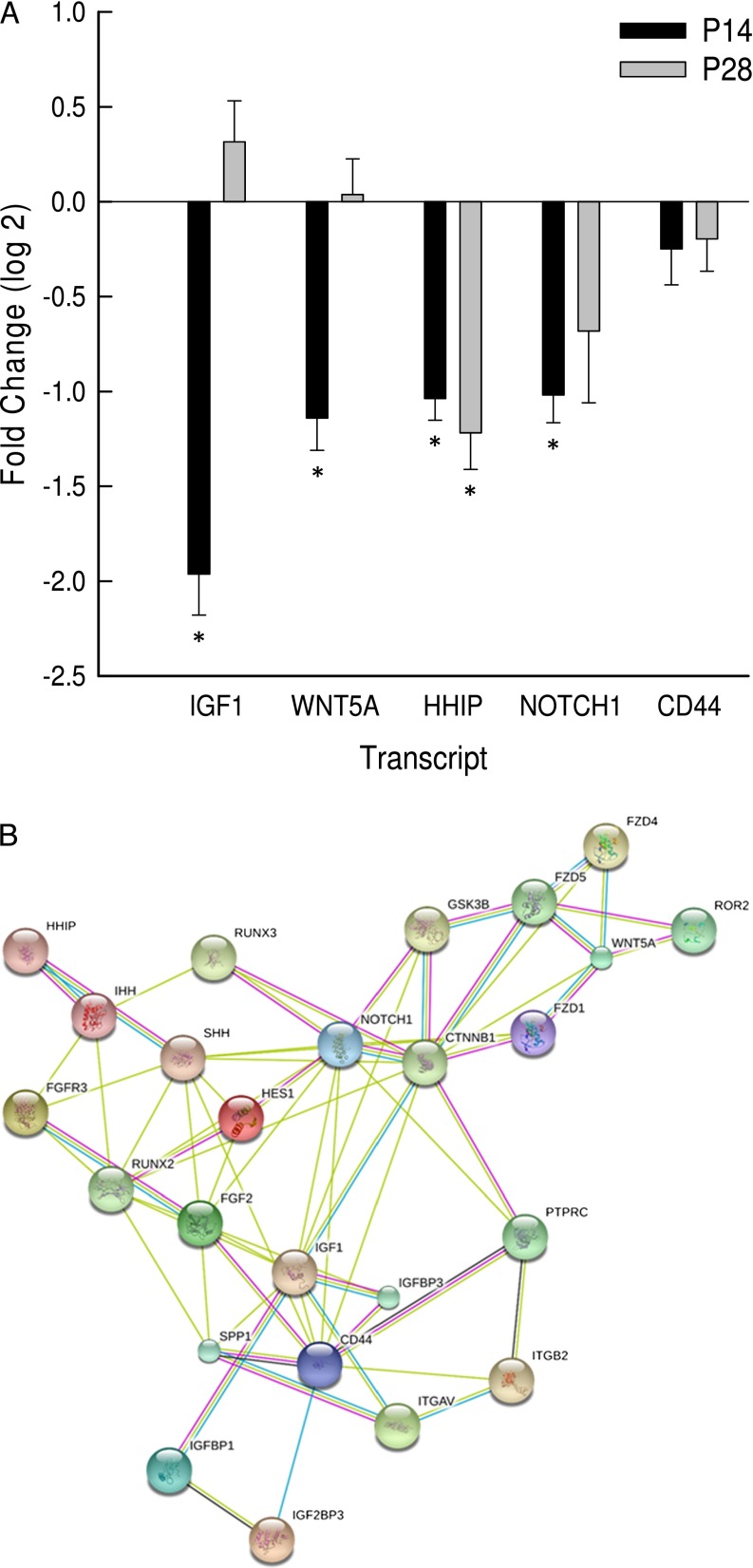
To further assess transcripts associated with lung development, transcripts encoding IGF1, WNT5A, HHIP, notch 1 (NOTCH1), and CD44 antigen (CD44) were assessed by qRT-PCR. As compared with P14 Spp1(+/+) mice, lung IGF1, WNT5A, HHIP, and NOTCH1 transcripts decreased in P14 Spp1(−/−) mice (Figure 6A). At P28, only HHIP was decreased in Spp1(−/−) compared with Spp1(+/+) mouse lung (Figure 6A).
Figure 6.
Transcripts associated with lung development are altered in Spp1(−/−) as compared with strain-matched control [Spp1(+/+)] mice at P14 or P28. (A) Lung mRNA was isolated, and transcript levels were determined by quantitative RT-PCR (qRT-PCR). Values are mean ± SE (n = 6–14 mice/strain). Statistical significance (*P < 0.05) was determined by ANOVA and by all pairwise comparisons procedure (Holm-Sidak method). (B) Protein–protein interaction network of SPP1 with proteins associated with lung development. CTNNB1, catenin (cadherin associated protein), β 1; CD44, CD44 antigen; FGF2, fibroblast growth factor 2; FGFR3, fibroblast growth factor receptor 3; FZD1, frizzled class receptor 1; FZD4, frizzled class receptor 4; FZD5, frizzled class receptor 5; GSK3B, glycogen synthase kinase 3 β; HES1, hes family bHLH transcription factor 1; HHIP, hedgehog interacting protein; IGF1, insulin-like growth factor 1; IGFBP1, insulin-like growth factor binding protein 1; IGFBP3, insulin-like growth factor binding protein 3; IGF2BP3, insulin-like growth factor 2 mRNA binding protein 3; IHH, Indian hedgehog; ITGAV, integrin α V; ITGB2, integrin β 2; NOTCH1, notch 1; PTPRC, protein tyrosine phosphatase, receptor type, C (aka CD45); ROR2, receptor tyrosine kinase-like orphan receptor 2; RUNX2, runt-related transcription factor 2; RUNX3,runt-related transcription factor 3; SHH, sonic hedgehog; WNT5A, wingless-related MMTV integration site 5A.
Discussion
Rapid identification of functional candidate genes in mice has been valuable in providing insights into human lung development. In this study, we assessed the functionality of Spp1 located within another QTL for lung function for its plausible role as a pulmonary function determinant in mice.
Mammalian lung development is a precisely orchestrated process that involves lung airway branching morphogenesis and alveolarization along with angiogenesis and vasculogenesis during embryonic and postnatal periods (81). Severe impairments during any developmental stage can result in bronchopulmonary dysplasia, neonatal respiratory failure, and death (82). However, mild structural or functional defects due to aberrant lung development (83) may increase susceptibility to respiratory diseases (COPD, cystic fibrosis, or asthma) that may be clinically detectable only during childhood or later in life through pulmonary function testing (84–87). Therefore, it is important to detect genetic abnormalities that can affect early fetal and postnatal lung development; postnatal lung growth and maturation; and lung injury, repair, and remodeling processes (84–90).
In mice, alveolarization takes place between P5 and P30 and is controlled by finely integrated and mutually regulated networks of transcriptional factors, growth factors, matrix components, and physical forces (9, 89–92). Factors that adversely affect the developing lung include premature birth, oxygen exposure, early corticosteroidal exposure, dysregulated growth factor (IGF, WNT, NOTCH, BMP/TGFB, FGF, PDGF, VEGFA) signaling, and abnormal regulation or injury of the pulmonary capillary vasculature. Individually and cumulatively, these factors can result in hypoplasia of the alveolar epithelial surface, with a resulting deficiency in pulmonary function (e.g., decreased TLC or increased CL).
As compared with C3H/HeJ mice, lung SPP1 transcript decreased in JF1/Msf mice, a strain with decreased lung function. This decrease was noted from P14 onward, which is the peak phase of alveologenesis in mice. Alveolization takes place by the process of septation of primitive saccules into smaller units during late gestation in humans and postnatally in mice. During this period secondary crests develop and extend to form alveoli, resulting in increased surface area for gaseous exchange. Alveolization defects result in large alveoli, reminiscent of the abnormality found in emphysema but with less overt destruction. Indicative of impaired alveologeneis, Spp1(−/−) mice had increased alveolar size (i.e., Lm) that was detectable as early as 4 weeks of age when the process is just completed. Increased alveolar size also implicates reduced alveolar surface area (S = 4V/Lm) for gas exchange (93). At around 4 weeks of age, the lung development is complete, and the lung assumes the structure of an adolescent lung. Thus, P28 is an important screening stage for evaluating postnatal lung development (21).
Several genetic variants in the Spp1 proximal promoter differ between C3H/HeJ and JF1/Msf mice. Previously, Sowa and colleagues (34) identified a 13-bp insertion (rs234069704) at position −130 located at the 3′ end of the RUNX2 binding site. This insertion increased transcriptional responsiveness to RUNX2 in the C3H/HeJ promoter as compared with that of the C57BL/6J promoter. Because JF1/Msf mice, similar to C57BL/6J mice, lack the poly-T insertions, the C3H/HeJ Spp1 promoter would also be more responsive to RUNX2 than the JF1/Msf promoter. In addition, we examined whether SNPs at position −158 (rs264140167) or −198 (rs47003578) could alter putative binding sites. The C3H/HeJ T rs264140167 allele at −158 in the Spp1 promoter enhanced nuclear protein–target DNA binding capacity. The C3H/HeJ T allele forms an additional putative RUNX2 binding site not present in the JF1/Msf G allele.
Several variants in the human SPP1 promoter have been identified and are functional. For example, variants at −66 (rs28357094), −156 (rs11439060), and −443 (rs11730582) bp from the transcriptional start site can modify activation by Sp1 transcription factor (SP1), RUNX2, and v-myb avian myeloblastosis viral oncogene homolog, respectively (94, 95). The −156 bp rs11439060 variant is an insertion (−/G) that provides a functional RUNX2 binding site and is near the −158 SNP in the mouse genome, which also provides a putative RUNX2 binding site in C3H/HeJ mice (Figure E5). Human promoter SNPs also have been reported as autoimmune risk variants for systemic lupus erythematosus (96, 97), systemic sclerosis (98), inflammatory bowel disease (99), and rheumatoid arthritis (100). RUNX2-mediated SPP1 promoter activity can be inhibited by histone deacetylase 1 (101), and RUNX transcription factors have been associated with increased risk of asthma in children with in utero smoke exposure (102, 103).
Similar to what we have reported previously with primary human normal lung fibroblast and with the A549 lung epithelial cell line (27), SPP1 induced mouse MLE-15 cell proliferation. SPP1 also alters fibroblast migration (27), further supporting its likely role in lung development. The smaller TLC and higher CL in Spp1(−/−) mice could be a result of impaired alveologenesis. Inasmuch as SPP1 can influence the proliferation of type II–like epithelial cells and lung fibroblasts, the altered lung function in Spp1(−/−) mice may be due to increased alveoloar size or diminished tissue elastic recoil of the lungs. Therefore, impaired alveologenesis could explain the decreased TLC and increased CL observed in SPP1-deficient mice.
Lung microarray analysis revealed numerous differences in transcripts critical to lung development in Spp1(−/−) mice as compared with strain-matched control mice. GO categories of molecular function, biological process, and cell component and KEGG pathways contained transcripts associated with lung development (P14 increased FGFR3, HIF1A; P14 decreased THBS1; P28 increased FOXP2, MTF1, SOX5, SOX9, NFIA, and SPAG17; P28 decreased CSNK1E, MAPK6, SMAD1, FGFR3, PPP2CA, CLDN3, CLDN4, CLDN5, CLDN7, and LLGL2) or lung diseases including asthma (P14 increased ITLN1; P14 decreased CHIL3L1 and IL12B; P28 increased MID1; P28 decreased MTOR, TLR6 and PPP2CA) and COPD (P14 increased HMOX1; P14 decreased MMP25; P28 increased SOX5). These altered transcripts suggest that SPP1 interacts with a wide range of proteins that regulate normal development and supports the hypothesis that abnormal development is a risk factor for chronic respiratory diseases.
Lung IGF1, HHIP, WNT5A, and NOTCH1 transcripts decreased in P14 Spp1(−/−) mice as determined by qRT-PCR analysis. These transcripts encoded proteins that formed an interactive network that included interactions of SPP1 with IGF1, RUNX2, CD44, FGF2, and integrin α V (Figure 6B). Other proteins were required to include HHIP, WNT5A, and NOTCH1 in the interactome that includes SPP1, suggesting that SPP1 is associated with these transcripts through indirect interactions. In addition, the validated transcripts encode proteins that have key roles in the other regulatory networks that control lung development. In mice, IGF1 regulates airspace formation by promoting an elastogenic lineage in undifferentiated mesenchymal cells (104) and is critical for lung development (105).
HHIP regulates the hedgehog pathway implicated in development and repair in multiple tissues (106). Gene-targeted HHIP-deficient mice display defective airway branching morphogenesis and lung hypoplasia that results in death due to respiratory failure at birth (107). In humans, SNPs located near HHIP have been associated with lung development and growth (108) and COPD (10–16, 107–111).
In mice, disruption of Wnt5a results in distinct truncation of the trachea and overexpansion of the distal respiratory airways (112), whereas overexpression of WNT5A interferes with epithelial–mesenchymal crosstalk, resulting in reduced airway branching and dilated distal airways (113). In addition, hedgehog and FGF signaling were altered in WNT5A overexpressing mice (113), clearly indicating its role in lung development.
NOTCH signaling is critical for normal balance of differentiated cell fates in the airway epithelium (114, 115). Transgenic mice expressing a constitutively activated NOTCH1 in the lung epithelium have fewer ciliated cells and more mucin-producing cells, suggesting its role in the lineage determination of secretory or nonsecretory cells (116, 117). The NOTCH1 pathway has been implicated in SPP1 transcription in HES1-transfected cells and can be inhibited by AML-1/ETO, an inhibitor of RUNX2 (33).
To summarize, mice with decreased SPP1 have smaller but more compliant lungs, which is likely due to impaired alveologenesis. This is accompanied by altered expression patterns of key lung developmental transcripts in P14 Spp1(−/−) mice (during peak alveologenesis phase) and increased alveolar airspace detectable in P28 Spp1(−/−) mice (when alveologenesis is nearly complete). Together, these findings support a key role for SPP1 in lung development, which adds to its known role in chronic lung disease.
Footnotes
This study was supported by the National Institutes of Health grants ES015675, HL077763, and HL085655 (G.D.L.); HL084932 and HL095397 (N.K.); and DST SERB: SB/SO/AS-026/2013 (K.G.).
Author Contributions: K.G., H.S., N.K., and G.D.L. conceived the project and designed the experiments. K.G., T.M.M., V.J.C., S.U., K.B., K.A.B., and L.J.V. performed the experiments. K.G., H.S., N.K., G.D.L., J.P.F., L.G., A.M., T.A.T., and C.F. analyzed the data. K.G., L.G., H.S., and G.D.L. wrote the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0471OC on May 9, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrows B, Knudson RJ, Lebowitz MD. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977;115:751–760. doi: 10.1164/arrd.1977.115.5.751. [DOI] [PubMed] [Google Scholar]
- 3.Strachan D. Ventilatory function, height, and mortality among lifelong non-smokers. J Epidemiol Community Health. 1992;46:66–70. doi: 10.1136/jech.46.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engstrom G, Hedblad B, Janzon L. Reduced lung function predicts increased fatality in future cardiac events: a population-based study. J Intern Med. 2006;260:560–567. doi: 10.1111/j.1365-2796.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 5.Bridevaux P, Gerbase M, Probst-Hensch N, Schindler C, Gaspoz J, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified gold stage 1 COPD. Thorax. 2008;63:768–774. doi: 10.1136/thx.2007.093724. [DOI] [PubMed] [Google Scholar]
- 6.Hirschhorn JN. Genomewide association studies: illuminating biologic pathways. N Engl J Med. 2009;360:1699–1701. doi: 10.1056/NEJMp0808934. [DOI] [PubMed] [Google Scholar]
- 7.Weiss ST. Lung function and airway diseases. Nat Genet. 2010;42:14–16. doi: 10.1038/ng0110-14. [DOI] [PubMed] [Google Scholar]
- 8.Beers MF, Morrisey EE. The three R's of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrisey EE, Cardoso WV, Lane RH, Rabinovitch M, Abman SH, Ai X, Albertine KH, Bland RD, Chapman HA, Checkley W, et al. Molecular determinants of lung development. Ann Am Thorac Soc. 2013;10:S12–S16. doi: 10.1513/AnnalsATS.201207-036OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, Myers RH, Borecki IB, Silverman EK, Weiss ST, et al. A genome-wide association study of pulmonary function measures in the framingham heart study. PLoS Genet. 2009;5:e1000429. doi: 10.1371/journal.pgen.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42:36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH, Barr RG, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42:45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imboden M, Bouzigon E, Curjuric I, Ramasamy A, Kumar A, Hancock DB, Wilk JB, Vonk JM, Thun GA, Siroux V, et al. Genome-wide association study of lung function decline in adults with and without asthma. J Allergy Clin Immunol. 2012;129:1218–1228. doi: 10.1016/j.jaci.2012.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao TC, Du G, Han L, Sun Y, Hu D, Yang JJ, Mathias R, Roth LA, Rafaels N, Thompson EE, et al. Genome-wide association study of lung function phenotypes in a founder population. J Allergy Clin Immunol. 2013;133:248–255. doi: 10.1016/j.jaci.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, Zhai G, Zhao JH, Smith AV, Huffman JE, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082–1090. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock DB, Artigas MS, Gharib SA, Henry A, Manichaikul A, Ramasamy A, Loth DW, Imboden M, Koch B, McArdle WL, et al. Genome-wide joint meta-analysis of snp and snp-by-smoking interaction identifies novel loci for pulmonary function. PLoS Genet. 2012;8:e1003098. doi: 10.1371/journal.pgen.1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11:446–450. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: genetic interactions create phantom heritability. Proc Natl Acad Sci USA. 2012;109:1193–1198. doi: 10.1073/pnas.1119675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, Schulz H. Inbred strain variation in lung function. Mamm Genome. 2002;13:429–437. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 20.Reinhard C, Meyer B, Fuchs H, Stoeger T, Eder G, Ruschendorf F, Heyder J, Nurnberg P, de Angelis MH, Schulz H. Genomewide linkage analysis identifies novel genetic loci for lung function in mice. Am J Respir Crit Care Med. 2005;171:880–888. doi: 10.1164/rccm.200409-1204OC. [DOI] [PubMed] [Google Scholar]
- 21.Ganguly K, Stoeger T, Wesselkamper SC, Reinhard C, Sartor MA, Medvedovic M, Tomlinson CR, Bolle I, Mason JM, Leikauf GD, et al. Candidate genes controlling pulmonary function in mice: transcript profiling and predicted protein structure. Physiol Genomics. 2007;31:410–421. doi: 10.1152/physiolgenomics.00260.2006. [DOI] [PubMed] [Google Scholar]
- 22.Ganguly K, Depner M, Fattman C, Bein K, Oury TD, Wesselkamper SC, Borchers MT, Schreiber M, Gao F, von Mutius E, et al. Superoxide dismutase 3, extracellular (SOD3) variants and lung function. Physiol Genomics. 2009;37:260–267. doi: 10.1152/physiolgenomics.90363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsey JY, Ganguly K, Brass DM, Li Z, Potts EN, Degan S, Chen H, Brockway B, Abraham SN, Berndt A, et al. c-Kit is essential for alveolar maintenance and protection from emphysema-like disease in mice. Am J Respir Crit Care Med. 2011;183:1644–1652. doi: 10.1164/rccm.201007-1157OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 24.Dahl M, Bowler RP, Juul K, Crapo JD, Levy S, Nordestgaard BG. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am J Respir Crit Care Med. 2008;178:906–912. doi: 10.1164/rccm.200804-549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siedlinski M, van Diemen CC, Postma DS, Vonk JM, Boezen HM. Superoxide dismutases, lung function and bronchial responsiveness in a general population. Eur Respir J. 2009;33:986–992. doi: 10.1183/09031936.00171507. [DOI] [PubMed] [Google Scholar]
- 26.Sorheim IC, DeMeo DL, Washko G, Litonjua A, Sparrow D, Bowler R, Bakke P, Pillai SG, Coxson HO, Lomas DA, et al. Polymorphisms in the superoxide dismutase-3 gene are associated with emphysema in COPD. COPD. 2010;7:262–268. doi: 10.3109/15412555.2010.496821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider DJ, Lindsay JC, Zhou Y, Molina JG, Blackburn MR. Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB J. 2010;24:70–80. doi: 10.1096/fj.09-140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 30.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 31.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 33.Shen Q, Christakos S. Vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem. 2005;280:40589–40598. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- 34.Sowa AK, Kaiser FJ, Eckhold J, Kessler T, Aherrahrou R, Wrobel S, Kaczmarek PM, Doehring L, Schunkert H, Erdmann J, et al. Functional interaction of osteogenic transcription factors Runx2 and Vdr in transcriptional regulation of Opn during soft tissue calcification. Am J Pathol. 2013;183:60–68. doi: 10.1016/j.ajpath.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein c/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen NM, Bai Y, Mochitate K, Senior RM. Laminin alpha-chain expression and basement membrane formation by MLE-15 respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1004–L1011. doi: 10.1152/ajplung.00379.2001. [DOI] [PubMed] [Google Scholar]
- 37.Schulz H, Johner C, Eder G, Ziesenis A, Reitmeier P, Heyder J, Balling R. Respiratory mechanics in mice: strain and sex specific differences. Acta Physiol Scand. 2002;174:367–375. doi: 10.1046/j.1365-201x.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 38.Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 39.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 40.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedmacher F, Doi T, Gosemann JH, Fujiwara N, Kutasy B, Puri P. Upregulation of fibroblast growth factor receptor 2 and 3 in the late stages of fetal lung development in the nitrofen rat model. Pediatr Surg Int. 2012;28:195–199. doi: 10.1007/s00383-011-2985-2. [DOI] [PubMed] [Google Scholar]
- 42.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–156. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pemberton AD, Rose-Zerilli MJ, Holloway JW, Gray RD, Holgate ST. A single-nucleotide polymorphism in intelectin 1 is associated with increased asthma risk. J Allergy Clin Immunol. 2008;122:1033–1034. doi: 10.1016/j.jaci.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 44.Gu N, Kang G, Jin C, Xu Y, Zhang Z, Erle DJ, Zhen G. Intelectin is required for IL-13-induced monocyte chemotactic protein-1 and -3 expression in lung epithelial cells and promotes allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L290–L296. doi: 10.1152/ajplung.90612.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raval CM, Lee PJ. Heme oxygenase-1 in lung disease. Curr Drug Targets. 2010;11:1532–1540. doi: 10.2174/1389450111009011532. [DOI] [PubMed] [Google Scholar]
- 47.Nie J, Pei D. Rapid inactivation of alpha-1-proteinase inhibitor by neutrophil specific leukolysin/membrane-type matrix metalloproteinase 6. Exp Cell Res. 2004;296:145–150. doi: 10.1016/j.yexcr.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Sozo F, Hooper SB, Wallace MJ. Thrombospondin-1 expression and localization in the developing ovine lung. J Physiol. 2007;584:625–635. doi: 10.1113/jphysiol.2007.138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Xiong Z, Lechner EJ, Klenotic PA, Hamburg BJ, Hulver M, Khare A, Oriss T, Mangalmurti N, Chan Y, et al. Thrombospondin-1 triggers macrophage il-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 2014;7:440–448. doi: 10.1038/mi.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wurfel MM, Gordon AC, Holden TD, Radella F, Strout J, Kajikawa O, Ruzinski JT, Rona G, Black RA, Stratton S, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med. 2008;178:710–720. doi: 10.1164/rccm.200803-462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson CM, Holden TD, Rona G, Laxmanan B, Black RA, O'Keefe GE, Wurfel MM. Toll-like receptor 1 polymorphisms and associated outcomes in sepsis after traumatic injury: a candidate gene association study. Ann Surg. 2014;259:179–185. doi: 10.1097/SLA.0b013e31828538e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blohmke CJ, Park J, Hirschfeld AF, Victor RE, Schneiderman J, Stefanowicz D, Chilvers MA, Durie PR, Corey M, Zielenski J, et al. TLR5 as an anti-inflammatory target and modifier gene in cystic fibrosis. J Immunol. 2010;185:7731–7738. doi: 10.4049/jimmunol.1001513. [DOI] [PubMed] [Google Scholar]
- 53.Lee CG, Dela Cruz CS, Ma B, Ahangari F, Zhou Y, Halaban R, Sznol M, Elias JA. Chitinase-like proteins in lung injury, repair, and metastasis. Proc Am Thorac Soc. 2012;9:57–61. doi: 10.1513/pats.201112-056MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ortega H, Prazma C, Suruki RY, Li H, Anderson WH. Association of CHI3L1 in African-Americans with prior history of asthma exacerbations and stress. J Asthma. 2013;50:7–13. doi: 10.3109/02770903.2012.733991. [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, Choi SJ, Choi JP, Jeon SG, Oh S, Lee BJ, Gho YS, Lee CG, Zhu Z, Elias JA, et al. IL-12-STAT4-IFN-gamma axis is a key downstream pathway in the development of IL-13-mediated asthma phenotypes in a Th2 type asthma model. Exp Mol Med. 2010;42:533–546. doi: 10.3858/emm.2010.42.8.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida M, Watson RM, Rerecich T, O'Byrne PM. Different profiles of T-cell IFN-gamma and IL-12 in allergen-induced early and dual responders with asthma. J Allergy Clin Immunol. 2005;115:1004–1009. doi: 10.1016/j.jaci.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 57.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- 58.Collison A, Hatchwell L, Verrills N, Wark PA, de Siqueira AP, Tooze M, Carpenter H, Don AS, Morris JC, Zimmermann N, et al. The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity. Nat Med. 2013;19:232–237. doi: 10.1038/nm.3049. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Wimmer U, Lichtlen P, Inderbitzin D, Stieger B, Meier PJ, Hunziker L, Stallmach T, Forrer R, Rulicke T, et al. Metal-responsive transcription factor-1 (MTF-1) is essential for embryonic liver development and heavy metal detoxification in the adult liver. FASEB J. 2004;18:1071–1079. doi: 10.1096/fj.03-1282com. [DOI] [PubMed] [Google Scholar]
- 60.Chang DR, Martinez Alanis D, Miller RK, Ji H, Akiyama H, McCrea PD, Chen J. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci USA. 2013;110:18042–18051. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hersh CP, Silverman EK, Gascon J, Bhattacharya S, Klanderman BJ, Litonjua AA, Lefebvre V, Sparrow D, Reilly JJ, Anderson WH, et al. SOX5 is a candidate gene for chronic obstructive pulmonary disease susceptibility and is necessary for lung development. Am J Respir Crit Care Med. 2011;183:1482–1489. doi: 10.1164/rccm.201010-1751OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene. 2000;249:31–45. doi: 10.1016/s0378-1119(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 63.Teves ME, Zhang Z, Costanzo RM, Henderson SC, Corwin FD, Zweit J, Sundaresan G, Subler M, Salloum FN, Rubin BK, et al. Sperm-associated antigen-17 gene is essential for motile cilia function and neonatal survival. Am J Respir Cell Mol Biol. 2013;48:765–772. doi: 10.1165/rcmb.2012-0362OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cruciat CM, Dolde C, de Groot RE, Ohkawara B, Reinhard C, Korswagen HC, Niehrs C. RNA helicase DDX3 is a regulatory subunit of casein kinase 1 in Wnt-beta-catenin signaling. Science. 2013;339:1436–1441. doi: 10.1126/science.1231499. [DOI] [PubMed] [Google Scholar]
- 65.Klinger S, Turgeon B, Levesque K, Wood GA, Aagaard-Tillery KM, Meloche S. Loss of ERK3 function in mice leads to intrauterine growth restriction, pulmonary immaturity, and neonatal lethality. Proc Natl Acad Sci USA. 2009;106:16710–16715. doi: 10.1073/pnas.0900919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramer EL, Hardie WD, Mushaben EM, Acciani TH, Pastura PA, Korfhagen TR, Hershey GK, Whitsett JA, Le Cras TD. Rapamycin decreases airway remodeling and hyperreactivity in a transgenic model of noninflammatory lung disease. J Appl Physiol. 1985;2011:1760–1767. doi: 10.1152/japplphysiol.00737.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mozaffarian A, Brewer AW, Trueblood ES, Luzina IG, Todd NW, Atamas SP, Arnett HA. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J Immunol. 2008;181:7243–7253. doi: 10.4049/jimmunol.181.10.7243. [DOI] [PubMed] [Google Scholar]
- 68.Nogueira-Silva C, Piairo P, Carvalho-Dias E, Veiga C, Moura RS, Correia-Pinto J. The role of glycoprotein 130 family of cytokines in fetal rat lung development. PLoS ONE. 2013;8:e67607. doi: 10.1371/journal.pone.0067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kormann MS, Depner M, Hartl D, Klopp N, Illig T, Adamski J, Vogelberg C, Weiland SK, von Mutius E, Kabesch M. Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol. 2008;122:86–92. doi: 10.1016/j.jaci.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 70.Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 2013;6:379–392. doi: 10.1038/mi.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leikauf GD, Borchers MT, Prows DR, Simpson LG. Mucin apoprotein expression in COPD. Chest. 2002;121:166S–182S. doi: 10.1378/chest.121.5_suppl.166s. [DOI] [PubMed] [Google Scholar]
- 72.Shi W, Chen H, Sun J, Chen C, Zhao J, Wang YL, Anderson KD, Warburton D. Overexpression of Smurf1 negatively regulates mouse embryonic lung branching morphogenesis by specifically reducing Smad1 and Smad5 proteins. Am J Physiol Lung Cell Mol Physiol. 2004;286:L293–L300. doi: 10.1152/ajplung.00228.2003. [DOI] [PubMed] [Google Scholar]
- 73.Xu B, Chen C, Chen H, Zheng SG, Bringas P, Jr, Xu M, Zhou X, Chen D, Umans L, Zwijsen A, et al. Smad1 and its target gene Wif1 coordinate BMP and Wnt signaling activities to regulate fetal lung development. Development. 2011;138:925–935. doi: 10.1242/dev.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor BK, Stoops TD, Everett AD. Protein phosphatase inhibitors arrest cell cycle and reduce branching morphogenesis in fetal rat lung cultures. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1062–L1070. doi: 10.1152/ajplung.2000.278.5.L1062. [DOI] [PubMed] [Google Scholar]
- 75.Kobayashi Y, Mercado N, Barnes PJ, Ito K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS ONE. 2011;6:e27627. doi: 10.1371/journal.pone.0027627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaarteenaho R, Merikallio H, Lehtonen S, Harju T, Soini Y. Divergent expression of claudin -1, -3, -4, -5 and -7 in developing human lung. Respir Res. 2010;11:59. doi: 10.1186/1465-9921-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, Dopico RA, Jr, Di YP, Knoell DL, Barchowsky A, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–490. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohta H, Chiba S, Ebina M, Furuse M, Nukiwa T. Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:L193–L205. doi: 10.1152/ajplung.00349.2010. [DOI] [PubMed] [Google Scholar]
- 79.Tao T, Lan J, Presley JF, Sweezey NB, Kaplan F. Nucleocytoplasmic shuttling of lgl2 is developmentally regulated in fetal lung. Am J Respir Cell Mol Biol. 2004;30:350–359. doi: 10.1165/rcmb.2003-0126OC. [DOI] [PubMed] [Google Scholar]
- 80.Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, Rozado D, Magen A, Canidio E, Pagani M, Peluso I, et al. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 2011;9:e1000582. doi: 10.1371/journal.pbio.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122:479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J. 2014;43:808–816. doi: 10.1183/09031936.00039513. [DOI] [PubMed] [Google Scholar]
- 84.Massaro D, Massaro GD. Critical period for alveologenesis and early determinants of adult pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2004;287:L715–L717. doi: 10.1152/ajplung.00166.2004. [DOI] [PubMed] [Google Scholar]
- 85.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, de Marco R, Norback D, Raherison C, Villani S, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 87.Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2013;7:161–173. doi: 10.1177/1753465813479428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132:651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 89.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 90.Warburton D, Bellusci S, Del Moral PM, Kaartinen V, Lee M, Tefft D, Shi W. Growth factor signaling in lung morphogenetic centers: automaticity, stereotypy and symmetry. Respir Res. 2003;4:5. doi: 10.1186/1465-9921-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K, Shi W. Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res. 2005;57:26R–37R. doi: 10.1203/01.PDR.0000159570.01327.ED. [DOI] [PubMed] [Google Scholar]
- 92.Shi W, Xu J, Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology. 2009;14:656–665. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitzner W. Use of mean airspace chord length to assess emphysema. J Appl Physiol. 2008;105:1980–1981. doi: 10.1152/japplphysiol.90968.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giacopelli F, Marciano R, Pistorio A, Catarsi P, Canini S, Karsenty G, Ravazzolo R. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiol Genomics. 2004;20:87–96. doi: 10.1152/physiolgenomics.00138.2004. [DOI] [PubMed] [Google Scholar]
- 95.Schultz J, Lorenz P, Ibrahim SM, Kundt G, Gross G, Kunz M. The functional -443T/C osteopontin promoter polymorphism influences osteopontin gene expression in melanoma cells via binding of c-Myb transcription factor. Mol Carcinog. 2009;48:14–23. doi: 10.1002/mc.20452. [DOI] [PubMed] [Google Scholar]
- 96.D'Alfonso S, Barizzone N, Giordano M, Chiocchetti A, Magnani C, Castelli L, Indelicato M, Giacopelli F, Marchini M, Scorza R, et al. Two single-nucleotide polymorphisms in the 5′ and 3′ ends of the osteopontin gene contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2005;52:539–547. doi: 10.1002/art.20808. [DOI] [PubMed] [Google Scholar]
- 97.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009;10:487–494. doi: 10.1038/gene.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barizzone N, Marchini M, Cappiello F, Chiocchetti A, Orilieri E, Ferrante D, Corrado L, Mellone S, Scorza R, Dianzani U, et al. Association of osteopontin regulatory polymorphisms with systemic sclerosis. Hum Immunol. 2011;72:930–934. doi: 10.1016/j.humimm.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 99.Glas J, Seiderer J, Bayrle C, Wetzke M, Fries C, Tillack C, Olszak T, Beigel F, Steib C, Friedrich M, et al. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn's disease. PLoS ONE. 2011;6:e29309. doi: 10.1371/journal.pone.0029309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gazal S, Sacre K, Allanore Y, Teruel M, Goodall AH, The CARDIOGENICS consortium. Tohma S, Alfredsson L, Okada Y, Xie G, Constantin A, Balsa A, Kawasaki A, Nicaise P, Amos C, Rodriguez-Rodriguez L, et al. Identification of secreted phosphoprotein 1 gene as a new rheumatoid arthritis susceptibility gene Ann Rheum DisIn press) [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z, Deepak V, Meng L, Wang L, Li Y, Jiang Q, Zeng X, Liu W. Analysis of HDAC1-mediated regulation of Runx2-induced osteopontin gene expression in C3h10t1/2 cells. Biotechnol Lett. 2012;34:197–203. doi: 10.1007/s10529-011-0756-8. [DOI] [PubMed] [Google Scholar]
- 102.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Haley KJ, Lasky-Su J, Manoli SE, Smith LA, Shahsafaei A, Weiss ST, Tantisira K. RUNX transcription factors: association with pediatric asthma and modulated by maternal smoking. Am J Physiol Lung Cell Mol Physiol. 2011;301:L693–L701. doi: 10.1152/ajplung.00348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Srisuma S, Bhattacharya S, Simon DM, Solleti SK, Tyagi S, Starcher B, Mariani TJ. Fibroblast growth factor receptors control epithelial-mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med. 2010;181:838–850. doi: 10.1164/rccm.200904-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 106.Briscoe J, Therond PP. The mechanisms of hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 107.Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17:342–347. doi: 10.1101/gad.1026303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kerkhof M, Boezen HM, Granell R, Wijga AH, Brunekreef B, Smit HA, de Jongste JC, Thijs C, Mommers M, Penders J, et al. Transient early wheeze and lung function in early childhood associated with chronic obstructive pulmonary disease genes. J Allergy Clin Immunol. 2014;133:68–76. doi: 10.1016/j.jaci.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5:e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou X, Baron RM, Hardin M, Cho MH, Zielinski J, Hawrylkiewicz I, Sliwinski P, Hersh CP, Mancini JD, Lu K, et al. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet. 2012;21:1325–1335. doi: 10.1093/hmg/ddr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lamontagne M, Couture C, Postma DS, Timens W, Sin DD, Pare PD, Hogg JC, Nickle D, Laviolette M, Bosse Y. Refining susceptibility loci of chronic obstructive pulmonary disease with lung eqtls. PLoS ONE. 2013;8:e70220. doi: 10.1371/journal.pone.0070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- 113.Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 114.Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol. 2004;14:357–364. doi: 10.1016/j.semcancer.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 115.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–664. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]