Abstract
Infection and undernutrition are prevalent in developing countries and demonstrate a synergistic relation. Undernutrition increases infection-related morbidity and mortality. The acute phase response (APR) is an innate, systemic inflammatory reaction to a wide array of disruptions in a host’s homeostasis, including infection. Released from immune cells in response to deleterious stimuli, proinflammatory cytokines act on distant tissues to induce behavioral (e.g., anorexia, weakness, and fatigue) and systemic effects of the APR. Cytokines act to increase energy and protein requirements to manifest fever and support hepatic acute phase protein (APP) production. Blood concentrations of glucose and lipid are augmented to provide energy to immune cells in response to cytokines. Additionally, infection decreases intestinal absorption of nutrients and can cause direct loss of micronutrients. Traditional indicators of iron, zinc, and vitamin A status are altered during the APR, leading to inaccurate estimations of deficiency in populations with a high or unknown prevalence of infection. Blood concentrations of APPs can be measured in nutrition interventions to assess the time stage and severity of infection and correct for the APR; however, standardized cutoffs for nutrition applications are needed. Protein-energy malnutrition leads to increased gut permeability to pathogens, abnormal immune cell populations, and impaired APP response. Micronutrient deficiencies cause specific immune impairments that affect both innate and adaptive responses. This review describes the antagonistic interaction between the APR and nutritional status and emphasizes the need for integrated interventions to address undernutrition and to reduce disease burden in developing countries.
Introduction
Undernutrition persists as a major global health concern, particularly in sub-Saharan Africa, South Asia, and regions of Latin America (1–3). Dietary intakes of populations in these areas are often chronically deficient in macronutrients [leading to protein-energy malnutrition (PEM)3], micronutrients (leading to specific micronutrient deficiencies), or both (2, 4–6). Poverty is the foundational cause of malnutrition and associated health determinants, such as infectious disease risk (7–9).
More than half of all deaths from infection are associated with malnutrition in children <5 y of age (10), and a synergistic relation exists between poor nutritional status and immunity and infectious disease. Malnutrition increases the vulnerability to infection through impaired immunity, and infection exacerbates the condition, further weakening the immune response (5). Even mild febrile infections negatively affect nutritional status; however, prior nutritional status of the host, extent of illness, and dietary intake during recovery determine the severity of the consequences (11–13). It is not surprising to find a high prevalence of microbial and parasitic diseases occurring in developing countries where undernutrition is common (6, 14).
PEM is associated with abnormal immune cell populations and impaired intestinal barrier function, which increases risk of microbial infection (5, 15–17). Micronutrient deficiencies can lead to specific immune impairments that negatively affect both innate and adaptive immune responses (16, 18). Innate immunity includes the acute phase response (APR), a systemic inflammatory reaction to a wide variety of imbalances in an individual’s homeostasis (19). During the APR to infection, metabolic processes and nutrient requirements are altered (19, 20). Furthermore, blood concentrations of micronutrients are affected, leading to inaccurate assessments of micronutrient status and misestimates of deficiency prevalence during infection, which are important in both public health and biochemical assessments of nutritional status (21–23).
We review here the relation between infection and nutritional status. We will first consider the effects of the APR to infection on nutrient metabolism and then the impact of infection on nutrient requirements and immune function. We will also consider acute phase protein (APP) measures in nutrition interventions and the impact of the APR on micronutrient status indicators.
Current Status of Knowledge
The APR to infection.
The APR is a systemic inflammatory reaction to disruptions in the body’s homeostasis due to infection, tissue damage, tumor development, certain chronic disease states, and immunologic disorders. Inflammation is believed to be protective to the host because it removes injurious stimuli and promotes the healing of damaged tissue. The magnitude of the APR varies with the severity of injury and by time.
Generally, the APR to infection initiates when a pathogen is detected by pattern recognition receptors, including Toll-like receptors, on a variety of immune cells. The activation of these receptors causes cells to secrete prostaglandins and cytokines that promote proinflammatory mechanisms, such as increased vascular permeability and the recruitment of more cytokine-secreting neutrophils and macrophages. Cytokines serve as short-lived, long-range mediators that act on various tissues to mount the systemic response, which is commonly characterized by fever, leukocytosis, increased secretion of adrenocorticotropic hormone (ACTH) and glucocorticoids, and alterations in plasma protein concentrations. Protein concentrations that are increased or decreased in the plasma during the APR are called positive or negative APPs, respectively, and are primarily regulated by the liver. The APR is limited by counter-regulatory mechanisms, including diffuse cell-mediated and targeted neural anti-inflammatory pathways. These mechanisms also promote tissue repair once the deleterious stimuli have been removed. The pathophysiologic changes that occur during the APR were previously reviewed in detail (19, 24–38). Aspects of the APR that relate to nutrient metabolism and nutritional status will be examined further herein.
Cytokine biology and nutrient metabolism.
Acute phase cytokines are multifunctional and can be divided into 3 categories: 1) proinflammatory cytokines, which include TNF-α and IL-1; 2) IL-6–type cytokines that promote systemic features of the APR; and 3) anti-inflammatory cytokines (26). These cytokines modulate the immune response and associated metabolic effects through complex interactions that can be additive, synergistic, or antagonistic. Cytokines regulate gene expression through intracellular signal transduction pathways that activate transcription factors (24, 39–41).
TNF-α and IL-1 are generated shortly after the initial stimulus, promoting the production of other cytokines, such as IL-6, and inducing sickness behavior by acting on distant tissue sites through cell-mediated and neural communication pathways (24, 42, 43). Cytokine-induced sickness behavior is characterized by symptoms of fever, anorexia, weakness, malaise, somnolence, and an inability to concentrate (19, 43–46). Fever increases energy expenditure by ∼7–11% per unit increase in temperature (°C) in children (Table 1) (47, 48). This, coupled with anorexia, produces a state of negative energy balance during infection. The fatigue and discomfort associated with illness are believed to have the adaptive advantage of energy conservation (43, 44).
TABLE 1.
Impact of the acute phase response to infection on energy expenditure and macronutrient metabolism1
| Metabolic indicator | Impact of infection |
| Energy | ↓ Dietary intake due to appetite suppression, ↑ energy expenditure to produce fever |
| Macronutrient | |
| Protein | ↑ Muscle protein wasting, ↑ hepatic protein synthesis |
| Carbohydrate | ↑ Blood glucose due to insulin resistance, glycogenolysis, and gluconeogenesis |
| Fat | ↑ Peripheral lipolysis, ↑ hepatic TG and VLDL synthesis, ↓ serum cholesterol |
↑, increase; ↓, decrease.
Proinflammatory cytokines shift metabolic processes to a catabolic state. TNF-α and IL-1 inhibit muscle protein synthesis and promote wasting (49–53). Through the stimulation of glucocorticoid release, IL-1 increases the whole-body flux of amino acids by promoting muscle catabolism and liver anabolism (54). During infection, mean losses are 0.6 g protein · kg body weight−1 · d−1, and can be as high as 1.14 g protein · kg body weight−1 · d−1 during peak febrile response (11). The high demands for aromatic amino acids in APP production are responsible for a substantial portion of the net loss in body nitrogen during the APR (55).
Hyperglycemia is directly induced by cytokines to fuel obligate glucose-consuming cells during infection. Proinflammatory cytokines act on the hypothalamic-pituitary-adrenal axis to increase production of ACTH and glucocorticoids, which promote glycogenolysis and insulin resistance (56). Additionally, TNF-α stimulates glucagon production, and both TNF-α and IL-1 promote gluconeogenesis (56, 57). The duration of hyperglycemia is dependent on the severity of infection and typically normalizes within 10 d (56); however, when infection is severe or septic, hypoglycemia can pose potentially lethal health risks (11).
Proinflammatory cytokines and IL-6 quickly increase serum TG concentrations by 1) promoting peripheral lipolysis, 2) increasing intracellular citrate concentrations to upregulate de novo FA synthesis and hepatic TG production, and 3) enhancing VLDL-cholesterol secretion (57, 58). Hepatic FA oxidation and ketogenesis are suppressed to ensure sufficient FA substrate for these processes. Serum cholesterol concentrations are decreased by TNF-α through suppression of LDL and HDL cholesterol. Alterations in lipid profile are protective to the host during infection because lipoproteins have the ability to bind bacterial endotoxin and viruses, resulting in rapid clearance by the liver and reduced activation of cytokine-induced inflammatory mechanisms. During inflammatory chronic disease states and sustained APR, the prolonged alteration of the lipid profile is associated with an increased risk of atherosclerosis (58).
IL-6 is the major regulator of the APR in hepatocytes and promotes the systemic effects of the APR by stimulating the growth and differentiation of many cell types. Although TNF-α and IL-1 can promote the production of certain APPs in the liver, IL-6 induces the production of the full spectrum of APPs in a dose-dependent manner (19, 24), which modulates the response to infection severity (24, 39). Additional APR functions facilitated by IL-6 include the maturation of B and T cells and hematopoiesis (24, 39, 59). IL-6 also displays anti-inflammatory attributes by suppressing TNF-α and IL-1 production in mononuclear cells and promoting ACTH and glucocorticoid production (24, 60, 61).
Infection and nutrient requirements.
Infection can lead to a reduction in food intake, impairment of nutrient absorption, and increased nutrient requirements (Fig. 1). Although breast-milk intake appears to be unaffected, food intake is suppressed in children during the APR according to the magnitude of infection. Caloric intake decreases between 8% and 22% in children with diarrheal diseases, malaria, and acute respiratory infection (62–64). During more severe infections, such as cholera and measles, decreases in caloric intake were reported to be 44% and 75%, respectively. In Peruvian infants with diarrhea or fever, breast-milk intake was unaffected; however, caloric intakes of non–breast-milk food decreased by 20–30%, resulting in an overall decrease in caloric intake of 5–6% (65). Infection-related caloric suppression results in both decreased macro- and micronutrient intake (63, 64).
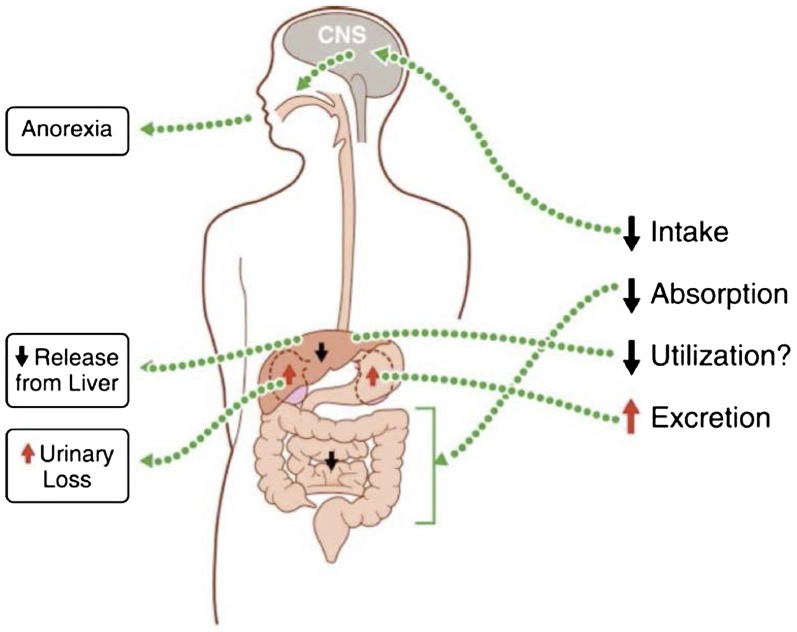
FIGURE 1.
Infections can alter micronutrient status. For example, for vitamin A, illness can negatively affect status through 1) decreased dietary intake caused by cytokine-induced sickness behavior, 2) reduced intestinal absorption during both enteric and nonenteric infection, and 3) increased urinary excretion. Infection also suppresses the release of vitamin A from the liver, reducing plasma concentrations and thus possibly altering utilization of liver stores. CNS, central nervous system. Adapted from reference 110 with permission.
Nutrient absorption can be decreased during enteric and nonenteric infection. Gut helminths and diarrheal diseases can impair nutrient absorption by damaging epithelial cells (20, 63, 66). The extent of malabsorption corresponds to the severity of enteric infection and diarrheal output (20). Nutrient absorption appears to also be reduced during nonenteric infection. Carbohydrate malabsorption was observed in hospitalized American children with HIV infection (67). By using labeled tracer doses of vitamin A, Sivakumar and Reddy (68, 69) found that uninfected children absorbed 99% of the dose, children with diarrhea and Ascaris infection absorbed 70% and 80%, respectively, and children with nonenteric infection absorbed 74% of the dose.
In addition to increased energy requirements due to the metabolic cost of fever, macro- and micronutrient demands may be augmented through greater direct loss. Leakage of low-molecular-weight proteins in the urine is common during febrile infection (20). This phenomenon leads to protein loss in the urine, as well as loss of vitamin A bound to retinol-binding protein (RBP), which can exceed the daily requirement for children (70, 71). Tissue damage from parasitic infection can lead to blood loss and iron-deficiency anemia (20).
Undernutrition and immune function.
PEM and micronutrient deficiencies lead to underweight and stunting. As of 2011, 16% of children <5 y of age were underweight and 26% of children were stunted, which marks a great achievement in combating global malnutrition (72). However, half of all infectious deaths in developing countries are associated with a low weight-for-age (10). Although many interventions and research studies focus on severe PEM because of the associated sharp increase in mortality, Pelletier et al. (73) demonstrated that mild-to-moderate PEM accounts for 80% of childhood mortality in developing countries. Stunting also appears to compromise immune function, but the effect has not been studied comprehensively (15, 20).
PEM leads to increased susceptibility to and mortality from infection (74–76). Gut barrier function is decreased, increasing the risk of microbial infection (16). Undernourished individuals also experience a reduction in leukotrienes, which promote leukocyte accumulation and enhance macrophage phagocytosis, negatively affecting the host’s ability to kill microbial, fungal, and parasitic agents (77). During PEM, the structure and function of the thymus gland is damaged, leading to abnormal T-cell populations and reduced antigen response (78, 79). Compared with healthy children, B-cell response is lowered during malnutrition (80). However, the humoral response appears to be well preserved during the short-term seroconversion to whole inactivated viral and polysaccharide vaccines (81). Additionally, the development of antibody titers to common pathogens appeared to be normal in Kenyan children (82).
Conflicting evidence exists regarding the ability of children with severe PEM to mount a complete immune response. Although Doherty et al. (83) reported decreased TNF-α and IL-6 in an in vitro study using whole blood from malnourished children, Sauerwein et al. (84) found that children with PEM mount a greater immune response than do healthy children. A study in Kenyan children supports PEM as a proinflammatory state with a generalized increase in IL-6 (85). The APP response is impaired in children with severe PEM, with a greater deficit occurring in kwashiorkor as compared with marasmus (85–87). In children with marasmus, rates of protein catabolism are similar to healthy children during infection and higher than in children with kwashiorkor (88). These comparative differences in immunity may reflect an inappropriate response during kwashiorkor and are consistent with observations of a lower fatality rate in marasmic children with infection (89).
A high burden of infectious disease is associated with delayed linear growth and stunting (90–94). Similar to underweight, stunted children display an abnormal lymphocyte profile (15). Rural children in developing countries tend to experience more severe stunting and have higher plasma concentrations of APPs than do children living in urban settings (73).
Severe deficiencies frequently occur for multiple micronutrients simultaneously and can complicate PEM (17, 18). Certain micronutrients, including vitamin A, iron, and zinc, are important immunomodulators and thereby are crucial to the host in mounting an effective response to infection. Iron deficiency is a major global health concern, affecting 3.5 billion people (95). Iron is essential for redox reactions, gene regulation, oxygen delivery to tissues, and cell growth (96–98). Iron supports immunity through its involvement with peroxide- and NO-producing enzymes, cytokine production and function, and lymphocyte proliferation (96–98). Iron homeostasis is paramount because deficiency and overload negatively affect immune function (96). Many pathogens require iron to thrive, and therefore sequestration during infection is protective to the host (96, 97). Iron supplementation is controversial because excess and timing of repletion may promote pathogenic infection (97). In deficient children, iron supplementation improves status and promotes related growth and development (99). However, iron supplementation in children does not affect the incidence of infection or associated mortality and may increase the risk of diarrheal diseases and malaria (99, 100).
Zinc is essential for highly proliferating cells in the immune system and influences both innate and acquired immune functions (101–103). Zinc is a coenzyme in many important reactions during the immune response and is essential for thymic hormone function (102, 104). Phagocyte and lymphocyte activity are impaired or completely suppressed in zinc deficiency, resulting in weakened cytokine and antibody responses (101–103). Zinc deficiency affects approximately one-third of the global population and often coexists with PEM (18, 105). Zinc supplementation in children promotes gains in weight and height and protects against respiratory infections (106–108). Although zinc supplementation protected against diarrheal disease in children <5 y of age (107), Negi et al. (109) recently reported supplements to be ineffective in children between 5 and 12 y of age.
Vitamin A deficiency compromises innate immunity through the degradation of mucosal epithelial barriers and impaired development of neutrophils, macrophages, and NK cells (110). Pathogen-specific immunity is also lessened during vitamin A deficiency via reduction in antibody production in T cells (110). Vitamin A deficiency affects 190 million children and is the leading cause of preventable blindness (111). Xerophthalmia is associated with respiratory tract infection, measles, and diarrhea in children (112). This is not surprising considering that infection can worsen deficiency through anorexia and increase direct loss of vitamin A in the urine. The semiannual vitamin A supplementation program recommended by the WHO (111, 113) reduced malnutrition, which was assessed by increases in mid–upper arm circumference, and was protective against infection in Nepalese children (114). Additionally, vitamin A supplementation reduced the frequency of malarial episodes by 30% in children in Papua New Guinea and promoted ponderal growth and protection against malaria-related death in Tanzanian children (115, 116). Provitamin A carotenoid–biofortified staple crops provide a potentially effective method for alleviating vitamin A deficiency in the rural poor, who are disproportionally missed by supplementation interventions (117, 118). However, biofortification target concentrations of provitamin A carotenoids should be increased in areas with a high prevalence of infection to account for reduced food intake and increased requirements (23).
APPs as markers of infection for nutrition interventions.
The liver plays an important role in modulating the immune response and removing pathogens (119, 120). In mice, ∼7% of hepatic genes respond to APR cytokines, inducing a wide array of functional changes, including those in macronutrient metabolism and APP production (121, 122). Positive APPs broadly work to mitigate the consequences of infection and modify the host’s immune response (19, 28, 30, 33). The role of negative APPs remains unclear, but the downregulation is speculated to increase the availability of amino acids for positive APP production (28, 30). Many negative APPs are transporters; the hepatic suppression of these proteins allows for a temporarily increased free availability of their associated nutrients and hormones in the plasma followed by decreases in plasma concentration due to sequestration (19, 28, 33). It is important to note that much of the research concerning APP responses was conducted in adults undergoing trauma rather than infection. Additionally, relatively little is known about APP responses in children, particularly those in developing countries who may have compromised immune function.
The manner in which the liver alters APP concentrations in the plasma is dependent on the time stage of the APR, such that positive APPs become elevated in the plasma at different time points poststimulus. In nutrition interventions, C-reactive protein (CRP) and serum amyloid A are used as early markers of infection because they become elevated within 24 h poststimulus and normalize rapidly as infection resolves (23, 24, 33, 123, 124). Antichymotrypsin (ACT) and α1-acid glycoprotein (AGP) are implemented as late-stage markers of infection because they increase after 48 h and can remain elevated after the infection convalesces (23, 33, 124, 125). By using these proteins, it is possible to identify different stages of infection including incubation (early-stage APP only increased), early convalescence (both early- and late-stage APPs increased), convalescence (late-stage APP only increased), and return to a healthy state (neither early- nor late-stage APP increased) (23, 124). Positive APPs provide a superior assessment of infection when compared with cytokines, which possess short half-lives.
The magnitude of the APP response is dependent on the severity of injury to the host. Contreras-Manzano et al. (126) reported positive APPs to be more elevated in children with respiratory infections than in children with diarrhea. Furthermore, direct correlations between plasma concentrations of positive APPs and indirect correlations between positive APP concentration and transporter-requiring micronutrients were found in children (23, 127–132). Duncan et al. (133) also found that alteration of several micronutrients assessed in the blood corresponded to the extent of the APR when assessed by CRP concentration in adults.
APP elevation cutoffs used to determine infection are not standardized for nutrition applications and may vary by age, sex, and nutrient. For example, elevation cutoffs for CRP of 5 or 10 mg/L are commonly reported in the literature to describe the APR (21, 23, 124, 134–138). Bresnahan et al. (23) found that a CRP concentration of 5 mg/L was an effective elevation cutoff to show the impact on traditional blood-based indicators of vitamin A and iron status in Zambian children, whereas Abraham et al. (139) found significant changes in the same micronutrient status indicators occurring at ≥0.6 mg/L with the use of high-sensitivity assays in apparently healthy German children. In adults, CRP cutoffs between 5 and 20 mg/L were necessary to detect changes in blood concentration for various vitamins and minerals (133). CRP concentrations increase with age and tend to be higher in women than in men (140). This increase is, in part, related to chronic inflammatory conditions, suggesting a higher CRP cutoff may be necessary to assess acute infection in adults (134, 141). Additionally, noninfectious inflammation can occur during pregnancy and lactation and is poorly understood (142–144). More research is imperative to establish APP cutoffs that are adequate for determining the impact of the APR on micronutrient status indicators.
Micronutrient status indicators during the APR.
The impact of the APR on micronutrient status indicators is well documented in the literature, yet no standardized method for adjustment during infection has been developed. In public health assessments, micronutrient status is commonly determined by using concentrations in blood as a surrogate for total body status. Blood concentrations of retinol, iron, and zinc can be altered by the APR to infection (Table 2) (21–23, 124, 127–134, 137, 145–160). Alterations in blood micronutrient concentrations result from hepatic suppression of transport proteins (RBP, transthyretin, albumin, and transferrin) and increases in serum ferritin and hepatic metallothionein, positive APPs that assist in iron and zinc sequestration, respectively (28, 98, 161–164). Although micronutrient demands can be increased during infection, significant changes in micronutrient status do not likely occur as early as decreases in blood concentrations suggest during the APR. Furthermore, blood concentrations are typically restored to preinfection values as the APR resolves, supporting measurements taken during febrile infection as inaccurate reflections of micronutrient status (127, 145, 146, 154).
TABLE 2.
Effect of inflammation on indicators of micronutrient status1
| Blood indicator | Impact of infection |
| Iron status | |
| Ferritin | ↑ |
| Serum iron | ↓ |
| Transferrin | ↓ |
| Transferrin receptors | NC to ↓ |
| Hemoglobin | NC to ↓ |
| Zinc status | |
| Serum zinc | ↓ |
| Vitamin A status | |
| Serum retinol | ↓ |
| MRDR test | NC |
MRDR, modified relative dose response; NC, no change; ↑, increase; ↓, decrease.
The APR rapidly affects common biomarkers for iron status, including serum iron, transferrin, and ferritin (164). Of the iron biomarkers, serum ferritin concentrations are the most significantly affected by infection, with reported increases of 30% to 1400% depending on the time stage and severity of infection in children (21, 23, 131, 132, 147, 152, 159). Serum iron and transferrin are suppressed by 50% and 30%, respectively, and the percentage of transferrin saturation is lowered to ∼20% (154, 164). The impacts of infection on soluble transferrin receptors and hemoglobin remain unclear. Although iron status appears to be the main factor affecting soluble transferrin receptor concentration, it was reported to be decreased in surgical patients and in individuals with malarial and HIV infection (154, 165–167). Hemoglobin was suppressed in British, Zambian, and Zanzibari children during the APR and found to be inversely related to serum ferritin concentration, supporting its role as a reactive iron status indicator (23, 131, 168). In Kenyan children, hemoglobin was negatively correlated to CRP and AGP and positively associated with serum ferritin (169). Upon adjusting for inflammation, the relation between hemoglobin and the APPs disappeared and that with serum ferritin was strengthened (169). However, Wieringa et al. (21) reported no effect of the APR on hemoglobin, and Das et al. (152) found no association between hemoglobin and serum ferritin.
Serum zinc concentration is suppressed by 12% in children during infection (21, 147). Similarly, in HIV-positive adults, a 12% decrease in serum zinc was observed (22). In children with malaria, CRP concentration and parasite density predicted low serum zinc concentration (158). It is important to also consider the definition of zinc deficiency when assessing the impact of infection on estimates of deficiency prevalence because cutoffs vary by sex, age, and time of day (170).
Serum retinol concentration is a widely used indicator for vitamin A status and is recommended for population assessment by the WHO when used in conjunction with another indicator (171, 172). The current deficiency cutoff is set at 0.7 μmol retinol/L serum (or plasma) regardless of inflammation or infection status (172). However, in children, serum retinol concentrations are suppressed by ∼25% during common infections (23, 124, 148) and up to 69% in children with Shigellosis (127). This decrease in serum retinol concentration is independent of vitamin A liver reserves (173). Therefore, individuals who are experiencing infection but have an adequate vitamin A status may be misclassified as deficient, leading to overestimation of deficiency prevalence in a population. The modified relative dose response (MRDR) test relies on the principle that RBP accumulates when vitamin A liver reserves are low (174). This phenomenon may continue during mild infection. The MRDR value (3,4-didehydroretinol:retinol) is measured by quantifying the rapid release of 3,4-didehydroretinol from the liver bound to RBP in the serum after a challenge dose of 3,4-didehydroretinyl acetate within a few hours (171). The 3,4-didehydroretinol:retinol is not appreciably altered by the APR during common infections, implying that the release of 3,4-didehydroretinol and retinol from the liver may be suppressed in a similar manner, such that the ratio between the 2 remains unchanged (21, 23). The MRDR test is more sensitive than serum retinol to changes in liver retinol reserves, supporting it as a superior tool for assessment, particularly in populations with a high or unknown prevalence of infection (21, 23, 171). Furthermore, it is recommended that all dose-response tests be administered to children without active fever to minimize effects that active illness may have on dose absorption (68). Plasma concentrations of provitamin A carotenoids are also suppressed during inflammation (150, 175). This may suggest an increased demand for vitamin A during infection, a reflection of alterations in blood lipid profile, or a decrease in dietary intake (175).
Developing a method for adjusting blood-based micronutrient status indicators that are reactive to the APR is challenging because the accurate measurement of micronutrient deficiency prevalence can be difficult in regions where dietary micronutrient intake is low and inflammation is high. Additionally, the relation between blood concentrations of micronutrients and APPs is not always linear (160, 176). The measurement of APP concentrations when assessing micronutrient status should become standard practice because APPs are elevated during subclinical inflammation (22, 177). Moreover, both early- and late-stage markers of infection should be evaluated because it is common to find more individuals in a population with elevated AGP or ACT than with elevated CRP (21–23). It is important to also consider sex differences when adjusting micronutrient status indicators for the APR. The precision of scientific studies and population-level estimates of micronutrient deficiency prevalence may be compromised by excluding individuals with elevated APPs. Therefore, Thurnham et al. (22) proposed a method of adjustment that modifies micronutrient status indicators by APP-determined infection stage with the use of a correction factor based on a healthy reference population. However, because individuals with infection are more likely to suffer micronutrient deficiency in at-risk populations, this conservative method may also result in misestimations of deficiency prevalence.
Conclusions
The synergism between nutritional status and the APR is apparent where malnutrition and infectious diseases are prevalent. The APR to infection increases energy expenditure to generate fever and accelerates catabolism of macronutrients. Furthermore, infection suppresses food intake, decreases nutrient absorption, and can increase requirements by augmenting direct loss of micronutrients. Malnutrition, in turn, increases susceptibility to infection by impairing both innate and adaptive immune responses. Integrated interventions are paramount to improve nutritional status and reduce disease burden in the developing world.
Blood-based micronutrient status indicators can be altered during the APR, leading to misestimates of deficiency prevalence. It is therefore paramount that the APR be measured and corrected for when blood-based micronutrient status indicators are assessed in populations with a high or unknown prevalence of infection. APP elevation cutoffs that are specific to nutrition applications are necessary, because current values reported in the literature vary widely. Additionally, it should become routine practice to assess both early- and late-stage APPs because micronutrient status indicators can remain altered into convalescence of the infection.
Acknowledgments
The authors thank Bryan Gannon for his critical review of the manuscript. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: ACT, antichymotrypsin; ACTH, adrenocorticotropic hormone; AGP, α1-acid glycoprotein; APP, acute phase protein; APR, acute phase response; CRP, C-reactive protein; MRDR, modified relative dose response; PEM, protein-energy malnutrition; RBP, retinol-binding protein.
References
- 1.Schofield C, Ashworth A. Why have mortality rates for severe malnutrition remained so high? Bull World Health Organ 1996;74:223–9. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. The World Health Report 2002. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.Food and Agriculture Organization. The state of food insecurity in the world 2010. Rome: Food and Agriculture Organization; 2010. [Google Scholar]
- 4.Millward DJ, Jackson AA. Protein/energy ratios of current diets in developing countries compared with a safe protein/energy ratio: implications for recommended protein and amino acid intakes. Public Health Nutr 2004;7:387–405. [DOI] [PubMed] [Google Scholar]
- 5.Neumann CG, Gewa C, Bwibo NO. Child nutrition in developing countries. Pediatr Ann 2004;33:658–74. [DOI] [PubMed] [Google Scholar]
- 6.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease control priorities in developing countries. 2nd ed. New York: Oxford University Press; 2006. [PubMed] [Google Scholar]
- 7.Fernandez ID, Himes JH, de Onis M. Prevalence of nutritional wasting in populations: building explanatory models using secondary data. Bull World Health Organ 2002;80:282–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Sachs JD, McArthur JW. The Millennium Project: a plan for meeting the Millennium Development Goals. Lancet 2005;365:347–53. [DOI] [PubMed] [Google Scholar]
- 9.Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, Bode L, Emenaker NJ, Haqq AM, Satia JA, Silver HJ, Stadler DD. Poverty, obesity, and malnutrition: an international perspective recognizing the paradox. J Am Diet Assoc 2007;107:1966–72. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier DL, Frongillo EA. Changes in child survival are strongly associated with changes in malnutrition in developing countries. J Nutr 2003;133:107–19. [DOI] [PubMed] [Google Scholar]
- 11.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr 1997;66 Suppl:464S–77S. [DOI] [PubMed] [Google Scholar]
- 12.Woodward B. Protein, calories, and immune defenses. Nutr Rev 1998;56:S84–92. [DOI] [PubMed] [Google Scholar]
- 13.Solomons NW. Malnutrition and infection: an update. Br J Nutr 2007;98:S5–10. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. World malaria report 2013. Geneva: World Health Organization; 2013. [Google Scholar]
- 15.Chandra RK, Sarchielli P. Body size and immune responses. Nutr Res 1996;16:1813–9. [Google Scholar]
- 16.Welsh FKS, Farmery SM, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Gut barrier function in malnourished patients. Gut 1998;42:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham-Rundles S, McNeely DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 2005;115:1119–28. [DOI] [PubMed] [Google Scholar]
- 18.Bhaskaram P. Immunobiology of mild micronutrient deficiencies. Br J Nutr 2001;85:S75–80. [DOI] [PubMed] [Google Scholar]
- 19.Gruys E, Toussaint MJM, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B 2005;6:1045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephensen CB. Burden of infection on growth failure. J Nutr 1999;129 Suppl:534S–8S. [DOI] [PubMed] [Google Scholar]
- 21.Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]
- 22.Thurnham DI, Mburu ASW, Mwaniki DL, Wagt AD. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc 2005;64:502–9. [DOI] [PubMed] [Google Scholar]
- 23.Bresnahan KA, Chileshe J, Arscott SA, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affects traditional measures of micronutrient status in rural Zambian children during a randomized controlled feeding trial. J Nutr 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumann H, Gauldie J. The acute phase response. Immunol Today 1994;15:74–80. [DOI] [PubMed] [Google Scholar]
- 26.Koj A. Initiation of the acute phase response and synthesis of cytokines. Biochim Biophys Acta 1996;1317:84–94. [DOI] [PubMed] [Google Scholar]
- 27.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP. New insights into the biology of the acute phase response. J Clin Immunol 1999;19:203–14. [DOI] [PubMed] [Google Scholar]
- 28.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:135–45. [DOI] [PubMed] [Google Scholar]
- 30.Ceciliani F, Giordano A, Spangnolo V. The systemic reaction during inflammation: the acute-phase proteins. Protein Pept Lett 2002;9:211–23. [DOI] [PubMed] [Google Scholar]
- 31.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002;20:197–216. [DOI] [PubMed] [Google Scholar]
- 32.Tracey KJ. The inflammatory reflex. Nature 2002;420:853–9. [DOI] [PubMed] [Google Scholar]
- 33.Raynes JG. The acute phase response. In: Kaufmann SHK, Steward MW, editors. Tropley and Wilson’s microbiology and microbial infection. 10th ed. London: Hodder Arnold; 2014. p. 1–22. [Google Scholar]
- 34.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783–801. [DOI] [PubMed] [Google Scholar]
- 35.Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–35. [DOI] [PubMed] [Google Scholar]
- 36.Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009;9:418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron 2009;64:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–20. [DOI] [PubMed] [Google Scholar]
- 39.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem J 1998;334:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev 2002;13:413–21. [DOI] [PubMed] [Google Scholar]
- 41.O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity 2008;28:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun 2003;17:S112–8. [DOI] [PubMed] [Google Scholar]
- 43.Dantzer R. Cytokine-induced sickness behavior: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 2004;500:399–411. [DOI] [PubMed] [Google Scholar]
- 44.Exton MS. Infection-induced anorexia: active host defense strategy. Appetite 1997;29:369–83. [DOI] [PubMed] [Google Scholar]
- 45.Bluthé RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol Behav 2000;70:367–73. [DOI] [PubMed] [Google Scholar]
- 46.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 2007;21:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stettler N, Shutz Y, Whitehead R, Jequier E. Effect of malaria and fever on energy metabolism in Gambian children. Pediatr Res 1992;31:102–6. [DOI] [PubMed] [Google Scholar]
- 48.Benhariz M, Goulet O, Salas J, Colomb V, Ricour C. Energy cost of fever in children on total parenteral nutrition. Clin Nutr 1997;16:251–5. [DOI] [PubMed] [Google Scholar]
- 49.Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol 1991;260:E727–30. [DOI] [PubMed] [Google Scholar]
- 50.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab 2002;282:E336–47. [DOI] [PubMed] [Google Scholar]
- 51.Cooney R, Owens E, Jurasinski C, Gray K, Vannice J, Vary T. Interleukin-1 receptor antagonist prevents sepsis-induced inhibition of protein synthesis. Am J Physiol 1994;267:E636–41. [DOI] [PubMed] [Google Scholar]
- 52.Zamir O, Hasselgren PO, Kunkel SL, Frederick J, Higashiguchi PO. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. Arch Surg 1992;127:170–4. [DOI] [PubMed] [Google Scholar]
- 53.Zamir O, O'Brien W, Thompson R, Bloedow DC, Fisher JE, Hasselgren PO. Reduced muscle protein breakdown in septic rats following treatment with interleukin-1 receptor antagonist. Int J Biochem 1994;26:943–50. [DOI] [PubMed] [Google Scholar]
- 54.Le Floc’h N, Melchior D, Obled C. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livest Prod Sci 2004;87:37–45. [Google Scholar]
- 55.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of the acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr 1994;124:906–10. [DOI] [PubMed] [Google Scholar]
- 56.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab 2001;15:533–51. [DOI] [PubMed] [Google Scholar]
- 57.Van der Poll T, Romijn JA, Endert E, Borm JJJ, Büller HR, Sauerwein HP. Tumor necrosis factor mimics the metabolic response to acute infection in healthy humans. Am J Physiol 1991;261:E457–65. [DOI] [PubMed] [Google Scholar]
- 58.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 2004;45:1169–96. [DOI] [PubMed] [Google Scholar]
- 59.Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol 2014;26:2–12. [DOI] [PubMed] [Google Scholar]
- 60.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarella CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 1990;75:40–7. [PubMed] [Google Scholar]
- 61.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an anti-inflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 1998;101:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martorell R, Yarbrough C, Yarbrough S, Klein RE. The impact of ordinary illnesses on the dietary intakes of malnourished children. Am J Clin Nutr 1980;33:345–50. [DOI] [PubMed] [Google Scholar]
- 63.Molla A, Molla AM, Rahim A, Sarker SA, Mozaffar Z, Rahaman M. Intake and absorption of nutrients in children with cholera and rotavirus infection during acute diarrhea and after recovery. Nutr Res 1982;2:233–42. [Google Scholar]
- 64.Bresnahan KA, Chileshe J, Tanumihardjo SA. Quantification of food and nutrient intakes in Zambian children with and without malaria under controlled feeding conditions. Exp Biol Med (Maywood) 2014;239:45–51. [DOI] [PubMed] [Google Scholar]
- 65.Brown KH, Stallings RY, de Kanashiro HC, de Romaña GL, Black RE. Effects of common illnesses on infants’ energy intakes from breast milk and other foods during longitudinal community-based studies in Huascar (Lima), Peru. Am J Clin Nutr 1990;52:1005–13. [DOI] [PubMed] [Google Scholar]
- 66.Rosenberg IH, Solomons NW, Schneider RE. Malabsorption associated with diarrhea and intestinal infections. Am J Clin Nutr 1977;30:1248–53. [DOI] [PubMed] [Google Scholar]
- 67.Miller TL, Orav EJ, Martin SR, Cooper ER, McIntosh K, Winter HS. Malnutrition and carbohydrate malabsorption in children with vertically transmitted human immunodeficiency virus 1 infection. Gastroenterology 1991;100:1296–302. [PubMed] [Google Scholar]
- 68.Sivakumar B, Reddy V. Absorption of vitamin A in children during infection. Br J Nutr 1972;27:299–304. [DOI] [PubMed] [Google Scholar]
- 69.Sivakumar B, Reddy V. Absorption of vitamin A in children with ascariasis. J Trop Med Hyg 1975;78:114–5. [PubMed] [Google Scholar]
- 70.Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI, Jr, Gammon B., Jr Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 1994;60:388–92. [DOI] [PubMed] [Google Scholar]
- 71.Mitra AK, Alvarez JO, Stephensen CB. Increased urinary retinol loss in children with severe infections. Lancet 1998;351:1033–4. [DOI] [PubMed] [Google Scholar]
- 72.United Nations Children’s Fund; World Health Organization; World Bank. UNICEF-WHO-World Bank joint childhood malnutrition estimates. New York: United Nations Children’s Fund; Geneva: World Health Organization; Washington: World Bank; 2012. [Google Scholar]
- 73.Pelletier DL, Frongillo EA, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ 1995;73:443–8. [PMC free article] [PubMed] [Google Scholar]
- 74.Müller O, Garenne M, Kouyaté B, Becher H. The association between protein-energy malnutrition, malaria morbidity and all-cause mortality in West African children. Trop Med Int Health 2003;8:507–11. [DOI] [PubMed] [Google Scholar]
- 75.Schneider SM, Veyres P, Pivot X, Soummer AM, Jambou P, Filippi J, van Obberghen E, Hébuterne X. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr 2004;92:105–11. [DOI] [PubMed] [Google Scholar]
- 76.Thomas JE, Dale A, Bunn JEG, Harding M, Coward WA, Cole TJ, Weaver LT. Early Helicobacter pylori colonization: the association with growth faltering in The Gambia. Arch Dis Child 2004;89:1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol 2005;174:589–94. [DOI] [PubMed] [Google Scholar]
- 78.McDade TW, Beck MA, Kuzawa CW, Adair LS. Prenatal undernutrition and postnatal growth are associated with adolescent thymic function. J Nutr 2001;131:1225–31. [DOI] [PubMed] [Google Scholar]
- 79.Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr 2002;56:S46–9. [DOI] [PubMed] [Google Scholar]
- 80.Nájera O, Gonzalez C, Toledo G, López L, Ortiz R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infection. Clin Diagn Lab Immunol 2004;11:577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore SE, Goldblatt D, Bates CJ, Prentice AM. Impact of nutritional status on antibody responses to different vaccines in undernourished Gambian children. Acta Paediatr 2003;92:170–6. [DOI] [PubMed] [Google Scholar]
- 82.Siekmann JH, Allen LH, Watnik MR, Nestel P, Neumann CG, Shoenfeld Y, Peter JB, Patnik M, Ansari AA, Coppel RL, et al. Titers of antibody to common pathogens: relation to food-based interventions in rural Kenyan schoolchildren. Am J Clin Nutr 2003;77:242–9. [DOI] [PubMed] [Google Scholar]
- 83.Doherty JF, Golden MHN, Remick DG, Griffin GE. Production of interleukin-6 and tumour necrosis factor-α in vitro is reduced in whole blood of severely malnourished children. Clin Sci 1994;86:347–51. [DOI] [PubMed] [Google Scholar]
- 84.Sauerwein RW, Mulder JA, Mulder L, Lowe B, Peshu N, Demacker PNM, van der Meet JWM, Marsh K. Inflammatory mediators in children with protein-energy malnutrition. Am J Clin Nutr 1997;65:1534–9. [DOI] [PubMed] [Google Scholar]
- 85.Dülger H, Arik M, Sekeroglu MR, Tarakçioglu M, Noyan T, Cesur Y, Balahoroglu R. Pro-inflammatory cytokines in Turkish children with protein-energy malnutrition. Mediators Inflamm 2002;11:363–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Doherty JF, Golden MHN, Raynes JG, Griffin GE. Acute-phase protein response is impaired in severely malnourished children. Clin Sci 1993;84:169–75. [DOI] [PubMed] [Google Scholar]
- 87.Reid M, Badaloo A, Forrester T, Morlese JF, Heird WC, Jahoor F. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am J Clin Nutr 2002;76:1409–15. [DOI] [PubMed] [Google Scholar]
- 88.Manary MJ, Broadhead RL, Yarasheski KE. Whole-body protein kinetics in marasmus and kwashiorkor during acute infection. Am J Clin Nutr 1998;67:1205–9. [DOI] [PubMed] [Google Scholar]
- 89.Friedland IR. Bacteraemia in severely malnourished children. Ann Trop Paediatr 1992;12:433–40. [DOI] [PubMed] [Google Scholar]
- 90.Neumann CG, Harrison GC. Onset and evolution of stunting in infants and children. Examples from the Human Nutrition Collaborative Research Support Program. Kenya and Egypt studies. Eur J Clin Nutr 1994;48:S90–102. [PubMed] [Google Scholar]
- 91.Bwibo NO, Neumann CG. The need for animal source foods by Kenyan children. J Nutr 2003;133 Suppl:3936S–40S. [DOI] [PubMed] [Google Scholar]
- 92.Assis AMO, Prado MS, Barreto ML, Reis MG, Pinheiro SMC, Parraga IM, Blanton RE. Childhood stunting in northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr 2004;58:1022–9. [DOI] [PubMed] [Google Scholar]
- 93.Mamiro PS, Kolsteren P, Roberfroid D, Tatala S, Opsomer AS, Van Camp JH. Feeding practices and factors contributing to wasting, stunting, and iron-deficiency anaemia among 3–23-month old children in Kilosa District, rural Tanzania. J Health Popul Nutr 2005;23:222–30. [PubMed] [Google Scholar]
- 94.Olney DK, Kariger PK, Stoltzfus RJ, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Pollitt E. Development of nutritionally at-risk young children is predicted by malaria, anemia, and stunting in Pemba, Zanzibar. J Nutr 2009;139:763–72. [DOI] [PubMed] [Google Scholar]
- 95.United Nations Children’s Fund; United Nations University; World Health Organization; Micronutrient Initiative. Preventing iron deficiency in women and children: technical consensus on key issues. Boston: International Nutrition Foundation; 1999. [Google Scholar]
- 96.Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest 2002;32:70–8. [DOI] [PubMed] [Google Scholar]
- 97.Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol 2004;2:946–53. [DOI] [PubMed] [Google Scholar]
- 98.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr 2010;30:105–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. Am J Clin Nutr 2006;84:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gera T, Sachdev HPS. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ 2002;325:1142–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68 Suppl:447S–63S. [DOI] [PubMed] [Google Scholar]
- 102.Prasad AS. Effects of zinc deficiency on immune functions. J Trace Elem Exp Med 2000;13:1–20. [Google Scholar]
- 103.Ibs KH, Rink L. Zinc-altered immune function. J Nutr 2003;133 Suppl:1452S–6S. [DOI] [PubMed] [Google Scholar]
- 104.Dardenne M. Zinc and immune function. Eur J Clin Nutr 2002;56:S20–3. [DOI] [PubMed] [Google Scholar]
- 105.Lopez A. Malnutrition and the burden of disease. Asia Pac J Clin Nutr 2004;13:S7. [Google Scholar]
- 106.Sazawal S, Black RE, Jalla S, Mazumdar S, Sinha A, Bhan MK. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics 1998;102:1–5. [DOI] [PubMed] [Google Scholar]
- 107.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, Khatun F, Martorell R, Ninh NX, Penny ME, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. J Pediatr 1999;135:689–97. [DOI] [PubMed] [Google Scholar]
- 108.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2002;75:1062–71. [DOI] [PubMed] [Google Scholar]
- 109.Negi R, Dewan P, Shah D, Das S, Bhatnagar S, Gupta P. Oral zinc supplements are ineffective for treating acute dehydrating diarrhea in five to 12-year-olds. Acta Paediatr. 2014; DOI: 10.111/apa.12645. [DOI] [PubMed] [Google Scholar]
- 110.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 111.World Health Organization. Guideline: vitamin A supplementation in infants and children 6–59 months of age. Geneva: World Health Organization; 2011. [Google Scholar]
- 112.Al-Kubaisy W, Al-Rubaiy MG, Nassief HA. Xerophthalmia among hospitalized Iraqi children. East Mediterr Health J 2002;8:496–502. [PubMed] [Google Scholar]
- 113.World Health Organization. Guideline: vitamin A supplementation for infants 1–5 months of age. Geneva: World Health Organization; 2011. [Google Scholar]
- 114.Grubesic RB, Selwyn BJ. Vitamin A supplementation and health outcomes for children in Nepal. J Nurs Scholarsh 2003;35:15–20. [DOI] [PubMed] [Google Scholar]
- 115.Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet 1999;354:203–9. [DOI] [PubMed] [Google Scholar]
- 116.Villamor E, Mbise R, Spiegelman D, Hertzmark E, Fataki M, Peterson KE, Ndossi G, Fawzi WW. Vitamin A supplements ameliorate the adverse effects of HIV-1, malaria, and diarrheal infections on growth. Pediatrics 2002;109:E6. [DOI] [PubMed] [Google Scholar]
- 117.United Nations Children’s Fund. Vitamin A supplementation: a decade of progress. New York: United Nations Children’s Fund; 2007. [Google Scholar]
- 118.Tanumihardjo SA. Food-based approaches for ensuring adequate vitamin A nutrition. Compr Rev Food Sci Food Saf. 2008;7:373–81. [Google Scholar]
- 119.Knolle P, Löhr H, Treichel U, Dienes HP, Lohse A, Schlaack H, Gerken G. Parenchymal and nonparenchymal liver cells and their interaction in the local immune response. Z Gastroenterol 1995;33:613–20. [PubMed] [Google Scholar]
- 120.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 2000;174:21–34. [DOI] [PubMed] [Google Scholar]
- 121.Desiderio S, Yoo JY. A genome-wide analysis of acute-phase response and its regulation by Stat3β. Ann N Y Acad Sci 2003;987:280–4. [DOI] [PubMed] [Google Scholar]
- 122.Yoo JY, Desiderio S. Innate and acquired immunity intersect in a global view of the acute phase response. Proc Natl Acad Sci. 2003;100:1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med 1999;17:1019–25. [DOI] [PubMed] [Google Scholar]
- 124.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency. Lancet 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 125.Thompson D, Milford-Ward A, Whicher JT. The value of the acute phase protein measurement in clinical practice. Ann Clin Biochem 1992;29:123–31. [DOI] [PubMed] [Google Scholar]
- 126. Contreras-Manzano A, Villalpando S, Lamadrid H, Shamah-Levy T. Acute phase proteins are more elevated in acute respiratory infections than in diarrhea. FASEB J 2013;27:886.3.
- 127.Mitra AK, Alvarez JO, Wahed MA, Fuchs GJ, Stephensen CB. Predictors of serum retinol in children with shigellosis. Am J Clin Nutr 1998;68:1088–94. [DOI] [PubMed] [Google Scholar]
- 128.Rosales FJ, Topping JD, Smith JE, Shankar AH, Ross AC. Relation of serum retinol to acute phase proteins in Papua New Guinea children. Am J Clin Nutr 2000;71:1582–8. [DOI] [PubMed] [Google Scholar]
- 129.Paracha PI, Jamil A, Northrop-Clewes CA, Thurnham DI. Interpretation of vitamin A status in apparently healthy Pakistani children by using markers of subclinical infection. Am J Clin Nutr 2000;72:1164–9. [DOI] [PubMed] [Google Scholar]
- 130.Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr 2006;84:1498–505. [DOI] [PubMed] [Google Scholar]
- 131.Kung’u JK, Wright VJ, Haji HJ, Ramsan M, Goodman D, Tielsch JM, Bickle QD, Raynes JG, Stoltzfus RJ. Adjusting for the acute phase response is essential to interpret iron status indicators among young Zanzibari children prone to chronic malaria and helminth infection. J Nutr 2009;139:2124–31. [DOI] [PubMed] [Google Scholar]
- 132.Brito A, Hertrampf E, Olivares M. Iron status biomarkers and C-reactive protein in children aged 19 to 72 months in Chile. Food Nutr Bull 2013;34:14–20. [DOI] [PubMed] [Google Scholar]
- 133.Duncan A, Talwar D, McMillan DC, Stafanowicz F, O’Reilly DS. Quantitative data on the magnitude of the systematic inflammatory response and its effects on micronutrient status based on plasma measurements. Am J Clin Nutr 2012;95:64–71. [DOI] [PubMed] [Google Scholar]
- 134.Stephensen CB, Gildengorin G. Serum retinol, the acute phase response, and the apparent misclassification of vitamin A status in the third National Health and Nutrition Examination Survey. Am J Clin Nutr 2000;72:1170–8. [DOI] [PubMed] [Google Scholar]
- 135.Dijkhuizen MA, Wieringa FT, West CE, Martuti S, Muhilal Effects of iron and zinc supplementation in Indonesian infants on micronutrient status and growth. J Nutr 2001;131:2860–5. [DOI] [PubMed] [Google Scholar]
- 136.Haskell MJ, Lembcke JL, Salazar M, Green MH, Peerson JM, Brown KH. Population-based plasma kinetics of an oral dose of [2H4]retinyl acetate among preschool-aged, Peruvian children. Am J Clin Nutr 2003;77:681–6. [DOI] [PubMed] [Google Scholar]
- 137.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 2008;45:18–32. [DOI] [PubMed] [Google Scholar]
- 138.Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, Lynch SR, Grummer-Strawn LM. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003–2006. Am J Clin Nutr 2009;89:1334–42. [DOI] [PubMed] [Google Scholar]
- 139.Abraham K, Müller C, Grüters A, Whan U, Schweigert FJ. Minimal inflammation, acute phase response and avoidance of misclassification of vitamin A and iron status in infants—importance of a high sensitivity C-reactive protein (CRP) assay. Int J Vitam Nutr Res 2003;73:423–30. [DOI] [PubMed] [Google Scholar]
- 140.Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med 2005;352:1611–3. [DOI] [PubMed] [Google Scholar]
- 141.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood 2005;105:2294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16:206–15. [DOI] [PubMed] [Google Scholar]
- 143.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 2011;1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.World Health Organization. Mastitis: Causes and management. Geneva: World Health Organization; 2000. [Google Scholar]
- 145.Reddy V, Bhaskaram P, Raghuramulu N, Milton RC, Rao V, Madhusudan J, Krishna KV. Relationship between measles, malnutrition, and blindness: a prospective study in Indian children. Am J Clin Nutr 1986;44:924–30. [DOI] [PubMed] [Google Scholar]
- 146.Louw JA, Werbeck A, Louw ME, Kotze JvW, Cooper R, Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit Care Med 1992;20:934–41. [DOI] [PubMed] [Google Scholar]
- 147.Brown KH, Lanata CF, Yuen ML, Peerson JM, Butron B, Lönnerdal B. Potential magnitude of the misclassification of a population’s trace element status due to infection: example from a survey of young Peruvian children. Am J Clin Nutr 1993;58:549–54. [DOI] [PubMed] [Google Scholar]
- 148.Filteau SM, Morris SS, Abbott RA, Tomkins AM, Kirkwood BR, Arthur P, Ross DA, Gyapong JO, Raynes JG. Influence of morbidity on serum retinol of children in a community-based study in northern Ghana. Am J Clin Nutr 1993;58:192–7. [DOI] [PubMed] [Google Scholar]
- 149.Salazar-Lindo E, Salazar M, Alvarez JO. Association of diarrhea and low serum retinol in Peruvian children. Am J Clin Nutr 1993;58:110–3. [DOI] [PubMed] [Google Scholar]
- 150.Das BS, Thurnham DI, Das DB. Plasma α-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am J Clin Nutr 1996;64:94–100. [DOI] [PubMed] [Google Scholar]
- 151.Friis H, Ndhlovu P, Kaondera K, Sandström B, Michaelsen KF, Vennervald BJ, Christensen NØ. Serum concentration of micronutrients in relation to schistosomiasis and indicators of infection: a cross-sectional study among rural Zimbabwean schoolchildren. Eur J Clin Nutr 1996;50:386–91. [PubMed] [Google Scholar]
- 152.Das BS, Thurnham DI, Das DB. Influence of malaria on markers of iron status in children: implications for interpreting iron status in malaria-endemic communities. Br J Nutr 1997;78:751–60. [DOI] [PubMed] [Google Scholar]
- 153.Christian P, Schulze K, Stoltzfus RJ, West KP., Jr Hyporetinolemia, illness symptoms, and acute phase protein in pregnant women with and without night blindness. Am J Clin Nutr 1998;67:1237–43. [DOI] [PubMed] [Google Scholar]
- 154.Feelders RA, Vreugdenhil G, Eggermont AMM, Kuiper-Kramer PA, van Eijk HG, Swaak AJG. Regulation of iron metabolism in the acute-phase response: interferon γ and tumor necrosis factor α induce hypoferraemia, ferritin production and a decrease in circulating transferrin receptors in cancer patients. Eur J Clin Invest 1998;28:520–7. [DOI] [PubMed] [Google Scholar]
- 155.Hautvast JLA, Tolboom JJM, Willems JL, Mwela CM, Monnens LAH. Consequences of infections for three-month length increment in young children in rural Zambia. Acta Paediatr 2000;89:296–301. [PubMed] [Google Scholar]
- 156.Stoltzfus RJ, Chwaya HM, Montresor A, Albonico M, Savioli L, Tielsch JM. Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-y old Zanzibari children and these relationships change with age. J Nutr 2000;130:1724–33. [DOI] [PubMed] [Google Scholar]
- 157.Baeten JM, Richardson BA, Bankson DD, Wener MH, Kreiss JK, Lavreys L, Mandaliya K, Bwayo JJ, McClelland RS. Use of serum retinol-binding protein for prediction of vitamin A deficiency: effects of HIV-1 infection, protein malnutrition, and the acute phase response. Am J Clin Nutr 2004;79:218–25. [DOI] [PubMed] [Google Scholar]
- 158.Duggan C, MacLeod WB, Krebs NF, Westcott JL, Fawzi WW, Premji ZG, Mwanakasale V, Simon JL, Yeboah-Antwi K, Hamer DH. Zinc Against Plasmodium Study Group. Plasma zinc concentrations are depressed during the acute phase response in children with Falciparum malaria. J Nutr 2005;135:802–7. [DOI] [PubMed] [Google Scholar]
- 159.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 160.Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr 2012;15:1688–96. [DOI] [PubMed] [Google Scholar]
- 161.Bremner I, Beattie JH. Metallothionein and the trace minerals. Annu Rev Nutr 1990;10:63–83. [DOI] [PubMed] [Google Scholar]
- 162.Min KS, Terano Y, Onosaka S, Tanaka K. Induction of hepatic metallothionein by nonmetallic compounds associated with acute-phase response in inflammation. Toxicol Appl Pharmacol 1991;111:152–62. [DOI] [PubMed] [Google Scholar]
- 163.Ilbäck NG, Glynn AW, Wikberg L, Netzel E, Lindh U. Metallothionein is induced and trace element balance changed in target organs of a common viral infection. Toxicology 2004;199:241–50. [DOI] [PubMed] [Google Scholar]
- 164.Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. Report on priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva: World Health Organization; 2012. [Google Scholar]
- 165.Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 1990;75:1870–6. [PubMed] [Google Scholar]
- 166.Beesley R, Filteau S, Tomkins A, Doherty T, Ayles H, Reid A, Ellman T, Parton S. Impact of acute malaria on plasma concentrations of transferrin receptors. Trans R Soc Trop Med Hyg 2000;94:295–8. [DOI] [PubMed] [Google Scholar]
- 167.Rawat R, Stoltzfus RJ, Ntozini R, Mutasa K, Iliff PJ, Humphrey JH. Influence of inflammation as measured by α-1-acid glycoprotein on iron status indicators among HIV-positive postpartum Zimbabwean women. Eur J Clin Nutr 2009;63:787–93. [DOI] [PubMed] [Google Scholar]
- 168. Ramdath DD, Simeon DT, Wong MS, Grantham-McGregor SM. Iron status of schoolchildren with varying intensities of Trichuris trichiura infection. Parasitology 1995;110:347–51. [DOI] [PubMed]
- 169.Grant FKE, Suchdev PS, Flores-Ayala R, Cole CR, Ramakrishnan U, Ruth LJ, Martorell R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr 2012;142:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hotz C, Brown KH, editors. International Zinc Consultative Group (IZiNCG) technical document 1: assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25:S99–203. [PubMed] [Google Scholar]
- 171.Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr 2011;94 Suppl:658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: World Health Organization; 2011. [Google Scholar]
- 173.Willumsen JF, Simmank K, Filteau SM, Wagstaff LA, Tomkins AM. Toxic damage to the respiratory epithelium induces acute phase changes in vitamin A metabolism without depleting retinol stores of South African children. J Nutr 1997;127:1339–43. [DOI] [PubMed] [Google Scholar]
- 174.Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol binding protein metabolism by vitamin A status in the rat. J Biol Chem 1972;247:2542–50. [PubMed] [Google Scholar]
- 175.Schweigert FJ. Inflammation-induced changes in the nutritional biomarkers serum retinol and carotenoids. Curr Opin Clin Nutr Metab Care 2001;4:477–81. [DOI] [PubMed] [Google Scholar]
- 176.Tomkins A. Assessing micronutrient status in the presence of inflammation. J Nutr 2003;133 Suppl:1649S–55S. [DOI] [PubMed] [Google Scholar]
- 177.Panter-Brick C, Lunn PG, Todd A. Elevated acute-phase protein in stunted Nepali children reporting low morbidity: different rural and urban profiles. Br J Nutr 2001;85:125–31. [DOI] [PubMed] [Google Scholar]