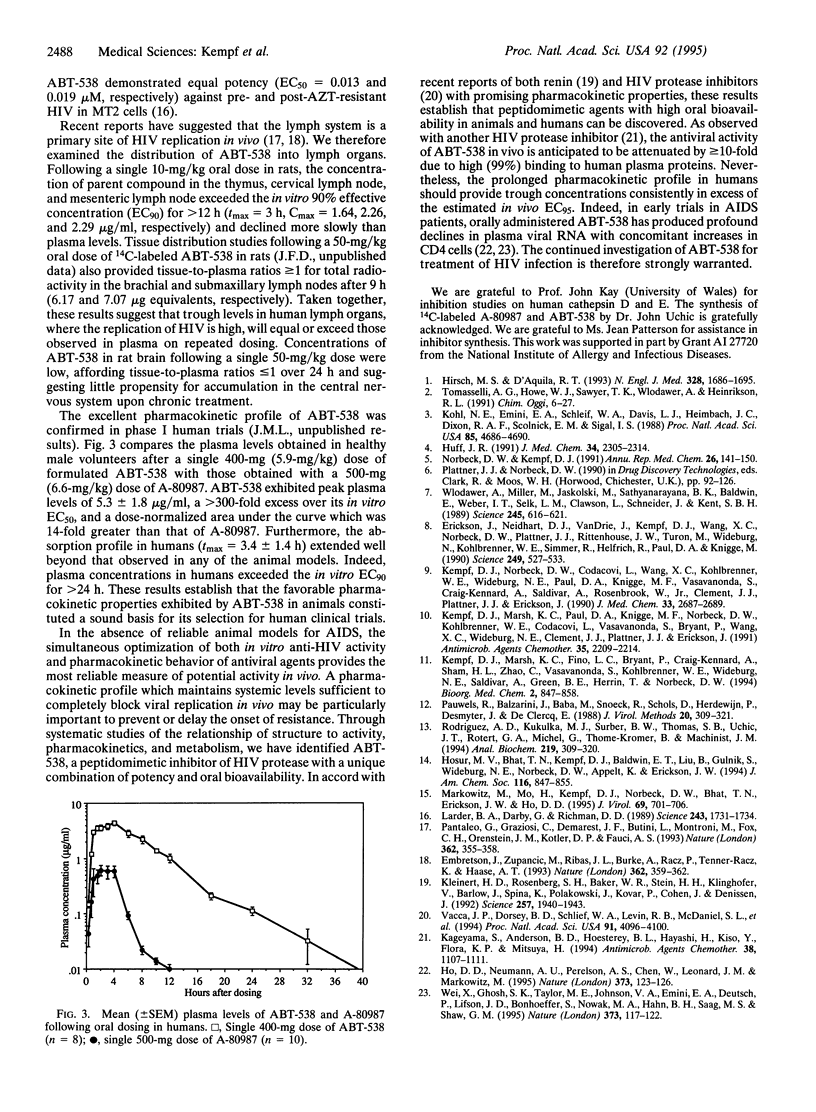
Abstract
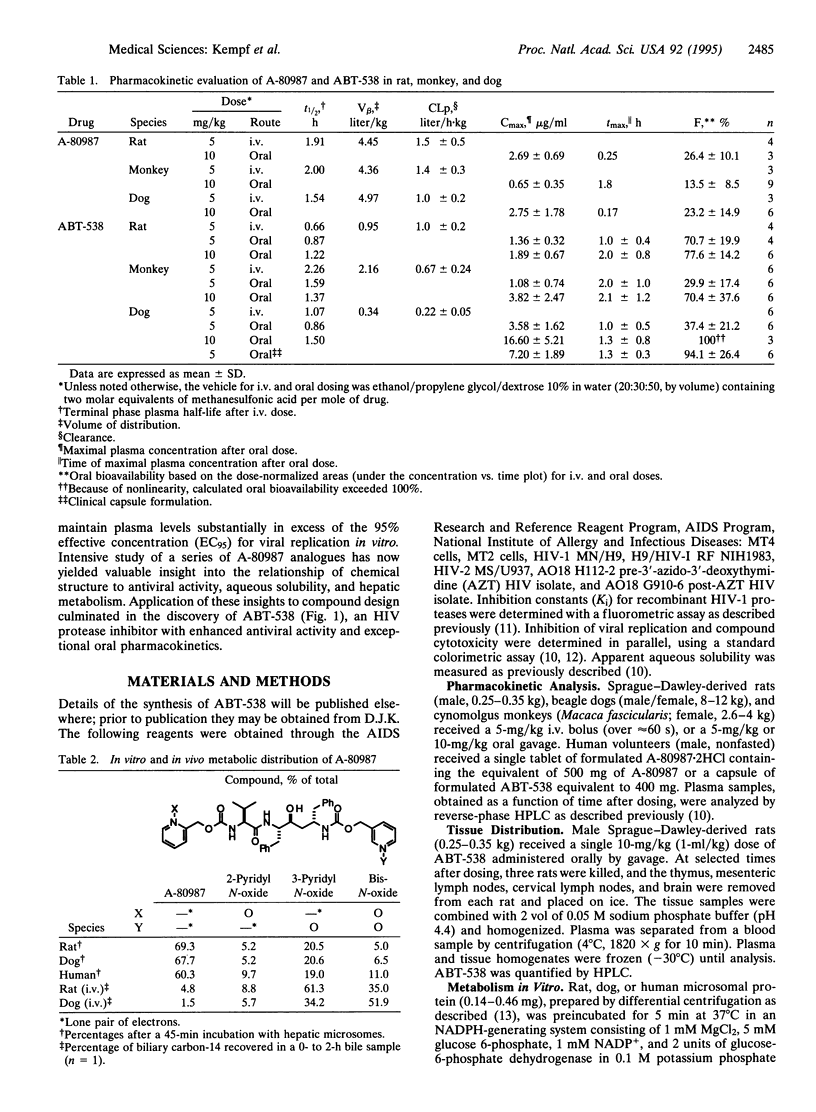
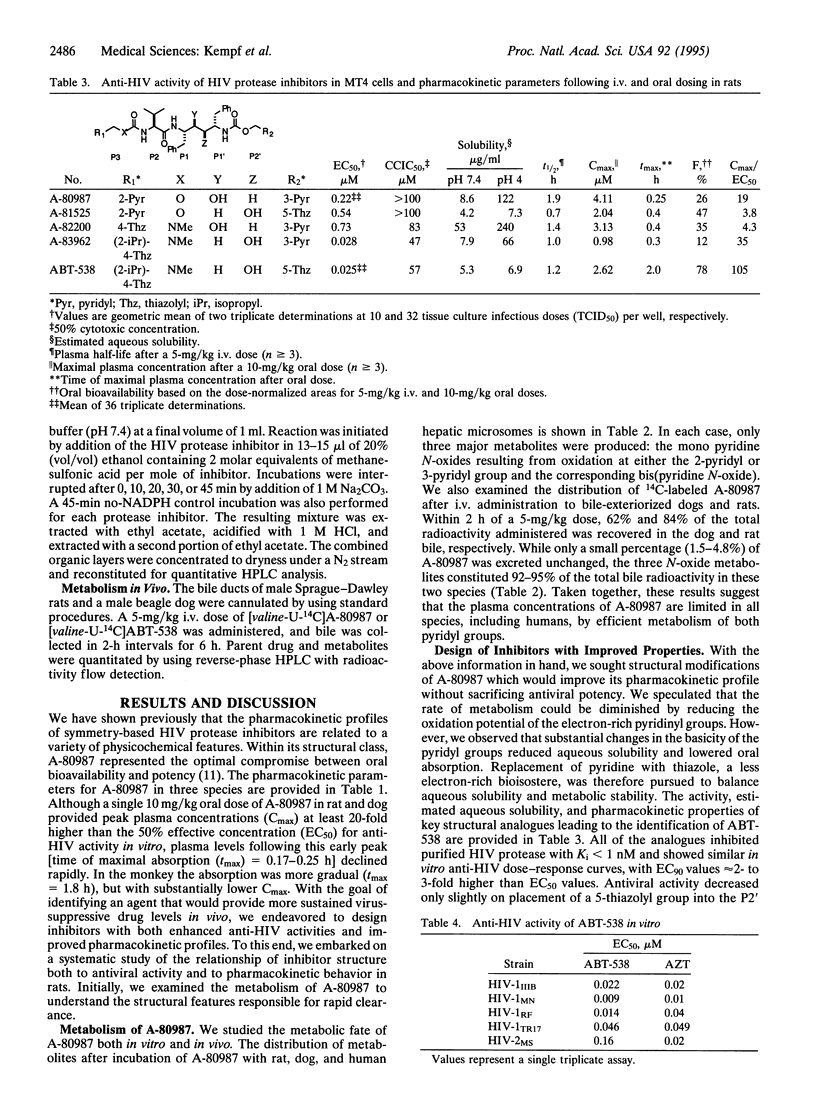
Examination of the structural basis for antiviral activity, oral pharmacokinetics, and hepatic metabolism among a series of symmetry-based inhibitors of the human immunodeficiency virus (HIV) protease led to the discovery of ABT-538, a promising experimental drug for the therapeutic intervention in acquired immunodeficiency syndrome (AIDS). ABT-538 exhibited potent in vitro activity against laboratory and clinical strains of HIV-1 [50% effective concentration (EC50) = 0.022-0.13 microM] and HIV-2 (EC50 = 0.16 microM). Following a single 10-mg/kg oral dose, plasma concentrations in rat, dog, and monkey exceeded the in vitro antiviral EC50 for > 12 h. In human trials, a single 400-mg dose of ABT-538 displayed a prolonged absorption profile and achieved a peak plasma concentration in excess of 5 micrograms/ml. These findings demonstrate that high oral bioavailability can be achieved in humans with peptidomimetic inhibitors of HIV protease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Erickson J., Neidhart D. J., VanDrie J., Kempf D. J., Wang X. C., Norbeck D. W., Plattner J. J., Rittenhouse J. W., Turon M., Wideburg N. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990 Aug 3;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., D'Aquila R. T. Therapy for human immunodeficiency virus infection. N Engl J Med. 1993 Jun 10;328(23):1686–1695. doi: 10.1056/NEJM199306103282307. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995 Jan 12;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Huff J. R. HIV protease: a novel chemotherapeutic target for AIDS. J Med Chem. 1991 Aug;34(8):2305–2314. doi: 10.1021/jm00112a001. [DOI] [PubMed] [Google Scholar]
- Kageyama S., Anderson B. D., Hoesterey B. L., Hayashi H., Kiso Y., Flora K. P., Mitsuya H. Protein binding of human immunodeficiency virus protease inhibitor KNI-272 and alteration of its in vitro antiretroviral activity in the presence of high concentrations of proteins. Antimicrob Agents Chemother. 1994 May;38(5):1107–1111. doi: 10.1128/aac.38.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf D. J., Marsh K. C., Fino L. C., Bryant P., Craig-Kennard A., Sham H. L., Zhao C., Vasavanonda S., Kohlbrenner W. E., Wideburg N. E. Design of orally bioavailable, symmetry-based inhibitors of HIV protease. Bioorg Med Chem. 1994 Sep;2(9):847–858. doi: 10.1016/s0968-0896(00)82036-2. [DOI] [PubMed] [Google Scholar]
- Kempf D. J., Marsh K. C., Paul D. A., Knigge M. F., Norbeck D. W., Kohlbrenner W. E., Codacovi L., Vasavanonda S., Bryant P., Wang X. C. Antiviral and pharmacokinetic properties of C2 symmetric inhibitors of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1991 Nov;35(11):2209–2214. doi: 10.1128/aac.35.11.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf D. J., Norbeck D. W., Codacovi L., Wang X. C., Kohlbrenner W. E., Wideburg N. E., Paul D. A., Knigge M. F., Vasavanonda S., Craig-Kennard A. Structure-based, C2 symmetric inhibitors of HIV protease. J Med Chem. 1990 Oct;33(10):2687–2689. doi: 10.1021/jm00172a002. [DOI] [PubMed] [Google Scholar]
- Kleinert H. D., Rosenberg S. H., Baker W. R., Stein H. H., Klinghofer V., Barlow J., Spina K., Polakowski J., Kovar P., Cohen J. Discovery of a peptide-based renin inhibitor with oral bioavailability and efficacy. Science. 1992 Sep 25;257(5078):1940–1943. doi: 10.1126/science.1411510. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Markowitz M., Mo H., Kempf D. J., Norbeck D. W., Bhat T. N., Erickson J. W., Ho D. D. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J Virol. 1995 Feb;69(2):701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Rodrigues A. D., Kukulka M. J., Surber B. W., Thomas S. B., Uchic J. T., Rotert G. A., Michel G., Thome-Kromer B., Machinist J. M. Measurement of liver microsomal cytochrome p450 (CYP2D6) activity using [O-methyl-14C]dextromethorphan. Anal Biochem. 1994 Jun;219(2):309–320. doi: 10.1006/abio.1994.1271. [DOI] [PubMed] [Google Scholar]
- Vacca J. P., Dorsey B. D., Schleif W. A., Levin R. B., McDaniel S. L., Darke P. L., Zugay J., Quintero J. C., Blahy O. M., Roth E. L-735,524: an orally bioavailable human immunodeficiency virus type 1 protease inhibitor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4096–4100. doi: 10.1073/pnas.91.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995 Jan 12;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]