Abstract
The Hippo pathway is a key signaling cascade in controlling organ size. The core components of this pathway are two kinases, Hippo (Hpo) and Warts (Wts), and a transcriptional coactivator, Yorkie (Yki). Yes-associated protein (YAP, a Yki homolog in mammals) promotes epithelial–mesenchymal transition and cell migration in vitro. Here, we use border cells in the Drosophila ovary as a model to study Hippo pathway functions in cell migration in vivo. During oogenesis, polar cells secrete Unpaired (Upd), which activates JAK/STAT signaling of neighboring cells and specifies them into outer border cells. The outer border cells form a cluster with polar cells and undergo migration. We find that hpo and wts are required for migration of the border cell cluster. In outer border cells, overexpression of hpo disrupts polarization of the actin cytoskeleton and attenuates migration. In polar cells, knockdown of hpo and wts or overexpression of yki impairs border cell induction and disrupts migration. These manipulations in polar cells reduce JAK/STAT activity in outer border cells. Expression of upd-lacZ is increased and decreased in yki and hpo mutant polar cells, respectively. Furthermore, forced expression of upd in polar cells rescues defects of border cell induction and migration caused by wts knockdown. These results suggest that Yki negatively regulates border cell induction by inhibiting JAK/STAT signaling. Together, our data elucidate two distinct mechanisms of the Hippo pathway in controlling border cell migration: (1) in outer border cells, it regulates polarized distribution of the actin cytoskeleton; (2) in polar cells, it regulates upd expression to control border cell induction and migration.
Keywords: Hippo pathway, JAK/STAT, Drosophila, oogenesis, border cell migration
EPITHELIAL–mesenchymal transition (EMT) and cell migration are fundamental for pattern formation during embryonic development (Thiery et al. 2009). In addition, these two cellular processes are critical steps of metastasis, a key event of cancer progression. Therefore, genes and signaling pathways involved in EMT or cell migration are of great interest for both basic and clinical research. To identify genes that are crucial for epithelial cells to become migratory, border cells in the Drosophila ovary provide an eligible in vivo model.
Border cells are a group of specialized follicle cells. During oogenesis, germline stem cells and follicle stem cells continue to divide and give rise to egg chambers, which are 16-cell germline cysts enwrapped by a single layer of follicle cells. The egg chamber buds off from the germarium and develops gradually until it becomes a mature egg. Polar cells located at the anterior and posterior ends of an egg chamber are specialized follicle cells important for patterning of the follicular epithelium. Based on polyploidization of germline cells, mitotic division of follicle cells, and the size of egg chambers, developmental egg chambers are categorized into different stages. At stage 8, anterior polar cells secrete Unpaired (Upd), a ligand of the JAK/STAT pathway. Upd activates JAK/STAT signaling of neighboring cells, leading to border cell induction (Silver and Montell 2001; Beccari et al. 2002). Activation of JAK/STAT signaling in outer border cells induces expression of slow border cells (slbo), which encodes a C/EBP transcription factor (Montell et al. 1992). Slbo induces expression of focal adhesion kinase (Fak), singed (sn, a homolog of mammalian Fascin), DE-cadherin, armadillo (arm, a Drosophila homolog of β-catenin), stathmin, and other genes involved in cell migration (Borghese et al. 2006; Wang et al. 2006). After being specified, outer border cells undergo partial EMT and form a cluster surrounding two polar cells. They detach from the follicular epithelium together and migrate toward the oocyte at stage 9 (Figure 1I). By stage 10, the border cell cluster arrives at the oocyte-nurse-cell border. Importantly, activation of JAK/STAT signaling is required throughout the migratory process, suggesting that JAK/STAT signaling is critical for both border cell induction and migration (Silver et al. 2005). As in all migratory cells, actin organization regulated by members of the Rho family GTPases, such as Rac, is crucial for border cell migration (Wang et al. 2010). Border cell migration is guided by Gurken (a Drosophila homolog of EGF) and PDGF/VEGF-related factor 1 (PVF1) secreted from the oocyte. In border cells, signaling through the PDGF/VEGF-related receptor (PVR) and the EGF receptor (EGFR) function together to control their migratory speed and direction (Duchek and Rorth 2001; Duchek et al. 2001; McDonald et al. 2003, 2006). Other signaling cascades, such as steroid hormones and the Notch pathway, also affect border cell migration (Bai et al. 2000; Wang et al. 2007; Jang et al. 2009). Importantly, homologs of these genes and the same signaling cascades in mammals play roles in regulating cell migration and cancer metastasis (Montell 2003; Naora and Montell 2005; Jang et al. 2007), demonstrating the relevance of studies of the Drosophila border cells to cancer biology. With powerful genetic tools, it is efficient to use border cells as a model to identify genes or signaling pathways involved in cell migration in vivo.
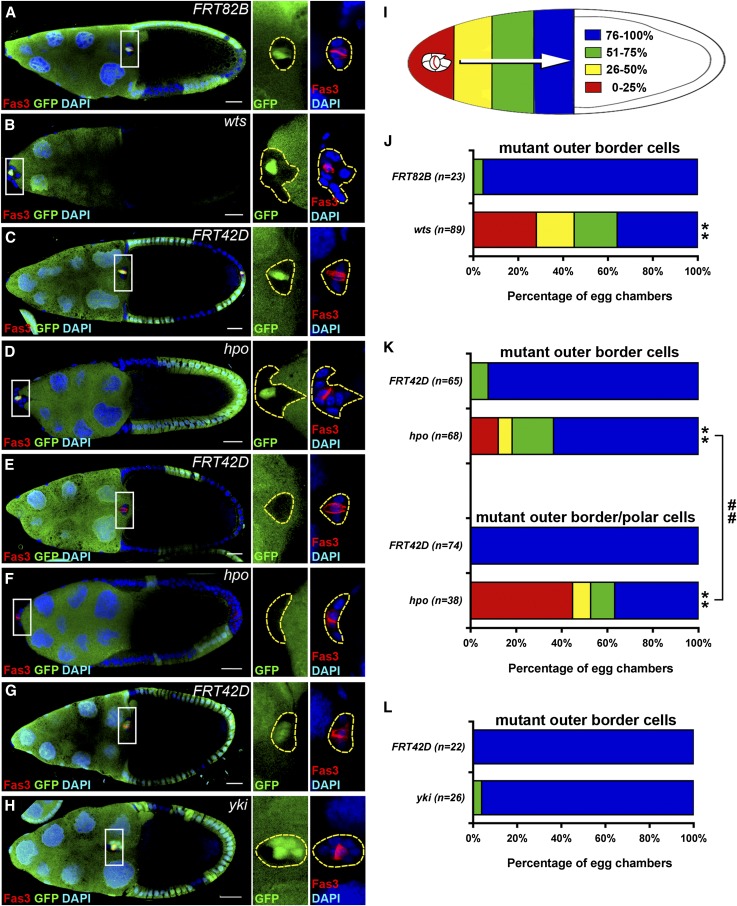
Figure 1.
hpo and wts are required for border cell migration. GFP-negative mitotic clones were generated in FRT82B (A), FRT82B wtsX1 (B), FRT42D (C and E), and FRT42D hpo42-47 (D and F) and examined 6 days after clone induction. Mitotic clones of FRT42D (G) and FRT42D ykiB5 (H) were examined 3 days after clone induction. The ovaries were immunostained with anti-Fas3 and anti-GFP antibodies. Cell nuclei were stained with DAPI. Stage-10 egg chambers were selected and oriented as anterior toward the left. The border cell cluster is composed of two Fas3-positive polar cells in the center surrounded by four to six outer border cells. High magnification views of border cell clusters are shown in the panels on the right in A–H. (A) A border cell cluster containing GFP-positive polar cells and GFP-negative FRT82B control outer border cells migrated normally and reached the oocyte-nurse-cell border. (B) A border cell cluster containing GFP-positive polar cells and GFP-negative wts mutant outer border cells failed to migrate. (C) A border cell cluster containing GFP-positive polar cells and GFP-negative FRT42D control outer border cells reached the oocyte-nurse-cell border. (D) A border cell cluster containing GFP-positive polar cells and GFP-negative hpo mutant outer border cells failed to migrate. (E) A border cell cluster containing GFP-negative FRT42D control polar cells and outer border cells reached the oocyte-nurse-cell border. (F) A border cell cluster containing GFP-negative hpo mutant polar cells and border cells failed to migrate. (G) A border cell cluster containing GFP-positive polar cells and GFP-negative FRT42D control outer border cells reached the oocyte-nurse-cell border. (H) A border cell cluster containing GFP-positive polar cells and some GFP-negative yki mutant outer border cells migrated >75%. (I) A diagram demonstrates colors representing the distance of border cell migration. (J) Quantification and percentage distribution of border cell migration. Only border cell clusters with GFP-positive polar cells and GFP-negative outer border cells were counted. wts mutation in outer border cells severely impaired migration. (K) Border cell clusters were categorized into two groups. The group with mutant outer border cells contained GFP-positive polar cells and GFP-negative mutant outer border cells; the group with mutant outer border/polar cells contained one or two GFP-negative mutant polar cells and GFP-negative mutant outer border cells. The migratory defect was more severe in the group with mutant outer border/polar cells than it was in the group with mutant outer border cells. (L) Only border cell clusters with GFP-positive polar cells and some GFP-negative outer polar cells were counted. yki mutation did not affect migration 3 days after clone induction. Wilcoxon rank-sum test, **P < 0.01 comparing with control; ##P < 0.01. Bar, 20 μm in A–H.
Recently, the Hippo pathway has been demonstrated to be crucial in multiple aspects of tumorigenesis, including cell migration. YAP, a mammalian homolog of Drosophila Yorkie), a key effector of this pathway, promotes EMT and cell motility in vitro (Overholtzer et al. 2006; Zhao et al. 2008a; Zhang et al. 2009). Furthermore, abnormal YAP activity associates with poor survival of ovarian cancer patients significantly (Hall et al. 2010; Zhang et al. 2011b). These results drew our attention to investigate roles of the Hippo pathway in cell migration. The Hippo pathway has recently emerged as a critical signaling cascade in controlling organ size by regulating cell proliferation and apoptosis (Lian et al. 2010; Lu et al. 2010; Oh and Irvine 2010; Pan 2010; Halder and Johnson 2011). The core components of this pathway in Drosophila are Hippo (Hpo), Warts (Wts), Salvador (Sav), Mob as a tumor suppressor (Mats), and Yorkie (Yki). Hpo is an Ste20-like kinase that forms a complex with the adaptor protein Sav and phosphorylates Wts, a nuclear Dbf2-related family kinase (Justice et al. 1995; Watson 1995; Harvey et al. 2003; Pantalacci et al. 2003; Wu et al. 2003). In association with the adapter protein Mats (Lai et al. 2005), Wts phosphorylates Yki, a transcriptional coactivator (Huang et al. 2005; Wei et al. 2007). Phosphorylated Yki interacts with 14-3-3 phosphopeptide-binding protein, resulting in cytoplasmic retention and repression of its transcriptional activity (Huang et al. 2005; Dong et al. 2007; Oh and Irvine 2008, 2009; Ren et al. 2010b). When Yki is not phosphorylated, it is localized to the nucleus and interacts with transcription factors such as Scalloped (Sd) to induce target gene expression (Goulev et al. 2008; Zhang et al. 2008; Zhao et al. 2008a). Most target genes of Yki, such as cyclin E, dm (dMyc), Diap1, and bantam, promote cell proliferation or survival (Wu et al. 2008; Ziosi et al. 2010). These core components are conserved from Drosophila to mammals, suggesting that Hippo pathway functions are essential (Harvey and Tapon 2007; Zeng and Hong 2008; Badouel et al. 2009; Pan 2010). During oogenesis in Drosophila, the Hippo pathway has also been shown to control proliferation of the follicle cell lineage (Zhao et al. 2008b; Huang and Kalderon 2014).
Although YAP has been shown to induce EMT and cell migration in cultured cells (Zhao et al. 2008a; Zhang et al. 2009; Xu et al. 2011), whether YAP/Yki or the Hippo pathway regulates cell migration in vivo remains unclear. In Drosophila, it has been demonstrated that Wts regulates invasion of follicle cells into egg chambers in coordination with basolateral junctional components, such as Fasciclin 2 and Discs large 1 (Zhao et al. 2008b). Wts has also been shown to be required for border cell migration (Zhao et al. 2008b). In addition, when we were conducting this study, Lucas et al. (2013) showed that Hpo and Wts controlled border cell migration through regulating localization of actin polymerization (see Discussion for details). In this study, we find that Hpo in outer border cells controls border cell migration and affects the actin cytoskeleton. In polar cells, the Hippo pathway controls the expression of upd, which activates JAK/STAT-signaling activity to promote border cell induction and migration. Together, our data show distinct mechanisms of the Hippo pathway in outer border cells and polar cells to regulate migration of the border cell cluster.
Materials and Methods
Fly stocks
Fly lines used for overexpression and knockdown experiments were the following: y w; UAS-hpo/CyO; MKRS/TM2, y w; Sp/CyO; UAS-hpo/TM2 (gift from Jin Jiang), P{UAS-yki.V5.O}attP2 (Bloomington Stock Center BLM28819), P{UAS-yki.S168A.V5}attP2 (BLM28818), P{UAS-yki.S111A.S168A.S250A.V5}attP2 (BLM28817), P{UAS-sd.S}V1 (BLM9373), UAS-H2B.RFP, UAS-GFP, P{KK101704}VIE-260B (Vienna Drosophila Resource Center, V104169), w1118; P{GD1570}v7823 (V7823), y1 sc* v1; P{TRiP.GL00046}attP2 (BLM35176), w1118; P{GD1563}v9928 (V9982), P{KK101055}VIE-260B (V106174), y1 v1; P{TRiP.HMS00026}attP2 (BLM34064), y1 v1; P{TRiP.JF02741}attP2 (BLM27662), P{KK109756}VIE-260B (V104523), w1118; P{GD11187}v40497/TM3 (V40497), y1 v1; P{TRiP.JF03119}attP2/TM3, Sb1 (BLM31965), y1 v1; P{TRiP.HMS00041}attP2 (BLM34067), P{KK104232}VIE-260B (V104197), upd-Gal4, upd-Gal4; tub-Gal80ts/CyO, slbo-Gal4 (gift from G. J. Liaw). Fly lines used for clonal analysis were the following: ey-flp; FRT42D, FRT42D ykiB5,whs/CyO, w−; FRT42D hpo42-47,w+/CyO, hs-flp; FRT42D ubi-GFP/CyO, FRT82B wtsx1/TM3,Sb, hs-flp;; FRT82B ubi-GFP/TM3,Sb, y w hs-flp; FRT42D arm-LacZ/CyO. upd-lacZ/FM6b and 10Xstat::GFP/TM3 were used for examining JAK/STAT-signaling activities. PZ80-LacZ and A101-LacZ were used for the identification of polar cells.
Antibodies and reagents
The following antibodies were used at the indicated dilutions: mouse anti-Fasciclin III 1:200 [7G10, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-β-galactosidase 1:200 (40-1A, DSHB), mouse anti-Arm 1:200 (N2 7A1, DSHB), mouse anti-Eya 1:200 (eya10H6, DSHB), mouse anti-cyclin B 1:200 (F2F4, DSHB), rabbit anti-GFP 1:1000 (Invitrogen), rat anti-Slbo 1:500 from Pernille Rorth (Borghese et al. 2006), Dylight-488 goat anti-rabbit IgG(H+L) 1:1000, Dylight-549 goat anti-mouse IgG(H+L) 1:1000 (Jackson ImmunoResearch Laboratories), Alexa-546 phalloidin 1:50 (Invitrogen).
Overexpression and RNAi knockdown
Offspring from crosses of UAS lines and slbo-Gal4; UAS-GFP, upd-Gal4; UAS-GFP, or upd-Gal4; tub-Gal80ts/CyO lines were cultured at 18° until eclosion. Newly eclosed adult flies were collected and grown at 29° for 40 or 54 hr (Gal80ts experiments) or 5–6 days before dissection.
Generation of mitotic clones
Mitotic clones were generated by using the FLP/FRT system (Xu and Rubin 1993). Adult flies eclosed in 3 days were collected and heat-shocked at 37° four times over the next 2 days. On the first day, they were heat-shocked twice for 30 min each time with at least a 3-hr interval. On the second day, they were heat-shocked once for 30 min and once for an hour with at least a 3-hr interval. Flies were incubated at 25° for 3 or 6 days before dissection.
Immunostaining and fluorescence microscopy
Flies were dissected in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS 20 min. After fixation, ovaries were washed with PBT (1× PBS with 0.2% Triton X-100) three times and then incubated with PBTB blocking solution (1× PBS, 0.5% Triton X-100, 5% goat serum, 2.5 mg/ml BSA, and 0.05% sodium azide) for an hour. Ovaries were incubated with primary antibodies in PBTB overnight at 4° and then with secondary antibodies in PBTB overnight at 4°. Ovaries were further stained with DAPI in PBT (1 μg/ml; Sigma) and then mounted with mounting solution [85% glycerol, 1× PBS, 3% propyl gallate (Sigma-Aldrich), and ProLong Gold Antifade reagents (Invitrogen)]. All images were taken by Zeiss LSM700 confocal microscope (Carl Zeiss AG) and processed with Photoshop CS3 (Adobe). For quantification of signal intensity, images of the brightest β-galactosidase optical slice were quantified by drawing an elliptic field of identical dimensions for each cell, and reading of the average intensity in the field was done by using ImageJ (National Institutes of Health, Bethesda, MD). A paired Student’s t-test was used to compare the signal intensities of the mutant and adjacent control cells.
Analysis of border cell migration
Stage-10 egg chambers were selected, and the position of border cell clusters was analyzed. We defined stage 10 as when the oocyte spanned the posterior half of the egg chamber. As an index for migration, these stage-10 egg chambers were categorized based on the location of the border cell cluster as depicted in Figure 1I. The Wilcoxon rank-sum test was used for analyzing the data of border cell migration. All graphs were plotted with Excel (Microsoft).
Results
hpo and wts are required for migration of the border cell cluster
To examine functions of the Hippo pathway in border cell migration, we generated homozygous wtsX1, hpo42-47, or ykiB5 mutant clones in border cell clusters using the FLP/FRT system (Xu and Rubin 1993). FRT82B or FRT42D clones were generated as controls. The border cell cluster usually arrives at the oocyte-nurse-cell border by stage 10, so we selected egg chambers at stage 10 and examined whether border cells migrated normally. Instead of using arrival of border cells at the oocyte-nurse-cell border as an indication of stage 10, we defined stage 10 as when the oocyte spanned the posterior half of the egg chamber. As an index for migration, these stage 10 egg chambers were further categorized based on the location of the border cell cluster (Figure 1I). Polar cells were identified based on the staining pattern of Fasciclin III (Fas3) (Ruohola et al. 1991). In comparison with the FRT82B control, migration of border cell clusters with wts mutant outer border cells was attenuated 6 days after clone induction (Figure 1, A, B, and J), suggesting that wts is required for border cell migration. No border cell cluster containing both wts mutant outer border cells and polar cells was observed (see Discussion). The migration of border cell clusters with hpo mutant outer border cells was attenuated compared with that in the FRT42D control (Figure 1, C, D, and K), suggesting that hpo is also required for border cell migration. Importantly, when both outer polar cells and border cells were mutant for hpo, the defect was more severe than it was when only outer border cells were mutant for hpo (Figure 1, E, F, and K). This result suggests that Hpo plays roles in both outer border cells and polar cells for migration of the border cell cluster. Although YAP has been demonstrated to induce EMT and cell migration in cultured cells (Zhao et al. 2008a; Zhang et al. 2009; Xu et al. 2011), migration of border cell clusters containing ykiB5 mutant outer border cells was normal compared with that in the FRT42D control 3 days after clone induction (Figure 1, G, H, and L), indicating that yki in outer border cells is not required for border cell migration. We did not examine yki mutant clones for border cell migration at day 6 after clone induction because most yki mutant cells differentiated into polar cells at day 6 as reported previously (Chen et al. 2011).
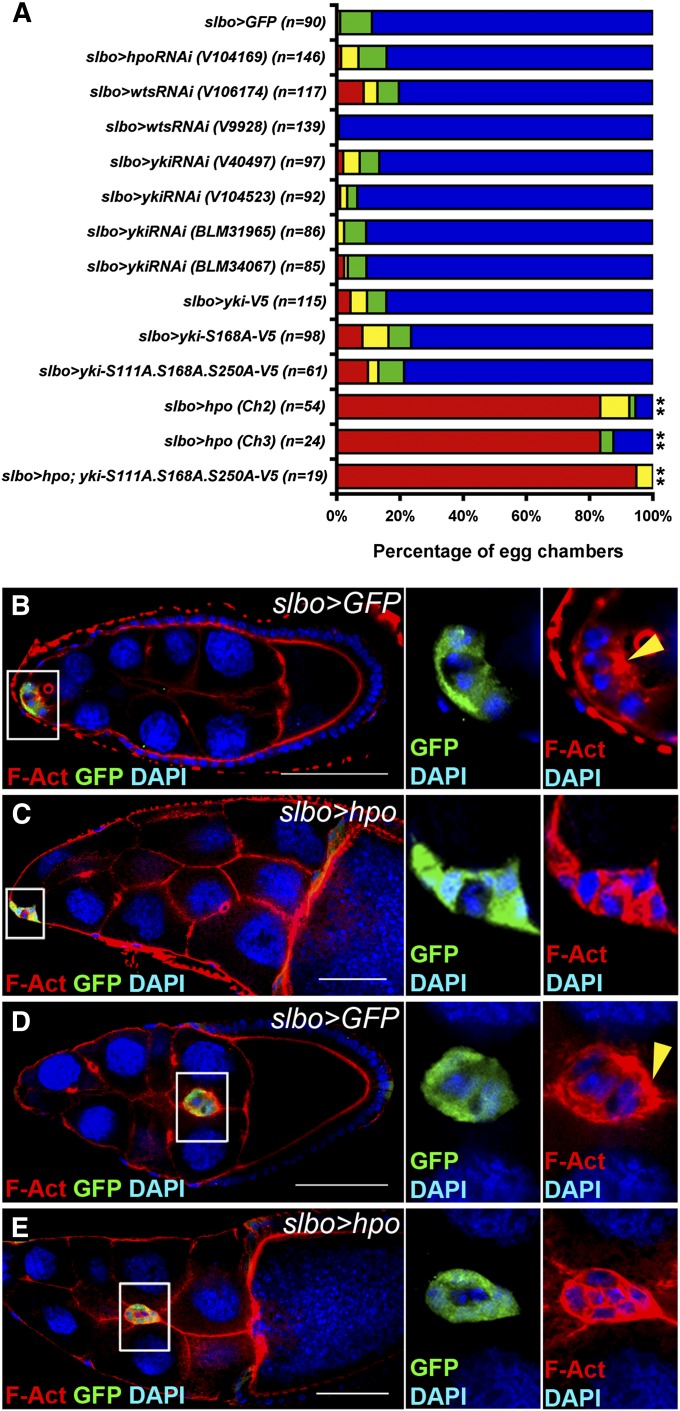
Overexpression of hpo in outer border cells disrupts border cell migration
To examine roles of the Hippo pathway in outer border cells, we used slbo-Gal4 driver to overexpress or knockdown Hippo pathway components in these cells specifically (Rorth et al. 1998; Pinheiro and Montell 2004; Inaki et al. 2012). RNA interference (RNAi) lines obtained from the Bloomington Drosophila Stock Center or Vienna Drosophila Resource Center have been tested in previous studies (Das Thakur et al. 2010; Genevet et al. 2010; Boggiano et al. 2011; Reddy and Irvine 2011; Chen and Verheyen 2012; Nagaraj et al. 2012; Poernbacher et al. 2012; Jukam et al. 2013). slbo-Gal4-driven UAS-GFP flies were used as controls. Unexpectedly, knockdown of hpo or wts in outer border cells by RNAi lines did not affect border cell migration significantly (Figure 2A). It is possible that knockdown of hpo and wts with slbo-Gal4 driver was not efficient enough to cause migratory defects in border cells compared with hpo and wts mutations (Figure 1). Phosphorylation of Yki on Ser residues at positions 111, 168, and 250 by Wts prevents nuclear localization of Yki. Yki with Ser-to-Ala mutation at residues 111/168/250 has been shown to stay in the nucleus to induce downstream targets constitutively (Oh and Irvine 2008, 2009). Neither overexpression of wild-type yki, yki-S168A, and yki-S111A, -S168A, -S250A (yki-3SA) nor knockdown of yki by various RNAi lines in outer border cells affected border cell migration (Figure 2A). Together with our result from yki mutant clones in Figure 1L, these data suggest that yki is dispensable in outer border cells for migration of the border cell cluster. Although knockdown of hpo, wts, and yki or overexpression of yki-3SA with slbo-Gal4 did not affect border cell migration, overexpression of hpo with slbo-Gal4 dramatically disrupted migration (Figure 2A). Canonically, Hpo acts by inhibiting Yki activity. However, overexpression of yki-3SA did not rescue the migratory defect caused by overexpression of hpo (Figure 2A), suggesting that hpo controls border cell migration in a yki-independent manner. The Hippo pathway has been demonstrated to control actin organization in the wing imaginal disc independently of Yki activity (Fernandez et al. 2011). Since rearrangement of the actin cytoskeleton is crucial for cell migration, we tested whether Hpo affected actin structures. In the control group, filamentous actin (F-actin) was distributed at the front edge of the border cell cluster and very little F-actin was localized to the boundary between border cells at stages 9 and 10 (Figure 2, B and D). Overexpression of hpo led to mis-localization of F-actin to the boundary between border cells and between outer border and polar cells (Figure 2, C and E), suggesting that hpo regulates localization of F-actin at the front edge during cell migration. While we were conducting this study, Lucas et al. (2013) showed that polarized distribution of F-actin was disrupted in hpo and wts mutant border cells, which is consistent with our finding.
Figure 2.
Overexpression of hpo with slbo-Gal4 increases filamentous actin between border cells and disrupts border cell migration. slbo-Gal4 was used to overexpress or knock down genes specifically in outer border cells. UAS-GFP driven by slbo-Gal4 was used as a control. The flies were dissected after being grown at 29° for 6 days. (A) Stage-10 egg chambers were selected for quantification. Colors of the graph represent the migratory distance depicted in Figure 1I. Numbers of egg chambers examined are indicated. Inhibition of hpo, wts, or yki in outer border cells did not affect border cell migration in comparison with that in the control. Overexpression of yki or constitutively active forms of yki, yki-S168A, and yki-S111A.S168A.S250A with slbo-Gal4 driver did not affect border cell migration. Overexpression of hpo with two different UAS-hpo lines severely disrupted border cell migration. The migratory defect was not alleviated when yki-S111A.S168A.S250A were overexpressed. Wilcoxon rank-sum test, **P < 0.01. (B–E) The ovaries were stained with anti-GFP, DAPI, and phalloidin for filamentous actin (F-actin). Border cell clusters were delaminating from the follicular epithelium (B and C) or migrating toward the oocyte (D and E). High magnification of border cell clusters is shown on the right. (B) In the UAS-GFP control, F-actin was enriched in the apical region of a border cell cluster indicated by a yellow arrowhead. (C) When hpo was overexpressed, F-actin was enriched between border cells. (D) In the UAS-GFP control, F-actin was enriched in the outer rim, especially in the front edge, of a migrating border cell cluster indicated by a yellow arrowhead. (E) When hpo was overexpressed, F-actin was enriched in boundaries between border cells as well as the outer rim. Bar, 20 μm in B–E.
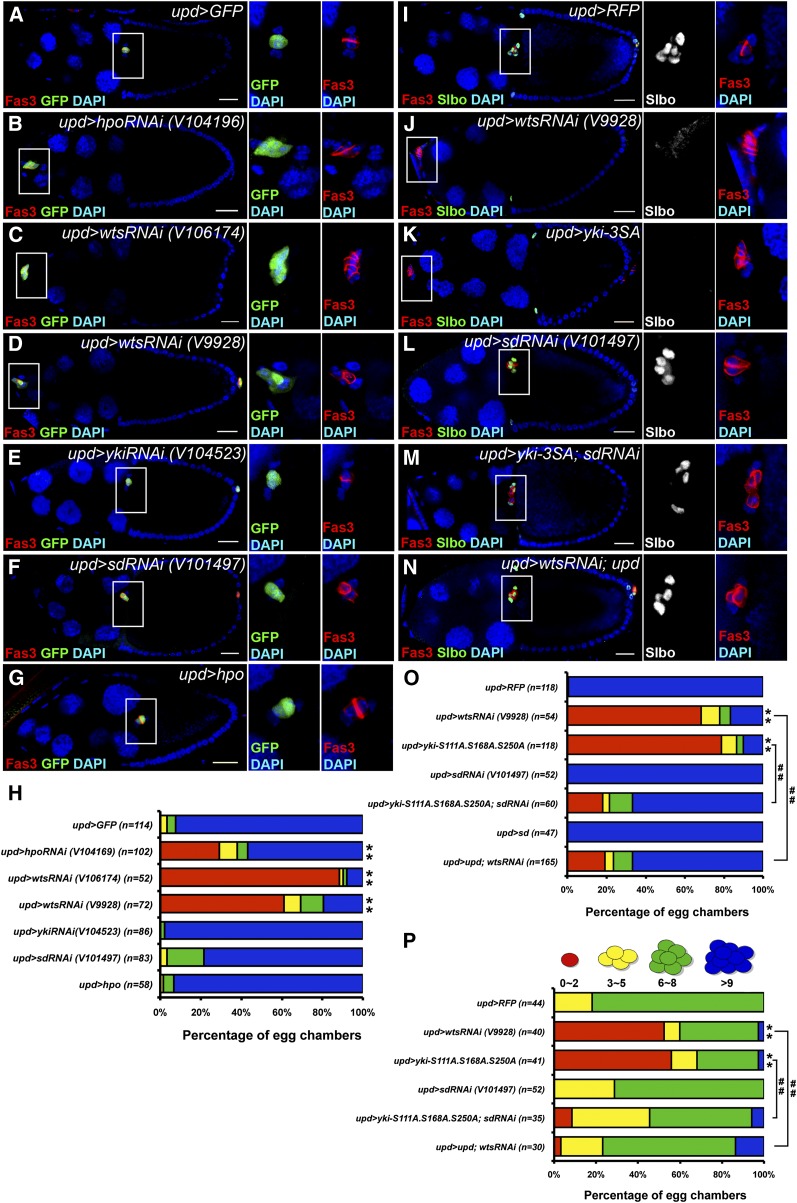
The Hippo pathway is required in polar cells for migration of the border cell cluster
Because the migratory defect of border cell clusters was more severe when hpo was mutant in both polar and outer border cells than it was when hpo was mutant in outer border cells alone (Figure 1K), we hypothesized that the Hippo pathway also played a role in polar cells to control the migration of border cell clusters. To examine this hypothesis, we used upd-Gal4 to overexpress or knockdown Hippo pathway components in polar cells specifically (Bai and Montell 2002). Because the 10Xstat::gfp (stat-GFP) reporter line was used for some experiments (see below), either UAS-GFP or UAS-RFP was used to visualize upd-Gal4 expression in polar cells and as a control (Figure 3, A and I). Knockdown of hpo or wts in polar cells severely disrupted border cell migration (Figure 3, B–D and H), suggesting that hpo and wts are required in polar cells for the migration of border cell clusters. In the canonical understanding of the Hippo pathway, Hpo and Wts phosphorylate and inactivate Yki. Consistently, overexpression of yki-3SA in polar cells showed a similar migratory defect as hpo or wts knockdown (Figure 3, J, K, and O). Knockdown of yki or overexpression of hpo in polar cells did not affect border cell migration (Figure 3, E, G, and H). Although Yki predominantly forms a transcription complex with Sd (Goulev et al. 2008; Ota and Sasaki 2008; Wu et al. 2008; Zhang et al. 2008; Zhao et al. 2008a), it may also interact with other transcription factors (Alarcon et al. 2009; Zhang et al. 2011a). We tested whether Sd is required for Yki function in polar cells. Knockdown or overexpression of sd alone in polar cells did not affect border cell migration (Figure 3, F, H, L, and O). Knockdown of sd in polar cells did alleviate the migratory defect caused by overexpression of yki-3SA (Figure 3, M and O), suggesting that sd is required for Yki in polar cells to regulate the migration of border cell clusters.
Figure 3.
hpo and wts are required in polar cells for the migration of border cell clusters and the induction of outer border cells. upd-Gal4 was used to overexpress or knock down genes specifically in polar cells. UAS-GFP or UAS-RFP driven by upd-Gal4 was used as a control (A and I). UAS-GFP was included in B–G. The flies were dissected after being grown at 29° for 5–6 days. The ovaries were immunostained with anti-Fas3 and anti-GFP (A–G) or anti-Slbo (I–N). Stage-10 egg chambers were selected and oriented as anterior toward the left (A–G and I–N). High magnification views of border cell clusters are shown on the right. (A) In the UAS-GFP control, a border cell cluster migrated normally and reached the oocyte-nurse-cell border. (B) Knockdown of hpo impaired border cell migration. (C and D) Knockdown of wts with two RNAi lines impaired border cell migration. Extra Fas3-positive cells were observed. (E) Knockdown of yki did not impair border cell migration. (F) Knockdown of sd did not impair border cell migration. (G) Overexpression of hpo did not impair border cell migration. (H) Quantification and percentage distribution of border cell migration. Wilcoxon rank-sum test, **P < 0.01. (I) In the UAS-RFP control, a border cell cluster with at least five Slbo-positive cells migrated normally and reached the oocyte-nurse-cell border. (J) No Slbo-positive cell was observed, and the border cell cluster did not migrate when wts was knocked down in polar cells. (K) No Slbo-positive cell was observed, and the border cell cluster did not migrate when yki-S111A.S168A.S250A (yki-3SA) was expressed in polar cells. (L) A border cell cluster with at least four Slbo-positive cells migrated normally when sd was knocked down in polar cells. (M) A border cell cluster with at least four Slbo-positive cells migrated normally and reached the oocyte-nurse-cell border when yki-3SA was expressed and sd was knocked down in polar cells. (N) A border cell cluster with at least four Slbo-positive cells migrated normally and reached the oocyte-nurse-cell border when wts was knocked down and upd was overexpressed in polar cells. (O) Quantification and percentage distribution of border cell migration. (P) Quantification and percentage distribution of Slbo-positive cell numbers in stage-10 egg chambers. Wilcoxon rank-sum test, **P < 0.01 comparing with control; ##P < 0.01. Bar, 20 μm in A–G and I–N.
The Hippo pathway controls the number of polar cells
Hippo signaling is a tumor-suppressing pathway inhibiting cell proliferation. When wts was knocked down or yki was overexpressed with upd-Gal4, 5–15 Fas3-positive cells were observed (Figure 3, C, J, K, and M). Since our previous study has demonstrated that the Hippo pathway controls polar cell fate during early oogenesis (Chen et al. 2011), it is crucial to examine whether modulation of the Hippo pathway disrupts polar cell fate determination, which in turn attenuates border cell migration. While there were usually two polar cells at the anterior end of an egg chamber (Figure 4, A, D, and F), knockdown of wts with upd-Gal4 led to more than two cells expressing polar cells markers PZ80-lacZ (a lacZ insertion in Fas3) and A101-lacZ (a lacZ insertion in neur) (Figure 4, A–E). In addition, elevated levels of Armadillo (Arm) at the apical side were observed in wts knockdown cells, which was similar to the Arm pattern of polar cells in the UAS-GFP control (Figure 4, F and G) (Peifer et al. 1993). These results suggest that knockdown of wts with upd-Gal4 increases polar cells instead of disrupting polar cell fate. Furthermore, polar cells usually withdraw from cell cycle by stage 2, but we observed Cyclin B (CycB)-positive polar cells when we knocked down wts with upd-Gal4 after stage 7 (Figure 4, H and I). This result suggests that wts-knockdown polar cells remain proliferating during mid-oogenesis. Interestingly, extra polar cells may not interfere with the migration of border cell clusters since many of them migrated normally (Figure 3M). To further confirm that the Hippo pathway is required for border migration in addition to its roles in polar cell fate determination during early oogenesis, we used the temperature-sensitive Gal80 system (Mcguire et al. 2003). Attenuation of border cell migration was observed after the flies were incubated at 29° for as few as 40 hr (Supporting Information, Figure S1). The effect of the 40-hr incubation was weaker than that of the 54-hr incubation at 29°, which could be a result of either continuous requirement of the Hippo signaling in polar cells or protein perdurance of Wts. Since polar cells are formed before stage 2 and it takes ∼36 hr for egg chambers to develop from stage 2 to stage 9 (Horne-Badovinac and Bilder 2005; Ma et al. 2014), this result demonstrates a role of the Hippo pathway in border cell migration after determination of polar cell fate during early oogenesis.
Figure 4.
The Hippo pathway controls the number of polar cells. wts was knocked down in polar cells by using upd-Gal4. The ovaries were immunostained with anti-Fas3, anti-β-Gal (A–E), anti-Arm (F and G), and anti-CycB (H and I). Stage-9 or stage-10 egg chambers (A–E) or stage 7–8 egg chambers (F–I) were selected and oriented as anterior toward the left. High magnification views of border cell clusters are shown in the panels on the right. (A) In the control, exactly two polar cells in the border cell cluster expressing PZ80-lacZ were observed. (B and C) Knockdown of wts with two different RNAi lines increased the number of cells expressing PZ80-lacZ. (D) In the control, exactly two polar cells in the border cell cluster expressing A101-lacZ were observed. (E) Knockdown of wts increased the number of cells expressing A101-lacZ. (F) In the control, Arm was enriched in the apical region of two polar cells indicated by yellow arrowheads. (G) Knockdown of wts increased the number of cells with Arm enriched in the apical region indicated by yellow arrowheads. (H) In the control, polar cells were not positive for CycB. (I) Knockdown of wts led to ectopic CycB-positive cells indicated by yellow arrowheads. Bar, 20 μm in A–I.
The Hippo pathway is required in polar cells for the induction of outer border cells
In addition to the migratory defect, border cell clusters usually failed to form when wts was knocked down or yki-3SA was overexpressed in polar cells (Figure 3, C, J, and K). We hypothesized that the Hippo pathway in polar cells might be essential for the induction of outer border cells. To test this hypothesis, we stained ovaries with an antibody against Slbo, a marker for border cells (Rorth et al. 2000), and we counted Slbo-positive cells. Stage 10 egg chambers were categorized based on the number of Slbo-positive cells in border cell clusters (Figure 3P). Most control egg chambers contained six to eight Slbo-positive cells in each border cell cluster (Figure 3, I and P). Knockdown of wts or overexpression of yki-3SA in polar cells strongly reduced the number of cells positive for Slbo (Figure 3, J, K, and P), demonstrating that the Hippo pathway in polar cells is crucial for the induction of outer border cells. Knockdown of sd alone in polar cells did not affect outer border cell induction (Figure 3, L and P). In contrast, knockdown of sd in yki-3SA overexpressing polar cells increased the cell number of Slbo-positive cells (Figure 3, M and P), suggesting that the function of Yki in outer border cell induction requires sd.
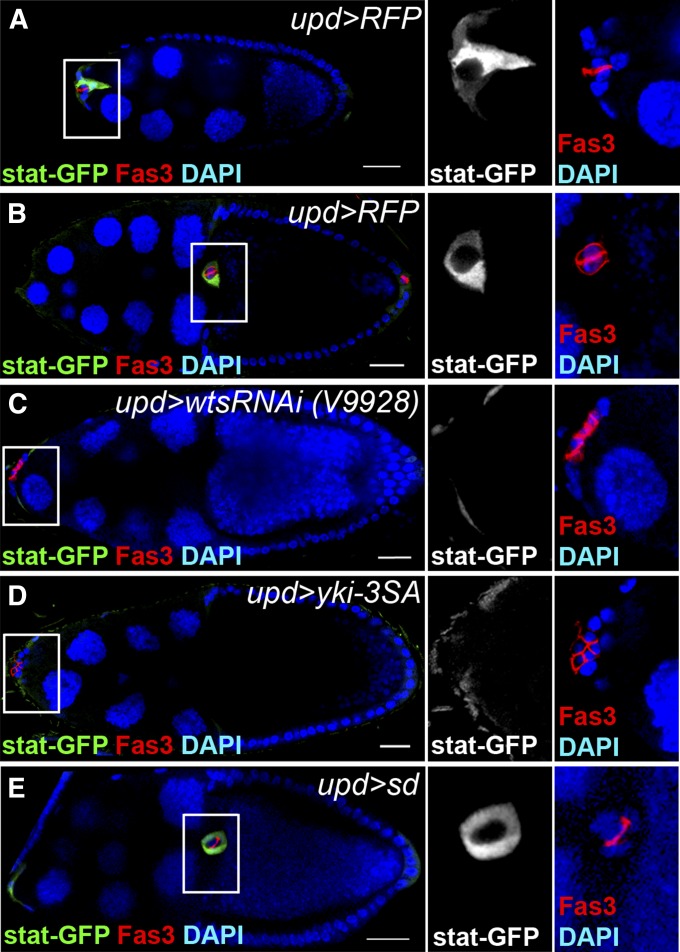
The Hippo pathway in polar cells is required for the activation of JAK/STAT signaling in outer border cells
Slbo acts downstream of JAK/STAT signaling in regulating border cell fate and migration (Beccari et al. 2002; Silver et al. 2005). Since the Hippo pathway in polar cells controls the number of Slbo-positive cells as well as the migration of border cell clusters, it is possible that it regulates JAK/STAT signaling activity in outer border cells. We examined JAK/STAT signaling activity using a reporter line 10Xstat::gfp (stat-GFP). In the control group, GFP was detected in outer border cells throughout the process of border cell induction and migration (Figure 5, A and B). Knockdown of wts or overexpression of yki-3SA in polar cells significantly reduced the level of stat-GFP in outer border cells (Figure 5, C and D). Overexpression of sd in polar cells did not affect the level of stat-GFP in outer border cells (Figure 5E). This result suggests that the Hippo pathway in polar cells is required for the activation of JAK/STAT signaling in outer border cells.
Figure 5.
The Hippo pathway in polar cells is required for the activation of the JAK/STAT signaling in outer border cells. upd-Gal4 was used to knockdown or overexpress genes in a 10Xstat::gfp (stat-GFP) reporter background (A–E). UAS-RFP driven by upd-Gal4 was used as a control (A and B). The flies were incubated at 29° for 5–6 days before dissection. The ovaries were immunostained with anti-GFP and anti-Fas3. Egg chambers at stage 9 (A) or stage 10 (B–E) were selected and oriented as anterior toward the left. High magnification views of border cell clusters are shown in the panels on the right. (A) In the UAS-RFP control, a border cell cluster with stat-GFP-positive outer border cells started to migrate at stage 9. (B) A border cell cluster with stat-GFP-positive outer border cells migrated normally and reached the oocyte-nurse-cell border at stage 10. (C) The stat-GFP level was reduced, and the border cell cluster did not migrate when wts was knocked down in polar cells. (D) The stat-GFP level was reduced, and the border cell cluster did not migrate when yki-3SA was overexpressed in polar cells. (E) A border cell cluster with stat-GFP-positive outer border cells migrated normally and reached the oocyte-nurse-cell border when sd was overexpressed in polar cells. Bar, 20 μm in A–E.
The Hippo pathway controls the expression of upd in polar cells
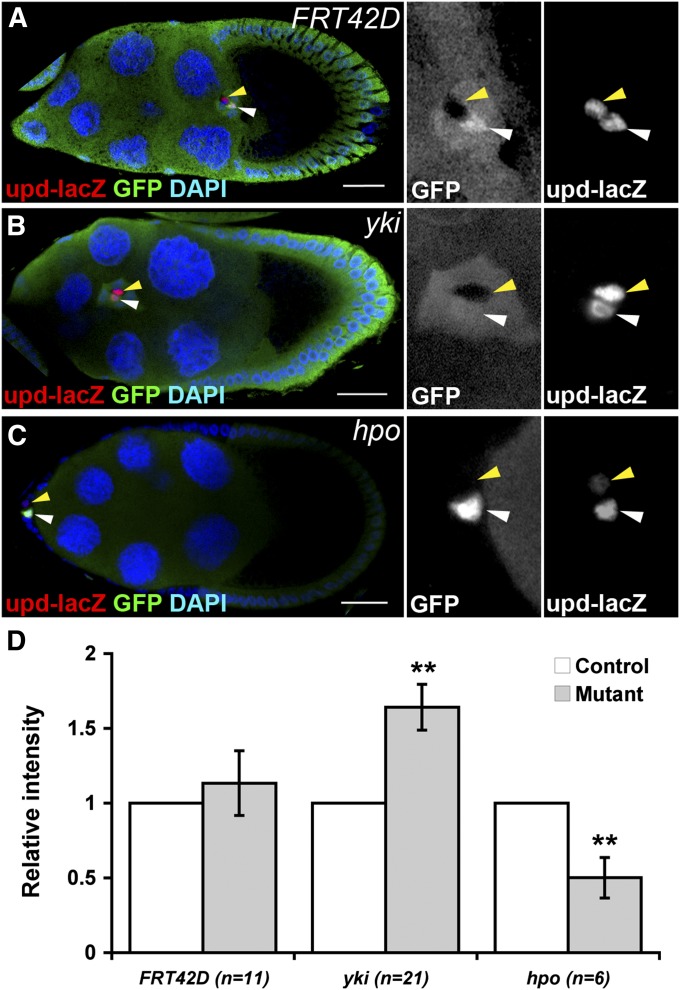
During border cell induction and migration, JAK/STAT signaling in outer border cells is activated by Upd secreted from polar cells (Silver and Montell 2001; Beccari et al. 2002; Ghiglione et al. 2002). Since the Hippo pathway is required in polar cells for border cell induction and migration, it is possible that the Hippo pathway controls upd expression cell-autonomously in polar cells. To test this, we generated mitotic clones mutant for yki or hpo in an upd-lacZ background. FRT42D clones were generated as controls. We specifically analyzed those pairs of polar cells with a GFP-negative yki or hpo mutant polar cell and a GFP-positive control polar cell (Figure 6, A–C). In FRT42D clones, expression of upd-lacZ was comparable between GFP-negative FRT42D and the adjacent GFP-positive polar cells (Figure 6, A and D). yki mutant polar cells expressed more upd-lacZ in comparison with the adjacent control polar cells; hpo mutant polar cells expressed less upd-lacZ in comparison with the adjacent control polar cells (Figure 6, B–D). This result shows that the Hippo pathway controls upd expression in polar cells. In addition, some yki mutant cells expressed ectopic upd-lacZ and induced ectopic border cell migration (Figure S2). Importantly, the levels of upd-lacZ in polar cells and stat-GFP in outer border cells were not affected when outer border cells were mutant for hpo (Figure S3, A and B). Outer border cells mutant for hpo remained positive for Slbo (Figure S3C). These results exclude the possibility that the Hippo pathway regulates upd expression in a non-cell-autonomous manner. Together, our data demonstrate that the Hippo pathway promotes upd expression in polar cells.
Figure 6.
The Hippo pathway controls the expression of upd in polar cells. GFP-negative mitotic clones were generated in FRT42D (A), FRT42D hpo42-47 (B), and FRT42D ykiB5 (C) in an upd-lacZ background. The ovaries were immunostained with anti-Fas3 and anti-β-galactosidase (β-Gal). Stage-9 and stage-10 egg chambers were selected and oriented as anterior toward the left. High magnification views of border cell clusters are shown on the right. (A) An FRT42D control polar cell (yellow arrowheads) was positive for β-Gal as the neighboring GFP-positive polar cell (white arrowheads). (B) A yki mutant polar cell (yellow arrowheads) showed a higher β-Gal level than that of the neighboring GFP-positive polar cell (white arrowheads). (C) A hpo mutant polar cell (yellow arrowheads) showed a lower β-Gal level than that of the neighboring GFP-positive polar cell (white arrowheads). (D) Quantification of β-Gal levels in FRT42D, yki, and hpo mutant polar cells in an upd-lacZ background. The bar graph is shown as mean ± SEM. Paired Student’s t-test, **P < 0.01. Bar, 20 μm in A–C.
The Hippo pathway in polar cells controls border cell induction and migration through regulating upd expression
If Upd functions downstream of the Hippo pathway in polar cells to promote outer border cell induction and migration, overexpression of upd in polar cells should rescue the defects caused by wts knockdown. Indeed, overexpression of upd in polar cells promoted border cell migration and increased the number of Slbo-positive cells in wts knockdown cells (Figure 3, N, O, and P), suggesting that Upd functionally acts downstream of the Hippo pathway in polar cells to induce border cell formation and promote migration.
Discussion
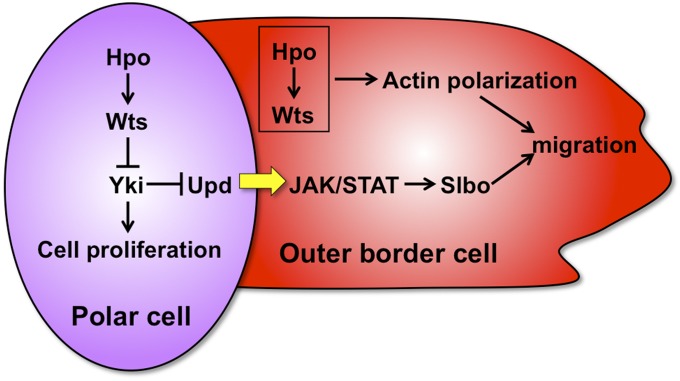
In this study, we identified two roles of the Hippo pathway in border cell migration: (1) in outer border cells, the Hippo pathway regulates localization of the actin cytoskeleton; (2) in polar cells, the Hippo pathway promotes upd expression to regulate the induction of outer border cells and border cell migration (Figure 7). A recent study reports that some Hippo pathway components regulate polarization of actin cytoskeleton of the border cell cluster (Lucas et al. 2013). They show that Wts is activated and localized to the interface between border cells, where Wts phosphorylates and inhibits Enabled, a crucial actin regulator. Thus actin polymerization is restricted to the outer rim of the migrating cluster instead of to the boundaries between border cells. In our study, overexpression of hpo led to abnormal actin polymerization between border cells and attenuated cell migration (Figure 2, B–E). Since asymmetric distribution and activation of Wts is crucial (Lucas et al. 2013), either gain- or loss-of-function of the Hippo pathway components may disrupt the distribution or activation of Wts, thereby attenuating cell migration. Both Lucas et al. (2013) and we show that Yki does not act downstream of the Hippo pathway in regulating actin polymerization as described in a previous study using the Drosophila wing imaginal disc as a model (Fernandez et al. 2011). While Lucas et al. did not dissociate roles of the Hippo pathway in polar cells and outer border cells during border cell migration, we additionally demonstrated here that the Hippo pathway controls JAK/STAT signaling through promoting upd expression in polar cells. Thus, we have identified a new role of the Hippo pathway in regulating border cell migration.
Figure 7.
The Hippo pathway controls the migration of border cell clusters through two mechanisms. In outer border cells, the Hippo pathway regulates polarized distribution of F-actin and controls border cell migration. In polar cells, the Hippo pathway induces upd expression. Upd from polar cells activates JAK/STAT signaling in outer border cells. Activation of JAK/STAT signaling induces slbo expression, which promotes the formation and migration of border cell clusters. The Hippo pathway also controls polar cell proliferation.
We did not observe any migratory defect when hpo or wts was knocked down in outer border cells with slbo-Gal4 (Figure 2A). On the contrary, border cell clusters with hpo or wts mutant border cells showed severe migratory defects (Figure 1). We did find strong migratory defects when we overexpressed hpo with slbo-Gal4, suggesting that the slbo-Gal4 driver indeed promoted transgene expression efficiently in outer border cells. Therefore, it is likely that driving RNAi with slbo-Gal4 may not efficiently knock down genes prior to or during border cell migration in outer border cells when the target protein is stable (Yang et al. 2012).
In our experimental conditions, border cell clusters of entirely wts mutant cells have never been observed. Furthermore, border cell clusters containing hpo or wts mutant polar cells and wild-type outer border cells have never been obtained (Figure 1). This phenomenon can be explained by our previous findings that the Hippo pathway is involved in polar cell determination (Chen et al. 2011). The Hippo pathway is critical to promote polar cell fate by suppressing Notch signaling during early oogenesis. Because mitotic mutant clones of hpo or wts were usually induced at early stages, we could not observe hpo or wts mutant polar cells. In addition to polar cell fate determination, we find that the Hippo pathway controls polar cell numbers by regulating cell proliferation. During cell fate determination in early oogenesis, the Hippo pathway promotes polar cell fate. Therefore, mutation of hpo or wts suppresses polar cell formation (Chen et al. 2011). Once polar cells are determined, the Hippo pathway suppresses proliferation of polar cells. The upd-Gal4 is expressed after polar cell fate is determined, so knockdown of wts or overexpression of yki-3SA with upd-Gal4 should increase polar cell numbers through promoting cell proliferation. Although these wts-knockdown cells resembled polar cells based on their expression of Fas3, PZ80-lacZ, and A101-lacZ and an increased level of Arm at the apical region (Figure 3 and Figure 4), they were positive for Eya after stage 7 (Figure S4). Eya should be detected only in follicle cell precursors and main-body follicle cells, but not in polar cells (Bai and Montell 2002). It is possible that the Hippo pathway controls eya expression because ectopic Eya is also detected in posterior follicle cells mutant for hpo or wts after stage 8 (Meignin et al. 2007; Polesello and Tapon 2007; Yu et al. 2008). Although knockdown of wts or overexpression of yki-3SA increased polar cells, these polar cells did not induce stat-GFP in neighboring cells (Figure 5, C and D). This result suggests that polar cell fate can be dissociated from upd expression.
In addition to polar cell fate determination and cell proliferation (Chen et al. 2011), the Hippo pathway plays roles in multiple steps of follicle cell development by interacting with various signaling cascades. In follicle stem cells, the Hippo pathway acts in parallel with Hedgehog signaling in regulating follicle stem cell proliferation (Huang and Kalderon 2014). In posterior follicle cells, the Hippo pathway is required for inducing their Gurken- and Notch-dependent differentiation (Meignin et al. 2007; Polesello and Tapon 2007; Yu et al. 2008). In this study, we find that the Hippo pathway controls JAK/STAT signaling through regulation of upd expression during border cell migration. All together, integration with other signaling cascades to control various cellular functions appears to be a particularly prominent feature of the Hippo pathway. In the Drosophila eye, the Hippo pathway has also been shown to regulate cell proliferation and apoptosis during early development as well as cell fate determination in postmitotic photoreceptors (Jukam et al. 2013). As to the regulation of actin cytoskeleton, the Hippo pathway has been demonstrated to respond to mechanical force and cell contact by acting downstream of cytoskeletal rearrangement (Sansores-Garcia et al. 2011). Here during cell migration, the Hippo pathway regulates actin polymerization (Figure 2) (Lucas et al. 2013). It will be interesting to examine whether cytoskeletal rearrangement also regulates the Hippo pathway to control cell migration.
Previous studies have shown that inactivation of the Hippo pathway or activation of Yki induces upd expression in midgut and certain regions of the wing imaginal discs where upd is endogenously expressed (Karpowicz et al. 2010; Ren et al. 2010a; Shaw et al. 2010; Staley and Irvine 2010). In our study, we found that upd expression was increased in yki mutant clones (Figure 6), suggesting that activation of the Hippo pathway or inactivation of Yki induces upd expression in polar cells. This difference may be caused by cell type- or tissue-specific functions or interactions of Yki with other proteins. Our results show that Yki requires Sd to function in polar cells (Figure 3, M, O, and P), so it is less likely that Yki directly represses upd expression through interacting with other transcription factors. It requires further analysis to demonstrate whether Yki and Sd directly or indirectly suppress upd expression in polar cells.
In conclusion, our data show that the Hippo pathway regulates border cell migration through controlling polarized distribution of F-actin and interacting with the JAK/STAT-signaling pathway. Both Hippo and JAK/STAT pathways are involved in various aspects of tumorigenesis, such as tumor growth, EMT, and metastasis of tumor cells (Hou et al. 2002; Pan 2010; Wang and Huang 2010; Harvey et al. 2013). It will be interesting to study the interplay of these two pathways in cancer formation.
Supplementary Material
Acknowledgments
We thank Y.-C. Tsai, G.-J. Liaw, H.-H. Lee, C. O. Brown, H.-J. Hsu, S.-J. Chou, and C.-H. Chen for discussion and critical reading of the manuscript; Y.-C. Tsai, H. Ruohola-Baker, Y. H. Sun, D. Pan, W.-M. Deng, J. Jiang, A. C.-C. Jang, the Fly Core in Taiwan, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, and the Drosophila Genomics Resource Center for fly stocks; and P. Rørth and the Developmental Studies Hybridoma Bank for antibodies. This research was funded by National Science Council grant 101-2311-B-010-007-MY3 and by the Brain Research Center, National Yang-Ming University.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.167346/-/DC1.
Communicating editor: L. Cooley
Literature Cited
- Alarcon C., Zaromytidou A. I., Xi Q., Gao S., Yu J., et al. , 2009. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 139: 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badouel C., Garg A., McNeill H., 2009. Herding Hippos: regulating growth in flies and man. Curr. Opin. Cell Biol. 21: 837–843. [DOI] [PubMed] [Google Scholar]
- Bai J., Montell D., 2002. Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development 129: 5377–5388. [DOI] [PubMed] [Google Scholar]
- Bai J., Uehara Y., Montell D. J., 2000. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 103: 1047–1058. [DOI] [PubMed] [Google Scholar]
- Beccari S., Teixeira L., Rorth P., 2002. The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech. Dev. 111: 115–123. [DOI] [PubMed] [Google Scholar]
- Boggiano J. C., Vanderzalm P. J., Fehon R. G., 2011. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell 21: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese L., Fletcher G., Mathieu J., Atzberger A., Eades W. C., et al. , 2006. Systematic analysis of the transcriptional switch inducing migration of border cells. Dev. Cell 10: 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. J., Wang C. M., Wang T. W., Liaw G. J., Hsu T. H., et al. , 2011. The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev. Biol. 357: 370–379. [DOI] [PubMed] [Google Scholar]
- Chen J., Verheyen E. M., 2012. Homeodomain-interacting protein kinase regulates Yorkie activity to promote tissue growth. Curr. Biol. 22: 1582–1586. [DOI] [PubMed] [Google Scholar]
- Das Thakur M., Feng Y., Jagannathan R., Seppa M. J., Skeath J. B., et al. , 2010. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr. Biol. 20: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N., et al. , 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek P., Rorth P., 2001. Guidance of cell migration by EGF receptor signaling during Drosophila oogenesis. Science 291: 131–133. [DOI] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jekely G., Beccari S., Rorth P., 2001. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107: 17–26. [DOI] [PubMed] [Google Scholar]
- Fernandez B. G., Gaspar P., Bras-Pereira C., Jezowska B., Rebelo S. R., et al. , 2011. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138: 2337–2346. [DOI] [PubMed] [Google Scholar]
- Genevet A., Wehr M. C., Brain R., Thompson B. J., Tapon N., 2010. Kibra is a regulator of the salvador/warts/hippo signaling network. Dev. Cell 18: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiglione C., Devergne O., Georgenthum E., Carballes F., Medioni C., et al. , 2002. The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis. Development 129: 5437–5447. [DOI] [PubMed] [Google Scholar]
- Goulev Y., Fauny J. D., Gonzalez-Marti B., Flagiello D., Silber J., et al. , 2008. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr. Biol. 18: 435–441. [DOI] [PubMed] [Google Scholar]
- Halder G., Johnson R. L., 2011. Hippo signaling: growth control and beyond. Development 138: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. A., Wang R., Miao J., Oliva E., Shen X., et al. , 2010. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 70: 8517–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K., Tapon N., 2007. The Salvador-Warts-Hippo pathway: an emerging tumour-suppressor network. Nat. Rev. Cancer 7: 182–191. [DOI] [PubMed] [Google Scholar]
- Harvey K. F., Pfleger C. M., Hariharan I. K., 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467. [DOI] [PubMed] [Google Scholar]
- Harvey K. F., Zhang X., Thomas D. M., 2013. The Hippo pathway and human cancer. Nat. Rev. Cancer 13: 246–257. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S., Bilder D., 2005. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 232: 559–574. [DOI] [PubMed] [Google Scholar]
- Hou S. X., Zheng Z., Chen X., Perrimon N., 2002. The Jak/STAT pathway in model organisms: emerging roles in cell movement. Dev. Cell 3: 765–778. [DOI] [PubMed] [Google Scholar]
- Huang J., Kalderon D., 2014. Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J. Cell Biol. 205: 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Wu S., Barrera J., Matthews K., Pan D., 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434. [DOI] [PubMed] [Google Scholar]
- Inaki M., Vishnu S., Cliffe A., Rorth P., 2012. Effective guidance of collective migration based on differences in cell states. Proc. Natl. Acad. Sci. USA 109: 2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang A. C., Starz-Gaiano M., Montell D. J., 2007. Modeling migration and metastasis in Drosophila. J. Mammary Gland Biol. Neoplasia 12: 103–114. [DOI] [PubMed] [Google Scholar]
- Jang A. C., Chang Y. C., Bai J., Montell D., 2009. Border-cell migration requires integration of spatial and temporal signals by the BTB protein Abrupt. Nat. Cell Biol. 11: 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D., Xie B., Rister J., Terrell D., Charlton-Perkins M., et al. , 2013. Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science 342: 1238016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice R. W., Zilian O., Woods D. F., Noll M., Bryant P. J., 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9: 534–546. [DOI] [PubMed] [Google Scholar]
- Karpowicz P., Perez J., Perrimon N., 2010. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137: 4135–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Z. C., Wei X., Shimizu T., Ramos E., Rohrbaugh M., et al. , 2005. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120: 675–685. [DOI] [PubMed] [Google Scholar]
- Lian I., Kim J., Okazawa H., Zhao J., Zhao B., et al. , 2010. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 24: 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Li Y., Kim S. M., Bossuyt W., Liu P., et al. , 2010. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl. Acad. Sci. USA 107: 1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E. P., Khanal I., Gaspar P., Fletcher G. C., Polesello C., et al. , 2013. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J. Cell Biol. 201: 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Xu H., O’Farrell P. H., 2014. Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster. Nat. Genet. 46: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Pinheiro E. M., Montell D. J., 2003. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 130: 3469–3478. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Pinheiro E. M., Kadlec L., Schupbach T., Montell D. J., 2006. Multiple EGFR ligands participate in guiding migrating border cells. Dev. Biol. 296: 94–103. [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L., 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768. [DOI] [PubMed] [Google Scholar]
- Meignin C., Alvarez-Garcia I., Davis I., Palacios I. M., 2007. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17: 1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell D. J., 2003. Border-cell migration: the race is on. Nat. Rev. Mol. Cell Biol. 4: 13–24. [DOI] [PubMed] [Google Scholar]
- Montell D. J., Rorth P., Spradling A. C., 1992. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell 71: 51–62. [DOI] [PubMed] [Google Scholar]
- Nagaraj R., Gururaja-Rao S., Jones K. T., Slattery M., Negre N., et al. , 2012. Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes Dev. 26: 2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naora H., Montell D. J., 2005. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat. Rev. Cancer 5: 355–366. [DOI] [PubMed] [Google Scholar]
- Oh H., Irvine K. D., 2008. In vivo regulation of Yorkie phosphorylation and localization. Development 135: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Irvine K. D., 2009. In vivo analysis of Yorkie phosphorylation sites. Oncogene 28: 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Irvine K. D., 2010. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 20: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Sasaki H., 2008. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development 135: 4059–4069. [DOI] [PubMed] [Google Scholar]
- Overholtzer M., Zhang J., Smolen G. A., Muir B., Li W., et al. , 2006. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 103: 12405–12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., 2010. The hippo signaling pathway in development and cancer. Dev. Cell 19: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S., Tapon N., Leopold P., 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5: 921–927. [DOI] [PubMed] [Google Scholar]
- Peifer M., Orsulic S., Sweeton D., Wieschaus E., 1993. A role for the Drosophila segment polarity gene armadillo in cell adhesion and cytoskeletal integrity during oogenesis. Development 118: 1191–1207. [DOI] [PubMed] [Google Scholar]
- Pinheiro E. M., Montell D. J., 2004. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development 131: 5243–5251. [DOI] [PubMed] [Google Scholar]
- Poernbacher I., Baumgartner R., Marada S. K., Edwards K., Stocker H., 2012. Drosophila Pez acts in Hippo signaling to restrict intestinal stem cell proliferation. Curr. Biol. 22: 389–396. [DOI] [PubMed] [Google Scholar]
- Polesello C., Tapon N., 2007. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17: 1864–1870. [DOI] [PubMed] [Google Scholar]
- Reddy B. V., Irvine K. D., 2011. Regulation of Drosophila glial cell proliferation by Merlin-Hippo signaling. Development 138: 5201–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F., Wang B., Yue T., Yun E. Y., Ip Y. T., et al. , 2010a Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc. Natl. Acad. Sci. USA 107: 21064–21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F., Zhang L., Jiang J., 2010b Hippo signaling regulates Yorkie nuclear localization and activity through 14–3-3 dependent and independent mechanisms. Dev. Biol. 337: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P., Szabo K., Bailey A., Laverty T., Rehm J., et al. , 1998. Systematic gain-of-function genetics in Drosophila. Development 125: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Rorth P., Szabo K., Texido G., 2000. The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation. Mol. Cell 6: 23–30. [DOI] [PubMed] [Google Scholar]
- Ruohola H., Bremer K. A., Baker D., Swedlow J. R., Jan L. Y., et al. , 1991. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell 66: 433–449. [DOI] [PubMed] [Google Scholar]
- Sansores-Garcia L., Bossuyt W., Wada K., Yonemura S., Tao C., et al. , 2011. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30: 2325–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. L., Kohlmaier A., Polesello C., Veelken C., Edgar B. A., et al. , 2010. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137: 4147–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D. L., Montell D. J., 2001. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell 107: 831–841. [DOI] [PubMed] [Google Scholar]
- Silver D. L., Geisbrecht E. R., Montell D. J., 2005. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 132: 3483–3492. [DOI] [PubMed] [Google Scholar]
- Staley B. K., Irvine K. D., 2010. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr. Biol. 20: 1580–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Acloque H., Huang R. Y., Nieto M. A., 2009. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890. [DOI] [PubMed] [Google Scholar]
- Wang X., Bo J., Bridges T., Dugan K. D., Pan T. C., et al. , 2006. Analysis of cell migration using whole-genome expression profiling of migratory cells in the Drosophila ovary. Dev. Cell 10: 483–495. [DOI] [PubMed] [Google Scholar]
- Wang X., Adam J. C., Montell D., 2007. Spatially localized Kuzbanian required for specific activation of Notch during border cell migration. Dev. Biol. 301: 532–540. [DOI] [PubMed] [Google Scholar]
- Wang X., He L., Wu Y. I., Hahn K. M., Montell D. J., 2010. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat. Cell Biol. 12: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. H., Huang M. L., 2010. Organogenesis and tumorigenesis: insight from the JAK/STAT pathway in the Drosophila eye. Dev. Dyn. 239: 2522–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. L., 1995. Drosophila warts–tumor suppressor and member of the myotonic dystrophy protein kinase family. BioEssays 17: 673–676. [DOI] [PubMed] [Google Scholar]
- Wei X., Shimizu T., Lai Z. C., 2007. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 26: 1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Huang J., Dong J., Pan D., 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456. [DOI] [PubMed] [Google Scholar]
- Wu S., Liu Y., Zheng Y., Dong J., Pan D., 2008. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14: 388–398. [DOI] [PubMed] [Google Scholar]
- Xu M. Z., Chan S. W., Liu A. M., Wong K. F., Fan S. T., et al. , 2011. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene 30: 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rubin G. M., 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237. [DOI] [PubMed] [Google Scholar]
- Yang N., Inaki M., Cliffe A., Rorth P., 2012. Microtubules and Lis-1/NudE/dynein regulate invasive cell-on-cell migration in Drosophila. PLoS ONE 7: e40632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Poulton J., Huang Y. C., Deng W. M., 2008. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE 3: e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., Hong W., 2008. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13: 188–192. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ji J. Y., Yu M., Overholtzer M., Smolen G. A., et al. , 2009. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat. Cell Biol. 11: 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ren F., Zhang Q., Chen Y., Wang B., et al. , 2008. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Zhou Q., Pignoni F., 2011a Yki/YAP, Sd/TEAD and Hth/MEIS control tissue specification in the Drosophila eye disc epithelium. PLoS ONE 6: e22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., George J., Deb S., Degoutin J. L., Takano E. A., et al. , 2011b The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 30: 2810–2822. [DOI] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J., Li L., Li W., et al. , 2008a TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22: 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Szafranski P., Hall C. A., Goode S., 2008b Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics 178: 1947–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziosi M., Baena-Lopez L. A., Grifoni D., Froldi F., Pession A., et al. , 2010. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 6: e1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.