Abstract
Actin dynamics plays an essential role in regulating airway smooth muscle contraction. The mechanisms that regulate actin dynamics in smooth muscle are not completely understood. Glia maturation factor (GMF) is a protein that has been reported to inhibit actin nucleation and to induce actin network debranching in vitro. The role of GMF in human smooth muscle cells and tissues has not been investigated. In this study, knockdown of GMF-γ by RNA interference enhanced actin polymerization and contraction in human airway smooth muscle (HASM) cells and tissues without affecting myosin phosphorylation (another important biochemical change during contractile activation). Activation of HASM cells and tissues with acetylcholine induced dissociation of GMF-γ from Arp2 of the Arp2/3 complex. Acetylcholine stimulation also increased GMF-γ phosphorylation at Tyr-104. GMF-γ phosphorylation at this residue was mediated by c-Abl tyrosine kinase. The GMF-γ mutant Y104F (phenylalanine substitution at Tyr-104) had higher association with Arp2 in HASM cells upon contractile activation. Furthermore, expression of mutant Y104F GMF-γ attenuated actin polymerization and contraction in smooth muscle. Thus, we propose a novel mechanism for the regulation of actin dynamics and smooth muscle contraction. In unstimulated smooth muscle, GMF-γ binds to the Arp2/3 complex, which induces actin disassembly and retains lower levels of F-actin. Upon contractile stimulation, phosphorylation at Tyr-104 mediated by c-Abl tyrosine kinase leads to the dissociation of GMF-γ from Arp2/3, by which GMF-γ no longer induces actin disassembly. Reduced actin disassembly renders F-actin in higher level, which facilitates smooth muscle contraction.
Keywords: tyrosine kinase, actin-associated protein, signal transduction, actin polymerization
Clinical Relevance
Glia maturation factor (GMF)-γ has been implicated in actin dynamics in vitro. The present study unveils a novel role of GMF-γ in actin dynamics and contraction in human airway smooth muscle. This new finding provides a groundwork to better understand the molecular and cellular mechanisms that induces airway hyperresponsiveness.
Airway smooth muscle contraction plays an essential role in regulating functions of the respiratory system, such as airway tone. Abnormal smooth muscle contraction contributes to the development of various diseases, such as asthma.
In smooth muscle, myosin activation by phosphorylation is critical for the regulation of force development (1, 2). Moreover, recent studies have shown that contractile stimulation induces actin polymerization. Blockage of actin dynamics by inhibitors and molecular approach diminishes smooth muscle contraction without affecting myosin phosphorylation (3–8). Reorganization of the actin cytoskeleton may facilitate force transmission between contractile units and extracellular matrix (3, 6, 7, 9). Thus, myosin activation and actin cytoskeletal remodeling are essential for smooth muscle contraction. Myosin may serve as an “engine” for smooth muscle contraction, whereas the actin cytoskeleton may function as a “transmission system” in smooth muscle. However, the mechanisms that regulate actin dynamics in smooth muscle are not completely understood.
Actin dynamics is largely regulated by the balance between polymerization and depolymerization. Actin polymerization is controlled by nucleation-promoting factors, such as neuronal Wiskott–Aldrich Syndrome protein (10, 11). Nucleation-promoting factors recruit the Arp2/3 complex and actin monomers, which promotes actin filament polymerization and branching. In addition, formins promote unbranched actin filament elongation. The activity of nucleation-promoting factors is regulated by many molecules, including small GTPases, phosphatidylinositol 4,5-bisphosphate, Nck, CrkII, Crk-associated substrate, paxillin, cortactin, and Abi1 (3, 6, 8, 10–13). Actin depolymerization is mediated by several factors, including cofilin, twinfilin, Abp1/drebrin, and coactosin (14–16). Other mechanisms may exist to regulate actin dynamics.
Glia maturation factor (GMF)-γ is a member of actin-depolymerizing factor (ADF)/cofilin family that is widely expressed in eukaryotes and plays a central role in reorganizing the actin cytoskeleton by disassembling actin filaments (14, 15). GMF-γ is able to inhibit actin nucleation in vitro (15). Moreover, in vitro biochemical studies show that GMF-γ may induce debranching of actin filament networks (14, 15). Nevertheless, the functional role of GMF-γ in human cells is largely unknown.
c-Abl (Abelson tyrosine kinase, Abl) is a nonreceptor tyrosine kinase that has a role in the regulation of actin dynamics and in cell adhesion, migration, proliferation, growth, and development (13, 17–19). In smooth muscle, contractile stimulation induces c-Abl phosphorylation. Knockdown (KD) of c-Abl attenuates actin polymerization and contraction in smooth muscle upon agonist activation (8, 20, 21). The interaction of c-Abl with GMF-γ in human cells has not been explored.
In the present study, we evaluated whether GMF-γ has a role in regulating actin dynamics and contraction in smooth muscle and assessed the potential mechanisms that regulate the function of GMF-γ in human smooth muscle.
Materials and Methods
Cell Culture
Human airway smooth muscle (HASM) cells were prepared and cultured as previously described (7, 13, 22). Details are provided in the online supplement.
Immunoblot Analysis and Coimmunoprecipitation Analysis
Immunoblot analysis and coimmunoprecipitation analysis were performed as previously described (8, 23, 24) with minor modifications as described in the online supplement. GMF-γ antibody and α-smooth muscle actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against phospho-myosin light chain (Ser-19), myosin light chain, Arp2, and c-Abl were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Fitzgerald (Acton, MA). Phospho–GMF-γ (Tyr-104) was custom made by Thermo Scientific (Pierce Rockford, IL). The sequence of the peptide for generating phospho-GMF-γ antibody was CKPEQQMMY(P)AGSKNRLVQTA (NCBI accession number NM_004877.2).
Lentivirus-Mediated RNA Interface in Cells
For GMF-γ KD, lentiviruses encoding GMF-γ short hairpin RNA (shRNA) or control shRNA were purchased from Santa Cruz Biotechnology. HASM cells were infected with control shRNA lentivirus or GMF-γ shRNA lentivirus for 12 hours and cultured for 3 to 4 days. Positive clones expressing shRNAs were selected by puromycin. Immunoblot analysis was used to determine the expression levels of GMF-γ in these cells. GMF-γ KD cells and cells expressing control shRNA were stable at least five passages after initial infection. The experimental procedures for generating c-Abl KD cells were previously described (13, 17).
Analysis of F-actin/G-actin Ratios by Fractionation Assay
The content of F-actin and G-actin in smooth muscle was measured as previously described (7, 8, 20).
Measurement of Smooth Muscle Contraction
Human bronchial rings (diameter, 5 mm) were placed in physiolgical saline solution at 37°C in a 25-ml organ bath and attached to a force transducer that had been connected to a computer with A/D converter (Grass). For lentivirus-mediated RNA interface (RNAi) in tissues, the thin epithelium layer of human bronchial rings was removed with forceps. Tissues were then transduced with lentivirus encoding GMF-γ shRNA or control shRNA for 3 days. Force development in response to contractile activation was compared before and after lentivirus transduction. For biochemical analysis, human tissues were frozen using liquid nitrogen and pulverized as previously described (25, 26).
Gene Synthesis and Site-Directed Mutagenesis
Human GMF-γ DNA (NCBI accession number, NM_004877.2) was synthesized by Life Technologies and subcloned into bacterial vectors (pEGFP or pGEX-4T). A Quickchange II site-directed mutagenesis kit (Stratagene) was used to generate Y104F mutant (phenylalanine substitution at Tyr-104). The 5′ primer was 5′-gccggaacaacagatgatgttcgcagggagtaaaaacagg-3′. The 3′ primer was 5′-cctgtttttactccctg cgaacatcatctgttgttccggc-3′.
Statistical Analysis
Statistical analyses were performed using Prism 6 software (Graph Pad Software, San Diego, CA). Comparison among multiple groups was performed by one-way ANOVA followed by Tukey’s multiple comparison test. Differences between pairs of groups were analyzed by Student-Newman-Keuls test. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered significant.
Results
GMF-γ Inhibits Actin Polymerization and Airway Smooth Muscle Contraction
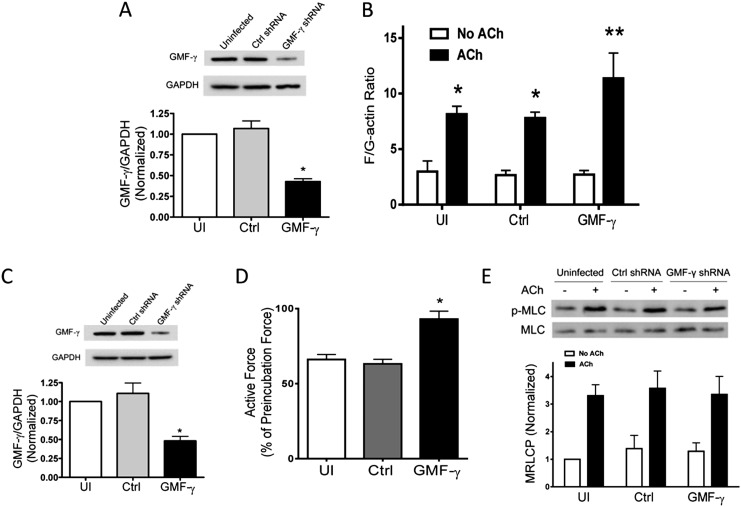
In vitro biochemical studies implicate GMF-γ in inhibiting actin filament nucleation (14, 15). The role of GMF-γ in human cells and tissues has not been investigated. We evaluated the effects of GMF-γ KD on actin polymerization in HASM cells. Stable GMF-γ KD cells were generated by using lentivirus-mediated RNAi. Immunoblot analysis showed that the protein level of GMF-γ in cells infected with virus for GMF-γ shRNA was lower compared with control cells. However, the expression level of GAPDH was similar in these cells. Ratios of GMF-γ/GAPDH were lower in GMF-γ KD cells than in control cells (Figure 1A). We then evaluated the effects of GMF-γ KD on actin dynamics by using the fractionation assay. Acetylcholine (ACh) stimulation induced increases in polymerization of actin in cells (Figure 1B). Furthermore, the increase in F/G actin ratios in response to ACh stimulation was higher in GMF-γ KD cells compared with uninfected cells and cells expressing control shRNA (Figure 1B). Moreover, we used fluorescent microscopy to evaluate F/G-actin ratios; GMF-γ KD enhanced the agonist-induced actin polymerization (see Figure E1 in the online supplement).
Figure 1.
Glia maturation factor (GMF)-γ has a negative role in regulating actin dynamics in response to contractile activation. (A) Human airway smooth muscle (HASM) cells were infected with lentivirus encoding control (Ctrl) or GMF-γ short hairpin RNA (shRNA) for 3 to 4 days. Blots of these cells were probed with use of antibodies against GMF-γ and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). *Significantly lower protein ratios in cells infected with virus for GMF-γ shRNA than in uninfected (UI) cells and cells infected with Ctrl shRNA (P < 0.05; n = 6). (B) Cells expressing Ctrl shRNA or GMF-γ shRNA were stimulated with acetylcholine (ACh) (10−4 M, 5 min) or left unstimulated. F/G actin ratios in these cells were evaluated as described in Materials and Methods. ACh-stimulated F/G actin ratios are higher than corresponding unstimulated cells (*P < 0.05). F/G actin ratios during ACh stimulation were higher in GMF-γ knockdown (KD) cells than in uninfected cells and cells expressing Ctrl shRNA (**P < 0.05). Values represent mean ± SE (n = 6). (C) Immunoblot analysis showing RNAi-mediated GMF-γ KD in human bronchial rings. Lentivirus encoding Ctrl shRNA or GMF-γ shRNA was transduced into the tissues and incubated for 3 days. Protein ratios of GMF-γ/GAPDH in tissues transduced with lentivirus encoding GMF-γ shRNA were significantly lower than those in uninfected tissues and tissues expressing Ctrl shRNA (*P < 0.05; n = 4). (D) Contractile responses of human bronchial rings to ACh (10−4 M) were determined, after which lentivirus encoding Ctrl shRNA or GMF-γ shRNA was transduced into the tissues for 3 days. Force development in bronchial rings was then determined. *Contractile response was enhanced in rings transduced with lentivirus encoding GMF-γ shRNA compared with uninfected tissues and tissues treated with Ctrl shRNA (P < 0.05; n = 4). (E) Myosin light chain phosphorylation at Ser-19 in cells under various treatments was assessed by immunoblot analysis. Myosin phosphorylation was similar in uninfected cells, cells expressing Ctrl shRNA and GMF-γ KD cells (P > 0.05). Values represent mean ± SE (n = 6). MLC, myosin light chain; p-MLC, phospho-myosin light chain.
To assess the role of GMF-γ in smooth muscle contraction, we developed a lentivirus-mediated RNAi to inhibit the expression of GMF-γ in smooth muscle tissues (7). Briefly, the contractile responses of human bronchial rings were determined and transduced with lentivirus-encoding control shRNA or GMF-γ shRNA for 3 days. Immunoblot analysis confirmed lower GMF-γ expression in these tissues (Figure 1C). Force development in bronchial rings was then determined. In uninfected tissues and tissues treated with virus encoding control shRNA, contractile response was 70% of preincubation force. However, contractile force in tissues transduced with virus for GMF-γ shRNA was increased to 95% of preincubation level (Figure 1D). Moreover, myosin light chain phosphorylation at Ser-19 was not different among uninfected cells, cells infected with virus for control shRNA, and GMF-γ KD cells (Figure 1E).
Agonist Stimulation Induces the Dissociation of GMF-γ from Arp2 in Smooth Muscle Cells and Tissues
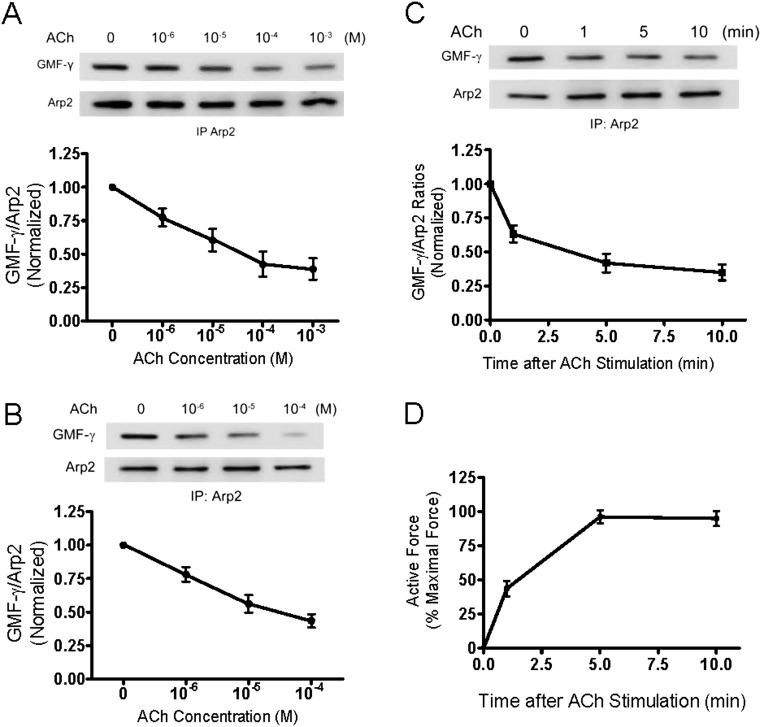
In vitro biochemical studies suggest that GMF-γ may bind to the Arp2/3 complex and induce debranching and disassembly of actin filament networks (15). We hypothesized that GMF-γ may bind to Arp2/3 and induce actin disassembly in resting cells. Contractile stimulation may induce the dissociation of GMF-γ from Arp2/3 and reduce actin disassembly. To test this, HASM cells were stimulated with ACh or left unstimulated. The interaction of GMF-γ with Arp2 component of the Arp2/3 complex was evaluated by coimmunoprecipitation analysis. The seven proteins of the Arp2/3 complex form a stable and tightly associated complex, which remains intact in cells (10). Thus, we can use individual Arp2/3 complex proteins as markers for the entire Arp2/3 complex (6, 8). ACh stimulation reduced ratios of GMF-γ to immunoprecipitated Arp2 in cells, which was dose dependent (Figure 2A). Moreover, the ACh-induced decrease in GMF-γ/Arp2 ratios was dose dependent in human bronchial tissues (Figure 2B). The ACh-induced decrease in the GMF-γ/Arp2 ratios was also time dependent (Figure 2C). More importantly, the decrease in GMF-γ/Arp2 ratios was inversely associated with force development in smooth muscle (Figures 2C and 2D). These results suggest that contractile stimulation induces GMF-γ disengagement from Arp2/3 in smooth muscle.
Figure 2.
Contractile activation induces the dissociation of GMF-γ from Arp2 in cells and tissues. (A) Stimulation with ACh induces the dissociation of GMF-γ from Arp2. Blots of Arp2 immunoprecipitates from unstimulated and stimulated (ACh, 5 min) cells were probed with use of antibodies against GMF-γ and Arp2. GMF-γ/Arp2 ratios in stimulated cells are normalized to those in unstimulated cells. (B) Stimulation with ACh (5 min) results in the disengagement of GMF-γ from Arp2 in human bronchial tissues in a dose-dependent manner. (C) Time dependence of ACh (10−4 M)-induced dissociation of GMF-γ from Arp2 from tissues. (D) Time course of contractile response of human bronchial rings. Force is expressed as percent of maximal response to ACh (10−4 M, 5 min). All values are means ± SE (n = 4).
GMF-γ Undergoes Phosphorylation at Tyr-104 upon ACh Stimulation
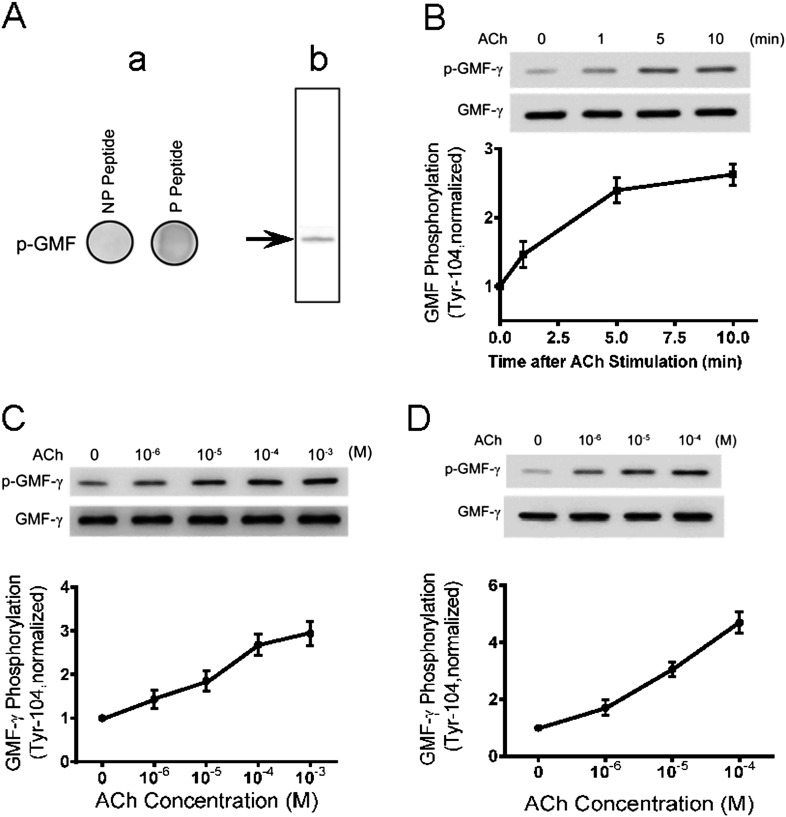
Structural analysis reveals that Tyr-104 is near the C-terminus of the protein. This raises the possibility that this site may be phosphorylated in response to external stimulation. To test this, we generated phospho–GMF-γ (Tyr-104) antibody. Dot blot analysis showed that the phospho–GMF-γ antibody reacted with phospho–GMF-γ peptide but not with nonphosphorylated peptide (Figure 3A, panel a). Moreover, the antibody reacted with phospho-GMF-γ from cell extracts (Figure 3A, panel b). The results demonstrate the specificity of the phospho–GMF-γ (Tyr-104) antibody.
Figure 3.
GMF-γ undergoes phosphorylation at Tyr-104 in HASM cells/tissues upon contractile activation. (A) Specificity of phospho–GMF-γ antibody (Tyr-104). Panel a: Phospho-GMF-γ peptide and nonphosphorylated GMF-γ peptide (5 μg) were immobilized on the membrane and then detected with use of phospho–GMF-γ antibody (Tyr-104). The antibody reacts with the phospho-peptide only. NP, nonphosphorylated; P, phosphorylated. Panel b: Phospho–GMF-γ antibody is able to detect phospho–GMF-γ from cell extracts. (B) HASM cells were stimulated with 10−4 M ACh for different periods of time or left unstimulated. GMF-γ phosphorylation at Tyr-104 was evaluated by immunoblotting. The phosphorylation level of GMF-γ in stimulated cells is normalized to unstimulated cells (n = 6). (C) GMF-γ phosphorylation in cells treated with different concentrations of ACh for 5 minutes was determined by immunoblot analysis (n = 6). (D) GMF-γ phosphorylation was assessed for human bronchial tissues treated with different concentrations of ACh for 5 minutes (n = 6). All values are means ± SE.
We then used this antibody to evaluate whether GMF-γ phosphorylation occurs in response to contractile activation. Cells were treated with ACh for different time periods or were left unstimulated. GMF-γ phosphorylation was assessed by immunoblot analysis. Stimulation with ACh induced GMF-γ phosphorylation at Tyr-104 in HASM cells, which was time dependent and dose dependent (Figures 3B and 3C).
To evaluate whether GMF-γ phosphorylation occurred at tissue level, we determined the effects of contractile activation on GMF-γ phosphorylation in human bronchial tissues. The phosphorylation level of GMF-γ at Tyr-104 was augmented in response to ACh stimulation (Figure 3D).
c-Abl Regulates GMF-γ Phosphorylation In Vitro and in Smooth Muscle Cells during Agonist Activation
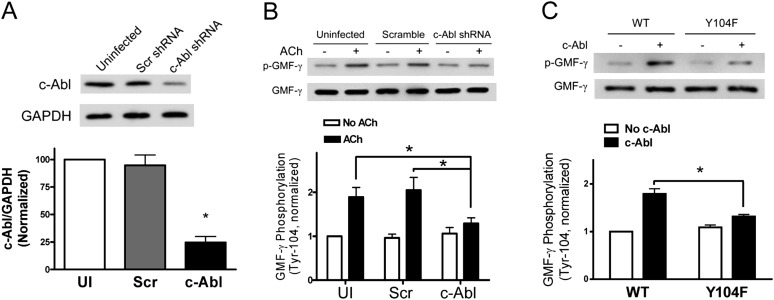
The kinase that mediates GMF-γ tyrosine phosphorylation has not been explored before. c-Abl is known to regulate actin filament polymerization in various cell types, including smooth muscle cells (8, 20, 27, 28). We hypothesized that c-Abl may mediate the phosphorylation of GMF-γ in human cells. To test the hypothesis, we determined the effects of c-Abl KD on GMF-γ phosphorylation. c-Abl KD cells were generated by lentivirus-mediated RNAi as previously described (7, 13, 17). Immunoblot analysis verified c-Abl KD in this cell line (Figure 4A).
Figure 4.
GMF-γ phosphorylation at Tyr-104 is mediated by c-Abl. (A) Immunoblot analysis showing c-Abl KD by lentivirus-mediated RNAi. Protein ratios of c-Abl/GAPDH in cells infected with lentivirus encoding c-Abl shRNA were significantly lower than those in uninfected (UI) cells and cells expressing scramble (Scr) shRNA (*P < 0.05; n = 5). (B) Uninfected HASM cells, cells expressing scramble shRNA, or c-Abl KD cells were stimulated with 10−4 M ACh for 5 minutes. GMF-γ phosphorylation at Tyr-104 was evaluated by immunoblotting. The phosphorylation levels of GMF-γ in cells under various treatments are normalized to the level in unstimulated and uninfected cells (P < 0.05; n = 6). (C) Wild-type (WT) GMF-γ or mutant Y104F GMF-γ was reacted with purified c-Abl protein. c-Abl catalyzes WT GMF-γ phosphorylation, but not mutant protein (*P < 0.05; n = 6). All values are means ± SE.
GMF-γ phosphorylation in c-Abl KD cells and control cells was evaluated by immunoblot analysis. Basal GMF-γ phosphorylation was similar in uninfected cells and in cells producing scramble shRNA or c-Abl shRNA. However, GMF-γ phosphorylation in response to stimulation with ACh was significantly lower in c-Abl KD cells compared with uninfected cells or cells producing scramble shRNA (Figure 4B). The results suggest that c-Abl may regulate the phosphorylation of GMF-γ in HASM during contractile activation.
We used in vitro kinase assay to determine whether c-Abl directly catalyzes GMF-γ phosphorylation. In the presence of c-Abl, wild-type (WT) GMF-γ underwent phosphorylation at Tyr-104. However, the phosphorylation level of mutant Y104F GMF-γ was lower compared with WT GMF-γ (Figure 4C). The result suggests that GMF-γ phosphorylation at Tyr-104 is catalyzed by c-Abl.
GMF-γ Phosphorylation Regulates its Association with Arp2
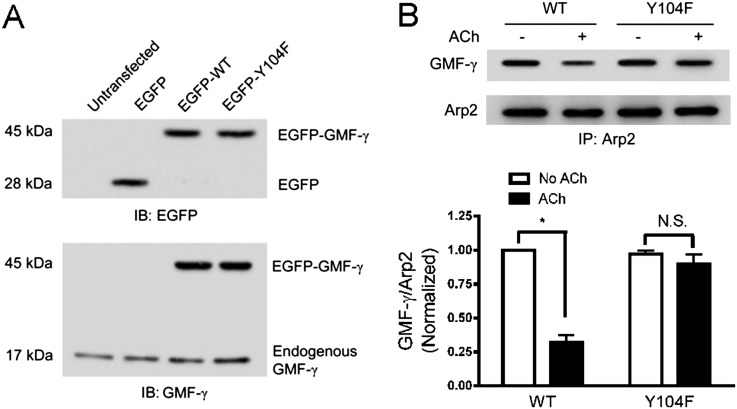
Thus far, we have shown that agonist stimulation induced the dissociation of GMF-γ from Arp2 and GMF-γ phosphorylation. To assess whether GMF-γ phosphorylation affects its interaction with Arp2, cells were transfected with plasmid encoding WT or Y104F GMF-γ. Immunoblot analysis verified the expression of the recombinant proteins (Figure 5A). In cells expressing WT GMF-γ, ACh stimulation significantly reduced the ratios of GMF-γ/Arp2 (P < 0.05) (Figure 5B). In contrast, ACh activation slightly diminished the ratios of GMF-γ/Arp2 in cells expressing Y104F mutant but without statistical difference (P > 0.05) (Figure 5B). The results suggest that GMF-γ phosphorylation at Tyr-104 promotes its dissociation from Arp2/3 in response to contractile activation.
Figure 5.
GMF-γ phosphorylation at Tyr-104 regulates its association with Arp2. (A) Cells were transfected with enhanced green fluorescent protein (EGFP) plasmid or plasmid encoding WT GMF-γ or mutant Y104F GMF-γ or left untransfected. Protein extracts of these cells were immunoblotted (IB) with EGFP antibody or GMF-γ antibody. EGFP-tagged proteins with 45-kD molecular weight were detected in the extracts of cells treated with the plasmid for WT and Y104F GMF-γ but not in untransfected cells or in cells transfected with empty vector, indicating effective expression of the fusion proteins in these cells. In cells transfected with constructs of WT or Y104F GMF-γ, ∼ 70% of total GMF-γ was the tagged recombinant proteins. Blots are representative of four identical experiments. (B) Cells expressing WT GMF-γ or mutant Y104F GMF-γ were treated with ACh or left untreated. Protein interaction was evaluated by coimmunoprecipitation. (*P < 0.05; n = 5–6). All values are means ± SE. N.S., not significant.
GMF-γ Phosphorylation at Tyr-104 Promotes Actin Polymerization and Smooth Muscle Contraction without Affecting Myosin Phosphorylation
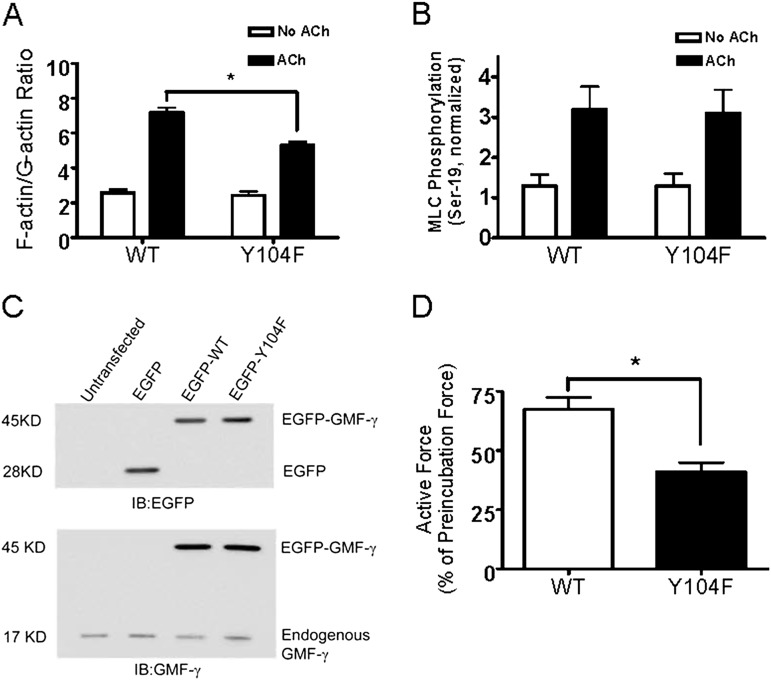
To reveal the functional role of GMF-γ phosphorylation in smooth muscle, we evaluated the effects of the expression of the nonphosphorylatable Y104F GMF-γ on actin dynamics and myosin activation by using the fractionation assay and immunoblot analysis, respectively. The increase in F/G-actin ratios in response to ACh stimulation was reduced in cells expressing Y104F GMF-γ as compared with cells expressing WT GMF-γ (Figure 6A). However, myosin light chain phosphorylation at Ser-19 was not different among cells expressing WT GMF-γ and cells expressing Y104F GMF-γ (Figure 6B).
Figure 6.
GMF-γ phosphorylation at Tyr-104 modulates actin polymerization and contraction in smooth muscle without affecting myosin activation. (A) Cells expressing WT GMF-γ or mutant Y104F GMF-γ were stimulated with 10−4 M ACh for 5 minutes or left unstimulated. F/G-actin ratios were evaluated as described in Materials and Methods (*P < 0.05; n = 6). (B) Myosin light chain (MLC) phosphorylation at Ser-19 was determined for cells expressing the recombinant proteins. (C) Verification of recombinant proteins in human bronchial tissues. Tissues were transfected with EGFP plasmid or plasmid encoding WT GMF-γ or mutant Y104F GMF-γ or left untransfected. Protein extracts of these cells were immunoblotted with EGFP antibody or GMF-γ antibody. Immunoblots are representative of four identical experiments. (D) Contractile responses of human bronchial rings to ACh (10−4 M) were determined, after which plasmid encoding WT or mutant Y104F GMF-γ was transduced into the tissues by reversible permeabilization and incubated for 3 days. Force development in bronchial rings was then determined. All values are means ± SE (*P < 0.05; n = 4).
We also assessed the role of GMF-γ phosphorylation in force development. Briefly, the contractile responses of human bronchial rings were determined, after which plasmid encoding WT GMF-γ or Y104F GMF-γ was transduced into the tissues by reversible permeabilization using methods we and others have previously established (8, 29, 30). Force development in bronchial rings was then determined. In tissues transfected with plasmid encoding WT GMF-γ, contractile response was 68% of preincubation force. However, contractile force in tissues transfected with plasmid encoding Y104F GMF-γ was reduced to 41% of preincubation level (Figures 6C and 6D).
Discussion
Actin polymerization/depolymerization has recently emerged as a critical cellular process that regulates contraction/relaxation cycle of smooth muscle (3–8). The mechanisms that regulate actin dynamics in smooth muscle are not well elucidated. GMF-γ is a member of the ADF/cofilin family, which has been implicated in regulating actin dynamics (14, 15). In vitro biochemical studies suggest that GMF-γ inhibits actin nucleation (15). To determine the role of GMF-γ, we evaluated the effects of GMF-γ KD on actin dynamics in human cells. GMF-γ KD significantly enhanced polymerization of actin and contraction in smooth muscle upon contractile activation. The results suggest that GMF-γ has a negative role in regulating F-actin level and smooth muscle contraction. Moreover, GMF-γ silencing did not affect myosin light chain phosphorylation. Thus, GMF-γ is not involved in the regulation of myosin activation.
In vitro studies suggest that GMF-γ may induce debranching and disassembly of actin filament networks by interacting with the Arp2/3 complex (14, 15). Structural analysis reveals that the ADF-H domain of GMF-γ binds the barbed end of Arp2, overlapping with the proposed binding site of the WASP-family proteins (31). Here, GMF-γ is able to bind to Arp2 in resting cells. Contractile activation induced a decrease in GMF-γ/Arp2 protein ratios. It is known that the level of F-actin is higher in stimulated smooth muscle than in unstimulated smooth muscle (3, 4, 6, 7, 12, 32). It is likely that GMF-γ binds to Arp2 in unstimulated cells, which induces debranching and disassembly of actin networks. Because F-actin level is regulated by the balance of actin disassembly and actin assembly, increased actin disassembly may retain lower levels of F-actin in unstimulated smooth muscle cells. Upon agonist stimulation, GMF-γ dissociates from Arp2, which leads to reduced actin disassembly and thus increases F-actin level. GMF-γ KD did not significantly affect basal F/G-actin ratios. This may be due to the lower exchange rate between actin assembly and actin disassembly in unstimulated cells. In contrast, the exchange rate between actin assembly and actin disassembly is higher in stimulated smooth muscle (3, 4, 6–8, 12). Thus, GMF-γ KD may affect the overall level of F-actin during contractile activation.
The regulation of GMF-γ functional state is largely unknown. In this study, we for the first time demonstrate that Tyr-104 is a pivotal phosphorylation site on GMF-γ. Furthermore, phosphorylation at this residue regulates its association with Arp2, actin dynamics, and cell contraction. Our result is supported by a previous report by others (33). Vasodilator-stimulated phosphoprotein is an actin-regulatory protein that has a role in modulating cell adhesion and migration. Phosphorylation of vasodilator-stimulated phosphoprotein by AMP-dependent kinase decreases its F-actin binding capability (33). Thus, it is likely that phosphorylation at Tyr-104 creates negative charges on the molecule, inducing conformational changes and the dissociation of GMF-γ from Arp2/3, which in turn modulates actin dynamics and cell contraction.
The nonreceptor tyrosine kinase c-Abl has a role in the regulation of smooth muscle contraction. c-Abl is activated in smooth muscle upon contractile activation. c-Abl has been shown to activate Crk-associated substrate and Abi1, which subsequently modulate actin dynamics and contraction (7, 8, 20, 21). However, other molecules may also be controlled by c-Abl. Here, c-Abl is required for GMF-γ phosphorylation at Tyr-104. Moreover, c-Abl directly catalyzes GMF-γ phosphorylation at this residue. Thus, agonist stimulation may activate c-Abl, which catalyzes GMF-γ phosphorylation at Tyr-104. This phosphorylation leads to the dissociation of GMF-γ from Arp2/3, by which GMF-γ no longer induces actin disassembly. Reduced actin disassembly renders F-actin in higher level, which promotes smooth muscle contraction.
Actin polymerization is regulated by polymerization, depolymerization, sequestration, G-actin transport, and barbed-end capping. Actin polymerization is controlled by the nucleation-promoting factors and their regulators (3, 6, 8, 10, 12, 13). On the other hand, actin depolymerization is mediated by several factors, including ADF/cofilin (14–16). Moreover, thymyosin-β4 is able to sequester actin monomers, whereas profilin facilitates G-actin transport (6, 10, 34). Here, we show that GMF-γ is able to interact with Arp2/3 in cells. More importantly, the activity of GMF-γ is regulated by phosphorylation at Tyr-104. To the best of our knowledge, this is the first evidence to suggest that function of a member of the ADF/cofilin family is regulated by tyrosine phosphorylation.
Actin polymerization may facilitate smooth muscle contraction by several mechanisms. Actin polymerization may enhance the linkage of actin filaments to integrins, strengthening the transduction of mechanical force between contractile units and extracellular matrix (3, 6, 35, 36). In addition, actin filament assembly may increase the numbers of contractile units and the length of actin filaments, providing more and efficient contractile elements for force development (37).
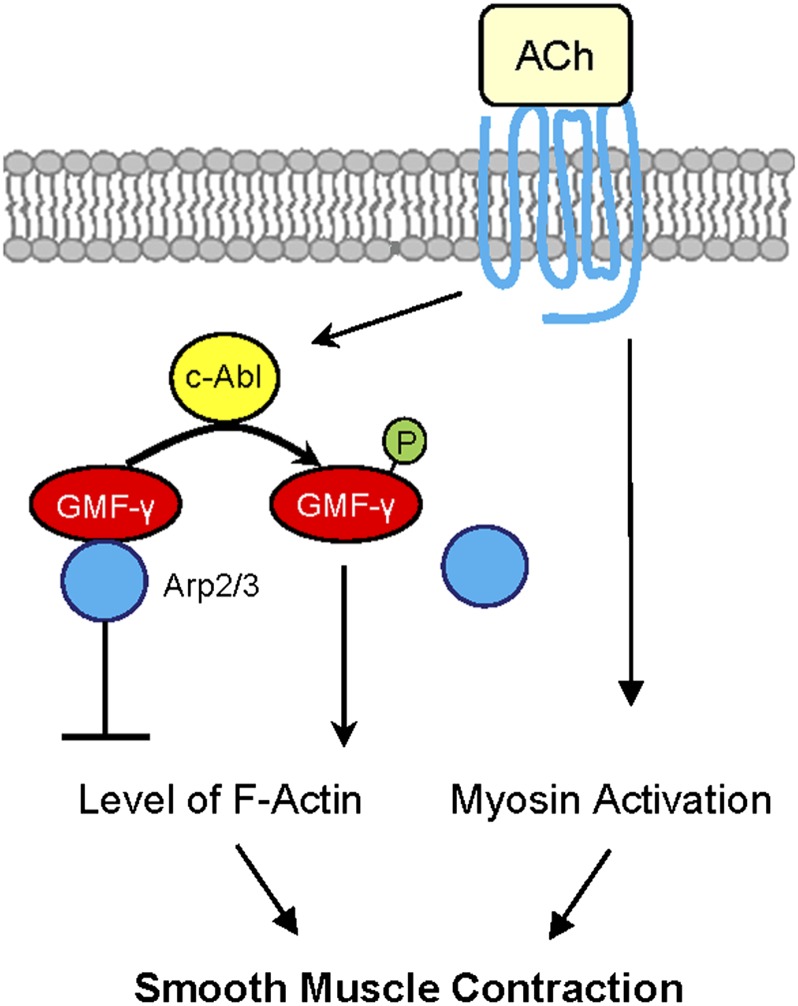
Allergic asthma is characterized by airway hyperresponsiveness, which largely stems from increased airway smooth muscle contraction (38). IL-13 has been implicated in the hyperactivity of airway smooth muscle (39). In addition, increases in intracellular Ca2+ signaling may contribute to the pathogenesis of smooth muscle hyperreactivity (40). Our recent studies suggest that c-Abl has an essential role in the development of airway hyperresponsiveness (41). In this study, we found that GMF-γ phosphorylation at Tyr-104 is critical for the regulation of HASM contraction. Furthermore, GMF-γ phosphorylation at this residue is mediated by c-Abl (Figure 7). Because c-Abl has been shown to mediate airway hyperresponsiveness (41), it is likely that c-Abl may induce airway hyperresponsiveness in part by modulating the phosphorylation of GMF-γ at this residue.
Figure 7.
Proposed mechanism. In unstimulated smooth muscle, GMF-γ functions as an actin network assembly suppressor. GMF-γ binds to Arp2/3, which induces actin disassembly. In addition to myosin activation, agonist stimulation activates c-Abl tyrosine kinase, which catalyzes GMF-γ phosphorylation at Tyr-104. This phosphorylation may induce conformation changes, which leads to the dissociation of GMF-γ from Arp2/3 (disinhibition) and promotes actin network assembly and force development in smooth muscle.
Footnotes
This work was supported by National Institutes of Health grants HL-110951 and HL-113208 (D.D.T.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0125OC on May 12, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gao N, Huang J, He W, Zhu M, Kamm KE, Stull JT. Signaling through myosin light chain kinase in smooth muscles. J Biol Chem. 2013;288:7596–7605. doi: 10.1074/jbc.M112.427112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He ZH, Sheng S, Shao Z, Somlyo AP. Smooth muscle myosin: regulation and properties. Philos Trans R Soc Lond B Biol Sci. 2004;359:1921–1930. doi: 10.1098/rstb.2004.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HR, Gallant C, Leavis PC, Gunst SJ, Morgan KG. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008;295:C768–C778. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol. 2007;293:C993–C1002. doi: 10.1152/ajpcell.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther. 2008;13:130–140. doi: 10.1177/1074248407313737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Cleary RA, Wang R, Tang DD. Role of the adapter protein abi1 in actin-associated signaling and smooth muscle contraction. J Biol Chem. 2013;288:20713–20722. doi: 10.1074/jbc.M112.439877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits cas-mediated process and constriction in resistance arteries. Circ Res. 2007;101:420–428. doi: 10.1161/CIRCRESAHA.107.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh MP, Cole WC. The role of actin filament dynamics in the myogenic response of cerebral resistance arteries. J Cereb Blood Flow Metab. 2013;33:1–12. doi: 10.1038/jcbfm.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollard TD. Regulation of actin filament assembly by arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 11.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–519. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang DD. P130 crk-associated substrate (cas) in vascular smooth muscle. J Cardiovasc Pharmacol Ther. 2009;14:89–98. doi: 10.1177/1074248409333490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Mercaitis OP, Jia L, Panettieri RA, Tang DD. Raf-1, actin dynamics and abl in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2013;48:172–178. doi: 10.1165/rcmb.2012-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi M, Smith BA, Bovellan M, Paavilainen V, Daugherty-Clarke K, Gelles J, Lappalainen P, Goode BL. Gmf is a cofilin homolog that binds arp2/3 complex to stimulate filament debranching and inhibit actin nucleation. Curr Biol. 2010;20:861–867. doi: 10.1016/j.cub.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ydenberg CA, Padrick SB, Sweeney MO, Gandhi M, Sokolova O, Goode BL. Gmf severs actin-arp2/3 complex branch junctions by a cofilin-like mechanism. Curr Biol. 2013;23:1037–1045. doi: 10.1016/j.cub.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poukkula M, Kremneva E, Serlachius M, Lappalainen P. Actin-depolymerizing factor homology domain: a conserved fold performing diverse roles in cytoskeletal dynamics. Cytoskeleton. 2011;68:471–490. doi: 10.1002/cm.20530. [DOI] [PubMed] [Google Scholar]
- 17.Jia L, Wang R, Tang DD. Abl regulates smooth muscle cell proliferation by modulating actin dynamics and erk1/2 activation. Am J Physiol Cell Physiol. 2012;302:C1026–C1034. doi: 10.1152/ajpcell.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Z, Cang Y, Goff SP. C-abl tyrosine kinase regulates cardiac growth and development. Proc Natl Acad Sci USA. 2010;107:1136–1141. doi: 10.1073/pnas.0913131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Bliss JM, Wang Y, Colicelli J. Rin1 is an abl tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr Biol. 2005;15:815–823. doi: 10.1016/j.cub.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Jia L, Tang DD. Abl activation regulates the dissociation of cas from cytoskeletal vimentin by modulating cas phosphorylation in smooth muscle. Am J Physiol Cell Physiol. 2010;299:C630–C637. doi: 10.1152/ajpcell.00095.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Wang R, Li QF, Tang DD. Abl knockout differentially affects p130 crk-associated substrate, vinculin, and paxillin in blood vessels of mice. Am J Physiol Heart Circ Physiol. 2009;297:H533–H539. doi: 10.1152/ajpheart.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Tang DD.C-abl tyrosine kinase regulates cytokinesis of human airway smooth muscle cells Am J Respir Cell Mol Biol 2014;50:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006;281:34716–34724. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J. 2005;388:773–783. doi: 10.1042/BJ20050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ach activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L240–L248. doi: 10.1152/ajplung.00199.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R, Li Q, Tang DD. Role of vimentin in smooth muscle force development. –Am J Physiol Cell Physiol. 2006;291:C483–C489. doi: 10.1152/ajpcell.00097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JY. Controlling abl: auto-inhibition and co-inhibition? Nat Cell Biol. 2004;6:3–7. doi: 10.1038/ncb0104-3. [DOI] [PubMed] [Google Scholar]
- 28.Burton EA, Oliver TN, Pendergast AM. Abl kinases regulate actin comet tail elongation via an n-wasp-dependent pathway. Mol Cell Biol. 2005;25:8834–8843. doi: 10.1128/MCB.25.20.8834-8843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YL, Zhang WW, Gunst SJ. Activation of vinculin induced by cholinergic stimulation regulates contraction of tracheal smooth muscle tissue. J Biol Chem. 2011;286:3630–3644. doi: 10.1074/jbc.M110.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem. 2008;283:36522–36531. doi: 10.1074/jbc.M805294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luan Q, Nolen BJ. Structural basis for regulation of arp2/3 complex by gmf. Nat Struct Mol Biol. 2013;20:1062–1068. doi: 10.1038/nsmb.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerthoffer WT. Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol. 2005;83:851–856. doi: 10.1139/y05-088. [DOI] [PubMed] [Google Scholar]
- 33.Thomson DM, Ascione MP, Grange J, Nelson C, Hansen MD. Phosphorylation of vasp by ampk alters actin binding and occurs at a novel site. Biochem Biophys Res Commun. 2011;414:215–219. doi: 10.1016/j.bbrc.2011.09.059. [DOI] [PubMed] [Google Scholar]
- 34.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 35.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J. 2000;15:600–616. doi: 10.1034/j.1399-3003.2000.15.29.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 37.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1161–L1168. doi: 10.1152/ajplung.00298.2003. [DOI] [PubMed] [Google Scholar]
- 38.Amrani Y, Tliba O, Deshpande DA, Walseth TF, Kannan MS, Panettieri RA., Jr Bronchial hyperresponsiveness: insights into new signaling molecules. Curr Opin Pharmacol. 2004;4:230–234. doi: 10.1016/j.coph.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Tliba O, Deshpande D, Chen H, Van BC, Kannan M, Panettieri RA, Jr, Amrani Y. Il-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br J Pharmacol. 2003;140:1159–1162. doi: 10.1038/sj.bjp.0705558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of cd38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol. 2004;31:36–42. doi: 10.1165/rcmb.2003-0313OC. [DOI] [PubMed] [Google Scholar]
- 41.Cleary RA, Wang R, Wang T, Tang DD. Role of abl in airway hyperresponsiveness and airway remodeling. Respir Res. 2013;14:105. doi: 10.1186/1465-9921-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]