Abstract
Eukaryotic gene expression occurs in the context of structurally distinct chromosomal domains such as the relatively open, gene-rich, and transcriptionally active euchromatin and the condensed and gene-poor heterochromatin where its specific chromatin environment inhibits transcription. To study gene silencing by heterochromatin, we created a minichromosome reporter system where the gene silencer elements were used to repress the URA3 reporter gene. The minichromosome reporters were propagated in yeast Saccharomyces cerevisiae at a stable copy number. Conduction of gene silencing through nucleosome arrays was studied by placing various repeats of clone-601 DNA with high affinity for histones between the silencer and reporter in the yeast minichromosomes. High-resolution chromatin mapping with micrococcal nuclease showed that the clone-601 nucleosome positioning downstream of the HML-E gene silencing element was not significantly altered by chromatin silencing. Using URA3 reporter assays, we observed that gene silencing was conducted through arrays of up to eight nucleosomes. We showed that the shorter nucleosome repeat lengths, typical of yeast (167 and 172 bp), were more efficient in conducting silencing in vivo compared to the longer repeats (207 bp) typical of higher eukaryotes. Both the longer and the shorter repeat lengths were able to conduct silencing in minichromosomes independently of clone-601 nucleosome positioning orientations vs. the silencer element. We suggest that the shorter nucleosome linkers are more suitable for conducting gene silencing than the long repeats in yeast due to their higher propensity to support native-like chromatin higher-order folding.
Keywords: transcriptional silencing, antisilencing, clone-601 nucleosomes, heterochromatin boundary, Saccharomyces cerevisiae
EUKARYOTIC DNA is repeatedly coiled by histone octamers into nucleosome cores, the primary structural units of chromatin (Richmond and Davey 2003). The nucleosome cores are connected by linker DNA-forming nucleosome arrays that fold into compact higher-order structures (Luger et al. 2012). One of the critical biological questions has been deciphering the chromatin structure–function relationship in epigenetic regulation of gene expression. Eukaryotic gene expression occurs mainly in the context of the structurally open and transcriptionally active state (euchromatin) while, in the repressive state (heterochromatin), its specific chromatin organization inhibits transcription (Grewal and Moazed 2003). A combination of transcription factors, DNA modifications, histone modifications, noncoding RNA, and chromatin compaction distinguishes heterochromatin from the transcriptionally active euchromatin (Moazed 2011). Recently, nucleosome positioning in the genome and intrinsic affinity of DNA to histones have received heightened interest, especially since they have been linked to regulation of gene expression in euchromatin and higher-order organization of chromatin (Brogaard et al. 2012; Eriksson et al. 2012; Hughes et al. 2012; Struhl and Segal 2013). Massive changes in nucleosome occupancy and positioning are associated with replicative aging (Hu et al. 2014). Whether the nucleosome positioning, DNA affinity to histones, and chromatin higher-order folding in heterochromatin are instrumental in creating and spreading of the repressive chromatin state remains an open question.
In Saccharomyces cerevisiae the two silent mating-type loci, HML and HMR, represent well-defined heterochromatin domains where genes are transcriptionally repressed. Transcriptional repression at the HML and HMR loci is a gene nonspecific mechanism that mediates epigenetic inheritance of the silent state of the heterochromatin region (Haber 2012; Motwani et al. 2012). The cis-acting E or I silencer elements of the HML locus are necessary and sufficient for initiating and mediating silencing by interacting with a large number of trans-acting factors to repress transcription (Moretti et al. 1994; Dillin and Rine 1995). Both the HML-E and HML-I elements are equally capable of silencing genes (Mahoney and Broach 1989; Haber 1998). Chromatin maps at nucleotide resolution following nuclease digestion and high-resolution DNA sequencing showed uniquely organized chromatin structures at the silent HML locus with arrays of precisely positioned pairs of nucleosomes with alternating short and long linkers abutting the E and I silencer elements (Weiss and Simpson 1998; Elgin and Workman 2000). The discontinuous, non-uniform nucleosome positioning of the HML locus perhaps is necessary for transcriptional repression and formation of higher-order repressive chromatin structures. Furthermore, it has been reported that DNA sequences that do not favor nucleosome formation and have the ability to disrupt chromatin structure can also function as barriers to the propagation of transcriptionally silent chromatin (Bi et al. 2004).
Here we used our recently established URA3-based yeast minichromosome reporter system containing silencer and antisilencer elements (Chakraborty et al. 2011) to investigate if arrays of nucleosomes with high DNA affinity to histones and varying nucleosome number and repeat lengths will conduct silencing from the HML-E and HML-I elements to a reporter gene. In this study, we employed the clone-601 DNA sequences that have the highest affinity for the histone octamer and positions the nucleosome core with a single-base precision (Lowary and Widom 1998). The clone-601 DNA previously served as an excellent tool for chromatin structure studies (Schlick et al. 2012) and for exploring the relationship between nucleosome structure and transcription in vitro (Bondarenko et al. 2006; Chen et al. 2013) and in vivo (Gaykalova et al. 2011; Perales et al. 2011). Using clone-601-based reconstituted nucleosome arrays, we have recently shown that chromatin higher-order structure is modulated by the length of DNA linkers (Correll et al. 2012). Now, by placing a number of different clone-601 repeats between the silencer and the URA3 reporter, we were able to examine their function in conducting silencing using in vivo genetic assays.
Here we show that the repeats of up to eight clone-601 nucleosomes are able to conduct silencing from both the HML-E and the HML-I silencers to repress the URA3 reporter and that there is an abrupt transition from silent chromatin to active chromatin between 8 and 10 nucleosomes. The efficiency of silencing is modulated by the nucleosome repeat length (NRL), but not by orientation of the clone-601 nucleosomes vs. the silencer element. We thus established a new S. cerevisiae minichromosome-based experimental system to study chromatin structure–function relationship between gene regulatory elements controlling the gene expression.
Materials and Methods
Yeast minichromosome constructs
The parent minichromosome shuttle vector (TRP1-ARS1) contains an autonomously replicating sequence, ARS1 [GenBank accession no. NC_001136.10; Saccharomyces Genome Database (SGD): chromosome IV, 462354–463192]; TRP1 as a selectable marker for selection in S. cerevisiae (Ducker and Simpson 2000) (GenBank accession no. NC_001136.10; SGD: chromosome IV, 461842–462516); and a pBR322 vector-derived sequence with an AmpR gene for propagation in Escherichia coli. The S. cerevisiae minichromosome constructs (Figure 1A) containing the URA3 reporter gene, the HML-E, and the HML-I silencer elements were generated as previously described (Chakraborty et al. 2011).
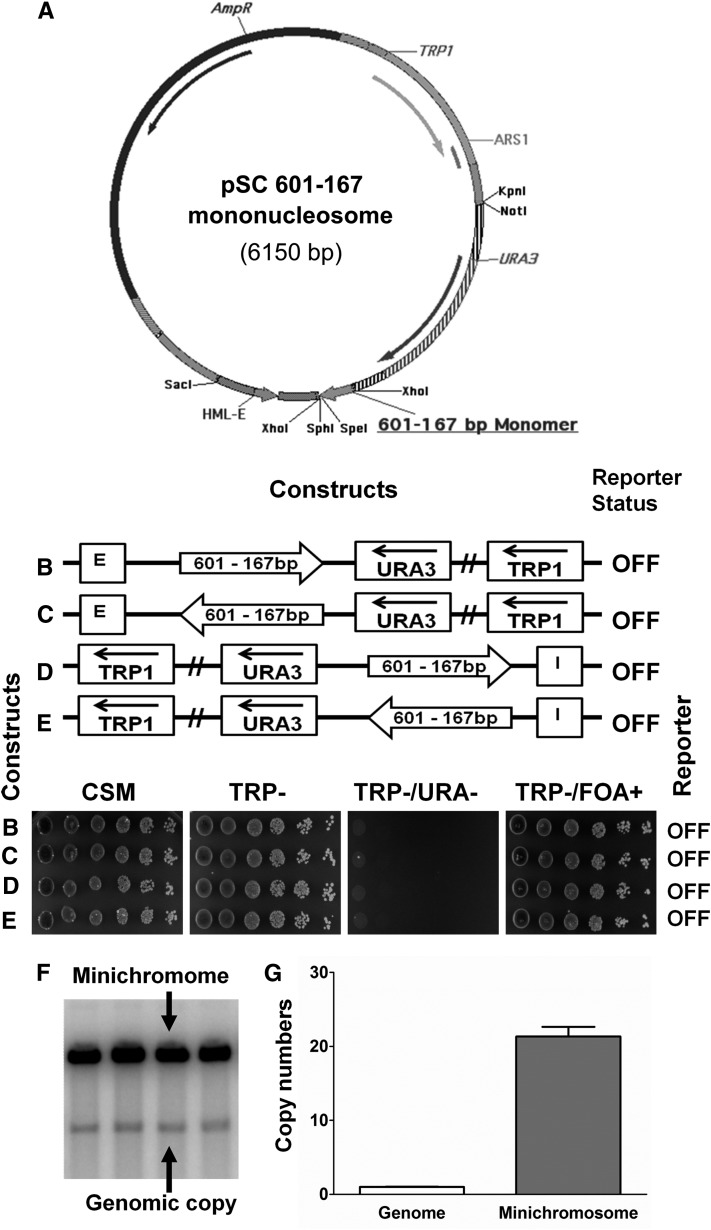
Figure 1.
A minichromosome reporter system to study silencing conductance through nucleosomes. (A) Physical map of one of the TRP1-ARS1-derived clone-601 DNA containing minichromosome constructs. This example contains a 167 bp clone-601 mononucleosome placed between XhoI and SpeI sites in a minichromosome containing the HML–E silencer element and the URA3 reporter gene. Other minichromosome constructs contain either monomers or nucleosome arrays of 167, 172, or 207 bp positioned between the E-silencer (placed between SacI and XhoI sites) or I-silencer (placed between NotI and KpnI sites) and the URA3 reporter gene in different orientations (see schemes in Table S2). (B–E) The 167 bp fragment containing nucleosome positioning sequence of clone-601 DNA (shown by arrows) was positioned between the HML–E silencer (B and C) or the HML–I silencer (D and E) and the URA3 reporter in different orientations. The URA3 reporter gene expression status (on or off) was assayed by the growth phenotypes of S. cerevisiae cells containing various minichromosome constructs tested in the indicated selective media by serial dilutions. The clone-601 monomer did not inhibit the silencing of the URA3 reporter gene by either the E-silencer or the I-silencer. (F) Southern hybridization of linearized minichromosomal and genomic DNA probed with a radiolabeled TRP1-ARS1-containing fragment. Four independent clones were transformed with a minichromosome construct containing the clone-601 mononucleosome DNA placed between the HML-E silencer and the URA3 reporter. (G) Histograms showing minichromosome copy numbers quantified by scanning of the Southern blots (such as shown in F) and normalized to the genomic TRP1-ARS1 signal. Error bars represent standard deviations. Where absent, error bars are too small to be visualized.
Nucleosome-positioning minichromosomes
The nucleosome-positioning minichromosome constructs contain the clone-601 nucleosome-positioning sequence (Lowary and Widom 1998) placed into the pUC19 vector at the XbaI–SphI restriction enzyme site to generate the 601-207 mononucleosome template (Grigoryev et al. 2009). A SpeI restriction enzyme site is positioned near the end of the mononucleosome for further modifications. The 601-167 (Figure 1A) and the 601-172 mononucleosome templates were generated from the 601-207 DNA by PCR using primer sets listed in Supporting Information, Table S1. Nucleosome oligomer templates were constructed as described (Grigoryev et al. 2009) and subcloned into the pSC E-silencer and pSC I-silencer constructs (Table S2) to provide a yeast shuttle vector containing repeats of clone-601 DNA. In some constructs, the boundary elements STAR and TEF2-UAS operating as antisilencers on the minichromosome were positioned upstream of the E-silencer at the SacI restriction site in different orientations. Schemes of all constructs containing clone-601 DNA are shown in Table S2.
The minichromosome constructs with mononucleosomes and oligonucleosomes were verified by DNA sequencing before transforming the yeast cells. The yeast strains containing minichromosome constructs with oligonucleosome repeats were tested by sequencing to ensure the absence of recombination events. Yeast plasmid DNA purified using the standard smash-and-grab method (Rose et al. 1990) were retransformed into E. coli DH5α cells and confirmed by DNA sequencing using the primers listed in Table S1.
Strains and media used
The minichromosome constructs were transformed into E. coli DH5α competent cells (Invitrogen, Carlsbad, CA), and bacterial colonies were screened with restriction enzyme digests, PCR analysis, and DNA sequencing. Purified minichromosome constructs were retransformed into S. cerevisiae a-cells YPH499 strain (MATa, ade2101°, his3-∆200, leu2-∆1, lys2-801a, trp1-∆63, ura3-52) (Sikorski and Hieter 1989). High-efficiency yeast transformations were carried out using lithium acetate and polyethylene glycol (Gietz and Schiestl 2007) or using an EZ yeast transformation kit (Qbiogene Inc.). Yeast colonies grown on −Trp media were selected for minichromosomes containing a TRP1 marker gene in the construct backbone. The expression of the URA3 reporter gene and function of the regulatory elements in the minichromosome constructs were determined in the presence or absence of the silencer elements using different selective media (Chakraborty et al. 2011).
Yeast colonies were grown on complete synthetic media (CSM), −Trp (lacking tryptophan), −Trp/−Ura (lacking both tryptophan and uracil), or −Trp/5-FOA+ (lacking tryptophan, but containing 5-fluroorotic acid). The yeast dropout media were made with yeast nitrogenous base without amino acids, 2% dextrose, and the CSM, −Trp, −Trp/−Ura media were made as per the manufacturer’s instructions. The −Trp/5-FOA+ media were made by adding 1 mg/ml 5-FOA (Toronto Research Chemicals) and uracil to a final concentration of 35 μg/ml. The functionality was tested using two complementary in vivo assays for URA3 expression: growing on uracil-deficient media and 5-FOA-dependent viability (Chakraborty et al. 2011).
Southern hybridization
The minichromosome construct DNA and copy numbers were examined by Southern hybridization. Yeast-purified DNA using standard smash-and-grab method (Rose et al. 1990) were linearized and subjected to electrophoretic separation on 1% agarose gel and transferred to Hybond-NX membrane (Amersham Biosciences) as per standard protocol (Mays Hoopes 1987). DNA was cross-linked with UV light and hybridized with probe specific to the TRP1-ARS1 backbone of the minichromosome constructs and random primer labeled with [α-32P]dATP. The membranes were exposed to a Bio-Rad imaging screen, and the signal intensities were analyzed using a Typhoon 9400 Phosphoimager (Amersham Biosciences). The signal intensities were quantified, and the copy numbers were determined by the ImageQuant 5.2 software (Molecular Dynamics) as previously described (Chakraborty et al. 2011).
Spotting assay
The yeast strains with various minichromosomal constructs were grown to mid-log phase in selective liquid −Trp media at 30° as described (Chakraborty et al. 2011). The mating-type a-cells without any minichromosome construct were grown in non-selective CSM media. The optical density of the yeast cultures were adjusted, and 10-fold serial dilutions or two 10-fold and three 5-fold serial dilutions up to ∼2 × 103 cells/ml were made for the spotting to assess URA3 expression (as described in Chakraborty et al. 2011) for assaying the silencing efficiency of the S. cerevisiae strains under different growth conditions (Fourel et al. 1999; Lebrun et al. 2003; Chakraborty et al. 2011).
For each strain, independent transformants were verified by Southern hybridization. Transformed cells were grown in −Trp liquid medium and spotted onto various selective media such as CSM, −Trp, −Trp/−Ura, and −Trp/5-FOA+. Cells with repressed URA3 reporter were able to grow in the presence of 5-FOA known to be toxic for cells expressing the functional URA3 gene product (Boeke et al. 1984). The selective media plates were spotted and grown for 2 days prior to imaging for studying the growth phenotypes as described (Chakraborty et al. 2011).
Yeast nuclei preparation and micrococcal nuclease digestion
The S. cerevisiae nuclei were prepared from 1 liter of cells grown at 30° to mid-log phase to an OD600 of ∼1.0 in minimal media. The yeast cell walls were lysed with Zymolyase 0.5 mg/ml (100T Seikagaku), and spheroplast formation was observed under the microscope. The samples were homogenized using a glass barrel on ice. The nuclei were isolated and digested with micrococcal nuclease (Worthington) in increasing concentrations from 0, 1, 2, 4, 8 units/ml of MNase at 37° for 10 min. The samples were treated with RNase A followed by Proteinase K, and the DNA was purified using standard phenol chloroform extraction procedures (Roth and Simpson 1991; Ravindra et al. 1999).
High-resolution micrococcal nuclease mapping with primer extension
The high-resolution chromatin mapping with micrococcal nuclease was performed with multi-cycle polymerase primer extension analysis. The purified oligonucleotide primers were end-labeled with radioisotope [32P ]γ-ATP using T4 Polynucleotide Kinase (NEB) and were purified. The primer sequences used for chromatin mapping are listed in Table S1. The primer extension was carried out with 32P-end-labeled primer and Taq DNA polymerase in a 20-cycle PCR (94°, 1 min; 55°, 1 min; 72°, 1.5 min) followed by 72° for a 5-min extension and cooling to 4°. The DNA was precipitated and then dissolved in sample buffer and denatured for 5 min at 95°. The gradient buffer system was used with the upper chamber containing 0.5× TBE buffer and the lower chamber containing 0.66× TBE and 1 M sodium acetate buffer. The samples were electrophoresed on a 6% polyacrylamide sequencing gel with 8 M urea in 0.5× TBE, and electrophoresis was carried out at 58 W for ∼3 hr after 20 min of pre-run. The gel was dried, covered with Saran Wrap at 80° for 60 min, and exposed to a phosphoimager screen (Bio-Rad). The signal intensities were analyzed using Typhoon 9400 Phosphoimager (Amersham Biosciences) and quantified by the ImageQuant 5.2 software (Molecular Dynamics). Nuclease cleavage sites were determined by a primer extension assay (Shimizu et al. 1991; Weiss and Simpson 1997; Simpson 1998).
Results
Clone-601 nucleosome-positioning sequence conducts silencing on yeast minichromosomes
To study the structure and function of positioned nucleosomes, we used the S. cerevisiae minichromosome system. In the prototype constructs, either the HML–E (positioned upstream of the URA3 reporter) or HML–I (positioned downstream of the URA3 reporter) alone were capable of silencing the expression of the URA3 reporter gene (Chakraborty et al. 2011). Here we examined if placing a clone-601 DNA sequence in combination with linker DNA of variable lengths between the silencer and the reporter would impede or facilitate silencing of the URA3 reporter gene. The clone-601 DNA sequence that directs positioning of the 147 bp nucleosome core (Nikitina et al. 2013) is referred to as the “nucleosome-positioning sequence.” We placed a 167 bp DNA fragment (601-167×1) containing one nucleosome-positioning sequence between the E-silencer and the URA3 reporter gene (as shown in Figure 1, A–C, and schematic representations) and between the I-silencer and the URA3 reporter gene in different orientations (Figure 1, D and E, and schematic representations). The nucleosome-positioning sequences were placed ∼250 bp downstream of the E- and the I-silencers to accommodate the silencer-flanking genomic sequences from the HML locus and thus to mimic native HML and negate a possible interference between the silencing-positioned nucleosome number 20 (see Weiss and Simpson 1998) and the clone-601 nucleosome by separating them with a DNA linker. We observed that the URA3 reporter was repressed by each of the silencer elements in the presence of the 601-167×1 DNA sequence. The cells were able to grow on CSM and –Trp, but were unable to grow on −Trp/−Ura media and were 5-FOA-resistant due to the absence of a functional URA3 gene product (Figure 1, B–E, spotting assays), showing that clone 601 did not perturb silencing. We also placed the 601-167×1 DNA sequences in different orientations to see if it exhibited any directionality and observed that the 601-167×1 DNA sequence did not inhibit silencing of the URA3 reporter in either of the two different orientations examined (Figure 1, B–E, schematic representations and spotting assays). Thus, clone-601 nucleosome DNA appeared to be permissive for silencing independently of its orientation vs. the silencer. The copy numbers of the multi-copy S. cerevisiae minichromosomes were tested by Southern hybridization with specific TRP1-ARS1 probe and quantified using the Image Quant software to be ∼20 copies compared to the genomic copy (Figure 1, F and G).
Clone-601 nucleosome-positioning sequence does not interfere with antisilencing activities of STAR and TEF2-UAS
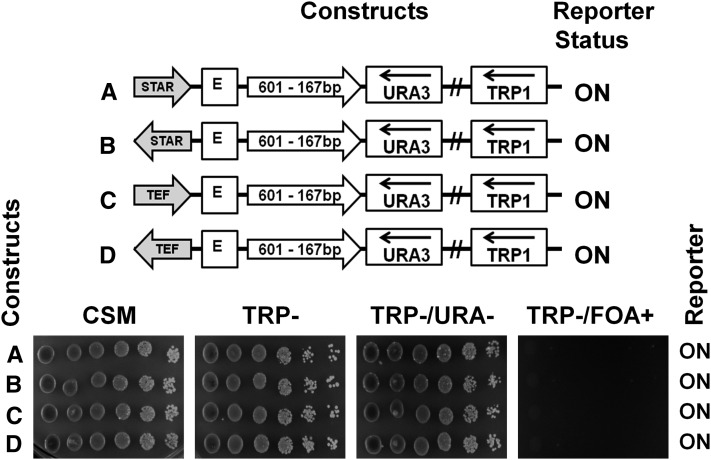
As the monomer of the nucleosome-positioning sequence did not interfere with silencing of the URA3 reporter by either E- or I-silencer elements, we examined if placing of the nucleosome-positioning sequences between the E-silencer and URA3 in the presence of antisilencing elements could affect URA3 transcription. Previously, we showed that the heterochromatin boundary and antisilencing elements STAR and TEF2-UASrpg could activate the URA3 reporter even if placed upstream of the silencing element in a minichromosome (Chakraborty et al. 2011; Bi and Broach 1999; Fourel et al. 1999). Here we placed STAR or TEF2UASrpg antisilencer elements upstream of the E-silencer in two different orientations in the minichromosome containing a monomer of clone 601-167×1 between the E-silencer and the URA3 reporter (Figure 2, A–D, schematic representations). We found that, in both orientations tested, the STAR and TEF2-UASrpg acted as antisilencer elements even in the presence of a monomer nucleosome-positioning sequence and were able to derepress URA3 (Figure 2, A–D, spotting assays). This experiment demonstrates that clone-601 DNA does not perturb the ability of an antisilencer placed upstream of the E-silencer to activate transcription of the reporter gene (Chakraborty et al. 2011). In further work, we used this property of the antisilencer elements to activate transcription without changing the sequence of chromatin block including the silencer, the array of clone-601 nucleosomes, and the reporter.
Figure 2.
Boundary elements STAR and TEF2-UAS block URA3 silencing in the presence of a clone-601 mononucleosome. Clone-601-167×1 DNA (167 bp NRL, open arrow) was positioned between the HML–E silencer and the URA3 reporter gene. The antisilencers STAR (A and B) or TEF2-UAS (C and D), shown by shaded arrows, were positioned upstream of the HML–E silencer in different orientations. The URA3 reporter status was tested by spotting assay as in Figure 1.
High-resolution micrococcal nuclease mapping reveals similar nucleosome organizations in the active and silenced states
Nuclei with yeast minichromosomes containing clone-601-167 monomer ∼250 bp downstream of the E silencer (construct 601-167×1) were digested with increasing amounts of MNase showing nucleosome ladder formation on agarose gel (Figure 3A). The chromatin structure of the MNase-digested samples was analyzed using high-resolution primer extension analysis for chromatin mapping. The strain YPH499 (untransformed a-cells) was used as a negative control. As expected, no significant DNA bands were observed in the control after digesting with MNase, as the end-labeled primers were specific only for the minichromosome and not for the genomic DNA (Figure 3B).
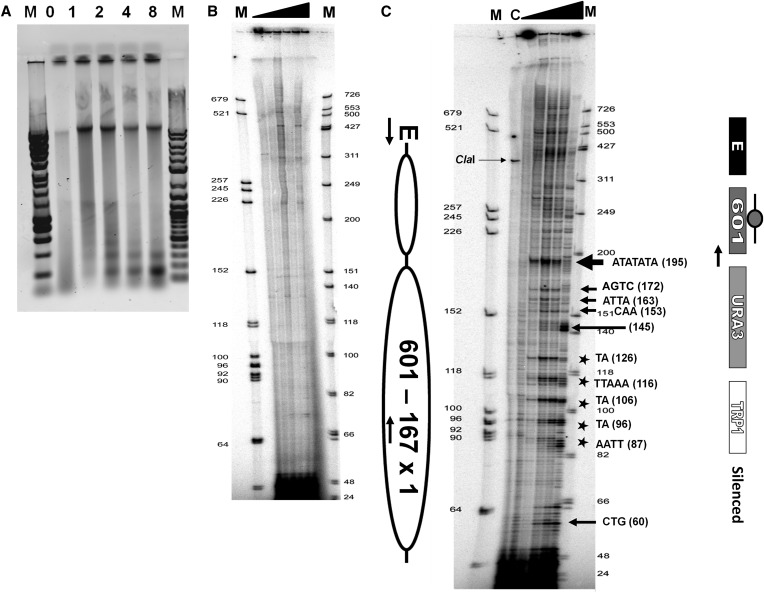
Figure 3.
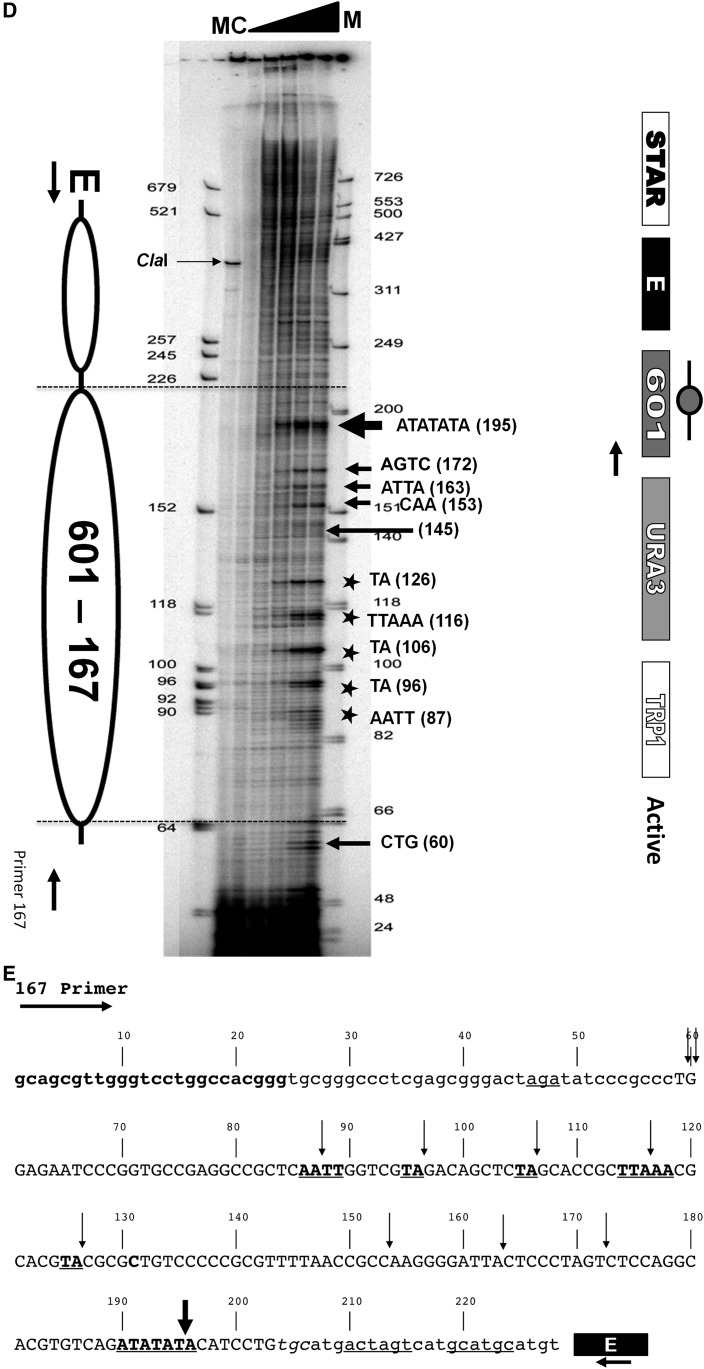
High-resolution MNase mapping of the clone-601 167 bp mononucleosome in the silent and the active states. (A) Ethidium-stained agarose gel showing nucleosome ladder of chromatin samples prepared from isolated nuclei of yeast cells transformed with 601-167×1 minichromosomes and digested with increasing amounts of micrococcal nuclease. “M” indicates the DNA size marker. Lanes marked 0, 1, 2, 4, and 8 represent units per milliliter of MNase used for digestion. (B) A control for the high-resolution chromatin mapping using the YPH499 (untransformed a-cells) and primer extension analysis of chromatin digested with increasing amounts of micrococcal nuclease from 0, 1, 2, 4, and 8 units/ml of MNase (shown by the solid triangle). In this phosphoimager image, the first and the last lane M is an AluI-digested dephosphorylated DNA marker and a Promega phiX174 DNA dephosphorylated marker respectively, end-labeled with a [32P ]γ-ATP radioisotope. (C and D) High-resolution chromatin maps obtained by a primer extension analysis of silent (C) and active (D) 601-167×1 mononucleosome digested with increasing amounts of micrococcal nuclease from 0, 1, 2, 4, and 8 units/ml of MNase as shown by the solid triangle. The first and the last lanes named M are the end-labeled AluI-digested DNA dephosphorylated marker and Promega phiX174 DNA dephosphorylated marker respectively. The lane C is a control showing a single ∼350 bp band of ClaI-digested chromatin. In the accompanying schemes, the ovals represent the predicted clone-601 nucleosome core positions and “E” denotes the HML-E silencer element. The nuclease hypersensitive cutting sites (A-T) are marked with arrows, and the sequence information is provided for the strongest MNase cutting. The five asterisks denote an ∼10 bp cutting pattern in the middle of the nucleosome core. The active state minichromosome construct (D) contains a STAR antisilencer element upstream of the HML-E silencer. (E) A nucleotide sequence scheme showing the primer sequence (in boldface), the clone-601 core nucleosome sequence (in uppercase letters), and the E-silencer as a rectangle. The nuclease hypersensitive cutting sites are marked by vertical arrows. The size of the arrows denotes the extent of MNase cutting. The asterisks in the chromatin mapping figures are shown by underlined and boldface letters. The lowercase letters are sequences from the nucleosome linker and the minichromosome backbone sequences, and the underlines are restriction enzyme sites used for multiple cloning.
A silent minichromosome construct containing a clone-601 mononucleosome in which the URA3 reporter was repressed by the E-silencer was mapped by MNase digestion (Figure 3C). The high-resolution mapping of clone-601 mononucleosome in the silent construct was conducted using primer SC-PE-N1 specific to the backbone of the minichromosome (Table S1). The first and the last lanes (M) are the end-labeled DNA size markers. As expected, a single band ∼350 bp can be seen in the second lane containing 0 unit/ml of MNase sample that was digested with ClaI restriction endonuclease as a hybridization control (lane C). The remaining lanes contain samples digested with 0, 1, 2, 4, and 8 units/ml of MNase. The MNase cutting pattern was prominent with increasing enzyme concentrations as expected and consistent with MNase preferentially cutting at A-T-rich nucleotides in the nucleosome linkers in vitro (Nikitina et al. 2013). The strongest MNase cutting sites are shown by boldface arrows (Figure 3C). The extent of cutting is denoted by the size of the arrows, where larger arrows correspond to more extensive cuts. The five asterisks denote ∼10 bp (A/T) nuclease cutting pattern in the nucleosome core (Figure 3C). The observed cutting pattern of the clone-601 nucleosome sequence is also schematized in Figure 3E. The dyad axis position at nucleotide 131 is according to nucleosome structural studies (Makde et al. 2010; Nikitina et al. 2013).
There are strong nuclease hypersensitive cutting sites at the AAT sequence at the beginning of the core and also at A-T-rich regions denoted by arrows within the core sequence of clone 601. A characteristic ∼10 bp cutting pattern (A/T) (shown by the asterisks) for every helical turn of the DNA was observed as five strong bands spanning a region of ∼40 bp in the core upstream of the dyad. There is a very strong nuclease hypersensitive cutting site at the ATATATA sequence at the end of the core (position 195 in Figure 3C). This position apparently corresponds to the end of a nucleosome core shifted by ∼8 bp from the predicted clone-601 core/linker boundary at position 203 (Figure 3E), suggesting that clone-601 DNA confers a unique nucleosome position in vivo that is different from in vitro.
We then mapped chromatin of a clone-601 mononucleosome in the active state. In this case, the URA3 reporter was not silenced by the E-silencer due to the presence of the upstream STAR antisilencer element (Figure 3D). The in vivo high-resolution chromatin structure of the clone-601 mononucleosome in the active state is shown in Figure 3D. As with the silent construct, the samples were digested with 0, 1, 2, 4, and 8 units/ml of MNase, and the cleavage patterns within the 601-167 bp mononucleosome of the active construct were determined to be very similar. Similar to the silent construct, in the active chromatin, the MNase cutting was observed at the linkers and the core including the strong cutting sites at the A-T-rich regions in the beginning and toward the end of the core shown by arrows within the active clone-601 mononucleosome. Although the characteristic ∼10 bp cutting pattern was seen, the active chromatin MNase cutting sites were less intense compared to the silent chromatin (Figure 3D). In particular, the digestion at sites 195 and 145 was notably slower compared to that of the silent chromatin (Figure 3C).
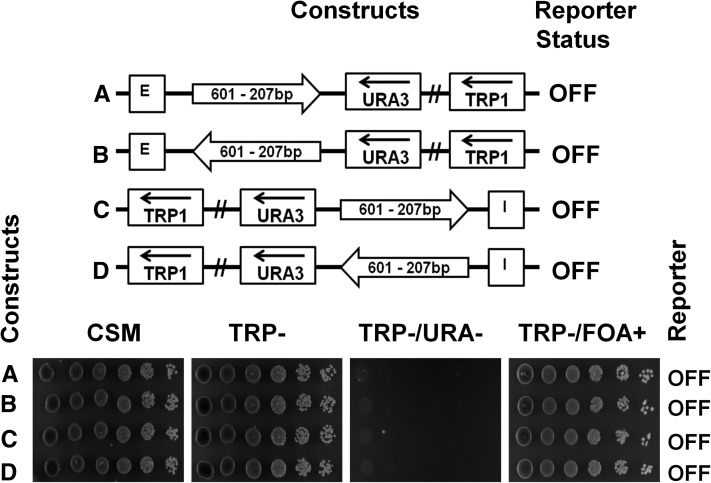
To examine if clone-601 DNA maintains its organization independently of its precise position vs. the E-silencer, we used a 601-207×1 monomeric template to place a clone-601 nucleosome starting ∼300 bp downstream of the E- or I-silencer, i.e., further downstream than the 601-167×1 nucleosome. We observed that, being placed at a longer distance from the silencer, the monomer of the 207 bp clone-601 nucleosome core did not hinder silencing of the URA3 reporter by either E- or I-silencers (Figure 4, A–D, spotting assays). High-resolution MNase mapping (Figure S2) shows that in this position clone 601 is separated by a longer linker from the E-silencer nucleosome number 20 (see Weiss and Simpson 1998). However, the pattern of internal MNase cleavage in the mononucleosome 601-207 is dramatically different, showing that clone-601 DNA organization depends on its position downstream of E-silencer. At the same time, the footprints of the E-silencer proximal nucleosomes numbers 20 and 21 (see Weiss and Simpson 1998) were preserved in the modified construct. We conclude that clone-601 nucleosome positioning is altered by its genomic context in the yeast minichromosome and does not disrupt the integrity of the yeast silencer in either active or inactive states.
Figure 4.
A monomer of clone-601 DNA (207 bp NRL) placed between the silencer and the reporter gene does not block silencing. Arrows indicate 207 bp clone-601 monomers placed in different orientations between the HML–E silencer (A and B) or the HML–I silencer (C and D) elements and the URA3 reporter gene. The URA3 reporter status was tested by spotting assay.
The number of nucleosome repeats influences silencing on minichromosomes
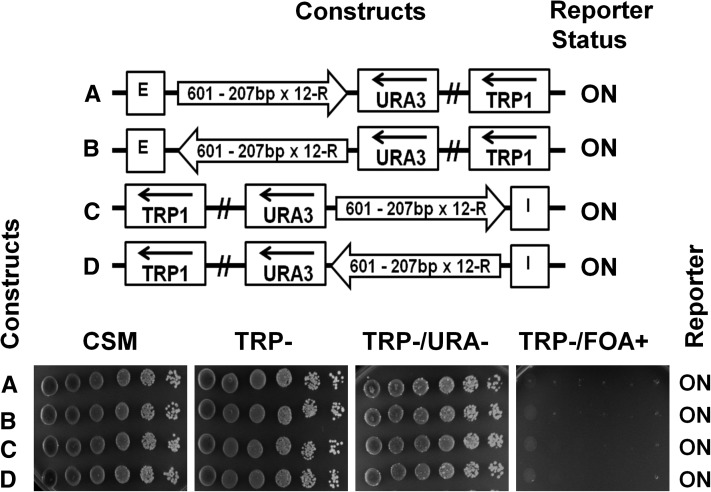
To relate in vitro studies of longer clone-601 nucleosome positioning sequence (Correll et al. 2012) to in vivo studies, we decided to test several longer nucleosome repeat lengths and repeat numbers of clone 601 in yeast minichromosomes. First, we used a 601-207×1 monomeric template to place a clone-601 nucleosome starting ∼300 bp downstream of the E- or I-silencer, i.e., further downstream than the 601-167×1 nucleosome to accommodate the longer linker and the silencer-flanking genomic sequences from the HML. We observed that, being placed at a longer distance from the silencer, the monomer of the 207 bp clone-601 nucleosome core did not hinder silencing of the URA3 reporter by either E- or I-silencers (Figure 4, A–D, spotting assays). In contrast, we observed 12-mer uniform arrays of 601-207×12 (Figure 5, A–D, spotting assays) and similar size arrays with variable linker DNA lengths [601-(207±2)×12] were able to block silencing of the reporter from both the E- and the I-silencer elements (Figure S1, schematic representations and spotting assays).
Figure 5.
An array of 12 regular repeats of clone-601 nucleosomes (207 bp NRL) blocks silencing of the URA3 reporter. Arrows indicate 207 bp clone-601 oligomers (regular 601-207×12 repeats) placed in different orientations between the HML–E silencer (A and B) or the HML–I silencer (C and D) elements and the URA3 reporter gene. The URA3 reporter status was tested by spotting assay.
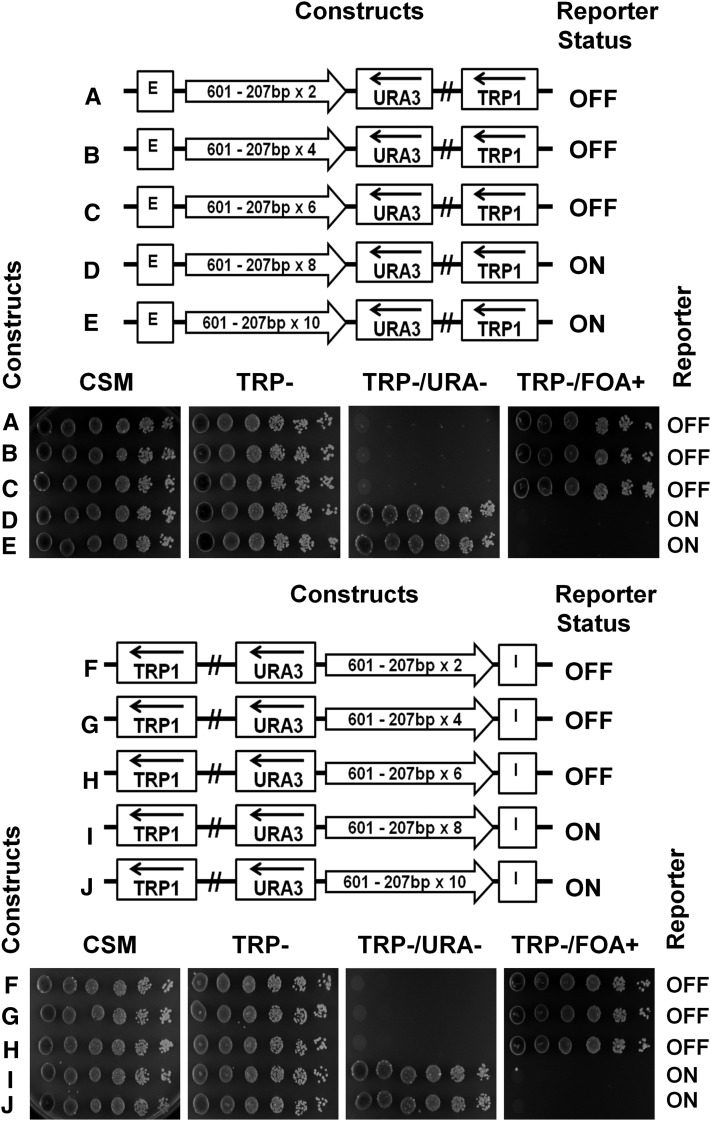
To identify the maximal number of repeats capable of conducting silencing on a minichromosome, we constructed and examined a dimer (601-207×2), tetramer (601-207×4), hexamer (601-207×6), octamer (601-207×8), and decamer (601207×10) positioned in between the E-silencer and the URA3 reporter (Figure 6, A–E, schematic representation). We have also conducted low-resolution mapping of 601-207 tetranucleosomes (Figure S3). We observed that the nucleosome repeats of the clone-601 oligonucleosomes were very similar to the bulk yeast nucleosome repeat.
Figure 6.
The 207 bp NRL arrays of up to six nucleosomes conduct silencing of the reporter gene by the E- or I-silencer elements. (A–E) The repeats of clone-601 DNA with 207 bp-long NRL (shown by open arrows) were positioned between the HML–E silencer and the URA3 reporter. The URA3 reporter status was tested by spotting assay. (F–J) The repeats of clone-601 DNA with 207 bp-long NRL (shown by open arrows) were positioned between the HML–I silencer and the URA3 reporter. The URA3 reporter status was tested by spotting assay.
Using our reporter assay, we observed that the first three constructs allowed the silencing of the URA3 reporter by the E-silencer (Figure 6, A–C, spotting assays). However, with the octamer and the decamer repeats, the silencing was disrupted and URA3 reporter was expressed (Figure 6, D and E, spotting assays). We thus concluded that more than six consecutive clone-601 nucleosomes were restricting the free conductance of silencing between the E- and I-silencers and the reporter gene. Similar results were obtained with the I-silencer, where the silencing was conducted through dimer up to hexamer (Figure 6, F–H) but not through the 8-mer and 10-mer (Figure 6, I and J).
Arrays with shorter spacing between the nucleosomes conduct silencing for a longer distance
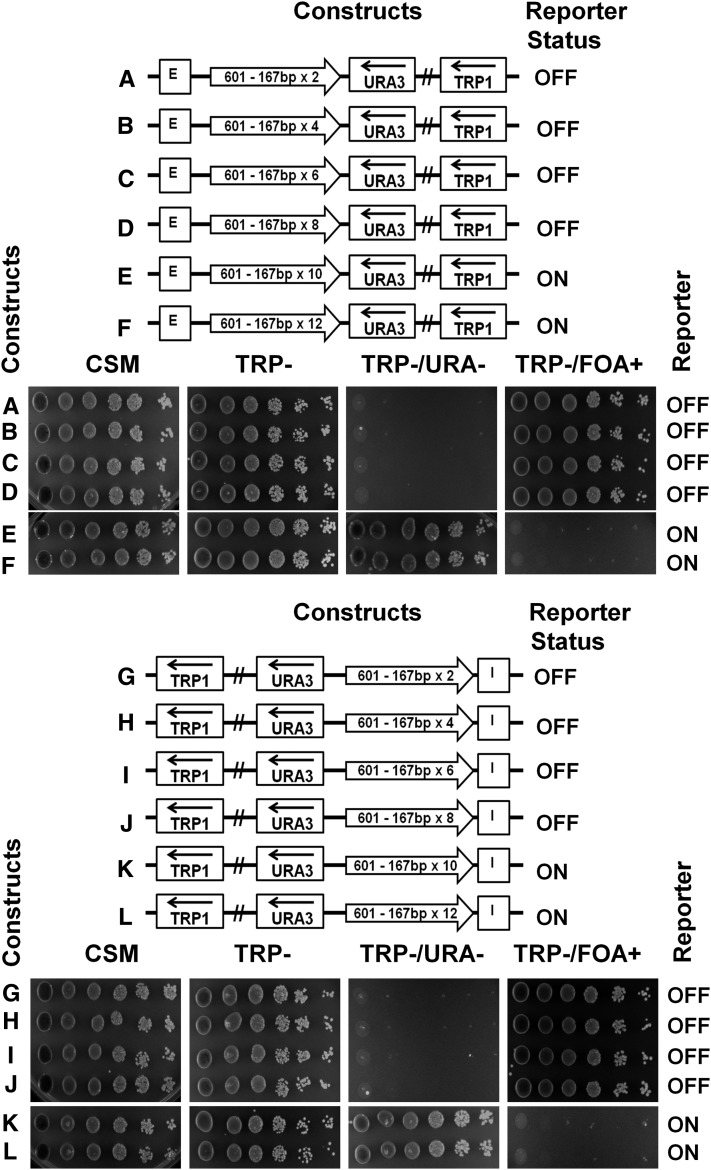
We then examined silencing conductance by shorter repeats of clone-601 DNA sequence containing 167 bp of nucleosome-positioning DNA sequences close to the predominant nucleosome repeats in yeast (Thomas and Furber 1976). Nucleosome arrays with 167 bp repeats had been shown to promote a tighter higher-order folding of chromatin fibers than arrays with 207 bp repeats in vitro (Routh et al. 2008; Correll et al. 2012). We observed that the repeats from the dimer to the octamer effectively conducted silencing emanating from the E-silencer (Figure 7, A–D, spotting assays). However, the decamer and the dodecamer were not able to conduct the silencing, which led to the expression of the URA3 reporter (Figure 7, E and F, spotting assays). We obtained very similar results when we placed the nucleosome-positioning sequences between the URA3 reporter and the I-silencer (Figure 7, G–L).
Figure 7.
The 167 bp NRL arrays of up to eight nucleosomes conduct silencing of the reporter gene by the E- or I-silencer elements. (A–F) The repeats of clone-601 DNA with 167 bp NRL (shown by open arrows) were positioned between the HML–E silencer and the URA3 reporter. The URA3 reporter status was tested by spotting assay. (G–L) The repeats of clone-601 DNA with 167 bp NRL (shown by open arrows) were positioned between the HML–I silencer and the URA3 reporter. The URA3 reporter status was tested by spotting assay.
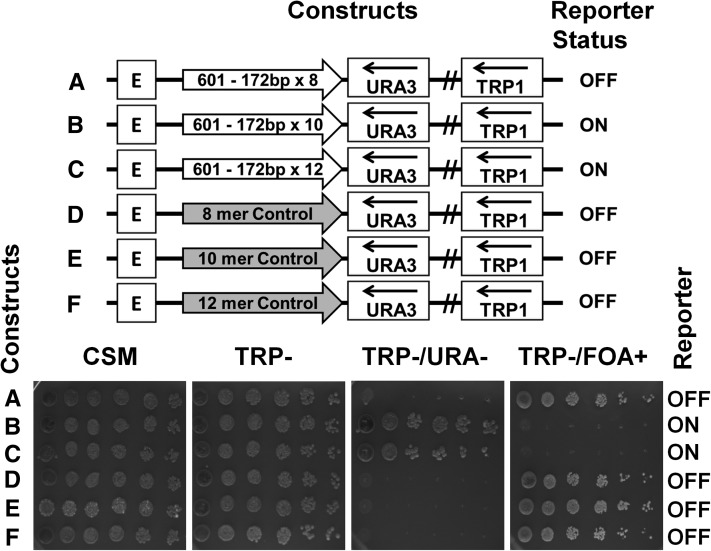
In a recent work we observed that the 172 bp nucleosome spacing, more abundant in S. cerevisiae than the 167 bp repeat (Wang et al. 2008), inhibits tight nucleosome folding as compared to the 167 bp repeats in vitro (Correll et al. 2012). To examine whether the difference observed between the NRL of 167 and 172 bp would affect chromatin silencing on a yeast minichromosome in vivo, we constructed and placed a series of 172 bp NRL nucleosome-positioning sequences between the E-silencer and the URA3 reporter. We observed, however, that substitution of the 167 bp repeat with the 172 bp repeat did not have a significant effect on yeast silencing conductance by the E-silencer (Figure 8, A–C, spotting assays). We also did not observe either an increased silencing by the 167 bp repeat as compared to the 172 bp repeat or a stronger protection of the silent minichromosomes by MNase. It thus appears that both shorter nucleosome repeat lengths (167 and 172 bp) are more efficient in promoting silencing in yeast minichromosomes in vivo than the longer 207 bp repeat, consistent with the more unfolded structure of the latter repeat in vitro.
Figure 8.
A native S. cerevisiae genomic DNA sequence is more efficient in conducting silencing than the arrays of the nucleosome-positioning sequence. (A–C) Octamer (A), decamer (B), and dodecamer (C) repeats of clone-601 DNA with 172 bp long NRL (shown by open arrows) were positioned between the HML–E silencer and the URA3 reporter. (E and F) A natural genomic sequence control equivalent to the length of an octamer, decamer, and dodecamer of 601–172 bp (from the S. cerevisiae LYS2 ORF shown by the shaded arrows) was placed between the HML–E silencer and the URA3 reporter. The URA3 reporter status was tested by spotting assay.
As an additional control, yeast strains with the minichromosome constructs containing different repeats (8-mers, 10-mers, and 12-mers oligonucleosomes) were isolated from yeast and sequenced. Yeast minichromosome DNA samples purified using standard protocols (Rose et al. 1990) were retransformed and grown in E. coli followed by DNA sequencing using the primers listed (Table S1). The sequences confirmed that no recombination events occurred.
Yeast genomic DNA sequence conducts silencing more efficiently than the clone-601 repeats
Since DNA repeats are known to promote heterochromatin formation in higher eukaryotes (Dorer and Henikoff 1994; Roshina et al. 2008; Kagansky et al. 2009), we examined whether silencing conductance is as efficient on a unique genomic DNA as on clone-601 repeats. Therefore, we placed natural DNA sequences from the LYS2 ORF of the length of octamer, decamer, and dodecamer between the E-silencer and the URA3 reporter as a control for the size comparable to the 601-172×8, 601-172×10, and 601-172×12 constructs (Figure 8, A–C, schematic representations). LYS2 DNA has been shown before not to interfere with silencing imposed by the E- and I-silencer elements (Maillet et al. 1996). We found that the silencing was not interrupted by the control sequences and that the URA3 reporter was repressed by the E-silencer (Figure 8, D–F, spotting assays). It thus appears that the silencing conducted by the yeast genomic sequences is more efficient compared to the repeats of clone-601 high DNA–histone affinity nucleosome arrays in vivo.
Discussion
Previous genetic screening revealed a number of nucleosome-size sequences acting in a position- and orientation-dependent manner to block spreading of heterochromatin silencing in yeast (Bi and Broach 2001). Previously, we showed that two boundary elements were specifically blocking silencing in our S. cerevisiae minichromosome system (Chakraborty et al. 2011). Here we used the same system as a tool to investigate the conductance of chromatin silencing in vivo through repeats of the clone-601 nucleosome-positioning sequence (Lowary and Widom 1998). We found that a single clone-601 mononucleosome was effectively conducting silencing of the URA3 reporter by either the HML-E or the HML-I silencer elements and hence had no specific barrier activity. This is in contrast to a number of other artificial DNA sequences such as short GC-rich DNA repeats and dA-dT tracts that did not support nucleosome formation and efficiently blocked conductance of silencing with only 40–80 bp of such sequences inserted between the E-silencer and URA3 reporter (Bi et al. 2004). However, we observed that the silencing was disrupted when repeats of more than six clone-601 nucleosomes were inserted between the silencer and URA3 reporter. Interestingly, the nucleosome arrays with shorter repeat lengths (167 and 172 bp) were more efficient in conducting silencing when compared to the longer (207 bp) nucleosome repeats. One potential explanation of this phenomenon could be the increasing distance between the silencer and the promoter, and the other one could be the formation of an alternative chromatin higher-order structure, as six nucleosomes are likely to be the minimal unit needed to form one turn of a helical 30 nm fiber (Ghirlando and Felsenfeld 2008). Whether the nucleosomes should fold into a helical fiber for conducting silencing signals across nucleosome arrays is of significant interest for further studies.
Since previous studies have demonstrated positional-dependent variegation of reporter genes regulated by telomeric heterochromatin (Gottschling et al. 1990; Smith et al. 2002), initially we expected that some of our constructs would impose a variegating phenotype. In this study and in our previous work (Chakraborty et al. 2011), we have genetically tested more than one hundred minichromosome constructs containing various nucleosome-positioning sequences, known silencing regulators, and DNA controls. However, there was not a single case of phenotypic instability or variegation in our minichromosomes (either promoting or inhibiting silencing). Previous data suggested that silencing could be spread over a distance of 7 kb through a Ty retrotransposon sequence inserted at the HML α-locus (Mastrangelo et al. 1992) and that the dose of an architectural factor Sir3 protein was limiting for a longer spreading of heterochromatin-mediated silencing at telomeres (Renauld et al. 1993; Hecht et al. 1996). Since in yeast cells our reporter minichromosomes are represented by ∼20 copies (Figure 1, F and G), it appears that an increased copy number of silent nucleosomes makes an efficient threshold to buffer natural dosage variation of the silencing factors. It is also known that plasmid-borne silencers are less constrained than those in the chromosomes and may interact in trans with multiple peritelomeric repressive compartments, rather than with just the nearby telomeric region (Lebrun et al. 2003), so that the variegating in cis spreading would be negated by the in trans interactions. In addition, if silencing is conducted via a distinct superhelical density (Bi and Broach 1997) or an altered looped topography as was proposed most recently (Thurtle and Rine 2014), then in the yeast minicromosomes the alternative looping could be facilitated by topological transitions in the covalently closed minichromosome DNA. Our system allows one to isolate yeast minichromosomes in their native state, so that future structural studies could clarify whether any topological or other conformational alterations distinguish the silent and active minichromosomes.
In vitro, the nucleosome repeats with short NRL such as 167–177 bp form a compact higher-order structure in the absence of linker histone H1 (Correll et al. 2012). In vivo, yeast S. cerevisiae has an average nucleosome repeat of 167 bp, which may represent a mixture of quantized repeats with maximal distribution of 162 and 172 bp (Wang et al. 2008; Brogaard et al. 2012; Eriksson et al. 2012). Yeast also have a relatively low level of the linker histone compared to higher eukaryotes (Freidkin and Katcoff 2001; Downs et al. 2003), which inhibits rather than promotes silencing (Yu et al. 2009). The decreased potential of the longer NRL (207 bp), and hence a lesser nucleosome density to fold the chromatin fiber and conduct silencing, is consistent with the global transcriptional upregulation observed as a result of decreased nucleosome density in yeast undergoing replicative aging (Hu et al. 2014).
Previous in vitro transcriptional experiments showed that positioned nucleosomes presented barriers for transcription depending on their sequence orientation vs. the transcription direction (Bondarenko et al. 2006). In our experiments, we observed no orientation dependency in mononucleosomes or oligonucleosomes inserted between the URA3 reporter and E- or I-silencers. Our results are consistent with the previous finding that the clone-601 and -603 mononucleosome-positioning sequences affected transcription from a yeast reporter gene in an orientation-independent manner in vivo (Gaykalova et al. 2011).
We also found no differences between the uniform and the variable nucleosome repeats, as both were equally strong in conducting silencing of the URA3 reporter by the silencer elements (Figure S1). The in vivo genetic results are consistent with recent in vitro experiments showing no difference in higher-order folding between the variable length and uniform repeats of clone-601 nucleosomes (Correll et al. 2012). However, with a natural yeast sequence, the silencing appeared to be conducted for a longer distance as the URA3 reporter was fully repressed by the longest fragment of LYS2 equal to a clone 12-mer. The weight of evidence, therefore, favors that the extent of silencing by yeast HML-E and HML-I is not promoted by the repeated nature of underlying DNA in contrast to higher eukaryotic systems (Dorer and Henikoff 1994; Roshina et al. 2008; Kagansky et al. 2009).
The design of oligonucleotide primer for the clone 601 allowed us to map the clone-601 mononucleosome in the E-silencer area with high precision. Here we observed that the chromatin organization of the HML-E silencer previously mapped in the yeast genome (Weiss and Simpson 1998) is retained in the minichromosomes and is very similar to the structure and function of the silencer elements in the genome. Our high-resolution mapping of the yeast minichromosomes in the silent and active states with micrococcal nuclease clearly shows that the complete minichromosome was readily accessible to the nuclease in sharp contrast to viral minichromosomes in human cells (Kumala et al. 2012). Moreover, in our experiments the silent chromatin appears to be digested notably faster by the MNase than the active chromatin (Figure 3C) although the MNase cleavage patterns of the active chromatin and the silent chromatin are similar (Figure 3, C and D). Thus, our minichromosome reporter that is remarkably robust in relation to copy number and recombination stability readily conducts repressive signals from HML silencers to the URA3 gene without any major chromatin unfolding and nucleosome losses or repositioning.
It thus appears that spreading of chromatin silencing does not require a higher DNA–histone affinity or a tighter chromatin folding. This is consistent with the previous findings (Sekinger and Gross 2001; Chen et al. 2005) that chromatin silencing does not present a strong hindrance for a transcriptional factor’s access to the silent promoters. Perhaps other aspects of chromatin structure such as special architectural factors, the intrinsic interaction of histone tails, and/or nucleosome chain topography (see, e.g., Wang et al. 2013; Thurtle and Rine 2014), rather than mere compaction, plays a critical role in the transmission of silencing signals from the silencer to the reporter. Future structural studies using the minichromosome reporters isolated from yeast cells in a native state should reveal the structural changes accompanying heterochromatin spreading in S. cerevisiae.
Supplementary Material
Acknowledgments
We thank the Department of Biochemistry and Molecular Biology, Pennsylvania State University, for providing equipment and reagents; the late Robert Simpson for insights; Evgenya Popova, Ralph Keil, Laura Carrel, and Avery August for helpful discussions; and Ji Qi and Ross Keller for technical assistance. We acknowledge funding support by National Science Foundation grant MCB–1021681 (to S.A.G.) and an American Association of University Women Doctoral Fellowship Award (to S.A.C.). This project was also funded, in part, by a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.169508/-/DC1.
Communicating editor: M. Hampsey
Literature Cited
- Bi X., Broach J. R., 1997. DNA in transcriptionally silent chromatin assumes a distinct topology that is sensitive to cell cycle progression. Mol. Cell. Biol. 17: 7077–7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., Broach J. R., 1999. UASrpg can function as a heterochromatin boundary element in yeast. Genes Dev. 13: 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., Broach J. R., 2001. Chromosomal boundaries in S. cerevisiae. Curr. Opin. Genet. Dev. 11: 199–204. [DOI] [PubMed] [Google Scholar]
- Bi X., Yu Q., Sandmeier J. J., Zou Y., 2004. Formation of boundaries of transcriptionally silent chromatin by nucleosome-excluding structures. Mol. Cell. Biol. 24: 2118–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R., 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197: 345–346. [DOI] [PubMed] [Google Scholar]
- Bondarenko V. A., Steele L. M., Ujvari A., Gaykalova D. A., Kulaeva O. I., et al. , 2006. Nucleosomes can form a polar barrier to transcript elongation by RNA polymerase II. Mol. Cell 24: 469–479. [DOI] [PubMed] [Google Scholar]
- Brogaard K., Xi L., Wang J. P., Widom J., 2012. A map of nucleosome positions in yeast at base-pair resolution. Nature 486: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S. A., Simpson R. T., Grigoryev S. A., 2011. A single heterochromatin boundary element imposes position-independent antisilencing activity in Saccharomyces cerevisiae minichromosomes. PLoS ONE 6: e24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Dundr M., Wang C., Leung A., Lamond A., et al. , 2005. Condensed mitotic chromatin is accessible to transcription factors and chromatin structural proteins. J. Cell Biol. 168: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Wilson M. A., Hirsch C., Watson A., Liang S., et al. , 2013. Stabilization of the promoter nucleosomes in nucleosome-free regions by the yeast Cyc8-Tup1 corepressor. Genome Res. 23: 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll S. J., Schubert M. H., Grigoryev S. A., 2012. Short nucleosome repeats impose rotational modulations on chromatin fibre folding. EMBO J. 31: 2416–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A., Rine J., 1995. On the origin of a silencer. Trends Biochem. Sci. 20: 231–235. [DOI] [PubMed] [Google Scholar]
- Dorer D. R., Henikoff S., 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77: 993–1002. [DOI] [PubMed] [Google Scholar]
- Downs J. A., Kosmidou E., Morgan A., Jackson S. P., 2003. Suppression of homologous recombination by the Saccharomyces cerevisiae linker histone. Mol. Cell 11: 1685–1692. [DOI] [PubMed] [Google Scholar]
- Ducker C. E., Simpson R. T., 2000. The organized chromatin domain of the repressed yeast a cell-specific gene STE6 contains two molecules of the corepressor Tup1p per nucleosome. EMBO J. 19: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. R., Workman J. L., 2000. Chromatin Structure and Gene Expression. Oxford University Press, Oxford. [Google Scholar]
- Eriksson P. R., Ganguli D., Nagarajavel V., Clark D. J., 2012. Regulation of histone gene expression in budding yeast. Genetics 191: 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel G., Revardel E., Koering C. E., Gilson E., 1999. Cohabitation of insulators and silencing elements in yeast subtelomeric regions. EMBO J. 18: 2522–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidkin I., Katcoff D. J., 2001. Specific distribution of the Saccharomyces cerevisiae linker histone homolog HHO1p in the chromatin. Nucleic Acids Res. 29: 4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykalova D. A., Nagarajavel V., Bondarenko V. A., Bartholomew B., Clark D. J., et al. , 2011. A polar barrier to transcription can be circumvented by remodeler-induced nucleosome translocation. Nucleic Acids Res. 39: 3520–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlando R., Felsenfeld G., 2008. Hydrodynamic studies on defined heterochromatin fragments support a 30-nm fiber having six nucleosomes per turn. J. Mol. Biol. 376: 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H., 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2: 31–34. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L., Zakian V. A., 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63: 751–762. [DOI] [PubMed] [Google Scholar]
- Grewal S. I., Moazed D., 2003. Heterochromatin and epigenetic control of gene expression. Science 301: 798–802. [DOI] [PubMed] [Google Scholar]
- Grigoryev S. A., Arya G., Correll S., Woodcock C. L., Schlick T., 2009. Evidence for heteromorphic chromatin fibers from analysis of nucleosome interactions. Proc. Natl. Acad. Sci. USA 106: 13317–13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32: 561–599. [DOI] [PubMed] [Google Scholar]
- Haber J. E., 2012. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics 191: 33–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A., Strahl-Bolsinger S., Grunstein M., 1996. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383: 92–96. [DOI] [PubMed] [Google Scholar]
- Hu Z., Chen K., Xia Z., Chavez M., Pal S., et al. , 2014. Nucleosome loss leads to global transcriptional up-regulation and genomic instability during yeast aging. Genes Dev. 28: 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Jin Y., Rando O. J., Struhl K., 2012. A functional evolutionary approach to identify determinants of nucleosome positioning: a unifying model for establishing the genome-wide pattern. Mol. Cell 48: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagansky A., Folco H. D., Almeida R., Pidoux A. L., Boukaba A., et al. , 2009. Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science 324: 1716–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumala S., Hadj-Sahraoui Y., Rzeszowska-Wolny J., Hancock R., 2012. DNA of a circular minichromosome linearized by restriction enzymes or other reagents is resistant to further cleavage: an influence of chromatin topology on the accessibility of DNA. Nucleic Acids Res. 40: 9417–9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun E., Fourel G., Defossez P. A., Gilson E., 2003. A methyltransferase targeting assay reveals silencer-telomere interactions in budding yeast. Mol. Cell. Biol. 23: 1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary P. T., Widom J., 1998. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276: 19–42. [DOI] [PubMed] [Google Scholar]
- Luger K., Dechassa M. L., Tremethick D. J., 2012. New insights into nucleosome and chromatin structure: An ordered state or a disordered affair? Nat. Rev. Mol. Cell Biol. 13: 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney D. J., Broach J. R., 1989. The HML mating-type cassette of Saccharomyces cerevisiae is regulated by two separate but functionally equivalent silencers. Mol. Cell. Biol. 9: 4621–4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet L., Boscheron C., Gotta M., Marcand S., Gilson E., et al. , 1996. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 10: 1796–1811. [DOI] [PubMed] [Google Scholar]
- Makde R. D., England J. R., Yennawar H. P., Tan S., 2010. Structure of RCC1 chromatin factor bound to the nucleosome core particle. Nature 467: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo M. F., Weinstock K. G., Shafer B. K., Hedge A. M., Garfinkel D. J., et al. , 1992. Disruption of a silencer domain by a retrotransposon. Genetics 131: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays Hoopes L. L., 1987. Nucleic acid blotting: Southern and Northern, 8.2.1-8.2.24 in Current Protocols: Essential Laboratory Techniques, edited by Ausubel F.. [Google Scholar]
- Moazed D., 2011. Mechanisms for the inheritance of chromatin states. Cell 146: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P., Freeman K., Coodly L., Shore D., 1994. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 8: 2257–2269. [DOI] [PubMed] [Google Scholar]
- Motwani T., Poddar M., Holmes S. G., 2012. Sir3 and epigenetic inheritance of silent chromatin in Saccharomyces cerevisiae. Mol. Cell. Biol. 32: 2784–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina T., Wang D., Gomberg M., Grigoryev S. A., Zhurkin V. B., 2013. Combined micrococcal nuclease and exonuclease III digestion reveals precise positions of the nucleosome core/linker junctions: implications for high-resolution nucleosome mapping. J. Mol. Biol. 425: 1946–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales R., Zhang L., Bentley D., 2011. Histone occupancy in vivo at the 601 nucleosome binding element is determined by transcriptional history. Mol. Cell. Biol. 31: 3485–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra A., Weiss K., Simpson R. T., 1999. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating-type locus HMRa. Mol. Cell. Biol. 19: 7944–7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renauld H., Aparicio O. M., Zierath P. D., Billington B. L., Chhablani S. K., et al. , 1993. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 7: 1133–1145. [DOI] [PubMed] [Google Scholar]
- Richmond T. J., Davey C. A., 2003. The structure of DNA in the nucleosome core. Nature 423: 145–150. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Hieter P. (Editors), 1990. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Roshina M. P., Loginova N. N., Devin A. B., Gvozdev V. A., 2008. [Heterochromatic DNA repeats in Drosophila and unusual gene silencing in yeast cells] Genetika 44: 752–760. [PubMed] [Google Scholar]
- Roth S. Y., Simpson R. T., 1991. Yeast minichromosomes. Methods Cell Biol. 35: 289–314. [PubMed] [Google Scholar]
- Routh A., Sandin S., Rhodes D., 2008. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proc. Natl. Acad. Sci. USA 105: 8872–8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlick T., Hayes J., Grigoryev S., 2012. Toward convergence of experimental studies and theoretical modeling of the chromatin fiber. J. Biol. Chem. 287: 5183–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekinger E. A., Gross D. S., 2001. Silenced chromatin is permissive to activator binding and PIC recruitment. Cell 105: 403–414. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Roth S. Y., Szent-Gyorgyi C., Simpson R. T., 1991. Nucleosomes are positioned with base pair precision adjacent to the alpha 2 operator in Saccharomyces cerevisiae. EMBO J. 10: 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P., 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., 1998. Chromatin structure and analysis of mechanisms of activators and repressors. Methods 15: 283–294. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Haimberger Z. W., Johnson C. O., Wolf A. J., Gafken P. R., et al. , 2002. Heritable chromatin structure: mapping “memory” in histones H3 and H4. Proc. Natl. Acad. Sci. USA 99(Suppl 4): 16454–16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Segal E., 2013. Determinants of nucleosome positioning. Nat. Struct. Mol. Biol. 20: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Furber V., 1976. Yeast chromatin structure. FEBS Lett. 66: 274–280. [DOI] [PubMed] [Google Scholar]
- Thurtle D. M., Rine J., 2014. The molecular topography of silenced chromatin in Saccharomyces cerevisiae. Genes Dev. 28: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Li G., Altaf M., Lu C., Currie M. A., et al. , 2013. Heterochromatin protein Sir3 induces contacts between the amino terminus of histone H4 and nucleosomal DNA. Proc. Natl. Acad. Sci. USA 110: 8495–8500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. P., Fondufe-Mittendorf Y., Xi L., Tsai G. F., Segal E., et al. , 2008. Preferentially quantized linker DNA lengths in Saccharomyces cerevisiae. PLOS Comput. Biol. 4: e1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K., Simpson R. T., 1997. Cell type-specific chromatin organization of the region that governs directionality of yeast mating type switching. EMBO J. 16: 4352–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K., Simpson R. T., 1998. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus HMLalpha. Mol. Cell. Biol. 18: 5392–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Kuzmiak H., Zou Y., Olsen L., Defossez P. A., et al. , 2009. Saccharomyces cerevisiae linker histone Hho1p functionally interacts with core histone H4 and negatively regulates the establishment of transcriptionally silent chromatin. J. Biol. Chem. 284: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.