Abstract
The potential cost-effectiveness and feasibility of dietary interventions aimed at reducing hypertension risk are of considerable interest and significance in public health. In particular, the effectiveness of restricted sodium or increased potassium intake on mitigating hypertension risk has been demonstrated in clinical and observational research. The role that modified sodium or potassium intake plays in influencing the renin-angiotensin system, arterial stiffness, and endothelial dysfunction remains of interest in current research. Up to the present date, no known systematic review has examined whether the sodium-to-potassium ratio or either sodium or potassium alone is more strongly associated with blood pressure and related factors, including the renin-angiotensin system, arterial stiffness, the augmentation index, and endothelial dysfunction, in humans. This article presents a systematic review and synthesis of the randomized controlled trials and observational research related to this issue. The main findings show that, among the randomized controlled trials reviewed, the sodium-to-potassium ratio appears to be more strongly associated with blood pressure outcomes than either sodium or potassium alone in hypertensive adult populations. Recent data from the observational studies reviewed provide additional support for the sodium-to-potassium ratio as a superior metric to either sodium or potassium alone in the evaluation of blood pressure outcomes and incident hypertension. It remains unclear whether this is true in normotensive populations and in children and for related outcomes including the renin-angiotensin system, arterial stiffness, the augmentation index, and endothelial dysfunction. Future study in these populations is warranted.
Introduction
High blood pressure, also known as hypertension, is 1 of the most well-known major risk factors for cardiovascular disease (CVD)5 and stroke (1). Estimates reported by the American Heart Association indicate that in 2007–2010, 33% of all adults aged ≥20 y in the United States (i.e., 78,000,000) had hypertension (2). According to the American Heart Association, prevalence estimates for hypertension are comparable between men and women, are highest among African Americans (44%), and show that only 53% of all hypertensive adults have their blood pressure under control (2). Given the established relation between hypertension and CVD and stroke, 2 leading causes of morbidity and mortality worldwide, it is critical that simple yet effective interventions for reducing blood pressure be identified. Potentially relevant indicators of CVD and stroke risk also include the renin-angiotensin system (3, 4), arterial stiffness (5) and the augmentation index (6), and endothelial dysfunction (7).
Dietary interventions, in particular those based on sodium or potassium intakes, have demonstrated their ability to reduce blood pressure in humans. For example, the Dietary Approaches to Stop Hypertension (DASH) diet, a U.S.-based multicenter randomized controlled trial (RCT), showed that a high-potassium and high-calcium dietary intervention was associated with significantly reduced mean blood pressure at low, intermediate, and high sodium intakes compared with the control diet (8). Two recently published meta-analyses reported that lower sodium intake resulted in lower levels of blood pressure (9, 10), whereas a third meta-analysis reported that higher potassium consumption was associated with a reduction in blood pressure in hypertensive populations only (11). Several mechanisms exist by which sodium and potassium can influence blood pressure, and evidence indicates that the interaction between these nutrients plays a dominant role in the development of primary hypertension (12). Specifically, diets characteristic of the modern Western diet—which is high in sodium and low in potassium—produce a biologic interaction with the kidneys, resulting in excessive sodium and insufficient potassium concentrations in the human body; these biologic changes result in vascular smooth muscle cell contraction, followed by an increase in peripheral vascular resistance and higher blood pressure, and finally hypertension (12). The influence of sodium or potassium intake on the renin-angiotensin system, arterial stiffness, and endothelial dysfunction remains under study (12, 13).
The joint effects of low sodium and high potassium intakes on blood pressure, hypertension, and related factors may be larger than the effects of either sodium or potassium alone (8, 14). Up to the present date, no known systematic review has been undertaken to determine if the sodium-to-potassium ratio is more strongly associated with blood pressure and related risk factors for CVD than either sodium or potassium alone. The goal of this review was to systematically evaluate and synthesize RCTs and observational research on this issue, to identify the current research gaps, and to make research recommendations on the basis of the published data regarding the evaluation of these determinants for blood pressure and related factors.
Methods
Literature search.
A literature search was performed in PubMed for publications through 21 August 2014. Citations were limited to those published in English; no other search limits were implemented. The search string referenced sodium, potassium, the sodium-to-potassium ratio, and the cardiovascular variables of interest (blood pressure, hypertension, the renin-angiotensin system, arterial stiffness, the augmentation index, and endothelial dysfunction).
The primary level of screening included a review of all titles and abstracts for relevance. A full-text review was performed on all studies not excluded at the primary level of screening to identify relevant studies. A supplementary literature search of the reference lists of all relevant studies with primary data and any pertinent review articles and meta-analyses was performed to identify eligible studies not retrieved through the initial PubMed query.
All RCTs regardless of publication date underwent a full-text review, whereas only observational studies, reviews, and meta-analyses published subsequent to 1999 were considered for full-text review. This approach was taken assuming that reviews and meta-analyses published within the past 15 y would capture all of the available literature published before 2000, and therefore only the most recent observational studies were of interest (published in 2000 to the present).
Eligibility.
Studies were eligible if they examined and reported on the relation between the sodium-to-potassium ratio or sodium or potassium alone and blood pressure, hypertension, endothelial dysfunction, arterial stiffness, the augmentation index, or the renin-angiotensin system, or a combination of these outcomes, in children and/or adults without acute illness. Studies were included even if they examined only the sodium-to-potassium ratio, mainly to provide additional information on the strength of associations with the outcomes of interest, even though these studies could not compare the relative strength of association between the ratio and the individual nutrients. Duplicate study populations were eligible if the studies reported unique findings; otherwise, the study with the most comprehensive and current results was included. RCTs were eligible if at least 1 group of participants was allocated to an intervention that strictly modified both sodium and potassium intakes and 1 group was allocated to a usual diet or a diet involving modification of either sodium or potassium alone. Dietary pattern evaluations were also eligible [e.g., the DASH diet (8)]. If concomitant interventions were examined (e.g., pharmacologic drugs), only studies that administered those interventions to all study groups were eligible.
Qualitative assessment.
All studies were qualitatively examined on the basis of their study design, geographic location, study duration, population demographic characteristics, baseline hypertensive status (or hypertension prevalence in the case of cross-sectional studies), other medically relevant conditions or behaviors (e.g., smoking), sample size, blinding (double, single, or neither), urinary exposure assessment (e.g., 24-h or first-void urine collection), dietary intake measurement (e.g., 24-h dietary recall or food weighing) and frequency, the nutrient database relied upon (e.g., food composition tables or the USDA Survey Nutrient Database), details on the intervention and control groups, the outcome or outcomes evaluated, whether the study examined sodium or potassium alone in addition to their ratio, the estimated exposure-outcome associations, and the control of potential confounders in observational studies.
Results
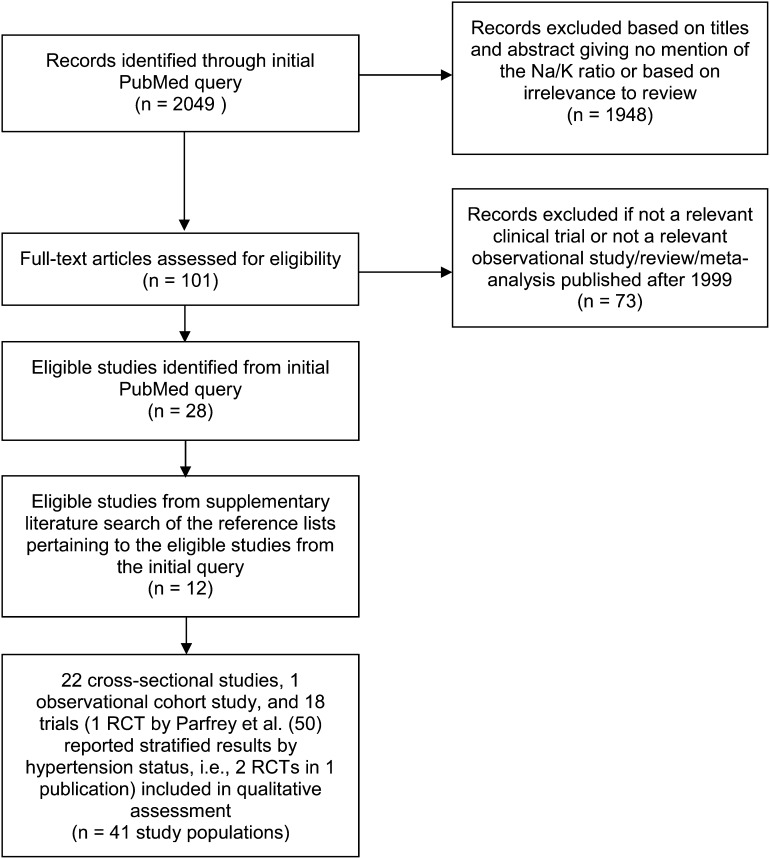
Twenty-two cross-sectional studies (15–36), 1 observational cohort study (37), and 18 RCTs [conducted in 13 hypertensive populations (38–50), 1 prehypertensive/hypertensive population (8), and 4 normotensive populations (50–53)] were included in this review. Of note, classification of prehypertension status in the DASH diet RCT was based on blood pressure >120/80 mm Hg. All other study populations among the RCTs reviewed examined effects in hypertensive or normotensive populations only. A flow diagram of the search strategy, including reasons for exclusion, is presented in Figure 1.
FIGURE 1.
Flow diagram of the literature search strategy. RCT, randomized controlled trial.
Study characteristics of RCTs
Study characteristics for each of the 18 RCTs were examined separately by hypertension status (Tables 1 and 2). One crossover RCT included both hypertensive and normotensive adults but reported the results by hypertension status (50).
TABLE 1.
Characteristics of the RCTs examining the hypotensive effect of the sodium-to-potassium ratio (Na:K) in hypertensive study populations1
| Intervention and comparator group details |
|||||||||||||
| First author, year, country (ref) | Age2 | Sex: M/F3 | Duration4 | Intervention type | Single or double blind | Na/K urine assessment | Dietary intake measurement during intervention/frequency | Nutrient database | Intervention | Dose (Na:K ratio)5 | Comparator | Dose (Na:K ratio)5 | Outcome |
| y | n | d | |||||||||||
| Sacks, 2001, U.S. (DASH)6 (8) | ≥22 (mean DASH/control: 47/49) | 179/233 | 90 (30 × 3)7 | Food | S | 24-h urine collection | Food records/daily | Estimated and monitored by using chemical analysis | High-Na + DASH diet | 144 mmol Na/d, 75 mmol K/d (NR; Na:K, 1.92) | High Na + control diet | 141 mmol Na/d, 40 mmol K/d (NR; Na:K, 3.53) | SBP, DBP |
| Intermediate-Na + DASH diet | 107 mmol Na/d, 81 mmol K/d (NR; Na:K, 1.32) | Intermediate Na + control diet | 106 mmol Na/d, 41 mmol K/d (NR; Na:K, 2.59) | ||||||||||
| Low-Na + DASH diet | 67 mmol Na/d, 81 mmol K/d (NR; Na:K, 0.83) | Low Na + control diet | 64 mmol Na/d, 42 mmol K/d (NR; Na:K, 1.52) | ||||||||||
| Singh, 1993, India (38) | 31–67 | 110/32 | 28 | Food | S | Urine assessment not performed; serum data evaluated | 24-h food records/daily; guava intake was weighed | Food composition tables | K-rich diet containing guava, Na restriction | 0.5–1.0 kg guava/d, 5–10 g salt/d (Na:K, 1.5) | Usual diet containing no guava, Na restriction | 5–10 g salt/d (Na:K, 4.7) | SBP, DBP |
| Langford, 1991, U.S. (39) | 21–65 (mean: 49) | 298 / 224 | 180 | Food | D | 24-h urine collection | 3-d food records/once every 6 mo (∼2 times) | NR | Low-Na/high-K + placebo, chlorthalidone, or atenolol | 52–100 mmol Na/d, 62–115 mmol K/d (NR; Na:K, 0.84) | Usual diet + placebo, chlorthalidone, or atenolol | NR (NR) | SBP, DBP |
| Bompiani, 1988, Italy (40) | Mean: 34 | 60 (M + F) | 28 (14 × 2) | Food | D | 24-h urine collection | NR | NR | Low-Na/high-K + standard diet | 100 mmol Na/d, 130 mmol K/d (Na:K, 0.86) | High Na diet + standard diet | 160 mmol Na/d, 80 mmol K/d (Na:K, 2.29) | SBP, DBP, PRA |
| Nowson, 1988, Australia (41) | NR | NR | 84 | Food | Neither S nor D | 24-h urine collection | 24-h recall/once every 2 wk (∼5 times; dietitian administered FFQ to facilitate recall upon review) | Modified British food tables | High-K | 120 mmol K/d (Na:K, 1.67) | Usual diet | NR (Na:K, 2.38) | SBP, DBP |
| Food | Low-Na | 50–70 mmol Na/d with minimal alteration in K (Na:K, 1.29) | |||||||||||
| Food | Low-Na/high-K | 50–70 mmol Na/d and ≥120 mmol K/d (Na:K, 0.95) | |||||||||||
| Suppa, 1988, Italy (42) | Mean: 47 | 202/120 | 28 | Food | D | 24-h urine collection | NR | NR | Dietary salt + metoprolol | 4 g/d dietary salt (50% NaCl, 25% KCl, 15% K citrate, equating to 34 mmol Na/d + 19.3 mmol K/d) (Na:K, 2.50) | Common salt + metoprolol | 4 g/d 100% NaCl (Na:K, 2.94) | SBP, DBP |
| Grobbee, 1987, The Netherlands (43) | 18–28 (mean: 24) | 34/6 | 126 (42 × 3) | Food+Su | D | 24-h urine collection | NR | NR | Slow-Na (normal-Na) | Dietary Na restriction + 90 mmol Na/d as slow-Na tablets (Na:K, 1.68) | Low Na (placebo) | Dietary Na restriction + placebo (Na:K, 0.77) | SBP, DBP, PR |
| Food+Su | Slow-K (low-Na/high-K) | Dietary Na restriction + 72 mmol K/d as slow-K tablets (Na:K, 0.53) | |||||||||||
| Valori, 1987, Italy (44) | Mean: 51 | 65/54 | 42 | Food | Neither S nor D | 24-h urine collection | NR | NR | Low-Na/high-K diet + low-Na + chlorthalidone or metoprolol | Dietary Na restriction + 4 g/d low-Na/high-K salt (equating to 17.1 mmol Na/d and 9.64 mmol K/d) (Na:K range, 2.38–2.75)8 | Low Na + chlorthalidone or metoprolol | Dietary Na restriction (NR) | SBP, DBP |
| Arzilli, 1986, Italy (45) | 28–53 (mean: 40) | 6/4 | 8 | Food | D | 24-h urine collection | NR | NR | Standard diet + low-Na/high-K salt | 20–40 mmol Na/d + 4 g/d low-Na/high-K salt (Na:K, 0.95) | Standard diet + common salt | 20–40 mmol Na/d + 4 g/d common salt (Na:K, 1.96) | BP, PRA |
| Smith, 1985, U.K. (46) | 30–66 (mean: 53) | 11/9 | 56 (28 × 2) | Food+Su | D | 24-h urine collection | NR | NR | K-supplemented diet, Na restriction | 64 mmol KCl/d as slow-K tablets + Na intake of 70 mmol/d (Na:K, 0.68) | Placebo, Na restriction | Placebo + Na intake of 70 mmol/d (Na:K, 1.09) | SBP, DBP, PRA |
| Fujita, 1984, Japan (47) | Mean: 49 | 17/6 | 6 | Su | NR | 24-h urine collection | NR | NR | High-Na/K | 250 mmol NaCl/d, 96 mmol KCl/d (NR; Na:K, 2.60) | High Na | 250 mmol NaCl/d (NR) | MBP, PRA |
| Richards, 1984, New Zealand (48) | 19–52 | 8/4 | 84–124 (28–42 × 3) | Su | Neither S nor D | 24-h urine collection | NR | NR | High-K | 180 mmol Na/d, 200 mmol K/d (NR; Na:K, 0.90) | Control | 180 mmol Na/d, 60 mmol K/d (NR; Na:K, 3.00) | SBP, DBP, PRA, angiotensin II |
| Food | Na-restricted | 80 mmol Na/d, 60 mmol K/d (NR; Na:K, 1.33) | |||||||||||
| Skrabal, 1984, Austria (49) | |||||||||||||
| Group 1 | 21–46 (mean: 32) | 8/1 | 56 (28 × 2) | Food+Su | Neither S nor D | 24-h urine collection | NR | NR | Low-Na/high-K | 80 mmol Na/d, 120 mmol K/d (Na:K, 0.85) | Low Na | 80 mmol Na/d (Na:K, 1.18) | SBP, DBP, MBP |
| Group 2 | 28–69 (mean: 45) | 9/3 | 56 (28 × 2) | Food+Su | Neither S nor D | 24-h urine collection | NR | NR | Low-Na/high-K | 80 mmol Na/d, 120 mmol K/d (Na:K, 1.17) | Low Na | 80 mmol Na/d (Na:K, 1.32) | SBP, DBP, MBP |
| Parfrey, 1981, U.K. (50) | 26–49 (mean: 36) | 10/6 | 168 (84 × 2) | Food+Su | S | 24-h urine collection | NR | NR | Low-Na/high-K | 100 mmol K/d as slow-K tablets or salt substitute (92% KCl, 6% K glutamate) (Na:K, 1.0) | High Na | Usual diet + 100 mmol Na/d as slow-Na tablets (Na:K, 4.0) | SBP, DBP, PRA |
BP, blood pressure; D, double-blind; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; MBP, mean blood pressure; NR, not reported; PR, plasma renin; PRA, plasma renin activity; RCT, randomized controlled trial; ref, reference; S, single-blind; SBP, systolic blood pressure; Su, supplement.
When the range is not provided, only the mean is shown.
The total sample size is shown plus “M + F” to indicate that both men and women were included when the distribution by sex was not provided [note: Bompiani et al. (40) reported only the number of enrollees and not the number of completers].
The number of control plus intervention regimens is provided and multiplied by the number of days per regimen.
The effect of the dietary regimen on the mean Na:K ratio as reported by study authors or calculated on the basis of the urinary excretion reported at the end of the dietary regimen; if none of these data were reported, then the Na:K ratio was classified as NR and calculated on the basis of the reported doses.
Participants in the DASH diet study included prehypertensive (blood pressure >120/80 mm Hg, as defined by the investigators at the time of the study) and hypertensive subjects. This is the only study in Table 1 that included subjects with prehypertension; 41% of subjects in each diet group were hypertensive (defined as an average SBP of 140–159 mm Hg or an average DBP of 90–95 mm Hg during the 3 screening visits).
Participants were randomly assigned to follow the DASH diet (a diet rich in fruits, vegetables, and low-fat dairy products, with relatively high potassium amounts) or a control diet (a typical American diet) according to a parallel-group design; participants consumed their assigned diet at each of the 3 sodium amounts (high, intermediate, or low) for 30 consecutive days in random order in a crossover design.
Based on the reported urinary excretions for the intervention group combined with each pharmacologic treatment separately.
TABLE 2.
Characteristics of the RCTs examining the hypotensive effect of the sodium-to-potassium (Na:K) ratio in normotensive study populations1
| Intervention and comparator group details |
|||||||||||||
| First author, year, country (ref) | Age2 | Sex: M/F | Duration3 | Intervention type | Single or double blind | Na/K urine assessment | Dietary intake measurement during intervention/frequency | Nutrient database | Intervention | Dose (Na:K ratio)4 | Comparator | Dose (Na:K ratio)4 | Outcome |
| y | n | d | |||||||||||
| Zoccali, 1985, Scotland (53) | 20–29 | 10/0 | 15 (5 × 3) | Food | Neither S nor D | 24-h urine collection | NR | NR | Fixed Na/K | Mean of 145 mmol Na/d, 76 mmol K/d (Na:K, 1.90) | Usual diet | NR (NR) | SBP, DBP, PRC, angiotensin II |
| Food+Su | Fixed Na/K diet + K (high-K diet) | Mean of 145 mmol Na/d, 76 mmol K/d + 100 mmol K/d as slow-K tablets + 4 g/d of salt substitute (92% KCl, 6% K gluconate, 1% calcium silicate, 1% glutamic acid)(Na:K, 0.77) | |||||||||||
| Parfrey, 1981, U.K. (50) | 26–39 (mean: 31) | 5/3 | 168 (84 × 2) | Food+Su | S | 24-h urine collection | NR | NR | Low-Na/high-K | 100 mmol K/d as slow-K tablets or salt substitute (92% KCl, 6% K glutamate) (Na:K, 1.0) | High-Na | Usual diet + 100 mmol Na/d as slow-Na tablets (Na:K, 4.0) | SBP, DBP, PRA |
| Skrabal, 1981, Austria (52) | 21–25 | 20/0 | 56 (14 × 4) | Food+Su | Neither S nor D | 24-h urine collection | NR | NR | High-K | 200 mmol Na/d, 200 mmol K/d (Na:K, 1.34) | Usual diet (high-Na/low-K) | 200 mmol Na/d, 80 mmol K/d (Na:K, 2.95) | SBP, DBP, PR |
| Food | Low-Na diet | 50 mmol Na/d, 80 mmol K/d (Na:K, 0.62) | |||||||||||
| Food+Su | Low-Na/high-K diet | 50 mmol Na/d, 200 mmol K/d (Na:K, 0.16) | |||||||||||
| Burstyn, 1980, U.K. (51) | |||||||||||||
| Group 1 | 20–35 | 4/3 | 24 (8 × 3)5 | Su | Neither S nor D | 24-h urine collection | NR | NR | Na diet | 25 slow-Na tablets/d (600 mg or 10 mmol NaCl); 2 men took 30 tablets/d (Na:K, 5.69) | Control | NR (period 1: Na:K, 2.16; period 2: Na:K, 2.40) | SBP, DBP |
| Group 2 | 20–47 | 10/11 | 54 (variable)6 | Su | Neither S nor D | 24-h urine collection | NR | NR | 2-stage: Na/K + slow-K diet | Commercial NaCl and KCl equal parts for 8 d followed by 14 d where women took 8 slow-K tablets/d (600 mg or 8 mmol KCl) and men took 10 tablets/d (stage I Na:K, 2.5; stage II Na:K, 1.48) | Control | NR (period 1: Na:K, 3.21; period 2: Na:K, 2.72) | SBP, DBP |
D, double-blind; DBP, diastolic blood pressure; NR, not reported; PR, plasma renin; PRA, plasma renin activity; PRC, plasma renin concentration; RCT, randomized controlled trial; ref, reference; S, single-blind; SBP, systolic blood pressure; Su, supplement.
When the range is not provided, only the mean is shown.
The number of control plus intervention regimens is provided and multiplied by the number of days per regimen.
The Na:K ratio as reported by study authors or calculated on the basis of the reported urinary excretion; if none of these data were reported, then the Na:K ratio was classified as NR and calculated on the basis of the reported doses.
Subjects underwent a control period for 8 d, followed by an 8-d period in which participants increased their sodium intakes, and then again followed by a control period.
Subjects in this trial first substituted their table salt with a compound that was equal parts commercial table salt and KCl for a period of 8 d, followed by a 22-d period in which potassium intakes were further increased by 8 mmol KCl; these 2 intervention periods were preceded and followed by 14-d and 10-d control periods, respectively.
There were 7 parallel-arm RCTs (38, 39, 41, 42, 44, 45, 47) and 6 crossover RCTs (40, 43, 46, 48–50) conducted in hypertensive populations only (Table 1). These RCTs enrolled subjects from Australia (41), Austria (49), India (38), Italy (40, 42, 44, 45), Japan (47), New Zealand (48), The Netherlands (43), the United Kingdom (46, 50), and the United States (39). All RCTs included adults who were at least 18 y of age. Sample sizes ranged from 9 (49) to 522 (39), with retention rates >89% (reported in 10 RCTs) (38, 39, 42, 43, 45–50). Six RCTs were conducted in a double-blind fashion (39, 40, 42, 43, 45, 46), 2 RCTs were single-blind (physician or study staff) (38, 50), 4 RCTs were not blind due to the nature of the intervention (41, 44, 48, 49), and 1 RCT did not report on blinding (47). Study durations ranged from 6 to 180 d in the parallel-arm RCTs (39, 47) and from 14 to 84 d per intervention/control period in the crossover RCTs. All but 1 RCT (38) in hypertensive subjects examined the excretion of sodium and potassium with the use of 24-h urine specimens. Methods for measuring dietary intake varied among the few RCTs that reported this information (38, 39, 41), and only 2 RCTs in hypertensive subjects reported the use of food composition tables (38, 41); information on the nutrient database was not reported in the remaining RCTs (Table 1). Six RCTs in hypertensive populations examined blood pressure outcomes only (38, 39, 41, 42, 44, 49), whereas 7 examined both blood pressure outcomes and renin-angiotensin variables (40, 43, 45–48, 50). None of the RCTs in hypertensive populations examined arterial stiffness, the augmentation index, or endothelial dysfunction.
One single-blind RCT was conducted in 412 prehypertensive and hypertensive adults combined (Table 1) (8). Fewer than half (41%) of all subjects assigned to each of the DASH and control diets—the former being a diet rich in fruits, vegetables, and low-fat dairy products, and the latter being a typical American diet—were hypertensive (defined by average blood pressure of 140/90–159/95 mm Hg during 3 screening visits). Participants were randomly assigned to follow the DASH or control diet based on a parallel-group design, and then consumed their assigned diet at each of 3 sodium amounts (high, intermediate, or low) for 30 successive days in random sequence based on a crossover design. Retention rates in both diet groups exceeded 93%. Urinary excretions and dietary intakes of sodium and potassium were measured by using 24-h urine collections and daily food records, respectively, and chemical analyses were performed to examine the nutrient composition of both diets. Systolic and diastolic blood pressure outcomes were evaluated (Table 1).
All 4 RCTs conducted in normotensive populations only were designed as crossover studies (Table 2) (50–53). These RCTs enrolled normotensive subjects from Austria (52), Scotland (53), and the United Kingdom (50, 51). Ages ranged from 20 to 47 y, and sample sizes ranged from 8 to 28 subjects. RCTs had a retention rate of 100%. Only 1 RCT was performed as a single-blind study (study staff) (50); the remaining 3 RCTs were not blind due to the nature of the intervention (51–53). Intervention and control periods across RCTs ranged from 5 to 84 d (50, 53). All 4 RCTs evaluated urinary excretions of sodium and potassium by using 24-h urine collections. Dietary intake measurements and nutrient databases were not reported. One RCT examined blood pressure outcomes only (51), whereas the remaining RCTs reported on renin-angiotensin variables in addition to blood pressure outcomes (50, 52, 53). None of the RCTs in normotensive populations examined arterial stiffness, the augmentation index, or endothelial dysfunction.
Findings on the sodium-to-potassium ratio, blood pressure, and related outcomes in RCTs
Hypertensive populations/DASH diet RCT.
Six of the 13 RCTs conducted in hypertensive populations only and published over the span of 12 y (1981–1993) reported that the sodium-to-potassium ratio was more strongly associated with blood pressure outcomes in adults than either sodium or potassium alone (Table 3) (41–43, 45, 47, 50). The findings reported in the 2001 DASH diet study provide additional support that the sodium-to-potassium ratio is more strongly associated with blood pressure outcomes than either nutrient alone among prehypertensive and hypertensive participants combined (8). Three RCTs in hypertensive populations did not detect such a difference (44, 46, 49), whereas 4 others found mixed results comparing a low-sodium/high-potassium diet (38–40) or regimens in which either sodium or potassium intakes were modified with a usual diet (48). The 7 RCTs that favored the sodium-to-potassium ratio over sodium or potassium alone are discussed first in this section.
TABLE 3.
Results from the RCTs examining the hypotensive effect of the sodium-to-potassium (Na:K) ratio1
| First author, year, country (ref) | Population | Na+K vs. Na and/or K only examined? | Findings | Is the Na:K ratio more strongly associated with a hypotensive effect than Na and/or K alone? |
| Sacks, 2001, U.S. (DASH) (8) | Hypertensive + prehypertensive | Yes | “The DASH diet, as compared with the control diet, resulted in a significantly lower systolic blood pressure at every sodium level and in a significantly lower diastolic blood pressure at the high and intermediate sodium levels.… It had a larger effect on both systolic and diastolic blood pressure at high sodium levels than it did at low ones (P < 0.001 for the interaction).” | Yes |
| “As compared with the control diet with a high sodium level, the DASH diet with a low sodium level led to a mean systolic blood pressure that was 7.1 mm Hg lower in participants without hypertension [prehypertension], and 11.5 mm Hg lower in participants with hypertension.” | ||||
| “…the combined effects on blood pressure of a low sodium intake and the DASH diet were greater than the effects of either intervention alone and were substantial…. We found that the reduction of dietary sodium significantly lowered the blood pressure of persons without hypertension who were eating a diet that is typical in the United States. These results should settle the controversy over whether the reduction of sodium has a worthwhile effect on blood pressure in persons without hypertension [prehypertensive status].” | ||||
| Singh, 1993, India (38) | Hypertensive | No | “After 4 weeks of follow-up on an increased consumption of dietary potassium and low sodium/potassium ratio, group A [intervention] patients were associated with 7.5/8.5 mm Hg net decrease in mean systolic and diastolic pressures compared with group B [comparator]…. It is possible that an increased consumption of guava fruit can cause a substantial reduction in BPs and blood lipids with a lack of decrease in HDL-cholesterol due to its higher potassium and soluble fiber content, respectively.” | Not applicable |
| Langford, 1991, U.S. (39) | Hypertensive | No | “Assignment to the sodium restriction/increased potassium diet group did not substantially affect blood pressure.” | Not applicable |
| Bompiani, 1988, Italy (40) | Hypertensive | No | “During the modest sodium (100 mmol/d)/high potassium (130 mmol/d) diet the blood pressure was significantly reduced (−17/−6 mm Hg) when compared to the normal diet (160 mmol Na/day and 80 mmol K/ day).” | Not applicable |
| Nowson, 1988, Australia (41) | Hypertensive | Yes | “In each diet group the most significant dietary alteration was the reduction in the dietary sodium/potassium ratio [lowest in the low Na/high K group], and this was confirmed by the reduction in the urinary sodium/potassium ratio. The significance of this reduction is supported by the finding that the best predictor for reduction in diastolic response was the change in urinary sodium/potassium ratio.” | Yes |
| Suppa, 1988, Italy (42) | Hypertensive | Yes | “Our study... shows that in patients treated with a β-blocker, a slight potassium load (∼20 mmol daily) attainable by using a dietary salt at table, may produce a slight antihypertensive response, although this is largely limited to systolic BP.” | Yes |
| Grobbee, 1987, The Netherlands (43) | Hypertensive | Yes | “In conclusion, our observations suggest that moderate restriction of dietary sodium intake has little effect on blood pressure in young subjects with mildly elevated blood pressure levels, but the combination of moderate sodium restriction with high potassium intake may have an antihypertensive effect.” | Yes |
| “Reducing the dietary sodium:potassium ratio may therefore be useful in the management of early primary hypertension.” | ||||
| “We found no significant change in plasma renin and catecholamines, nor was the change in blood pressure related to a change in these hormones.” | ||||
| Valori, 1987, Italy (44) | Hypertensive | Yes | "The slight blood pressure decline over time when moderate Na restriction, or moderate Na restriction + low Na-high K salt, was added to the previous therapy should be viewed as an uncontrolled finding, not proving the antihypertensive efficacy of the dietary regimens tested.... no significant differences were found between moderate sodium restriction and moderate sodium restriction combined with the low Na-high K salt in their effects on blood pressure, either in patients on chlorthalidone or in patients on slow-release metoprolol.” | No |
| Arzilli, 1986, Italy (45) | Hypertensive | Yes | “In conclusion, our data show that a reduction in sodium intake from about 100 to 50 mmol/day, linked to a small (20 mmol/day) potassium supplementation, further and significantly decreases blood pressure in essential hypertensives, whose blood pressure is already reduced but not normalized by a relatively low sodium diet and/or hospitalization.” | Yes |
| Smith, 1985, U.K. (46) | Hypertensive | Yes | “Our findings showed that in patients with mild to moderate essential hypertension who were already moderately restricting their sodium intake to around 70 mmol/day doubling potassium intake as a chloride salt (64 mmol potassium chloride a day) did not cause any further significant fall in blood pressure.” | No |
| “The lack of effect of potassium chloride on blood pressure when sodium intake is restricted could be due to potassium chloride having either less of a natriuretic effect or less effect on renin secretion.” | ||||
| Fujita, 1984, Japan (47) | Hypertensive | Yes | “The results suggest that KCl may prevent a rise in blood pressure with NaCl loads in hypertensive patients by attenuating the increase in cardiac output, mainly as a result of the natriuresis.” | Yes |
| ”...KCl-induced natriuresis with the resultant decrease in body Na content may change the pressor responses to angiotensin II and catecholamines, which, in turn, promote the reduction of blood pressure.” | ||||
| Richards, 1984, New Zealand (48) | Hypertensive | No | “The results show that moderate restriction of sodium intake or supplementation of dietary potassium has variable effects on arterial pressure in individuals with mild essential hypertension, and that overall the blood-pressure changes induced are very small. Responsiveness of the renin-angiotensin system may limit the fall in blood-pressure induced by sodium restriction.” | Not applicable |
| Skrabal, 1984, Austria (49) | Hypertensive | Yes | “It is concluded that sodium restriction to 80 mmol/day is effective in lowering systolic blood pressure but that a combined low-sodium/high-potassium diet does not further improve blood pressure control if the usual potassium intake is at least 80 mmol/day.” | No |
| Parfrey, 1981, U.K. (both hypertensive and normotensive populations included) (50) | Hypertensive | Yes | “The BP of mildly hypertensive patients responds to moderate alterations in the dietary intake of sodium and potassium continued for up to 12 wk and, in contrast to normotensive subjects, a high K/low Na intake significantly lowers BP. The mechanism of this depressor response remains unknown but there is no clear evidence that it is mainly dependent on a difference in the responses of the sympathetic or of the renin-angiotensin-aldosterone systems to the changes in electrolyte intake.” | Yes |
| “Although PRA was lower in both groups during the high Na, and higher during the high K/low Na diet, none of the changes was significant within or between groups.” | ||||
| Zoccali, 1985, Scotland (53) | Normotensive | No | “Although mean systolic pressure after potassium treatment was 4 mm Hg lower in the lying position and 6–8 mm Hg lower in the standing position than after the 2 control measurements (Days 5 and 15 [normal Na/K and unrestricted diets]), these differences were not significant. There were no significant differences in diastolic pressure between the 3 phases of the study.” | Not applicable |
| “Plasma concentrations of renin and angiotensin II showed no significant changes after potassium treatment but there was the expected increase in plasma concentrations of renin and angiotensin II on standing (P < 0.001).” | ||||
| Skrabal, 1981, Austria (52) | Normotensive | Yes | “The results of this study suggest that moderate salt restriction combined with a high potassium intake helps to prevent hypertension.…" | Yes |
| “Of the many feed-back loops regulating blood pressure, at least 4 are influenced beneficially by moderate salt restriction and high potassium intake: (1) the renin-angiotensin system and (2) the aldosterone system which are brought back into their regulatory range where they can help to control sodium balance; (3) the sympathetic nervous system... and (4) the baroreceptor reflex which becomes more sensitive.” | ||||
| Burstyn, 1980, U.K. (51) | Normotensive | Yes | “The relatively small reductions in sodium intake and increases in potassium intake that might be achieved through propaganda and changes in food processing are unlikely to lower mean blood pressure in Western societies. Such maneuvers may be useful to people who are genetically susceptible to salt-induced hypertension or whose salt intake is unusually high.” | No |
| “A weak negative correlation was found between the sodium:potassium ratio and systolic pressure [among all patients combined].” |
BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; PRA, plasma renin activity; RCT, randomized controlled trial; ref, reference.
One study examined the effect of potassium chloride (KCl) supplementation on mean blood pressure and plasma renin activity (PRA) in 23 sodium-loaded subjects from Japan with idiopathic hypertension (mean age: 49 y), and concluded that the natriuresis brought on by KCl supplementation may have affected, among other factors, the renin-angiotensin system, thereby resulting in the observed reduction in mean blood pressure (47). More specifically, subjects were maintained on their “normal sodium” diet [165 mmol sodium chloride (NaCl)/d] during the first 3 d of the RCT. Subjects were administered 40 mg furosemide i.v. on day 4 and then placed on a “low sodium” diet (25 mmol NaCl/d) for 3 d. Then, to investigate the influence of short-term KCl supplementation on the increase in blood pressure induced by high NaCl, subjects were divided into 2 groups and administered 250 mmol NaCl/d (“high sodium”) or 96 mmol KCl/d in addition to the high-sodium diet on days 7–12 (mean Na:K ratio = 2.60). The results showed that PRA was significantly greater in the KCl-supplemented subjects given a high-sodium diet than in the non–KCl-supplemented subjects consuming the high-sodium diet (1.30 vs. 0.88 μg · L−1 · h−1, respectively), and a significantly reduced mean blood pressure was reported in the KCl-supplemented subjects (−3.5 ± 1.6 mm Hg), whereas the opposite was observed in the non–KCl-supplemented subjects (+10.0 ± 2.0 mm Hg) (47).
A second and larger RCT in The Netherlands reported that moderate sodium restriction coupled with a high potassium intake (mean sodium-to-potassium ratio = 0.53) resulted in a greater blood pressure reduction than moderate restriction of sodium alone (mean Na:K ratio = 1.68) in 40 hypertensive subjects (mean age: 24 y) over a 6-wk period (Table 3) (43). No significant results were reported for plasma renin, leading the authors to conclude that the observed reduction in blood pressure was not mediated by this hormone. These results are supported by 2 earlier and smaller studies from Western Europe (Table 3) (45, 50).
The remaining 2 RCTs in hypertensive populations that provide support for the sodium-to-potassium ratio as a superior metric in the evaluation of blood pressure outcomes were large in study size and conducted more recently than the other RCTs (Table 3) (41, 42). Suppa et al. (42) conducted a double-blind, 4-wk RCT in 358 hypertensive subjects [322 subjects (90%) completed the study] and reported that those randomly assigned to receive dietary salt (34 mmol Na/d, 19.3 mmol K/d as 4 g/d NaCl 50%, KCl 25%, potassium citrate 15%; mean Na:K ratio = 2.50), which was added to subjects’ food at lunch and dinner, exhibited greater systolic and diastolic blood pressure reductions (which were significant and not significant, respectively) than subjects administered common salt (4 g/d 100% NaCl; mean Na:K ratio = 2.94) at lunch and dinner. All subjects in this study were also treated with a β-blocker for 4 wk before and concomitantly with dietary intervention. Nowson and Morgan (41) randomly assigned 108 subjects to 1 of 4 interventions: the control group (usual diet; mean Na:K ratio = 2.38), a high-potassium diet (120 mmol K/d; mean Na:K ratio = 1.67), a low-sodium diet (50–70 mmol Na/d with minimal alteration in potassium intake; mean Na:K ratio = 1.29), and a low-sodium/high-potassium diet (50–70 mmol Na/d and at least 120 mmol K/d; mean Na:K ratio = 0.95). Subjects were followed for 12 wk. The authors reported that the “best predictor” for the reduction in diastolic blood pressure was the sodium-to-potassium ratio, which was lowest in the low-sodium/high-potassium diet group.
One additional RCT included in this review offers support that the joint effects on blood pressure from a low sodium intake in conjunction with the DASH diet, which is high in potassium and other nutrients, are larger than the effects of either intervention alone (Table 3) (8). Blood pressure outcomes in DASH were examined in 412 prehypertensive and hypertensive adults randomly assigned to 1 of 6 sodium-diet groups as follows: a high-sodium plus DASH diet (144 mmol Na/d and 75 mmol K/d; mean Na:K ratio = 1.92), an intermediate-sodium plus DASH diet (107 mmol Na/d and 81 mmol K/d; mean Na:K ratio = 1.32), a low-sodium plus DASH diet (67 mmol Na/d and 81 mmol K/d; mean Na:K ratio = 0.83), a high-sodium plus control diet (141 mmol Na/d and 40 mmol K/d; mean Na:K ratio = 3.53), an intermediate-sodium plus control diet (106 mmol Na/d and 41 mmol K/d; mean Na:K ratio = 2.59), and a low-sodium plus control diet (64 mmol Na/d and 42 mmol K/d; mean Na:K ratio = 1.52). Sacks et al. (8) reported that in comparison with the high-sodium plus control diet, the largest difference in mean systolic blood pressure—7.1 mm Hg lower in prehypertensive participants and 11.5 mm Hg lower in hypertensive subjects—was observed with the low-sodium plus DASH diet. In the overall study population combined (i.e., prehypertensive/hypertensive subjects), the DASH diet resulted in a significantly reduced systolic blood pressure at each sodium amount compared with the control diet, and it also resulted in a significantly lower diastolic blood pressure at the high and intermediate sodium amounts.
Three RCTs performed in hypertensive populations did not find that the sodium-to-potassium ratio was more strongly associated with blood pressure outcomes in adults than sodium alone (Table 3) (44, 46, 49). Two of these studies in particular suggested that a threshold for potassium intake may be necessary given a modest restriction in sodium intake (70 or 80 mmol Na/d corresponding to 64 mmol KCl/d or 120 mmol K/d, respectively, in both RCTs) (46, 49). These studies, however, were limited in sample size [Smith et al. (46) and Skrabal et al. (49) reported on 20 and 21 hypertensive subjects, respectively], giving them low statistical power to detect any differences in outcomes, if present, between intervention groups. The potassium intake in 1 study specifically may have been too low to identify any meaningful effect as well (46). The third study reported no significant between-group reductions in blood pressure after a 6-wk period in 119 hypertensive men and women randomly assigned to receive either a low-sodium diet (intake per day was not reported) or moderate sodium restriction combined with a low-sodium/high-potassium diet (17.1 mmol Na/d, 9.64 mmol K/d added each day to food; mean Na:K ratio >2.35) (44). Twenty-four-hour urinary potassium excretion increased in the low-sodium/high-potassium group but not in the low-sodium group. Despite the blood pressure reductions observed in both groups, no significant between-group effects were reported. The dietary interventions were administered after subjects had been receiving pharmacologic treatment for 4 wk, at which point a moderate reduction in mean blood pressure was observed (from 165.0–168.9/105.6–105.9 to 145.2–149.0/91.8–94.6 mm Hg); as a result, the antihypertensive effects observed could not be attributed to the dietary interventions under study (Table 3). Likewise, it is unclear from this study whether the lack of a difference in outcomes between dietary intervention groups is related in part to the prior pharmacologic treatment of subjects. One other, larger RCT reviewed was designed to evaluate dietary interventions as ancillary treatment and reported significant blood pressure reductions (primarily systolic) between dietary interventions after a 4-wk period (42).
Four RCTs in hypertensive subjects that reported on the effects of the sodium-to-potassium ratio compared a low-sodium/high-potassium diet (38–40), or regimens where either sodium or potassium intakes were modified (48), with a control group who consumed a usual diet (Table 3). The single multicenter RCT conducted in the United States (39), which was based on the Trial of Antihypertensive Interventions and Management and is the largest study included in this review, randomly assigned hypertensive men and women to a low-sodium/high-potassium diet [n = 258; 52–100 mmol Na/d (mean: 87 mmol/d); 62–115 mmol K/d (mean: 103 mmol/d); mean Na:K ratio = 0.84], a weight-loss group (n = 265; not relevant for the present review), or to a control group who consumed a usual diet (n = 264; mean Na:K ratio was not reported and unable to be calculated). All subjects received the same combination of pharmacologic medication at randomization; therefore, the only difference between groups was the dietary intervention. No significant differences in diastolic blood pressure levels were reported between the low-sodium/high-potassium and usual-diet groups at the 6-mo follow-up, with the authors reporting that dietary intervention underperformed compared with pharmacologic treatment. The authors reported, however, that subjects assigned to the low-sodium/high-potassium diet attained only a moderate reduction in sodium intake/excretion and exhibited a very small alteration in potassium intake/excretion during the study (39). All analyses were conducted on the basis of an intention-to-treat approach. A small crossover RCT in 12 subjects from New Zealand (48) that examined blood pressure outcomes and renin-angiotensin variables over a 4- to 6-wk period reported very small changes in the blood pressure outcomes evaluated. In this study, the 2 dietary interventions (Na-restricted: 80 mmol Na/d, 60 mmol K/d, mean Na:K ratio = 1.33; high-K-supplemented: 180 mmol Na/d, 200 mmol K/d, mean Na:K ratio = 0.90) were compared with a usual diet (180 mmol Na/d, 60 mmol K/d; mean Na:K ratio = 3.00). An increase in PRA was observed in some patients, but not all. The Trial of Antihypertensive Interventions and Management results contrast with those reported from a crossover RCT in Italy in 60 hypertensive men and women who underwent an experimental 14-d period consisting of a low-sodium/high-potassium diet (100 mmol Na/d, 130 mmol K/d; mean Na:K ratio = 0.86) as well as a 14-d control period that consisted of the usual diet (160 mmol Na/d, 80 mmol K/d; mean Na:K ratio = 2.29) (40). In this study, the low-sodium/high-potassium diet resulted in a significant reduction in both systolic and diastolic blood pressures (−17 and −6 mm Hg, respectively) compared with the usual diet. Finally, a single-blind, 4-wk study conducted in India by Singh et al. (38) randomly assigned 145 hypertensive subjects to receive either a potassium-rich diet containing guava fruit in a “food to eat” approach with sodium restriction (0.5–1.0 kg/d of guava fruit, 5–10 g/d salt intake; mean Na:K ratio = 1.5) or to a control group consisting of a usual diet with no guava fruit and sodium restriction (usual diet, 5–10 g/d salt intake; mean Na:K ratio = 4.7). The authors reported that the increased potassium intakes and low sodium-to-potassium ratio in the intervention group resulted in significant reductions in both systolic and diastolic blood pressures (−7.5 and −8.5 mm Hg, respectively) than the control group.
Normotensive populations.
Among 4 crossover RCTs in normotensive populations that were published over 5 y (1980–1985), only 1 reported that the sodium-to-potassium ratio was more strongly associated with blood pressure outcomes than either sodium or potassium alone (n = 20 subjects; age range: 21–25 y; Table 3) (52). In this RCT, 3 14-d experimental periods were compared with a 14-d control period that consisted of a usual diet (200 mmol Na/d, 80 mmol K/d; mean Na:K ratio = 2.95). The high-potassium diet consisted of 200 mmol Na/d and 200 mmol K/d (mean Na:K ratio = 1.34), the low-sodium diet consisted of 50 mmol Na/d and 80 mmol K/d (mean Na:K ratio = 0.62), and the low-sodium/high-potassium diet consisted of 50 mmol Na/d and 200 mmol K/d (mean Na:K ratio = 0.16). The authors reported that the greatest beneficial effect on blood pressure outcomes occurred in the low-sodium/high-potassium diet intervention compared with the control, and that 4 regulatory systems for blood pressure including the renin-angiotensin system, the aldosterone system, the sympathetic nervous system, and the baroreceptor reflex were positively affected by the combined effect of a low-sodium and high-potassium diet (52). Two additional studies conducted in normotensive populations reported no effect of low sodium combined with high potassium intakes (50, 51). The fourth crossover RCT conducted in 10 men (age range: 20–29 y) in Scotland reported no significant differences between a fixed 5-d sodium/potassium diet (mean: 145 mmol Na/d and 76 mmol K/d) and a 5-d control period consisting of a usual diet, although this small study had limited statistical power to detect any significant difference (53).
Summary of findings from RCTs
Hypertensive populations/DASH diet RCT.
In summary, 7 RCTs from Australia, Italy, Japan, The Netherlands, the United Kingdom, and the United States that were conducted in hypertensive or prehypertensive/hypertensive populations supported the sodium-to-potassium ratio as being more strongly associated with blood pressure outcomes than either sodium or potassium alone (8, 41–43, 45, 47, 50). Four of these studies were large RCTs that followed subjects for at least 4 wk (8, 41–43). Methodologic heterogeneity across these RCTs is evident on the basis of the study durations and the different sodium and potassium intakes examined. A paucity of information on dietary intake measurements and the nutrient databases used is apparent; however, each of these RCTs used the gold-standard method for assessing sodium and potassium intakes via the collection of 24-h urine specimens. In addition, some RCTs described plausible techniques for maintaining subject blinding [e.g., use of salt packets composed of different electrolyte contents (40)], although variation across RCTs by the level of blinding was evident.
The RCTs that did not provide support for the sodium-to-potassium ratio as being more strongly associated with blood pressure outcomes than either sodium or potassium alone were small in study size (46, 49) or evaluated the dietary interventions as ancillary treatment and reported no significant difference between dietary interventions (44). Additionally, no conclusion regarding the sodium-to-potassium ratio as a better determinant of blood pressure outcomes than either sodium or potassium alone can be drawn from studies comparing a low-sodium/high-potassium diet with a usual diet (38–40).
Mixed results based on a small number of studies were identified regarding dietary intervention effects on the renin-angiotensin system. As a result, no firm conclusion regarding the effects of the sodium-to-potassium ratio on this blood pressure regulatory system can be made. Other risk factors for CVD, including arterial stiffness, the augmentation index, and endothelial dysfunction, were not examined in the reviewed studies.
Normotensive populations.
Only 1 crossover RCT conducted in a normotensive population supports the sodium-to-potassium ratio as being more strongly associated with blood pressure outcomes, specifically, than either sodium or potassium alone; this RCT, however, was based on only 20 subjects who were followed for 2-wk intervention or control periods (52). Overall, future study in larger populations of normotensive adults, with longer study durations, may clarify the role of sodium and potassium in determining blood pressure outcomes. However, given that reducing blood pressure in normotensive adults is not a public health priority, the utility of such dietary interventions in this group is questionable.
Study characteristics of observational cohort and cross-sectional studies (2000 to present)
Only 1 observational cohort study was identified (Table 4) (37). This ongoing, prospective, open-cohort study used data from 16,869 participants aged 20–60 y in the China Health and Nutrition Survey. Baseline data were collected in 1989, and participants were followed up in 1991, 1993, 1997, 2000, 2004, 2006, and 2009. Only 10.4% of subjects had hypertension at baseline. A proportion of previous participants were sampled at each follow-up beginning in 1993; this proportion ranged from 61% to 88% (37). Twenty-four-hour urine specimens were collected for validation of dietary intake data measured via 3 successive 24-h dietary recalls plus food weighing at each survey. The Chinese Food Composition Table was used as the nutrient database. Incident hypertension was evaluated in association with sodium intake, potassium intake, and the sodium-to-potassium ratio over the follow-up period.
TABLE 4.
Characteristics of the observational cohort and cross-sectional studies (2000–2014) examining the hypotensive effect of the sodium-to-potassium (Na:K) ratio1
| First author, year, country (ref) | Study design | Population | Sex: M/F | Age2 | Hypertension | Na/K assessment | Dietary intake measurement | Nutrient database | Exposure (Na:K ratio) | Outcomes | Adjustment factors |
| n | y | ||||||||||
| Du, 2014, China (37) | Ongoing open-cohort | China Health and Nutrition Survey (1991–2009) participants from all regions (north, south, central) | 8097/8772 | 20–60; mean: 37 at baseline | 10.4% at baseline | 24-h urine collection (used for validation purposes, authors report a lack of data for Na excretion) | Food and condiment weighing + 24-h dietary recall/3 consecutively in each survey | Chinese Food Composition Table3 | Dietary Na:K (mean in 1991–2009: 4.9–2.8) | Incident hypertension | Energy intake, age, sex, education, income, region, BMI, physical activity, smoking status, alcohol intake |
| Grubler, 2014, Austria (33) | Cross-sectional | Hypertensive patients derived from a tertiary care center (data from the Styrian Hypertension Study) | 102/109 | Mean: 60 | 100% | 24-h urine collection | Authors report use of urinary metrics rather than dietary metrics (“dietary salt intake reflected by 24-h urinary Na+/K+”) | NR | Urinary Na:K (mean: NR) | Nighttime SBP, nighttime DBP | SBP: sex, glycated hemoglobin, aldosterone:active renin ratio |
| DBP: age, sex, aldosterone:active renin ratio | |||||||||||
| Kim, 2014, Korea (34) | Cross-sectional | Korean adults who were recruited in the baseline surveys of the MRCohort | 2443/3840 | 40–89; mean: 62/60 (M/F) | 0 | FFQ used to estimate intakes | 106-item FFQ | Food Composition Table of Korea, 7th edition | Dietary Na:K (median in 1st–5th quintile: 0.8–1.94 in men; 0.69–1.91 in women) | SBP, DBP | Sex-stratified models: age, education, farmer status, physical activity, marital status, alcohol intake (men only), waist circumference (women only), fiber and calcium intakes |
| Mente, 2014, multiple (PURE study) (35) | Cross-sectional | Cross-sectional assessment of PURE study participants from 667 communities in 18 countries on 5 continents (42% from China) | 43,752/58,464 | 35–70; mean: 51 | Self-reported hypertension, 42%; not self-reported, 35% | Fasting morning urine collection and applying a formula-derived estimate of 24-h urinary excretion | Authors report use of urinary metrics rather than dietary metrics | NR | Urinary Na:K (mean: 2.33) | SBP, DBP | Age, sex, BMI, education, alcohol intake, geographic region |
| Millen, 2013, South Africa (15) | Cross-Sectional | Community sample of nuclear families with siblings aged >16 y | 137/194 | Mean: 40 | 26% | 24-h urine collection | Authors report use of urinary metrics rather than dietary metrics (“salt intake was indexed as urinary Na+/K+ in all analyses”) | 24-h urine excretion rates were calculated from the product of urine volume and urine electrolyte concentration | Urinary Na:K (mean: 4.18) | SBP, DBP | Age, sex, diabetes mellitus or glycosylated hemoglobin >6.5%, regular alcohol consumption, regular tobacco use |
| P values adjusted for nonindependence of family members | |||||||||||
| Zhang, 2013, U.S. (16) | Cross-sectional | NHANES, 2005–2010 | 5295/5268 | Mean: 41/50 (hypertensive/nonhypertensive) | 21% | Dietary recall used to estimate intakes (urine collections not available in NHANES 2005–2010 | 24-h dietary recall /supplements and antacids not included | USDA Survey Nutrient Database | Dietary Na, K, Na:K (mean: 1.41) | SBP, DBP, hypertension | Age, sex, race/ethnicity, BMI, education, use of table salt, smoking status, history of cardiovascular disease, self-reported chronic kidney disease, diabetes mellitus, alcohol use, physical activity; mutually adjusted for Na and K except in model for Na:K ratio |
| Hedayati, 2012, U.S. (17) | Cross-sectional | Probability sample of Dallas county residents | 1457/1846 | 30–65 (mean: 43) | 36% | First-void morning urine specimen | Authors report use of urinary metrics “rather than indirect measures such as dietary recall” | NR | Urinary Na:K (mean/median: 4.4/3.7 in AAs; 4.1/3.6 in non-AAs) | SBP, DBP, hypertension | Age, sex, race, diabetes mellitus, smoking, BMI, total cholesterol, estimated glomerular filtration rate, urine albumin:creatinine ratio |
| Michel, 2012, South Africa (18) | Cross-sectional | Community sample of nuclear families with siblings aged >16 y | 317/579 | Mean: 42/47 (Na:K ratio ≥median of 3.7 and <median of 3.7) | 46%/49% [Na:K ratio ≥3.7 (median) and <3.7] | 24-h urine collection | NR | 24-h urine excretion rates were calculated from the product of urine volume and urine electrolyte concentration | Urinary Na:K (median: 3.71) | SBP, DBP, PR, plasma angiotensin | Age, sex, BMI, regular tobacco use, regular alcohol use, diabetes mellitus/glycated hemoglobin >6.1%, antihypertension treatment |
| P values further adjusted for nonindependence of family members | |||||||||||
| Tzoulaki, 2012, multiple (INTERMAP) (36)4 | Cross-sectional | INTERMAP study: 17 population samples from Japan (4 samples), China (3 samples), United Kingdom (2 samples), United States (8 samples) | 2359/2321 | 40–59 (mean: 49) | 24% overall (Western/East Asian subjects: 27–32%/14–15% | 24-h urine collection | 24-h dietary recall (dietary supplements, foods, drinks recorded) | U.S. dietary data computerized using the Nutrition Data System, Nutrition Coordinating Center, University of Minnesota; in all other countries, dietary data entered onto standard forms, coded, and computerized | Urinary Na:K (mean Japan: 6.31; mean China: 4.23; mean U.K.: 2.23; mean U.S.: 3.04) | SBP, DBP | Age, sex, reported special diet, use of dietary supplements, moderate or heavy physical activity, doctor-diagnosed cardiovascular disease and diabetes mellitus, family history of hypertension, height, weight, and total energy intake |
| Huggins, 2011, Australia (19) | Cross-sectional | Subgroup of cohort study participants recruited opportunistically, with oversampling of southern European immigrants | 376/407 | Mean: 64 | 43% | 24-h urine collection | Discretionary salt use determined by a 2-question survey | NR | Urinary Na, K, Na:K (mean: 1.99) | SBP, DBP, hypertension | Continuous models adjusted for age, sex, BMI, country of birth, antihypertension medication use |
| Quintile models adjusted for age, sex, BMI, country of birth | |||||||||||
| Yang, 2011, U.S. (20) | Cross-sectional | Participants in the third NHANES | 5899/6368 | ≥20 | 21%/19% (M/F) | Dietary recall used to estimate intakes (urine collections not available in NHANES III) | 24-h dietary recall | USDA Survey Nutrient Database | Dietary Na, K, Na:K (mean M: 1.31; F: 1.23) | SBP, DBP, hypertension | None |
| Redelinghuys, 2010, South Africa (21) | Cross-sectional | Community sample of nuclear families with siblings aged >16 y | 221/414 | Mean: 45 | 43.6% | 24-h urine collection | NR | 24-h urine excretion rates were calculated from the product of urine volume and urine electrolyte concentration | Urinary Na:K (mean: 4.27) | PP, SBP, DBP, central hemodynamics | Age, BMI, sex, diabetes mellitus or glycosylated hemoglobin >6.1%, regular tobacco intake, regular alcohol intake, treatment for hypertension; mean arterial pressure adjusted for only in analyses of PP and central hemodynamics |
| P values further adjusted for nonindependence of family members | |||||||||||
| Ruixing, 2008, China (22) | Cross-sectional | Stratified randomized cluster sample of residents of 7 villages in Napo County, Guangxi | 834/835 | 15–84 (mean: 46) | 34%/19% (M/F) | Dietary recall analyzed to determine each subject’s dietary sodium and potassium intake (g/d) | 24-h dietary recall | 2002 Chinese Food Composition Table | Dietary Na, K, Na:K (mean: 1.1 in nonhypertensive M; 1.5 in hypertensive M; 1.4 in nonhypertensive F; 1.8 in hypertensive F) | Hypertension | NR |
| Polónia, 2006, Portugal (23) | Cross-sectional | Sample of 4 different adult populations living in northern Portugal | 187/239 | 20–71 (mean: 50) | NR | Average of 2 24-h urine collections for 162 participants/single 24-h urine collection for 264 participants | Daily salt intake based on calculation of 24-h urinary Na and assuming that all Na ingested was as NaCl | NR | Urinary Na, Na:K (mean: 1.9 in hypertensives; mean: ≤1.5 in nonhypertensives) | SBP, DBP, pulse wave velocity | None for SBP and DBP; age and SBP adjusted for in analysis of pulse wave velocity |
| Kwok, 2003, China (24) | Cross-sectional | Long-term (≥10 y) vegetarians recruited from religious organizations or old-age hostels | 0/111 | Mean: 78 | 64% | Fasting 20-mL urine specimen | 24-h dietary recall | Region-specific (Hong Kong) food composition tables | Urinary Na:K, dietary K (mean: 4.7 in hypertensives; mean, 3.4 in nonhypertensives) | SBP, DBP, hypertension | None in dietary K-hypertension analysis |
| Adjusted for urinary Na:creatinine ratio in urinary Na/K and SBP, DBP analyses | |||||||||||
| Age-adjusted in urinary Na/K and SBP, DBP, and hypertension analyses | |||||||||||
| Schröder, 2002, Spain (25) | Cross-sectional | Stratified randomized sample of general population of Gerona according to 1991 census | 758/809 | 45/59/62 (normotensives, untreated hypertensives, treated hypertensives) | 37% | Dietary recall analyzed to determine each subject’s dietary sodium and potassium intake (mg/d) | 72-h dietary recall | Diet Analysis Nutritionist IV; the database of this software was supplemented with 130 food items from Spanish food composition tables | Dietary Na, K, Na:K (mean: 0.60 in nonhypertensives; 0.62 in untreated hypertensives; 0.59 in treated hypertensives) | SBP, DBP, hypertension | Dietary Na/K and SBP, DBP analyses: age, sex, BMI, smoking and drinking status |
| Dietary Na analyses and SBP, DBP: age, sex, BMI, smoking and drinking status, calcium, K, magnesium | |||||||||||
| Dietary K analyses and SBP, DBP: age, sex, BMI, smoking and drinking status, calcium, Na, magnesium | |||||||||||
| Hypertension analyses: age, sex, BMI, alcohol and smoking status | |||||||||||
| Yamori, 2002, China (26) | Cross-sectional | Randomized cluster sample of male residents in Daping District of Chongqing, China | 177/0 | 43–55 (mean: 51) | 20% | 24-h urine collection | Questionnaire (NR; data to be presented in subsequent reports by the authors) | NR | Urinary Na, K, Na:K (mean: 4.55) | SBP, DBP, hypertension | Urinary Na and K analyses: none |
| Urinary Na/K: age, occupation, smoking, alcohol consumption, education, BMI, magnesium, total:HDL-cholesterol ratio, total protein, TGs | |||||||||||
| Hajjar, 2001, U.S. (27) | Cross-sectional | Participants in third NHANES | 8004/9026 | ≥20 (mean: 49) | NR | Dietary recall used to estimate intakes (urine collections not available in NHANES III) | 24-h dietary recall | USDA Survey Nutrient Database | Dietary Na, K, Na:K (mean: 1.22) | PP, SBP, DBP | Adjusted: age, sex, ethnicity, BMI |
| Multivariate: stepwise selection of demographic variables, BMI, dietary factors, and dietary factor interactions; model based on a random subsample of 8529 participants, then tested on remaining participants | |||||||||||
| Hu, 2001, China (28) | Cross-sectional | Two random stratified cluster samples, 3 y apart, in 6 districts in Tianjin | 990/1078 | 35–64 (mean: 47/54) (normotensive/hypertensive) | 37% | Food records or recall analyzed to determine each child‘s dietary sodium and potassium intake (mg/d) | Food weighing + 3-d food records or 24-h recall | Chinese Food Composition Tables | Dietary Na, K, Na:K (mean: 2.99 in nonhypertensive M; 3.13 in hypertensive M; 3.08 in nonhypertensive F; 3.34 in hypertensive F) | Hypertension | Age, energy, BMI, time of survey |
| Xie, 2001, China (29) | Cross-sectional | Stratified random sample of residents of an isolated farming village in Hubai Province | 191/162 | 14–75 (mean: 40/37) (M/F) | NR | 24-h urine collection | 24-h recall | 1991 Chinese Food Composition Table | Urinary Na, K, Na:K (mean: 6.1) | PP, SBP, DBP | Age, sex, height, weight, heart rate, serum total cholesterol |
| Caputo, 2000, U.S. (30) | Cross-sectional | Urban public 6th-grade students | 30/39 | 11–13 (mean: 12) | 0% | FFQs analyzed to determine each child‘s dietary sodium and potassium intake (mg/d) | FFQ jointly completed by parents and children | Nutritionist IV: Diet Analysis and Nutritional Evaluation | Dietary Na:K (mean: 0.96) | SBP, DBP | BMI, % body fat, family history of hypertension, physical activity, waist-to-hip ratio |
| Mosley, 2000, Egypt (31) | Cross-sectional | Household clusters in 6 governorates | 370/465 | Mean: 51/47 (M/F) | NR | 12-h urine collection | NR | NR | Urinary Na:K (median: 2.9 in M; median: 2.5 in F) | SBP | Male-only analysis: age, melanin index, and BMI |
| Female-only analysis: age | |||||||||||
| Mufunda, 2000, Zimbabwe (32) | Cross-sectional | Household clusters in Dombotombo township; restricted to residents in area for ≥3 y | 384/391 | ≥25 | 28%/41% (M/F) | Spot urine collection | NR | NR | Urinary Na:K (mean: 3.3 in M; mean: 3.4 in F) | SBP, hypertension | Hypertension analysis: age |
| SBP analysis: age, BMI, Shona ethnicity, tobacco use, alcohol use, months in rural area per year |
AA, African American; DBP, diastolic blood pressure; INTERMAP, International Study of Macro/Micronutrients and Blood Pressure; MRCohort, Multi-Rural Communities cohort; NR, not reported; PP, pulse pressure; PR, plasma renin; PURE, Prospective Urban Rural Epidemiology; SBP, systolic blood pressure; ref, reference.
The mean only is shown when the range is not provided.
The sodium and potassium compositions of a few foods imported or unavailable in Chinese markets were adopted from the food composition tables of Hong Kong, Taiwan, Japan, or the USDA.
NHANES data (4 cross-sectional cohorts: 1999–2000, 2001–2002, 2003–2004, 2005–2006) were used for external validation of the INTERMAP data.
Twenty-two cross-sectional studies were identified (Table 4) (15–36). These studies were conducted in various countries including Australia (19), Austria (33), China (22, 24, 26, 28, 29), Egypt (31), Korea (34), Portugal (23), South Africa (15, 18, 21), Spain (25), the United States (16, 17, 20, 27, 30), and Zimbabwe (32). One cross-sectional study based on the PURE (Prospective Urban Rural Epidemiology) cohort enrolled participants from 667 communities in 18 income-diverse countries on 5 continents (35). Another cross-sectional study, the International Study of Macro/Micronutrients and Blood Pressure (INTERMAP), included 17 population samples across Japan, China, the United Kingdom, and the United States (36). Sample sizes ranged from 69 (in a study in children) (30) to 102,216 (in the INTERMAP study). The only study conducted in children was located in the United States (30). The prevalence of hypertension in adult study populations ranged from ∼20% (16, 20, 26) to 64% (24). One study included only hypertensive subjects (33). Reporting on the prevalence of important risk factors including hyperlipidemia, diabetes, smoking, and medication use was inconsistent, with several studies not reporting these estimates or selectively reporting some medically relevant risk factors but not others. Fourteen studies (15, 17–19, 21, 23, 24, 26, 29, 31–33, 35, 36) examined urinary exposure assessments of sodium and potassium excretion in association with the outcomes of interest, whereas the other 8 studies (16, 20, 22, 25, 27, 28, 30, 34) evaluated dietary intake in relation to the outcomes of interest. Two studies examined hypertension only (22, 28), 6 studies examined systolic and diastolic blood pressure only (15, 30, 33–36), 1 study evaluated systolic blood pressure only (31), 1 study reported only on systolic blood pressure and hypertension (32), and 7 studies reported on hypertension and systolic and diastolic blood pressure outcomes (16, 17, 19, 20, 24–26). The 5 remaining cross-sectional studies evaluated, in addition to systolic and diastolic blood pressure, outcomes related to the renin-angiotensin system (18), central hemodynamics (21), pulse wave velocity (23), and pulse pressure (21, 27, 29).
Findings on the sodium-to-potassium ratio, blood pressure, and related outcomes in the observational cohort and cross-sectional studies
Results on the sodium-to-potassium ratio as evaluated in the observational cohort and cross-sectional studies are shown in Table 5. In the ongoing open-cohort study with ∼20 y of follow-up, Du et al. (37) reported that the sodium-to-potassium ratio was more strongly associated with incident hypertension than either sodium or potassium alone, although the strength of the association with the sodium-to-potassium ratio varied significantly by geographic region (Table 5). Eight of the 22 cross-sectional studies identified, which derived from Australia, China, Portugal, Spain, and multiple countries in the PURE study, reported that the sodium-to-potassium ratio was more strongly associated with hypertension and/or systolic and diastolic blood pressure outcomes than either sodium or potassium alone (Table 5) (19, 22, 23, 25, 26, 28, 29, 35). Among these, Pólonia et al. (23) also evaluated arterial stiffness (via pulse wave velocity), but only in association with sodium intake. Additionally, Kim et al. (34) reported a stronger association between the sodium-to-potassium ratio and systolic and diastolic blood pressure outcomes in Korean men but not women (Table 5).
TABLE 5.
Results from the observational cohort and cross-sectional studies (2000–2014) examining the hypotensive effect of the sodium-to-potassium (Na:K) ratio1
| First author, year, country (ref) | Na+K vs. Na and/or K only examined? | Findings | Is the Na:K ratio more strongly associated with a hypotensive effect than Na and/or K alone? |
| Du, 2014, China (37)2 | Yes | “Compared with the lowest group, recent intakes of sodium had strong dose-response associations with incident hypertension for the third to fifth quintiles. The second to fifth quintiles of potassium intake had a significantly lower HR adjusted for baseline covariates. The results show no evidence of a regional interaction with sodium or potassium intake. In contrast, we found that region had a significant interaction with the effect of the Na/K ratio on the risk of hypertension, with significant effects in both the overall sample and in each region. In the central and southern regions, the HR for the fourth and fifth quintiles of Na/K ratio were significantly different from the first quintile, and in all regions the fifth quintile was significantly different from the first quintile (Figure 3); a higher risk was found in the southern region (Table 4).” | Yes |
| “We found that the Na/K ratio has a stronger association with incident hypertension than does sodium or potassium intake solely; this result is consistent with that of previous studies. Furthermore, the interaction between region of residence and Na/K ratio is significant.” | |||
| Grubler, 2014, Austria (33) | No | In adjusted analyses using backward linear regression, the urinary Na/K ratio was positively associated with both nighttime SBP and DBP [note: not a direct quote from the authors] | Not applicable |
| Kim, 2014, Korea (34) | Yes | “… [S]odium and sodium to potassium ratio were positively related with blood pressure among men (DBP, 78.8 mm Hg in the lowest quintile vs 80.6 mm Hg in the highest quintile, P for trend = 0.0079 for sodium; DBP, 79.0 mm Hg in the lowest quintile vs 80.7 mm Hg in the highest quintile, P for trend = 0.0199 and SBP, 123.8 mm Hg in the lowest quintile vs 125.9 mm Hg in the highest quintile for sodium/potassium). Kimchies consumption was positively related to DBP for men (78.2 mm Hg in the lowest quintile vs 80.9 mm Hg in the highest quintile for DBP, P for trend = 0.0003). Among women, these relations were not found.” | Yes, in men only |
| Mente, 2014, multiple (PURE study) (35) | Yes | “For each 1-g increment in estimated sodium excretion, there was an increment of 1.46 mm Hg in systolic blood pressure (P < 0.001) and an increment of 0.54 mm Hg in diastolic blood pressure (P < 0.001). After correcting for regression dilution bias and adjusting for covariates, we observed a steeper slope (a larger increment in blood pressure for a 1-g increment in estimated sodium excretion) for the association between estimated usual sodium excretion and blood pressure, with an increment of 2.11 mm Hg in systolic blood pressure per gram and an increment of 0.78 mm Hg in diastolic blood pressure per gram (P < 0.001 for both comparisons).” | Yes |
| “For each increment of 1 g in estimated potassium excretion per day, there was a decrement of 0.75 mm Hg in systolic blood pressure (P < 0.001) and a decrement of 0.06 mm Hg in diastolic blood pressure (P = 0.33). The decrements were larger after correction for regression dilution bias (1.08 mm Hg and 0.09 mm Hg, respectively).” | |||
| “A 1-SD increment in the estimated sodium-to-potassium ratio (of 3.26) was associated with increments of 2.30 mm Hg in systolic blood pressure and 0.78 mm Hg in diastolic blood pressure (P < 0.001 for both comparisons). The highest blood pressures were observed in the group with the highest estimated sodium excretion and the lowest estimated potassium excretion (difference from group with lowest sodium excretion and highest potassium excretion, 12 mm Hg in systolic pressure and 5 mm Hg in diastolic pressure; P < 0.001 for interaction).” | |||
| Millen, 2013, South Africa (15) | No | "[T]his study is the first to show that insulin resistance, although not independently related to BP, modifies salt intake (urinary Na+/K+)–BP relationships in a group of African descent, an ethnic group that has been well documented as having a high prevalence of salt sensitivity.” | Not applicable |
| Zhang, 2013, U.S. (16) | Yes | In this large nationally representative sample of U.S. adults who were not taking anti-hypertensive medications, we found that usual sodium intake and sodium-to-potassium ratio were positively associated and usual potassium intake was negatively associated with blood pressure and hypertension.” | No |
| “Our results provide population-based evidence that concurrent higher sodium and lower potassium consumption are associated with hypertension.” | |||
| Hedayati, 2012, U.S. (17) | No | “In summary, this analysis supports the hypothesis that dietary Na+ excess and K+ deficiency may play an important role in hypertension pathogenesis and extends these findings across both sexes and racial groups in a multiethnic sample. It further demonstrates that this association may be independent of other traditional cardiovascular risk factors and measures of kidney function.” | Not applicable |
| Michel, 2012, South Africa (18) | No | “The main findings of the present study are that, in the context of a high-Na+ and low-K+ diet (indexed by urinary Na+/K+), which suppresses renin release (resulting in a negative relationship between renin and BP), angiotensinogen is an important determinant of [renin-angiotensin-aldosterone system] activation (as indexed by aldosterone concentrations) and of systolic BP.” | Not applicable |
| Tzoulaki, 2012, multiple (INTERMAP) (36) | No | “… [W]e identified and validated inverse associations between BP and intake of B vitamins (folacin, riboflavin, and thiamin) previously poorly studied or unconfirmed, as well as previously established direct [positive] associations of sodium-to-potassium ratio and alcohol with BP.” | Not applicable |
| Huggins, 2011, Australia (19) | Yes | “This is 1 of the few within-population studies and the first study in Australian adults to demonstrate a positive association between urinary sodium or sodium-to-potassium ratio and SBP. Our findings provide supporting evidence that the current high intake of sodium in older adults in Australia is related to higher BP.” | Yes, in highest quintile only |
| Yang, 2011, U.S. (20) | Yes | Descriptive analysis of the exposure and outcomes of interest precluded drawing any conclusion related to the relationship of interest. | Not applicable |
| The authors report: “Our findings suggest that a higher sodium-potassium ratio is associated with significantly increased risk of CVD and all-cause mortality, and higher sodium intake is associated with increased total mortality in the general US population.” | |||
| Redelinghuys, 2010, South Africa (21) | No | “In the present study we show that urinary Na+/K+ ratios are independently associated with central PP, as well as peripheral 24-h, day, and night PP independent of [mean arterial pressure], and that the central PP effect is mediated by both forward and augmented pressure wave effects but not through changes in aortic [pulse wave velocity]. Thus, the present study suggests that alterations in salt intake may modify cardiovascular risk at a population level through an impact on central and 24-h peripheral dynamic rather than static BPs.” | Not applicable |
| Ruixing, 2008, China (22) | Yes | “Hypertension was positively associated with… sodium intake, and sodium/potassium ratio, and negatively associated with … potassium intake… in men (P < 0.05). Hypertension was positively associated with … sodium intake, sodium/potassium ratio… and negatively associated with… potassium… in women (P < 0.05).” | Yes |
| Polónia, 2006, Portugal (23) | Yes | “Fig. 1 shows 24-h urinary sodium excretion and estimated daily salt intake for each subpopulation, with no significant differences between the 4 groups. Fig. 2 gives the sodium/potassium excretion ratio for each subpopulation. This ratio was significantly higher in the hypertensives compared to the other groups [stroke spouses, university students, factory workers], in which there were no statistically significant differences.” | Yes |
| “The present study found that hypertensives presented a significantly more unfavorable sodium/potassium excretion ratio than the other 3 groups [stroke spouses, university students, factory workers].” | |||
| Kwok, 2003, China (24) | No | “The dietary variables which correlated significantly with systolic blood pressure were calcium intake (r = 70.40), urinary sodium/creatinine ratio (r = 0.39), and urinary sodium/potassium ratio (r = 0.30). The only variable which correlated significantly with diastolic blood pressures was urinary sodium = creatinine (r = 0.29).” | Not applicable |
| Schröder, 2002, Spain (25) | Yes | “In conclusion, the sodium to potassium ratio and dietary intakes of calcium and sodium were related to blood pressure independently of hypertension drug treatment. Furthermore, moderate sodium (< 2400 mg Na/d) intake reduced the risk of hypertension in normotensive and non-medicated hypertensive subjects.” | Yes |
| “In the present study, sodium intake and the sodium to potassium ratio were directly related to diastolic blood pressure in normotensive and non-medicated hypertensive subjects. Additionally, in medicated hypertensive patients (on an average 65 y) a direct association between sodium intake, sodium to potassium ratio and systolic and diastolic blood pressures was observed.” | |||
| Yamori, 2002, China (26) | Yes | “In conclusion, BP was strongly associated with BMI, salt intake and other diet-related factors in the present sample. Subjects with higher educational levels had a lower risk of HT and lower HT risk profiles [including lower Na/K ratio]. These results emphasize that education plays an important role in public health for controlling high BP in the Chinese population.” | Yes |
| The urinary Na/K ratio was significantly associated with HTN (relative risk = 1.30, 95% confidence interval: 1.06 to 1.58). The relative risk of HTN in association with urinary Na or K was not reported. | |||
| Hajjar, 2001, U.S. (27) | Yes | In multivariate analysis, risk estimates were not provided for the Na/K ratio in association with SBP, DBP, or pulse pressure; however, Na and K were significantly associated and in the expected direction with SBP. Only K was significantly associated with DBP and in the expected direction, and only Na was significantly associated with pulse pressure in the expected direction. | No |
| Hu, 2001, China (28) | Yes | “There were higher dietary sodium intake and dietary sodium-to-potassium ratio in the hypertensive group than normal BP group. The dietary potassium intake showed no differences between hypertension and the normal BP population, but this Chinese population had lower potassium intake than desirable.” | Yes |
| The dietary Na/K ratio was significantly and more strongly associated with HTN among both men and women than either dietary Na or K alone. | |||
| Xie, 2001, China (29) | Yes | “In conclusion, the study established a significant positive correlation between systolic and diastolic blood pressure and the urinary Na/K ratio. If a Na/K ratio of 1 is considered optimal, by achieving that goal mean SBP could be reduced by about 6 mm Hg and mean DBP by 3 mm Hg in this normotensive population.” | Yes |
| Caputo, 2000, U.S. (30) | No | "[R]egression analyses revealed that with the exception of BMI and the sodium-to-potassium ratio, no other risk factors were independent predictors of systolic or diastolic blood pressure.” | Not applicable |
| Mosley, 2000, Egypt (31) | No | No specific conclusions on the relationship between the Na/K ratio and blood pressure; the Na/K ratio was examined as a confounder of the association between skin color and blood pressure. | Not applicable |
| The authors reported no statistically significant association between urinary Na/K and SBP in boys or girls. For DBP, a statistically significant positive association was reported among boys only. | |||
| Mufunda, 2000, Zimbabwe (32) | No | “In men, but not in women, the Na/K ratio was positively associated with SBP. The lack of a correlation between Na/K ratio and SBP in women may result from the relatively small sample size and/or from the uncertainty introduced by use of spot urine samples.” | Not applicable |
BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; INTERMAP, International Study of Macro/Micronutrients and Blood Pressure; PP, pulse pressure; PURE, Prospective Urban Rural Epidemiology; ref, reference; SBP, systolic blood pressure.
The study by Du et al. (37), based on the China Health and Nutrition Survey, is the only observational cohort study included in this table; all other studies, including the INTERMAP, are based on cross-sectional evaluations.
Two cross-sectional studies did not find a stronger association of the sodium-to-potassium ratio with blood pressure outcomes than sodium or potassium alone (16, 27), whereas the remaining 11 cross-sectional studies (15, 17, 18, 20, 21, 23, 30–33, 36), including the large INTERMAP study by Tzoulaki et al. (36), did not evaluate both the ratio and the individual nutrients, and therefore did not provide information on the relative strength of their associations (Table 5). Given the limitations of cross-sectional studies, described below, these results must be interpreted with caution.
Limitations of cross-sectional data
Cautious interpretation of the relation between the sodium-to-potassium ratio and blood pressure and related outcomes in cross-sectional studies is warranted. These data are limited by the assessment of nutrient intake and blood pressure at the same point in time; consequently, cross-sectional studies are unable to determine whether the exposure preceded the outcome. Because many or most hypertensive patients are counseled to reduce their sodium intake and perhaps to increase their potassium intake, it is highly plausible that hypertension could be associated with lower intake of sodium and a lower sodium-to-potassium ratio due to reverse causality. Intake estimates based on dietary recall may also underestimate typical sodium and potassium intakes due to the exclusion or limited quantification of important dietary sources such as table salt or supplements (16). As a result, 24-h urine collections provide the optimal data source for measuring sodium and potassium intakes in observational studies (54). Exposure misclassification may be nondifferential due to knowledge of hypertension status at the time of exposure assessment, thereby leading to bias either away from or toward a null association, depending on misclassification patterns. The adjustment for relevant confounders such as smoking and medically relevant conditions varied considerably across studies as well; therefore, residual and unmeasured confounding, an issue inherent in observational studies generally, limits the inferences that can be made from these data. Nevertheless, the fact that 7 of 9 cross-sectional studies with relevant data found that the sodium-to-potassium ratio was more strongly associated with hypertension or blood pressure outcomes than sodium or potassium alone is consistent with findings from the majority of relatively large RCTs reviewed with relevant data.
Conclusions and Research Recommendations
In summary, this study aimed to systematically review the literature to determine if the sodium-to-potassium ratio is more strongly associated with blood pressure and related risk factors for CVD in humans than either sodium or potassium alone. This review found that the strongest support for evaluating the relative predictive value of the sodium-to-potassium ratio stemmed from 4 relatively large (40 subjects) to large (>400 subjects) RCTs conducted in hypertensive or prehypertensive/hypertensive adults, with follow-up periods of ≥4 wk. The minority of RCTs that reported no beneficial effect from low sodium combined with high potassium intakes in hypertensive subjects derived from 2 small RCTs (≤21 subjects) and from 1 RCT that evaluated dietary intervention as ancillary to pharmacologic treatment (no differential between-group effects were reported). Beneficial effects from a low-sodium/high-potassium diet, thus leading to a reduced sodium-to-potassium ratio, were predominantly reported for blood pressure, with few studies reporting on other related outcomes.
Future research on the sodium-to-potassium ratio and blood pressure and related factors may be warranted to fill some of the research gaps identified. No recent RCTs on this topic have been published in the past 13 y, perhaps in part because scientists and clinicians believe that important questions in this area have already been answered. Indeed, the authors of the DASH diet RCT reported that their findings should put to rest the debate over the role of reduced sodium intake for lowering blood pressure in persons with prehypertension. The findings from this study, however, may not be generalizable to populations outside the United States. Additional research is warranted in populations outside the United States, especially those who mostly adhere to a modern Western diet.
Other key public health questions remain to be addressed. For example, RCTs of long duration (e.g., ≥6 wk) in hypertensive subjects could meaningfully contribute to understanding of the effects of urinary and dietary sodium and potassium and their ratio on CVD and all-cause mortality (55). Because none of the RCTs included in the present study examined arterial stiffness, the augmentation index, or endothelial dysfunction, an assessment of these cardiovascular variables in association with the sodium-to-potassium ratio and sodium and potassium intakes alone would also contribute new knowledge. In addition, very few RCTs included in the present study considered outcomes pertinent to the renin-angiotensin system including PRA, plasma renin concentration, plasma renin, and angiotensin II. A focused evaluation of the renin-angiotensin system, and an understanding of the variables most sensitive to the sodium-to-potassium ratio (e.g., PRA vs. plasma renin concentration), would lend greater insight into the biologic pathways underlying the regulation of blood pressure and fluid balance.
Only 1 observational cohort study was eligible for inclusion in the present review. This study, set in China, found that the sodium-to-potassium ratio was more strongly associated with incident hypertension than either sodium or potassium alone (37). Future observational cohort studies in ethnically diverse populations should evaluate this question. The incorporation of 24-h urinary sodium excretion, which was lacking in the Du et al. (37) study, is needed.
This review identified a lack of scientific evaluation on the effects of a low sodium-to-potassium ratio on blood pressure and related cardiovascular variables in normotensive populations. However, an abundance of data has informed on the effects of reduced sodium intake alone on blood pressure outcomes in normotensive subjects (10, 56). For example, a recent systematic Cochrane review and meta-analysis on the effects of a low-sodium diet compared with a high-sodium diet concluded that restriction of sodium intake resulted in a significant blood pressure reduction of 1% in normotensive subjects (56). Although the public health importance of reducing blood pressure in normotensive patients is unclear, identifying the additional effect, if any, of a low-sodium/high-potassium diet on blood pressure outcomes in normotensive subjects could aid future research and clinical recommendations for preventing the onset of hypertension.
This review also identified a paucity of research in both RCT and observational studies regarding the effects of a low sodium-to-potassium ratio on blood pressure and related cardiovascular variables in children and adolescents. Given that elevated blood pressure and primary hypertension are becoming more common in children and adolescents (57), with potentially lifelong implications for risk of CVD, chronic kidney disease, and overall life expectancy, this is an area of high public health importance. Simple, yet effective, dietary interventions for reducing high blood pressure in children and adolescents require critical attention. Joint modification of reduced sodium consumption and increased potassium intake in pediatric populations can be a feasible and cost-effective approach in mitigating premature hypertension; therefore, future research in this area is warranted.
Acknowledgments
The authors thank Nancy Rivera for editorial assistance and the Dairy Research Institute, McCain Foods, and the Alliance for Potato Research and Education for providing feedback and review of the manuscript. V.P. conceptualized the study, conducted the literature review, extracted study data, designed the analysis plan, and drafted the manuscript with support from E.C.; E.C. conducted a separate data extraction for quality control; and V.P. and E.C. interpreted the results. Both authors critically reviewed the manuscript for intellectual content and read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; INTERMAP, International Study of Macro/Micronutrients and Blood Pressure; KCl, potassium chloride; NaCl, sodium chloride; PRA, plasma renin activity; RCT, randomized controlled trial.
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambers Heerspink HJ, Perkovic V, de Zeeuw D. Renal and cardio-protective effects of direct renin inhibition: a systematic literature review. J Hypertens 2009;27:2321–31. [DOI] [PubMed] [Google Scholar]
- 4.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet 2007;369:1208–19. [DOI] [PubMed] [Google Scholar]
- 5.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: emerging concepts. Hypertension 2010;55:9–14. [DOI] [PubMed] [Google Scholar]
- 6.Nürnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schafers RF. Augmentation index is associated with cardiovascular risk. J Hypertens 2002;20:2407–14. [DOI] [PubMed] [Google Scholar]
- 7.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 9.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 11.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 2007;356:1966–78. [DOI] [PubMed] [Google Scholar]
- 13.Drenjančević-Perić I, Jelakovic B, Lombard JH, Kunert MP, Kibel A, Gros M. High-salt diet and hypertension: focus on the renin-angiotensin system. Kidney Blood Press Res 2011;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure: results for 24 hour urinary sodium and potassium excretion. BMJ 1988;297:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millen AM, Norton GR, Majane OH, Maseko MJ, Brooksbank R, Michel FS, Snyman T, Sareli P, Woodiwiss AJ. Insulin resistance and the relationship between urinary Na+/K+ and ambulatory blood pressure in a community of African ancestry. Am J Hypertens 2013;26:708–16. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Cogswell M, Gillespie C, Fang J, Loustalot F, Dai S, Carriquirry AL, Kuklina EV, Hong Y, Merritt R, et al. Association between usual sodium and potassium intake and blood pressure and hypertension among U.S. adults: NHANES 2005–2010. PLoS ONE 2013;8:e75289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedayati SS, Minhajuddin AT, Ijaz A, Moe OW, Elsayed EF, Reilly RF, Huang CL. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol 2012;7:315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michel FS, Norton GR, Majane OH, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contribution of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of African ancestry depends on salt intake. Hypertension 2012;59:62–9. [DOI] [PubMed] [Google Scholar]
- 19.Huggins CE, O'Reilly S, Brinkman M, Hodge A, Giles GG, English DR, Nowson CA. Relationship of urinary sodium and sodium-to-potassium ratio to blood pressure in older adults in Australia. Med J Aust 2011;195:128–32. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2011;171:1183–91. [DOI] [PubMed] [Google Scholar]
- 21.Redelinghuys M, Norton GR, Scott L, Maseko MJ, Brooksbank R, Majane OH, Sareli P, Woodiwiss AJ. Relationship between urinary salt excretion and pulse pressure and central aortic hemodynamics independent of steady state pressure in the general population. Hypertension 2010;56:584–90. [DOI] [PubMed] [Google Scholar]
- 22.Ruixing Y, Jinzhen W, Shangling P, Weixiong L, Dezhai Y, Yuming C. Sex differences in environmental and genetic factors for hypertension. Am J Med 2008;121:811–9. [DOI] [PubMed] [Google Scholar]
- 23.Polónia J, Maldonado J, Ramos R, Bertoquini S, Duro M, Almeida C, Ferreira J, Barbosa L, Silva JA, Martins F. Determinacao do consumo del sal numa amostra da populacao Portuguesa adulta pela exercao urnaria de sodio. Sua realcao com rigidez arterial. Rev Port Cardiol 2006;25:801–17. [PubMed] [Google Scholar]
- 24.Kwok TC, Chan TY, Woo J. Relationship of urinary sodium/potassium excretion and calcium intake to blood pressure and prevalence of hypertension among older Chinese vegetarians. Eur J Clin Nutr 2003;57:299–304. [DOI] [PubMed] [Google Scholar]
- 25.Schröder H, Schmelz E, Marrugat J. Relationship between diet and blood pressure in a representative Mediterranean population. Eur J Nutr 2002;41:161–7. [DOI] [PubMed] [Google Scholar]
- 26.Yamori Y, Liu L, Mu L, Zhao H, Pen Y, Hu Z, Kuga S, Negishi H, Ikeda K, Japan-China Cooperative Study Group: Chongqing P. Diet-related factors, educational levels and blood pressure in a Chinese population sample: findings from the Japan-China Cooperative Research Project. Hypertens Res 2002;25(4):559–64. [DOI] [PubMed] [Google Scholar]
- 27.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med 2001;161:589–93. [DOI] [PubMed] [Google Scholar]
- 28.Hu G, Tian H. A comparison of dietary and non-dietary factors of hypertension and normal blood pressure in a Chinese population. J Hum Hypertens 2001;15:487–93. [DOI] [PubMed] [Google Scholar]
- 29.Xie J, Liu L, Kesteloot H. Blood pressure and urinary cations in a low-fat intake Chinese population sample. Acta Cardiol 2001;56:163–8. [DOI] [PubMed] [Google Scholar]
- 30.Caputo JL, Gill DL, Tseh W, Jamurtas AZ, Morgan DW. Perceived stress and blood pressure in early adolescent children. Ann Behav Med 2000;22:65–70. [DOI] [PubMed] [Google Scholar]
- 31.Mosley JD, Appel LJ, Ashour Z, Coresh J, Whelton PK, Ibrahim MM. Relationship between skin color and blood pressure in Egyptian adults: results from the National Hypertension Project. Hypertension 2000;36:296–302. [DOI] [PubMed] [Google Scholar]
- 32.Mufunda J, Scott LJ, Chifamba J, Matenga J, Sparks B, Cooper R, Sparks H. Correlates of blood pressure in an urban Zimbabwean population and comparison to other populations of African origin. J Hum Hypertens 2000;14:65–73. [DOI] [PubMed] [Google Scholar]
- 33.Grübler MR, Kienreich K, Gaksch M, Verheyen N, Fahrleitner-Pammer A, Schmid J, Grogorenz J, Ablasser K, Pieske B, Tomaschitz A, et al. Aldosterone to active renin ratio is associated with nocturnal blood pressure in obese and treated hypertensive patients: the Styrian Hypertension Study. J Clin Hypertens (Greenwich) 2014;16:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim MK, Kim K, Shin MH, Shin DH, Lee YH, Chun BY, Choi BY. The relationship of dietary sodium, potassium, fruits, and vegetables intake with blood pressure among Korean adults aged 40 and older. Nutr Res Pract 2014;8:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 2014;371:601–11. [DOI] [PubMed] [Google Scholar]
- 36.Tzoulaki I, Patel CJ, Okamura T, Chan Q, Brown IJ, Miura K, Ueshima H, Zhao L, Van Horn L, Daviglus ML, et al. A nutrient-wide association study on blood pressure. Circulation 2012;126:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du S, Batis C, Wang H, Zhang B, Zhang J, Popkin BM. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr 2014;99:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh RB, Rastogi SS, Singh NK, Ghosh S, Gupta S, Niaz MA. Can guava fruit intake decrease blood presure and blood lipids? J Hum Hypertens 1993;7:33–8. [PubMed] [Google Scholar]
- 39.Langford HG, Davis BR, Blaufox D, Oberman A, Wassertheil-Smoller S, Hawkins M, Zimbaldi N. Effect of drug and diet treatment of mild hypertension on diastolic blood pressure. The TAIM Research Group. Hypertension 1991;17:210–7. [DOI] [PubMed] [Google Scholar]
- 40.Bompiani GD, Cerasola G, Morici ML, Condorelli M, Trimarco B, De Luca N, Leonetti G, Sampieri L, Cuspidi C, Cottone S, et al. Effects of moderate low sodium/high potassium diet on essential hypertension: results of a comparative study. Int J Clin Pharmacol Ther Toxicol 1988;26:129–32. [PubMed] [Google Scholar]
- 41.Nowson CA, Morgan TO. Change in blood pressure in relation to change in nutrients effected by manipulation of dietary sodium and potassium. Clin Exp Pharmacol Physiol 1988;15:225–42. [DOI] [PubMed] [Google Scholar]
- 42.Suppa G, Pollavini G, Alberti D, Savonitto S. Effects of a low-sodium high-potassium salt in hypertensive patients treated with metoprolol: a multicentre study. J Hypertens 1988;6:787–90. [PubMed] [Google Scholar]
- 43.Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens 1987;5:115–9. [DOI] [PubMed] [Google Scholar]
- 44.Valori C, Bentivoglio M, Corea L, Fabrizi G, Lieberati R, Porcellati C, Timio M, Tini-Brunozzi L, Toso M, Bichisao E, et al. Dietary sodium restriction versus low-sodium/high-potassium salt as ancillary treatment in hypertension. J Hypertens 1987;5:S315–7. [Google Scholar]
- 45.Arzilli F, Taddei S, Graziadei L, Bichisao E, Giovannetti R, Salvetti A. Potassium-rich and sodium-poor salt reduces blood pressure in hospitalized patients. J Hypertens Suppl 1986;4:S347–50. [PubMed] [Google Scholar]
- 46.Smith SJ, Markandu ND, Sagnella GA, MacGregor GA. Moderate potassium chloride supplementation in essential hypertension: is it additive to moderate sodium restriction? Br Med J (Clin Res Ed) 1985;290:110–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujita T, Ando K. Hemodynamic and endocrine changes associated with potassium supplementation in sodium-loaded hypertensives. Hypertension 1984;6:184–92. [PubMed] [Google Scholar]
- 48.Richards AM, Nicholls MG, Espiner EA, Ikram H, Maslowski AH, Hamilton EJ, Wells JE. Blood-pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet 1984;1:757–61. [DOI] [PubMed] [Google Scholar]
- 49.Skrabal F, Gasser RW, Finkenstedt G, Rhomberg HP, Lochs A. Low-sodium diet versus low-sodium/high-potassium diet for treatment of hypertension. Klin Wochenschr 1984;62:124–8. [DOI] [PubMed] [Google Scholar]
- 50.Parfrey PS, Vandenburg MJ, Wright P, Holly JM, Goodwin FJ, Evans SJ, Ledingham JM. Blood pressure and hormonal changes following alteration in dietary sodium and potassium in mild essential hypertension. Lancet 1981;1:59–63. [DOI] [PubMed] [Google Scholar]
- 51.Burstyn P, Hornall D, Watchorn C. Sodium and potassium intake and blood pressure. BMJ 1980;281:537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skrabal F, Aubock J, Hortnagl H. Low sodium/high potassium diet for prevention of hypertension: probable mechanisms of action. Lancet 1981;2:895–900. [DOI] [PubMed] [Google Scholar]
- 53.Zoccali C, Cumming A, Hutcheson MJ, Barnett P, Semple PF. Effects of potassium on sodium balance, renin, noradrenaline and arterial pressure. J Hypertens 1985;3:67–72. [DOI] [PubMed] [Google Scholar]
- 54.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 55.Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol 2007;22:763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens 2012;25:1–15. [DOI] [PubMed] [Google Scholar]
- 57.Ellis D, Miyashita Y. Primary hypertension and special aspects of hypertension in older children and adolescents. Adolesc Health Med Ther 2011;2:45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]