Abstract
The association between small intestinal epithelium and enteropathogenic Escherichia coli (EEC) was studied in ligated intestinal loops of pigs and rabbits. The association indexes (degree of association) for each of two porcine EEC strains varied widely among pigs and independently of each other. Significant litter-to-litter variations in association indexes among colostrum-deprived newborn pigs were interpreted to be the result of congenital resistance to association with specific EEC in some pigs. Since enterosorption occurred in loops with low association indexes, it was not necessary for EEC to establish a high association index for them to cause enterosorption in ligated intestinal loops. Two strains of EEC which are enteropathogenic for humans caused enterosorption in ligated loops in pigs 3 weeks old or less but not in 6-week-old pigs.
Full text
PDF
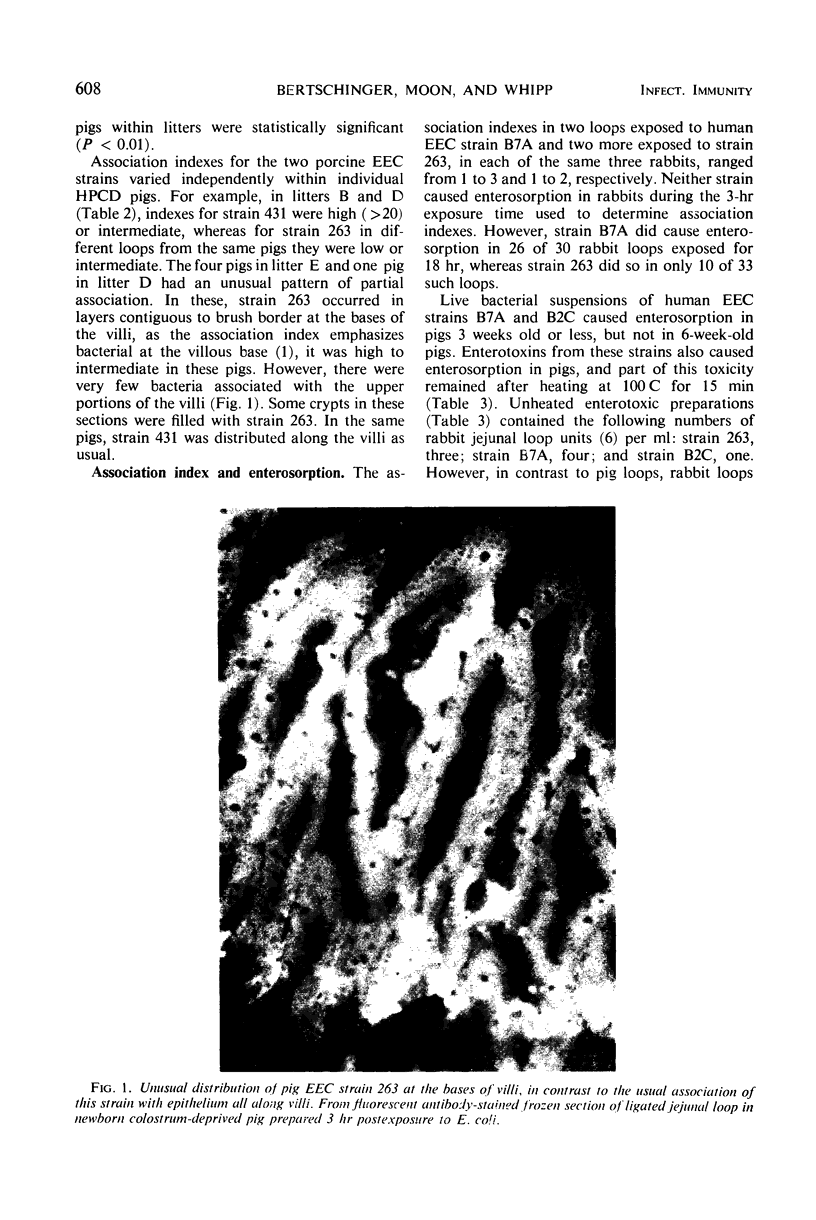


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L., Barnum D. A. Escherichia coli in ligated segments of pig intestine. J Pathol Bacteriol. 1967 Jul;94(1):189–194. doi: 10.1002/path.1700940124. [DOI] [PubMed] [Google Scholar]
- MYERS W. L., SEGRE D. THE IMMUNOLOGIC BEHAVIOR OF BABY PIGS. III. TRANSPLACENTAL TRANSFER OF ANTIBODY GLOBULIN IN SWINE. J Immunol. 1963 Nov;91:697–700. [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C. Development of resistance with age by swine intestine to effects of enteropathogenic Escherichia coli. J Infect Dis. 1970 Sep;122(3):220–223. doi: 10.1093/infdis/122.3.220. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Engstrom G. W., Baetz A. L. Response of the rabbit ileal loop to cell-free products from Escherichia coli enteropathogenic for swine. J Infect Dis. 1970 Feb;121(2):182–187. doi: 10.1093/infdis/121.2.182. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Observations by the ligated intestinal segment and oral inoculation methods on Escherichia coli infections in pigs, calves, lambs and rabbits. J Pathol Bacteriol. 1967 Apr;93(2):499–529. doi: 10.1002/path.1700930211. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Halls S. The production of oedema disease and diarrhoea in weaned pigs by the oral administration of Escherichia coli: factors that influence the course of the experimental disease. J Med Microbiol. 1968 Aug;1(1):45–59. doi: 10.1099/00222615-1-1-45. [DOI] [PubMed] [Google Scholar]
- Ujiye A., Kobari K. Protective effect on infections with Vibrio cholerae in suckling mice caused by the passive immunization with milk of immune mothers. J Infect Dis. 1970 May;121(Suppl):50+–50+. doi: 10.1093/infdis/121.supplement.s50. [DOI] [PubMed] [Google Scholar]