Abstract
Background. Protecting young children from pandemic influenza should also reduce transmission to susceptible adults, including pregnant women.
Methods. An open study assessed immunogenicity and reactogenicity of a heterologous booster dose of A/turkey/Turkey/1/2005(H5N1)-AS03B (AS03B is an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion [5.93 mg tocopherol]) in infants and children aged 6 to < 36 months that was given 6 months following 2-dose primary vaccination with A/Indonesia/05/2005(H5N1)-AS03B. Vaccines contained 1.9 µg of hemagglutinin antigen and AS03B. Hemagglutinin inhibition (HI) responses, microneutralization titers, and antineuraminidase antibody levels were assessed for 6 months following the booster vaccination.
Results. For each age stratum (defined on the basis of the subject's age at first vaccination as 6 to < 12 months, 12 to < 24 months, and 24 to < 36 months) and overall (n = 113), European influenza vaccine licensure criteria were fulfilled for responses to A/turkey/Turkey/1/2005(H5N1) 10 days following the booster vaccination. Local pain and fever increased with consecutive doses. Anamnestic immune responses were demonstrated for HI, neutralizing, and antineuraminidase antibodies against vaccine-homologous/heterologous strains. Antibody responses to vaccine-homologous/heterologous strains persisted in all children 6 months following the booster vaccination.
Conclusions. Prevaccination of young children with a clade 2 strain influenza A(H5N1) AS03-adjuvanted vaccine followed by heterologous booster vaccination boosted immune responses to the homologous strain and a related clade, with persistence for at least 6 months. The results support a prime-boost vaccination approach in young children for pandemic influenza preparedness.
Clinical Trials Registration. NCT01323946.
Keywords: pandemic, influenza, H5N1, children, booster
Avian influenza A(H5N1) was identified as a cause of death in poultry in 1996, with the first human cases of infection recorded in 1997 [1]. From 2003 until January 2014, influenza A(H5N1) has caused 650 cases of influenza in humans [2]. Around 90% of cases have occurred in individuals <40 years of age, with a case-fatality rate of 60% [3]. Although not yet able to spread efficiently between humans, influenza A(H5N1) is considered to be a potential threat for a future influenza pandemic [4]. Globally, preparatory activities are being undertaken to develop vaccines and vaccination strategies that could provide widespread protection in the event of a pandemic due to influenza A(H5N1) [5].
Pandemic preparedness strategies include the prepandemic vaccination of a population, to reduce attack rates in the event of a pandemic, or the release of stockpiled prepandemic vaccine at the start of a pandemic, to prime or protect recipients until strain-matched vaccine becomes available. Use of a prepandemic vaccine could be successful if the vaccine strain induces a broad immune response that includes a response to the pandemic strain. In the event of a pandemic, subsequent vaccination with a pandemic-strain-specific vaccine would boost the immune response, improving protection against the pandemic strain.
An inactivated, split-virion recombinant influenza A(H5N1) vaccine with 3.75 µg hemagglutinin (HA) combined with the proprietary Adjuvant System 03 (hereafter, “H5N1-AS03”; Prepandrix™ GlaxoSmithKline Vaccines, Dresden, Germany) is licensed for use as a prepandemic vaccine for adults aged ≥18 years of age in the European Union and other countries. A second version of H5N1-AS03 (with the same formulation but manufactured by GlaxoSmithKline Vaccines, Laval, Canada) has been approved for use in pandemic response in the European Union (as Pumarix™), Canada (as Arepanrix™ H5N1), and in the United States as of November 2013 [6].
Children play an important role in influenza outbreaks, having high attack rates and contributing to transmission among their families, schools, and day care centers [7, 8]. Thus, strategies to mitigate influenza virus infection among children could have important effects not only in protecting children, but in reducing transmission to adults, including pregnant women.
H5N1-AS03 had an acceptable clinical safety profile [9] and showed good immunogenicity, with broad clade and subclade antibody cross-reactivity after 2 doses in adults [10–13]. In children aged 3–9 years, 2 doses of H5N1-AS03 (full dose or half dose) were immunogenic for homologous and heterologous vaccine strains [14]. While H5N1-AS03 candidates have been studied extensively in older children and adults, they had not been assessed in infants and younger children, who are particularly vulnerable to influenza. We evaluated the immunogenicity, reactogenicity, and safety of H5N1-AS03, using a heterologous prime-boost vaccination schedule, in young children aged 6 to <36 months.
METHODS
Study Design and Objectives
This phase 2 study was open in design and conducted at 6 centers in Australia and 2 centers in Singapore between 18 April 2011 and 2 November 2012 (clinical trials registration: NCT01323946). The study was conducted according to good clinical practice and in accordance with the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by local institutional review boards. Written informed consent was obtained from the parents/guardians of children before enrollment.
The primary study objective was to assess whether a heterologous booster dose of A/turkey/Turkey/1/2005(H5N1)-AS03B (AS03B contains half the dose of α-tocopherol contained in AS03) given 6 months following a 2-dose primary vaccination series with A/Indonesia/05/2005(H5N1)-AS03B elicited an antibody response that met the European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) targets for influenza vaccine seroconversion rate, seroprotection rate, and mean geometric increase based on the hemagglutinin inhibition (HI) responses to A/turkey/Turkey/1/2005(H5N1) 10 days following the booster vaccination. The CHMP criteria were fulfilled if the point estimate for the seroconversion rate was >40%, the point estimate for the seroprotection rate was >70%, or the point estimate for the mean geometric increase was >2.5.
Secondary and tertiary study objectives included the assessment of immunogenicity in age-based subgroups, immunogenicity in terms of microneutralization and antineuraminidase (anti-NA) antibody titers, reactogenicity and safety of the study vaccines, and assessment of persistence of the immune response to day 364.
All participants received 2 priming doses 21 days apart of the A/Indonesia/05/2005(H5N1)- AS03B candidate vaccine and a booster dose of the A/turkey/Turkey/1/2005(H5N1)-AS03B candidate vaccine on day 182.
Participants were stratified into 3 age strata (defined on the basis of the subject's age at first vaccination as 6 to < 12 months, 12 to <24 months, and 24 to <36 months) in a 2:1:1 ratio. The planned ratio could not be achieved because of difficulties in recruiting infants. A protocol amendment allowed the age stratification ratio to differ from the ratio specified in the original protocol.
Study Participants
Participants were healthy children aged 6 to <36 months. Children were excluded from participation if they were immunosuppressed or had received immunosuppressants or other immune-modifying drugs for >14 days during the 6-month period before receipt of the first vaccine dose or if they had a history of allergy or hypersensitivity to any component of the vaccines, such as egg protein or thimerosal. Children were excluded if administration of any vaccine was planned ≤30 days before or 21 days after any study vaccine administration; if they had a history of any neurological disorders or seizures or a clinically significant pulmonary, cardiovascular, hepatic, or renal functional abnormality; or if they had received immunoglobulins or other blood products ≤3 months before enrollment. Children who had received an influenza A(H5N1) vaccine at any time and those with a history of physician-confirmed influenza A(H5N1) infection were also excluded.
Vaccines
The pandemic influenza vaccines (A/Indonesia/05/2005 and A/turkey/Turkey/1/2005) were 2-component vaccines presented in 2 multidose vials, one containing antigen and the other containing adjuvant. One pediatric dose of the vaccine, after mixing (0.25 mL), contained 1.9 µg of hemagglutinin antigen (HA) and AS03B (which contained 5.93 mg of α-tocopherol and squalene in an oil-in-water emulsion). Vaccines were administered intramuscularly into the anterior thigh (for children <12 months of age) or deltoid (for those ≥12 months of age), alternating sides for each dose.
Immunogenicity Assessment
The humoral immune response to vaccination was assessed on days 0, 42, 182, 192 (10 days following booster vaccination), and 364.
HI antibodies for the A/Indonesia/05/2005 and A/turkey/Turkey/1/2005 strains were measured as previously described but were modified by using horse erythrocytes rather than avian erythrocytes [15–18]. The lowest dilution tested was 1:10. The titration end point was the highest dilution step that showed complete inhibition (100%) of hemagglutination. HI antibody titers of ≥1:40 were considered indicative of seroprotection [19, 20].
The viral microneutralization assay for A/Indonesia/05/2005 and A/turkey/Turkey/1/2005 strains was performed as previously described [15, 17]. In brief, a standardized amount of virus was mixed with serial 2-fold dilutions of serum samples to allow antibody/virus binding. The mixture containing bound antibody was added to Madin-Darby canine kidney cell cultures and incubated for 7 days at 33°C. Viral replication was visualized by hemagglutination of chicken red blood cells. The 50% neutralization titer of a serum was calculated. The assay cutoff was 1:28.
Anti-NA antibodies for the A/Indonesia/05/2005(H5N1) strain were measured in a randomly selected subset of 50% of children as previously described [21]. The test is based on enzymatic activity of NA, which releases neuraminic acid from fetuin. After cleavage of the terminal neuraminic acid by NA, β-D-galactose-N-acetylgalactosamine is unmasked, and peanut agglutinin can bind to this galactose residue. By using peroxidase-labeled peanut agglutinin, the reaction can be detected and quantified in a substrate reaction. The intensity of the substrate reaction is inversely proportional to the quantity of antibodies in the serum.
All serological tests were performed at a GlaxoSmithKline Vaccines' central laboratory, using standardized, validated procedures.
Safety and Reactogenicity Assessment
Local (injection site pain, redness, and swelling) and general (drowsiness, fever [temperature ≥38°C by any measurement route], irritability/fussiness, loss of appetite, diarrhea, and vomiting) symptoms were recorded on diary cards for 7 days after each dose. All other (unsolicited) adverse events (AEs) were recorded for 21 days after each dose and from the first dose until day 84. AEs were graded on a 3-point scale in which “0” denoted no AEs, “1” denoted mild AEs, “2” denoted moderate AEs, and “3” denoted severe AEs. All injection site symptoms were considered to be vaccine related. For all other symptoms, potential causal relationships with vaccination were determined by the site investigator. Serious AEs (SAEs), potential immune-mediated diseases (pIMDs) and medically attended AEs, defined as hospitalization, an emergency department visit, or a visit to or from medical personnel for any reason, were recorded throughout the study until the day 364 contact.
The use of medications, including antipyretic medication, was recorded until day 203.
Statistical Analyses
The primary cohorts for the assessment of immunogenicity at each time point were the per-protocol cohorts, which included all eligible children who complied with protocol-defined procedures and who had HI assay results for the specific time point under evaluation.
The seroprotection rate was defined as the percentage of children with a serum influenza A(H5N1) HI antibody titer of ≥1:40. The seroconversion rate was defined as the percentage of initially seronegative participants with a postvaccination titer of ≥1:40 or the percentage of initially seropositive participants with a ≥4-fold increase in titer. The mean geometric increase was defined as the geometric mean of the within-subject ratios of the postvaccination reciprocal HI titer to the prevaccination (day 0) reciprocal HI titer. The booster seroconversion rate was defined as the seroconversion rate relative to the prebooster (day 182) blood sample. The booster factor was defined as the geometric mean of the within-subject ratios of the postbooster reciprocal HI titer to the prebooster (day 182) reciprocal HI titer. A vaccine/booster response for serum neutralizing antibody titers was defined a ≥4-fold increase in postvaccination titer relative to day 0 (day 182 for a booster response). Anti-NA antibodies were assessed in terms of seropositivity and geometric mean titers (GMTs).
The primary analysis of safety was conducted on the total vaccinated cohort, which included all children who received at least 1 vaccine administration during the study.
Analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC), and StatXact-8.1.
RESULTS
Study Participants
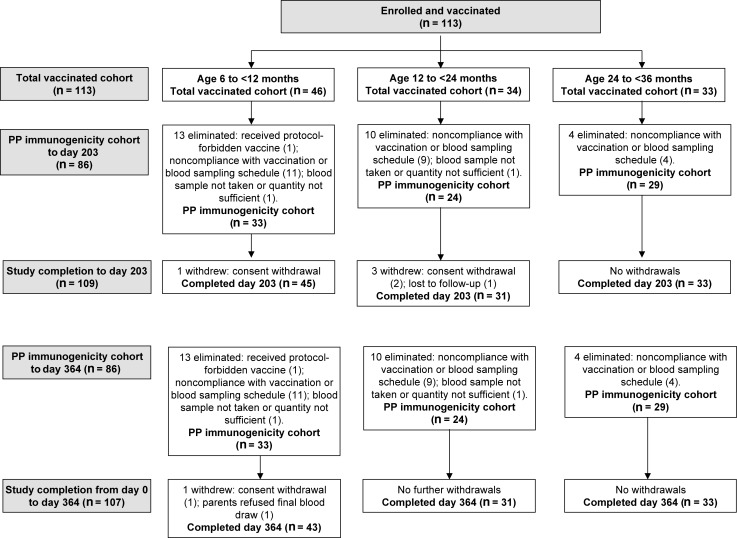
There were 113 children enrolled and vaccinated, of whom 109 (96.5%) completed the vaccination phase (to day 203) of the study. No child was withdrawn due to an AE (Figure 1). Sex and racial heritage of participants were similar across age strata (Table 1).
Figure 1.
Participant flow through the study. Abbreviation: PP, per protocol.
Table 1.
Demographic Characteristics, by Age, for the Total Vaccinated Cohort at Dose 1
| Characteristic | 6 to < 12 mo (n = 46) | 12 to < 24 mo (n = 34) | 24 to < 36 mo (n = 33) |
|---|---|---|---|
| Age, mo | |||
| Mean ± SD | 8.3 ± 1.56 | 16.1 ± 3.44 | 29.6 ± 3.38 |
| Median (range) | 8 (6–11) | 15 (12–23) | 30 (24–35) |
| Sex, no. (%) | |||
| Female | 25 (54.3) | 19 (55.9) | 19 (57.6) |
| Male | 21 (45.7) | 15 (44.1) | 14 (42.4) |
| Ethnicity, no. (%) | |||
| Southeast Asian | 34 (73.9) | 26 (76.5) | 22 (66.7) |
| White | 12 (26.1) | 8 (23.5) | 9 (27.3) |
| Other | 0 (0) | 0 (0) | 2 (6.1) |
Immunogenicity
Primary Objective
The primary objective was met: for each age stratum and overall, all CHMP criteria were fulfilled for responses to A/turkey/Turkey/1/2005(H5N1) 10 days after receipt of a heterologous booster dose of A/turkey/Turkey/1/2005(H5N1)-AS03B that was given 6 months after 2-dose primary vaccination with A/Indonesia/05/2005(H5N1)-AS03B (Table 2).
Table 2.
Hemagglutinin Inhibition (HI) Antibodies Against A/Turkey/Turkey/01/2005(H5N1) and A/Indonesia/5/2005(H5N1), by Age, in the Per Protocol Cohorts for Immunogenicity
| Vaccine, Age, Time Pointa | SC Rate/BSC Rateb |
SP Ratec |
MGI/BFd |
|
|---|---|---|---|---|
| Subjects, No. | Subjects, % (95% CI) | Subjects With a Titer ≥ 1:40, % (95% CI) | Value (95% CI) | |
| A/turkey/Turkey/01/2005(H5N1) | ||||
| 6 to <12 mo | ||||
| Before | 33 | … | 0.0 (0.0; 10.6) | … |
| Day 182 | 33 | 93.9 (79.8; 99.3) | 93.9 (79.8; 99.3) | 16.5 (12.8; 21.4) |
| Day 192 | 33 | 97.0 (84.2; 99.9) | 100 (89.4; 100) | 25.7 (18.4; 35.7) |
| Day 364 | 41 | 100 (91.4; 100) | 100 (91.4; 100) | 19.9 (15.2; 26.1) |
| 12 to <24 mo | ||||
| Before | 24 | … | 0.0 (0.0; 14.2) | … |
| Day 182 | 21 | 95.2 (76.2; 99.9) | 100 (83.9; 100) | 14.8 (10.8; 20.1) |
| Day 192 | 21 | 100 (83.9; 100) | 100 (83.9; 100) | 21.5 (16.4; 28.2) |
| Day 364 | 26 | 100 (86.8; 100) | 100 (87.2; 100) | 16.4 (12.6; 21.4) |
| 24 to <36 mo | ||||
| Before | 29 | … | 3.4 (0.1; 17.8) | … |
| Day 182 | 29 | 96.6 (82.2; 99.9) | 100 (88.1; 100) | 15.6 (12.5; 19.5) |
| Day 192 | 29 | 100 (88.1; 100) | 100 (88.1; 100) | 20.5 (16.2; 26.1) |
| Day 364 | 32 | 87.5 (71.0; 96.5) | 100 (89.1; 100) | 9.5 (7.2; 12.5) |
| All | ||||
| Before | 86 | … | 1.2 (0.0; 6.3) | … |
| Day 182 | 83 | 95.2 (88.1; 98.7) | 97.6 (91.6; 99.7) | 15.8 (13.6; 18.2) |
| Day 192 | 83 | 98.8 (93.5; 100) | 100 (95.7; 100) | 22.7 (19.3; 26.8) |
| Day 364 | 99 | 96.0 (90.0; 98.9) | 100 (96.4; 100) | 14.9 (12.6; 17.6) |
| A/Indonesia/5/2005(H5N1) | ||||
| 6 < 12 mo | ||||
| Before | 33 | … | 0.0 (0.0; 10.6) | … |
| Day 42 | 32 | 100 (89.1; 100) | 100 (89.1; 100) | 189.0 (149.2; 239.6) |
| Day 182 | 33 | 97.0 (84.2; 99.9) | 97.0 (84.2; 99.9) | 32.0 (25.1; 40.8) |
| Day 192 | 33 | 100 (89.4; 100) | 100 (89.4; 100) | 432.8 (354.5; 528.3) |
| Day 364 | 41 | 100 (91.4; 100) | 100 (91.4; 100) | 293.1 (233.6; 367.6) |
| 12 < 24 mo | ||||
| Before | 24 | … | 0.0 (0; 14.2) | … |
| Day 42 | 24 | 100 (85.8; 100) | 100 (85.8; 100) | 267.3 (198.1; 360.8) |
| Day 182 | 21 | 100 (83.9; 100) | 100 (83.9; 100) | 29.5 (22.6; 38.3) |
| Day 192 | 21 | 100 (83.9; 100) | 100 (83.9; 100) | 306.9 (221.6; 425.1) |
| Day 364 | 27 | 100 (87.2; 100) | 100 (87.2; 100) | 211.2 (153.8; 289.8) |
| 24 < 36 mo | ||||
| Before | 29 | … | 0.0 (0; 11.9) | … |
| Day 42 | 29 | 100 (88.1; 100) | 100 (88.1; 100) | 208.9 (166.5; 262.2) |
| Day 182 | 29 | 100 (88.1; 100) | 100 (88.1; 100) | 26.7 (22.6; 31.6) |
| Day 192 | 29 | 100 (88.1; 100) | 100 (88.1; 100) | 321.2 (250.9; 411.4) |
| Day 364 | 32 | 100 (89.1; 100) | 100 (89.1; 100) | 133.7 (101.0; 176.8) |
| All | ||||
| Before | 86 | … | 0.0 (0.0; 4.2) | … |
| Day 42 | 85 | 100 (95.8; 100) | 100 (95.8; 100) | 215.7 (187.1; 248.7) |
| Day 182 | 83 | 98.8 (93.5; 100) | 98.8 (93.5; 100) | 29.4 (25.9; 33.4) |
| Day 192 | 83 | 100 (95.7; 100) | 100 (95.7; 100) | 357.5 (310.5; 411.7) |
| Day 364 | 100 | 100 (96.4; 100) | 100 (96.4; 100) | 208.7 (177.2; 245.7) |
Abbreviations: BF, booster factor; BSC, booster seroconversion; CI, confidence interval; MGI, mean geometric increase; SC, seroconversion.
a “Before” denotes before vaccination; “day 42,” 21 days after dose 2; “day 182,” before booster; “day 192,” 10 days after the booster; and “day 364,” 6 months after the booster.
b A/turkey/Turkey/01/2005(H5N1) only: percentage of seronegative participants with a postvaccination/booster titer of ≥1:40 or the percentage of initially seropositive participants with a ≥4-fold increase in titer.
c The seroprotection (SP) rate is defined as the percentage with HI antibody titer of ≥1:40.
d A/turkey/Turkey/01/2005(H5N1) only: geometric mean of the within-subject ratios of the postvaccination/postbooster reciprocal HI titer to the prevaccination/prebooster reciprocal HI titer.
Immunogenicity to A/Turkey/Turkey/01/2005(H5N1) Before and After the Booster Dose
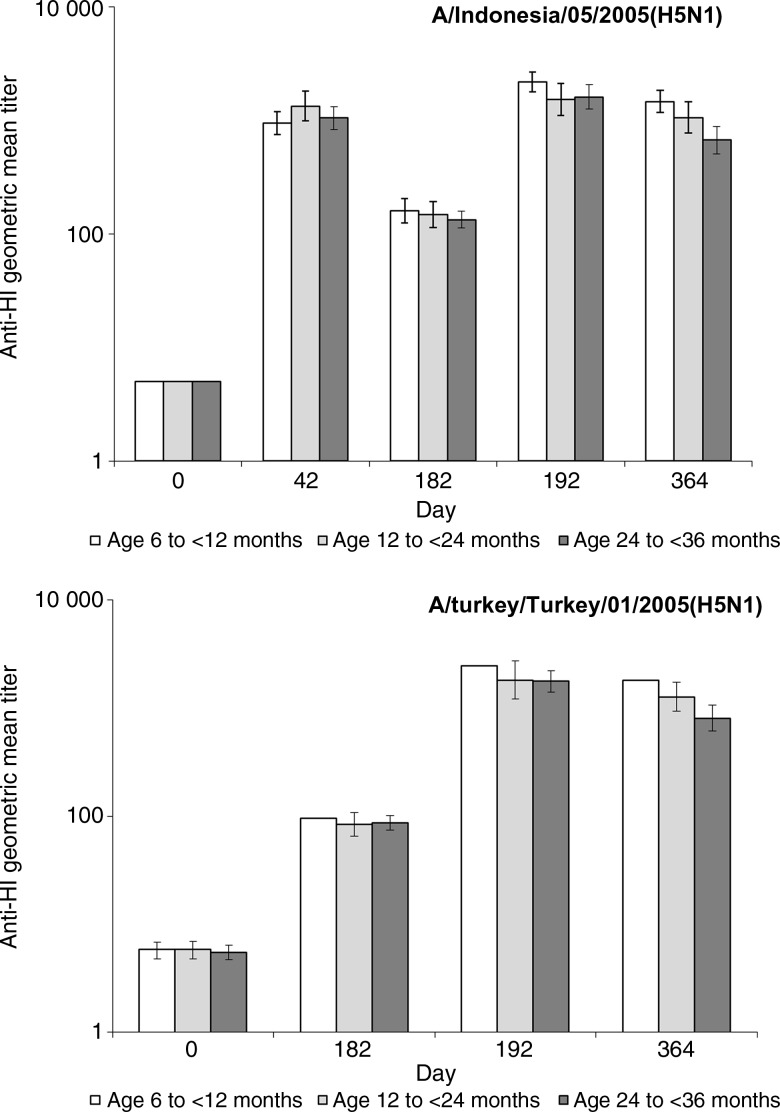
The seroprotection rate for the heterologous strain (A/turkey/Turkey/1/2005[H5N1]) was 1.2% before priming but 97.6% at day 182 after 2 doses of A/Indonesia/05/2005(H5N1)-AS03B (Table 2). Ten days after administration of the heterologous booster vaccination (A/turkey/Turkey/1/2005[H5N1]-AS03B), all children had HI titers of ≥1:40, and compared with the prebooster time point, 98.8% of all children seroconverted. HI GMTs were higher at day 182 than before vaccination and increased markedly in all age strata 10 days following booster vaccination (Figure 2). The booster factor was 25.7 for children aged 6 to < 12 month olds, 21.5 for those aged 12 to < 24 months, and 20.5 for those aged 24 to < 36 months (Table 2).
Figure 2.
Hemagglutinin inhibition (HI) geometric mean titers against A/Indonesia/05/2005(H5N1) and A/turkey/Turkey/01/2005(H5N1), by age, in the per protocol cohorts for immunogenicity. Vaccination occurred on days 0, 21, and 18.
All participants had neutralizing antibody responses to the heterologous strain (A/turkey/Turkey/01/2005[H5N1]) before the booster dose. Neutralizing antibody responses persisted until day 364 (Supplementary Table 1). GMTs of neutralizing antibodies increased markedly following booster vaccination, and at least 96.4% of children in each age stratum had a booster response to the A/turkey/Turkey/01/2005(H5N1)-AS03B strain (Supplementary Table 1).
Immunogenicity to A/Indonesia/05/2005(H5N1) Following Primary and Heterologous Booster Vaccinations
After 2 doses of A/Indonesia/05/2005(H5N1)-AS03B, all children seroconverted, and all had HI titers of ≥1:40 to A/Indonesia/05/2005(H5N1) (Table 2). The mean geometric increase after vaccination was at least 189.0 for each age stratum. Postvaccination HI GMTs were similar across age strata (Figure 2).
A/Indonesia/05/2005(H5N1) HI GMTs decreased over time but remained above baseline levels at day 182. Ten days after receipt of the heterologous A/turkey/Turkey/1/2005-H5N1-AS03B booster dose, A/Indonesia/05/2005(H5N1) HI GMTs increased to at least postprimary (day 42) levels (Figure 2). The post-booster mean geometric increase for A/Indonesia/05/2005(H5N1) was 432.8 for children aged 6 to < 12 months, 306.9 for those aged 12 to < 24 months, and 321.2 for those aged 12 to < 36 months (Table 2).
All participants were seropositive and had a vaccine response for neutralizing antibodies to A/Indonesia/05/2005(H5N1) at day 42 (Supplementary Table 1). All participants remained seropositive before and after the heterologous booster vaccination, until day 364. Neutralizing GMTs were similar across age strata. The A/turkey/Turkey/1/2005(H5N1)-AS03B booster dose induced postbooster A/Indonesia/05/2005(H5N1) HI titers that were at least as high as titers observed after primary vaccination (day 42).
All participants were seropositive for anti-NA antibodies against A/Indonesia/05/2005(H5N1) at day 42 (Supplementary Table 2). Prior to the booster vaccination, 97.6% of all participants were seropositive for anti-NA antibodies; the percentage increased to 100% after the booster vaccination and persisted until day 364.
Reactogenicity and Safety
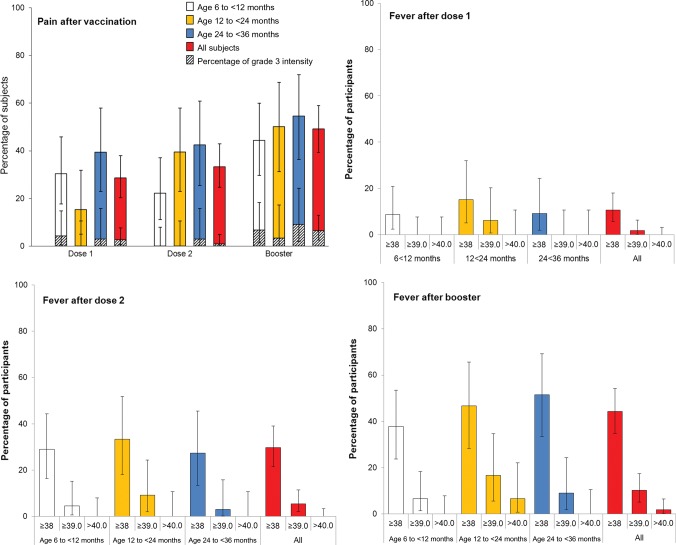
Pain was the most frequently reported local solicited symptom after each dose and appeared to increase in frequency with consecutive doses (Figure 3). Redness was reported for 3.6% of children after dose 1, 5.4% after dose 2, and 16.7% after the booster dose. Swelling was reported for 2.7% of children after dose 1, 3.6% after dose 2, and 10.2% after the booster dose. No grade 3 redness or swelling was reported during the study.
Figure 3.
Injection site pain and fever after vaccination by age and dose, in the total vaccinated cohort. Grade 3 pain is defined as “cried when limb was moved/spontaneously painful.”
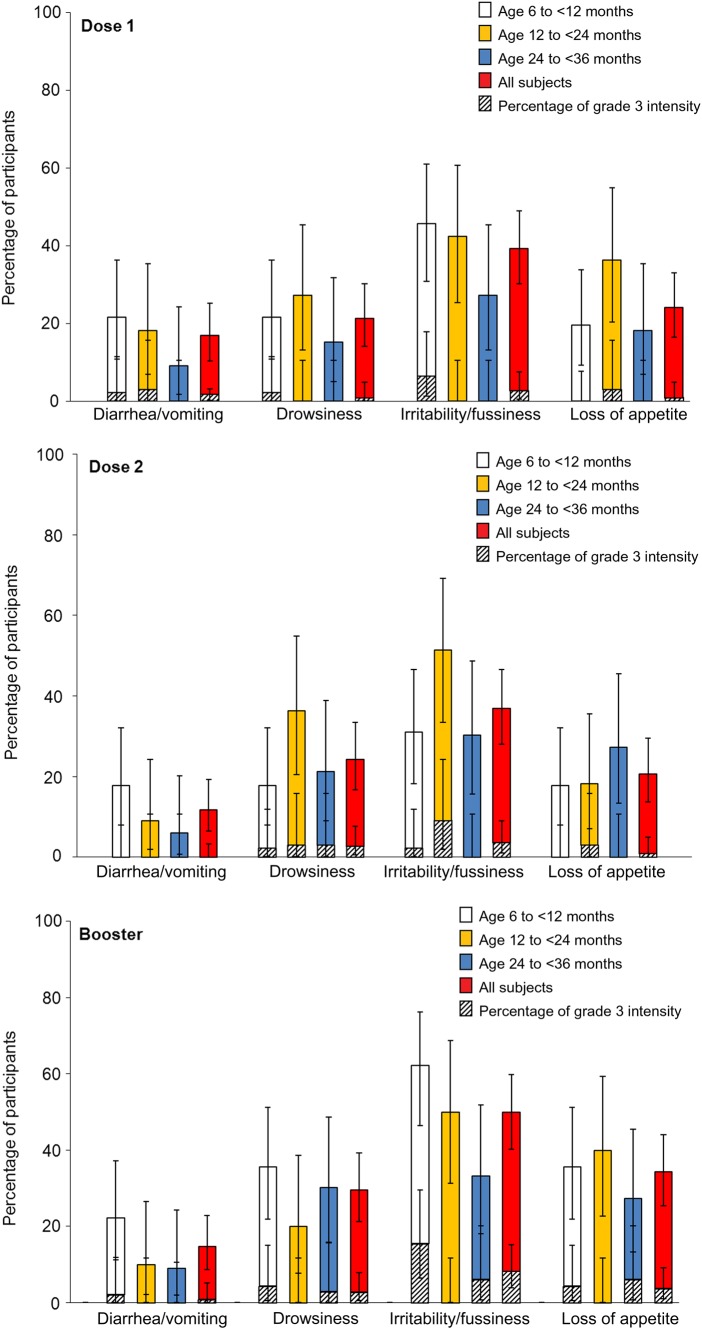
Irritability/fussiness was the most frequently reported general symptom (Figure 4). The incidence of fever appeared to increase with consecutive doses but was similar across age strata (Figure 3). After the booster vaccination, fever was reported in 50% of all participants, and grade 3 fever (temperature, ≥39.0°C) was reported in 10.2% of participants. There were no cases of fever involving a temperature of ≥40.0°C after dose 1 or 2, and 2 participants (1.9%) reported fever with a temperature of ≥40.0°C following the booster vaccination. No febrile convulsions were reported.
Figure 4.
General symptoms following vaccination, by age and dose, in the total vaccinated cohort. Grade 3 is defined as “preventing normal activity for diarrhea/vomiting, drowsiness, and loss of appetite and as crying that could not be comforted/prevented normal activity for irritability/fussiness.”
Between days 0 and 203, 79.6% subjects received an antipyretic medication, with the majority (94%) receiving it for therapeutic purposes. The frequency of antipyretic use increased with each dose, with 31.0% receiving treatment following dose 1, 50.0% receiving treatment following dose 2, and 65.7% receiving treatment following dose 3. Other unsolicited AEs considered to be causally related to study vaccination were reported ≤21 days after any dose for 28.3% (95% confidence interval [CI], 16.0%–43.5%) of infants aged 6 to < 12 months, 14.7% (95% CI, 5.0%–31.1%) aged 12 to < 24 months, and 21.2% (95% CI, 9.0%–38.9%) aged 24 to < 36 months. The most frequently reported related symptoms were rhinorrhea, cough, nasopharyngitis, rhinitis, and upper respiratory tract infection. There were 2 grade 3 causally related unsolicited events reported ≤21 days after any vaccine dose: nasopharyngitis (onset 2 days following dose 2) and swelling of the face (onset on the day of dose 1, with no recurrence after subsequent doses). A similar distribution of AEs was reported from days 0 through 84.
Between days 0 and 364, SAEs were reported for 9 children, none of which were considered to be vaccine related. All participants recovered. During the same period, unsolicited medically attended AEs were reported in 60.2% (95% CI, 50.5%–69.3%) of all participants. No pIMDs or deaths were reported.
DISCUSSION
In young children primed with a 2-dose series of A/Indonesia/05/2005(H5N1)-AS03B and boosted with a single dose of A/turkey/Turkey/1/2005(H5N1)-AS03B, an anamnestic response was demonstrated in terms of HI, neutralizing, and anti-NA antibodies against both strains. The booster vaccination induced HI antibody responses for the A/turkey/Turkey/1/2005(H5N1) strain that fulfilled CHMP criteria overall and for each age stratum. Six months after the booster dose, all children had HI titers of ≥1:40 and remained seropositive for neutralizing antibodies against homologous and heterologous strains and for anti-NA for the homologous strain. These data suggest that, as observed in older populations, a 2-dose series of H5N1-AS03B confers a primary immune response and induces broad cross-reactive immunity in young children, with immune responses that persist for at least 6 months.
We administered a booster vaccination after 2 priming doses to assess priming by demonstration of an anamnestic response. Reactogenicity and fever, as well as antipyretic use, were observed to increase with consecutive doses, particularly following the dose 3 booster vaccination. An increase in fever and local injection site symptoms after the second vaccination has been reported in some studies of AS03B-adjuvanted 2009 pandemic influenza A(H1N1) vaccine in young children [22; Kosalaraksa et al., submitted]. No related SAEs or pIMDs were reported during the study.
Recent reports have suggested that receipt of a 2009 pandemic influenza A(H1N1) vaccine (using a strain distinct from the one we used) or natural infection with influenza virus was linked with subsequent onset of narcolepsy [23–33]. Recently, CD4+ T cells from narcoleptic individuals with the HLA DQ0602 allele have been shown to recognize an epitope unique to the influenza A(H1N1) HA protein that mimics an epitope of the hypocretin protein [34]. Further research will help to elucidate the chain of events that resulted in narcolepsy and the potential roles of genetic and environmental factors. No cases of narcolepsy were detected in this study.
Prime-boost schedules using H5N1-AS03 vaccines in adults showed that rapid and robust immune responses to vaccine-homologous and heterologous strains could be induced up to 15 months after priming [10, 11, 35]. A previous study established that a lower dose of HA (1.9 μg) was immunogenic for vaccine-homologous and heterologous strains in children 3–9 years of age, with acceptable reactogenicity and safety, compared with unadjuvanted trivalent seasonal influenza vaccine [14]. Our study extends these results to the population of infants and young children from 6 months of age. Moreover we demonstrate the effectiveness of primary vaccination using H5N1-AS03B in infants and young children and the value of a heterologous booster dose in boosting the immune response to related clade strains. These results confirm the potential usefulness of H5N1-AS03B for children either in a prepandemic setting, to reduce attack rates early on in a pandemic, or in an early pandemic setting, where vaccination could prime or protect until a pandemic-strain-matched vaccine becomes available.
A potential limitation of this study is the absence of a control group, which precludes evaluation of common events such as fever in a pediatric population. Another limitation is the absence of efficacy data against influenza A(H5N1) disease that confirms current regulatory cutoffs for influenza vaccines. The heterologous booster was given 6 months after priming. Immunogenicity of a booster dose after a prolonged period after priming, as might occur should prepandemic vaccination be performed, has been assessed in adults but not yet in young children. A potential single-dose priming approach in young children also warrants further investigation in view of the possible pressures of vaccine supply in a pandemic setting, with putatively adequate immune responses following 2 doses of vaccine and increased reactogenicity with a third dose of vaccine. Finally, A/Indonesia/05/2005 and A/turkey/Turkey/1/2005 are related clade 2 strains (subclade 2.1 and 2.2, respectively) [36]. Although currently the majority of human cases have been due to clade 2 strains [36], broader immunity would be needed should infecting strains emerge from more distantly related clades.
In summary, this first study of H5N1-AS03 in infants and young children showed that a 2-dose series induced effective priming in this age group. Subsequent vaccination with a heterologous booster vaccine was immunogenic for both the homologous and heterologous strains. Reactogenicity appeared to increase with increasing doses of adjuvanted vaccine. Significant fever (temperature, ≥39.0°C) was reported for 1 in 10 children following booster vaccination, but no safety concerns were identified. The results support a prime-boost strategy for pandemic preparedness. Prepandemic vaccination with a clade 2 strain could be used in young children in the expectation that subsequent heterologous booster vaccination would boost immune responses to a related clade 2 pandemic-specific virus strain.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the children and their families who participated in this study; the dedicated clinical study staff in Melbourne (Jodie McVernon, Marita Kefford, Judith Spotswood, Clare Brophy, and AnnMarie McEvoy), Singapore (Charlotte Chua), Adelaide (Susan Lee, Verity Hill, Christine Heath, Trinh Tran, Kirsten Zyhajlo, Mary Walker, Sue Evans, Michelle Clarke, Jane Tidswell, and Natalie Thomas), and Sydney (Helen Knight), for important contributions; the global and regional clinical operations and safety teams, the scientific writer for clinical protocol and clinical report writing, the laboratory technicians and managers, and the statisticians of GlaxoSmithKline Vaccines, for their contribution to the study (particularly Jennifer Gearhart, Catena Lauria, Carline Vanden Abeele, Laurence Hollinger, Karl Walravens, Dorothy Slavin, Sara Van de Voorde, Pam Kalodimos, and Murtaza Shipchandler); Anne Schuind, for critically reviewing the manuscript; Joanne Wolter (medical writer on behalf of GlaxoSmithKline Vaccines), for assistance in preparing the first draft of the manuscript; and Vincent Laporte (of Business & Decision Life Sciences, on behalf of GlaxoSmithKline Vaccines), for coordination and editorial assistance.
All authors participated in the design, implementation, or analysis; the interpretation of data; and the development of this manuscript. All authors had full access to the data and gave final approval before submission. GlaxoSmithKline Biologicals was involved in all stages of the study conduct and analysis.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA. H. M. was supported by the National Health and Medical Research Council (career development fellowship 1016272).
Potential conflicts of interest. The institutions of T. N., H. M., P. C. C., B.-W. L., and R. B. received funding from the GlaxoSmithKline group of companies to complete the work disclosed in this manuscript. T. N. received fees from the GlaxoSmithKline group of companies for participation in review activities (data monitoring boards) and expert testimony outside the submitted work, is a member of the WHO SAGE committee (nonremunerated position), and chairs the Australian Government Technical Advisory Group on Immunisation (remunerated position). The institution of H. M. received fees from the GlaxoSmithKline group of companies for participation in an advisory board on a topic not related to this study, support for travel to meetings for the study, and support for travel to present scientific data. The institution of R. B. has received funding from CSL, Hoffmann–La Roche, Sanofi, the GlaxoSmithKline group of companies, Novartis, Baxter, and Pfizer to conduct sponsored research, educational grants, or to attend and present at scientific meetings. R. B. received honorarium for delivering educational presentations. Any funding received is not personally accepted by R. B. but is directed to a research account at The Children's Hospital at Westmead. P. I., M. D. and D. V. are employed by the GlaxoSmithKline group of companies. D. V. has restricted shares ownership in the GlaxoSmithKline group of companies.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.World Health Organization. Geneva: WHO; 2011. H5N1 avian influenza: Timeline of major events 13 December 2011. http://www.who.int/influenza/human_animal_interface/avian_influenza/H5N1_avian_influenza_update.pdf. Accessed 2 October 2013. [Google Scholar]
- 2.WHO. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/ Accessed 12 February 2014.
- 3.Update on human cases of influenza at the human–animal interface, 2012. Wkly Epidemiol Rec. 2013;88:137–44. [PubMed] [Google Scholar]
- 4.World Health Organization. WHO activities in avian influenza and pandemic influenza preparedness. http://www.who.int/influenza/resources/documents/WHO_CDS_EPR_GIP_2006_6.pdf. Accessed 1 April 2012.
- 5.Sellwood C, Asgari-Jirhandeh N, Salimee S. Bird flu: if or when? Planning for the next pandemic. Postgrad Med J. 2007;83:445–50. doi: 10.1136/pgmj.2007.059253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.H5N1 vaccine approved by the U.S. FDA as pandemic influenza preparedness measure. 2013. http://www.gsk.com/media/press-releases/2013/h5n1-vaccine-approved-by-the-u-s--fda-as-pandemic-influenza-prep.html. Accessed 5 December 2013.
- 7.Viboud C, Boëlle P-Y, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract J R Coll Gen Pract. 2004;54:684–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Glatman-Freedman A, Portelli I, Jacobs SK, et al. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One. 2012;7:e50228. doi: 10.1371/journal.pone.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rümke HC, Bayas J-M, de Juanes J-R, et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine. 2008;26:2378–88. doi: 10.1016/j.vaccine.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 10.Gillard P, Caplanusi A, Knuf M, et al. An assessment of prime-boost vaccination schedules with AS03A-adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults. Influenza Other Respir Viruses. 2013;1:55–65. doi: 10.1111/j.1750-2659.2012.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leroux-Roels I, Roman F, Forgus S, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine. 2010;28:849–57. doi: 10.1016/j.vaccine.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–53. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 13.Leroux-Roels I, Bernhard R, Gérard P, Dramé M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díez-Domingo J, Garcés-Sanchez M, Baldó J-M, et al. Immunogenicity and Safety of H5N1 A/Vietnam/1194/2004 (clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29:e35–46. doi: 10.1097/INF.0b013e3181daf921. [DOI] [PubMed] [Google Scholar]
- 15.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103:163–71. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Kendal A, Pereira M, Skehel J. Hemagglutination inhibition. Concepts and procedures for laboratory-based influenza surveillance. Atlanta, GA: Centers for Disease Control and Prevention and Pan-American Health Organization; 1985. [Google Scholar]
- 17.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–5. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–8. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989;7:385–94. doi: 10.1016/0264-410x(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 21.A revised system of nomenclature for influenza viruses. Bull World Health Organ. 1971;45:119–24. [PMC free article] [PubMed] [Google Scholar]
- 22.Waddington CS, Walker WT, Oeser C, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller E, Andrews N, Stellitano L, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 25.Dauvilliers Y, Arnulf I, Lecendreux M, et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain J Neurol. 2013;136:2486–96. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 26.Heier MS, Gautvik KM, Wannag E, et al. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013;14:867–71. doi: 10.1016/j.sleep.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Szakács A, Darin N, Hallböök T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. 2013;80:1315–21. doi: 10.1212/WNL.0b013e31828ab26f. [DOI] [PubMed] [Google Scholar]
- 28.Wijnans L, Lecomte C, de Vries C, et al. The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1)pdm09 pandemic and vaccination campaigns. Vaccine. 2013;31:1246–54. doi: 10.1016/j.vaccine.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Investigation of an increase in the incidence of narcolepsy in children and adolescents in 2009 and 2010 final report of National Narcolepsy Study Steering Committee. 2012. http://healthupdate.gov.ie/wp-content/uploads/2012/04/Final_Report_of_National_Narcolepsy_Study_Steering_Committee-latest1.pdf. Accessed 6 December 2013.
- 30.European Centre for Disease Prevention and Control. Stockholm: ECDC; 2012. Narcolepsy in association with pandemic influenza vaccination. A multi-country European epidemiological investigation. [Google Scholar]
- 31.Pitkänen M. Increased risk of narcolepsy observed also among adults vaccinated with Pandemrix in Finland. 2013. http://www.thl.fi/en_US/web/en/pressrelease?id=33516. Accessed 31 July 2013.
- 32.Uppsala, Sweden: Medical Products Agency; 2013. Registry study confirms increased risk of narcolepsy after vaccination with Pandemrix in children and adolescents and shows an increased risk in young adults. http://www.lakemedelsverket.se/english/All-news/NYHETER-2013/Registry-study-confirms-increased-risk-of-narcolepsy-after-vaccination-with-Pandemrix-in-children-and-adolescents-and-shows-an-increased-risk-in-young-adults/ Accessed 31 July 2013. [Google Scholar]
- 33.Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 34.De la Herrán-Arita AK, Kornum BR, Mahlios J, et al. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2013;5:216ra176. doi: 10.1126/scitranslmed.3007762. [DOI] [PubMed] [Google Scholar]
- 35.Risi G, Frenette L, Langley JM, et al. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: results of a randomised single heterologous booster dose study at 15 months. Vaccine. 2011;29:6408–18. doi: 10.1016/j.vaccine.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 36.Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness. Wkly Epidemiol Rec. 2011;86:469–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.