Abstract
Human milk is a source of bacteria to the infant gut; however, the origin of milk bacteria, as well as their impact on neonatal gut microbiota establishment, remains largely unknown. In the past years, results provided by different research groups suggest that certain bacteria from the maternal gastrointestinal tract could translocate through a mechanism involving mononuclear immune cells, migrate to the mammary glands via an endogenous cellular route (the bacterial entero-mammary pathway), and subsequently colonize the gastrointestinal tract of the breast-fed neonate. If such findings are confirmed in the future, we could exert a positive influence on infant health by modulating the maternal gut microbiota.
Introduction
In the past decade, some culture-dependent studies revealed that colostrum and milk from healthy women contain bacteria, including staphylococci, streptococci, corynebacteria, lactic acid bacteria, propionibacteria, and bifidobacteria (1). Later, the application of culture-independent techniques, including microbiome approaches, confirmed the presence of DNA from these and other bacterial genera (1). Therefore, such biologic fluids are continuous sources of live bacteria to the infant gastrointestinal tract and, in fact, different studies have shown that there is a mother-to-infant transfer of bacterial strains through breastfeeding (2–7). Traditionally, it was believed that any bacterial cell found in human milk was the result of contamination from the infant’s oral cavity or the mother’s skin (8). However, the detection of live bacterial cells and/or DNA from anaerobic species that are usually related to gut environments and that cannot survive in aerobic locations has fueled a scientific debate on the origin of milk-associated bacteria.
Infant’s Mouth and Maternal Skin as Potential Sources of Bacteria to Mammary Ducts and Milk
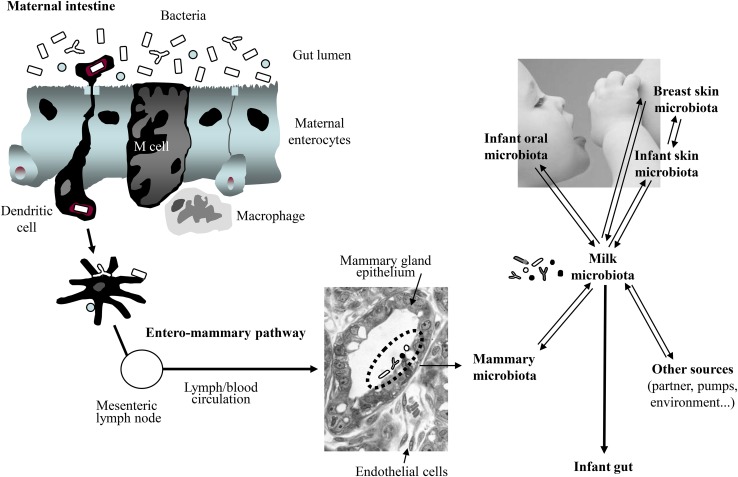
The microbiome of the different human body locations constitutes a dynamic network of interrelated communities (9). Therefore, the possibility that the infant’s mouth or maternal skin may provide some bacteria to the milk is not incompatible with the role of human milk as a source of bacteria to the infant’s mouth, maternal skin, and other infant/mother locations (Fig. 1).
FIGURE 1.
Sources of the bacteria present in human milk, including a model to explain how some maternal bacterial strains could be transferred to the infant gut through an entero-mammary pathway.
Some bacteria from the infant’s oral cavity may contaminate milk during suckling because of milk flow back into the mammary ducts (10); however, this mechanism does not explain why precolostrum secreted by some women before delivery (and obviously before any contact with the infant’s mouth) already contains the microbiota that characterizes human milk (11). Although the human salivary microbiome is still widely unknown, Streptococcus species seem to be dominant both in adults (12–14) and in edentulous infants (15–18). Streptococci are also among the dominant phylotypes in human milk (3, 19, 20), suggesting a potential role in the shaping of the salivary microbiota. The origin of the oral microbiota is far from elucidated and deserves research attention because of its relevant implications for human health.
Some of the bacteria that are commonly isolated from skin, such as Staphylococcus, Corynebacterium, and Propionibacterium (21, 22), are also frequent in human milk. However, it should be highlighted that although staphylococci, corynebacteria, and propionibacteria have been traditionally associated with the skin, they are widespread in most, if not all, human mucosal surfaces; in fact, the populations of such bacterial groups reach their highest concentrations in the mucosal layers of the digestive and genitourinary tracts. In addition, such bacteria have been detected in samples of chorioamnion and amniotic fluid from pregnant women and in umbilical cord blood obtained from healthy neonates born either by vaginal or cesarean delivery (17, 19, 23, 24); this suggests that they may colonize the fetal skin and digestive tract in utero and raises the possibility that the presence of bacteria in chorioamnion, amniotic fluid, colostrum, and milk may share a common or similar mechanism in healthy hosts.
Streptococci and staphylococci have received marginal attention regarding their role in the early colonization of the infant gastrointestinal tract despite being the dominant bacteria in human milk (3, 6). Interestingly, an abundant presence of Staphylococcus epidermidis seems to be a differential feature of the feces of healthy breast-fed infants when compared with those of formula-fed infants (3, 25–28). Such bacteria seem to have coevoluted with mammary glands and usually display specific properties that favor their growth in the mammary environment during lactation. For example, some staphylococci (e.g., S. epidermidis and Staphylococcus aureus) are typically associated with catheters and indwelling medical devices in hospital settings, and mammary glands develop an extraordinary complex net of “catheter-like" ducts during late pregnancy and lactation, providing an excellent physical support to these microorganisms. Second, lactose and galactose metabolism of staphylococci is highly efficient through the d-tagatose-6-phosphate pathway (29). Finally, these bacteria readily metabolize human milk oligosaccharides (30).
Indeed, it has been proposed that some coagulase-negative staphylococci and some streptococci from the mitis and salivarius groups may have a beneficial function by preventing colonization of the host by more severe pathogens, such as S. aureus (31–35). A previous study showed that cows’ udders that contained coagulase-negative staphylococci were less susceptible to mastitis after experimental challenge with S. aureus (36).
Despite sharing of some phylotypes, the comparison between the bacterial communities detected in milk and those found on breast skin reveals that there are major differences between them (20). As an example, Bifidobacterium is a strictly anaerobic genus and therefore skin is a highly improbable source of such microorganisms in milk (37). Sharing of Bifidobacterium longum DNA in maternal feces, human milk, and neonatal feces within the same mother-neonate pair has been reported (38). More recently, pyrosequencing allowed identifying gut-associated obligate anaerobic genera, such as Bifidobacterium, Bacteroides, Parabacteroides, and members of the Clostridia class (Blautia, Clostridium, Collinsella, and Veillonella), shared between maternal feces, human milk, and neonatal feces (7). Furthermore, several butyrate-producing members of Clostridia (Coprococcus, Faecalibacterium, Roseburia, and Subdoligranulum) were shared between maternal feces and human milk. A major drawback of culture-independent studies is the lack of information about the viability of the detected populations and the lack of possibility for strain-level discrimination, which is necessary for demonstrating that the same bacterial strain was shared between mother and neonate. Thus, without confirming the presence of these populations by culture, isolation, and strain level discrimination, it remains unclear whether human milk is a source of viable gut-associated obligate anaerobes or if dead cells or parts thereof are transferred to the breast-fed neonate (7). However, transfer of bifidobacteria, lactobacilli, and/or other bacteria at the strain level from the maternal gastrointestinal tract to the neonatal gut (39–41), from the maternal gastrointestinal tract to human milk (42–44), from milk to the neonatal gastrointestinal tract (3, 6), and from the maternal gastrointestinal tract to milk and the infant gastrointestinal tract (5, 7) has also been demonstrated by using culture and strain-level discrimination. Such studies reinforce the hypothesis that at least some bacteria, including obligate anaerobes, may be vertically transferred from mother to neonate via breastfeeding.
Gut Bacterial Translocation during Late Pregnancy and Lactation as a Physiologic Event
Recent findings suggest that selected bacteria of the maternal gastrointestinal microbiota can access the mammary glands through an entero-mammary pathway (11). Previous studies indicated that certain bacteria from the maternal digestive tract may spread to extradigestive locations in healthy hosts (23, 45–48).
Although this is a controversial issue, some studies have offered a scientific basis for such physiologic translocation [reviewed in (1)]. The mechanism would involve dendritic cells (DCs) and CD18+ cells (49–51), which would be able to take up nonpathogenic bacteria from the gut lumen and subsequently carry them to other locations, including the lactating mammary gland (52) (Fig. 1). It must be highlighted that there is an important efflux of intestinal immune cells to the mammary glands during late pregnancy and lactation (53) and that, in fact, the existence of an entero-mammary circulation of IgA-producing cells is long known (54).
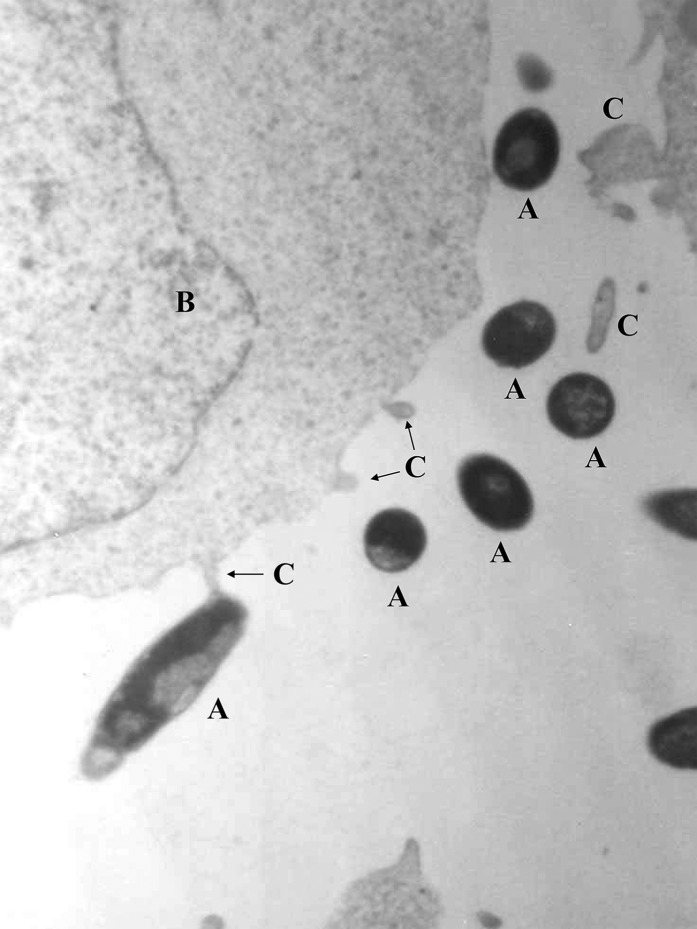
Research carried out by 2 independent groups obtained in vitro and in vivo data reinforcing the hypothesis that at least some human milk bacteria may reach the mammary glands through an internal route, involving maternal DCs and macrophages (38, 55, 56). As an example, 2 lactobacilli strains isolated from human milk (Lactobacillus salivarius CECT 5713 and Lactobacillus gasseri CECT 5713) were able to translocate across a Caco-2 cell monolayer through a DC-mediated mechanism (55) (Fig. 2). In addition, oral inoculation of pregnant mice with a genetically labeled Enterococcus faecium M1a strain led to the isolation and PCR detection of the labeled strain in the amniotic fluid (23) and milk (Jiménez E, Fernández L, Martín R, Rodríguez JM, 2005, unpublished results) of the inoculated animals. In contrast, it could not be detected in the respective samples obtained from a noninoculated control group. Similarly, oral administration of lactobacilli strains isolated from human milk led to their presence in the milk of >50% of the recruited women (42, 44).
FIGURE 2.
Specific interactions between cells of a Lactobacillus gasseri strain isolated from human milk (“A”) and dendritic (“B”) cells, as assessed by transmission electron microscopy (55). The interactions were studied by using trans-well bicompartmental assays in which bacterial cells and immature dendritic cells were initially separated by a monolayer of Caco-2 cells. C, dendritic cell dendrites.
An increased bacterial translocation from the gut to mesenteric lymph nodes and mammary glands in pregnant and lactating mice was observed in another study (38). Bacteria could be observed histologically in the subepithelial dome and interfollicular regions of Peyer’s patches, in the lamina propria of the small bowel, and associated with cells in the glandular tissue of the mammary gland. The Peyer’s patches of pregnant and lactating mice were macroscopically larger than those of control mice and had a more prominent subepithelial dome and more dilated draining lymphatic vessels, containing mononuclear cells. The same study showed that human milk contains viable bacteria, including Streptococcus, Lactobacillus, and Bifidobacterium, whereas acridine orange staining of milk and blood cytopreparations identified bacterial cells in association with maternal mononuclear cells. Globally, these results strongly suggest the involvement of mononuclear cells in the transport of intestinal bacteria to the mammary glands in late pregnancy.
The passage of viable bacteria through the intact intestinal mucosa is known as bacterial translocation. This phenomenon was postulated >60 y ago (57), although the term “translocation” was first used to describe the passage of Serratia marcescens from the duodenum of rats, where it had been inoculated, to the lymph (58). Later, the term bacterial translocation was defined as the passage of viable bacteria from the gastrointestinal tract into the lamina propria and then to the mesenteric lymph nodes and other extraintestinal organs such as spleen, liver, kidneys, peritoneal cavity, or bloodstream (59). Traditionally, gut bacteria translocation has been associated with pathogenic conditions and therefore it has been mainly studied in patients (with, e.g., severe burns, transplants, pancreatitis, cardiopulmonary diseases, AIDS) in whom pathogenic bacteria had spread throughout the body causing sepsis, multiple organ failure, and, sometimes, death (60).
However, it is known that a low rate of bacterial translocation also occurs in healthy individuals without causing detrimental effects in the host (60–62). In a study involving 132 patients who underwent laparotomy, 5 showed positive culture results in their blood samples, but the isolated bacteria lacked pathogenic traits and were not related to the patient morbidity (63). Langa (55) reported that the rates of translocation of some lactic acid bacteria (0.002–0.009% after 2 h) through a trans-well system, involving interactions between immune cells and Caco-2 cells, were notably lower than those (>20%) reported for Vibrio cholerae (64) and invasive Salmonella (65). In fact, it has been suggested that bacterial translocation to extraintestinal tissues is a beneficial physiologic event in healthy hosts because it may be associated with immunomodulation, including the initial maturation of the neonatal immune system (38, 66, 67).
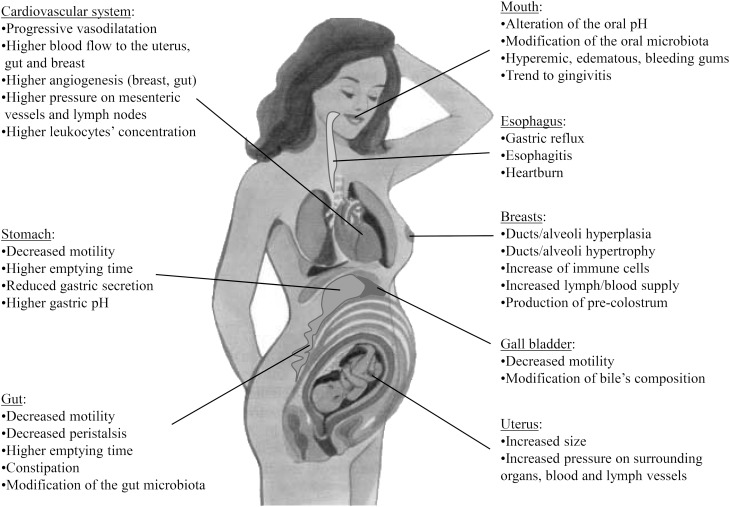
Many transient anatomic and physiologic changes occur during pregnancy and lactation to provide a suitable framework for the development of the fetus first, and the neonate later. These changes affect virtually all systems, including the cardiovascular, respiratory, genitourinary, and digestive systems. Interestingly, such adaptations may favor an increased bacterial translocation during late pregnancy and early lactation (Fig. 3). Adequate cardiovascular adaptations must secure good placental development and appropriate fetal growth. Therefore, changes in the cardiovascular system are characterized by a progressive and generalized vasodilatation state and an increase in several variables or processes, including blood volume, stroke volume, cardiac output, heart rate, regional blood flow to various organs (e.g., uterus, kidneys, gastrointestinal tract, skin, breasts), angiogenesis, and blood concentration of coagulation factors and leukocytes.
FIGURE 3.
Physiologic adaptations of the body during pregnancy that may favor an increased bacterial translocation.
The hormonal action induces relevant oral changes during pregnancy, affecting the mouth's pH and microbiota; the gums become hyperemic and edematous and tend to bleed. The main effects of gestation on the gastrointestinal system are associated with the displacement of the abdominal organs by the progressive growth of the uterus and to a decreased motility, presumably because of the effect of progesterone on smooth muscle contractility. This causes an increase in the gastric emptying time, whereas a decreased gastric secretion results in a more basic gastric pH. The decreased gut motility and peristalsis, particularly in the last trimester of pregnancy, along with the increased pressure of the uterus, can cause problems of constipation and hemorrhoids. In addition, the maternal mesenteric blood vessels are exposed to estrogens and to an increasing fetal pressure, leading to transient vascular engorgements and blood stasis. In addition, 1 of the body’s most dramatic adaptations to late pregnancy and lactation is a large increase in the size and complexity of the maternal intestine (68). Globally, the digestive tract is characterized by weakened barriers against bacterial growth, increased permeability, and reduced peristalsis, 3 factors that are closely associated to bacterial translocation (61).
Finally, several anatomic and physiologic changes in the mammary system, including ducts, areola, and nipples (69), during pregnancy facilitate the formation of a specific mammary microbiota (1). Interestingly, it has been considered that there is an obvious functional relation between the intestinal tract and the mammary glands during late pregnancy and lactation (68).
If the existence of the bacterial entero-mammary pathway is confirmed, this novel form of mother-neonate communication could influence the current understanding of neonatal gut development and provide future opportunities for manipulating an aberrant microbiota establishment and reduce the risk of disease, such as by administering probiotics to the neonate and/or to the breastfeeding mother (70).
Acknowledgments
The sole author read and approved the final manuscript.
References
- 1.Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013;69:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Martín R, Langa S, Reviriego C, Jiménez E, Marín ML, Xaus J, Fernández L, Rodríguez JM. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr 2003;143:754–8. [DOI] [PubMed] [Google Scholar]
- 3.Jiménez E, Delgado S, Maldonado A, Arroyo R, Albújar M, García N, Jariod M, Fernández L, Gómez A, Rodríguez JM. Staphylococcus epidermidis: a differential trait of the fecal microbiota of breast-fed infants. BMC Microbiol 2008;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martín R, Jiménez E, Heilig HG, Fernández L, Marín ML, Zoetendal EG, Rodríguez JM. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol 2009;75:965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albesharat R, Ehrmann MA, Korakli M, Yazaji S, Vogel RF. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst Appl Microbiol 2011;34:148–55. [DOI] [PubMed] [Google Scholar]
- 6.Martín V, Maldonado A, Moles L, Rodríguez-Baños M, Del Campo R, Fernández L, Rodríguez JM, Jiménez E. Sharing of bacterial strains between breast milk and infant feces. J Hum Lact 2012;28:36–44. [DOI] [PubMed] [Google Scholar]
- 7.Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol 2014;16:2881–904. [DOI] [PubMed] [Google Scholar]
- 8.West PA, Hewitt JH, Murphy OM. The influence of methods of collection and storage on the bacteriology of human milk. J Appl Bacteriol 1979;46:269–77. [DOI] [PubMed] [Google Scholar]
- 9.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon J, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009;326:1694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics 2004;113:361–7. [DOI] [PubMed] [Google Scholar]
- 11.Martín R, Langa S, Reviriego C, Jiménez E, Marín ML, Olivares M, Boza J, Jiménez J, Fernández L, Xaus J, et al. The commensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics. Trends Food Sci Technol 2004;15:121–7. [Google Scholar]
- 12.Nasidze I, Li J, Quinque D, Tang K, Stoneking M. Global diversity in the human salivary microbiome. Genome Res 2009;19:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005;43:5721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang F, Zeng X, Ning K, Liu KL, Lo CC, Wang W, Chen J, Wang D, Huang R, Chang X, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J 2012;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Teague E, Zhuang Z, Caufield PW. Screening for the spaP gene of Streptococcus mutans in predentate infants. J Dent Res 1997;76:102. [Google Scholar]
- 16.Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun 2000;68:4018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br J Obstet Gynaecol 2002;109:527–33. [DOI] [PubMed] [Google Scholar]
- 18.Cephas KD, Kim J, Mathai RA, Barry KA, Dowd SE, Meline BS, Swanson KS. Comparative analysis of salivary bacterial microbiome diversity in edentulous infants and their mothers or primary care givers using pyrosequencing. PLoS ONE 2011;6:e23503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiménez E, Fernández L, Delgado S, García N, Albújar M, Gómez A, Rodríguez JM. Assessment of the bacterial diversity of human colostrum by cultural-based techniques: analysis of the staphylococcal and enterococcal populations. Res Microbiol 2008;159:595–601. [DOI] [PubMed] [Google Scholar]
- 20.Hunt KM, Foster JA, Forney LJ, Schütte UME, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011;6:e21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao Z, Tseng C, Pei Z, Blaser MJ. Molecular analysis of human forearm superficial skin bacterial biota. Proc Natl Acad Sci USA 2007;104:2927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, NISC Comparative Sequencing Program. Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. Topographical and temporal diversity of the human skin microbiome. Science 2009;324:1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodríguez JM. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by caesarean section. Curr Microbiol 2005;51:270–4. [DOI] [PubMed] [Google Scholar]
- 24. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237ra65. [DOI] [PMC free article] [PubMed]
- 25.Sakata H, Yoshioka H, Fujita K. Development of the intestinal flora in very low birth weight infants compared to normal full-term newborns. Eur J Pediatr 1985;144:186–90. [DOI] [PubMed] [Google Scholar]
- 26.Lundequist B, Nord CE, Winberg J. The composition of the faecal microflora in breastfed and bottle-fed infants from birth to eight weeks. Acta Paediatr Scand 1985;74:45–51. [DOI] [PubMed] [Google Scholar]
- 27.Balmer SE, Wharton BA. Diet and faecal flora in the newborn: breast milk and infant formula. Arch Dis Child 1989;64:1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adlerberth I, Lindberg E, Aberg N, Hesselmar B, Saalman R, Strannegård IL, Wold AE. Reduced enterobacterial and increased staphylococcal colonization of the infantile bowel: an effect of hygienic lifestyle. Pediatr Res 2006;59:96–101. [DOI] [PubMed] [Google Scholar]
- 29.Schleifer KH, Hartinger A, Götz F. Occurrence of D-tagatose-6-phosphate pathway of D-galactose metabolism among staphylococci. FEMS Microbiol Lett 1978;3:9–11. [Google Scholar]
- 30.Hunt KM, Preuss J, Nissan C, Davlin CA, Williams JE, Shafii B, Richardson AD, McGuire MK, Bode L, McGuire MA. Human milk oligosaccharides promote the growth of staphylococci. Appl Environ Microbiol 2012;78:4763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto M, Sussmuth R, Vuong C, Jung G, Gotz F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett 1999;450:257–62. [DOI] [PubMed] [Google Scholar]
- 32.Otto M. Staphylococcus epidermidis; the 'accidental’ pathogen. Nat Rev Microbiol 2009;7:555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010;465:346–9. [DOI] [PubMed] [Google Scholar]
- 34.Park B, Iwase T, Liu GY. Intranasal application of S. epidermidis prevents colonization by methicillin-resistant Staphylococcus aureus in mice. PLoS ONE 2011;6:e25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehara Y, Kikuchi K, Nakamura T, Nakama H, Agematsu K, Kawakami Y, Maruchi N, Totsuka K. H2O2 produced by viridans group streptococci may contribute to inhibition of methicillin-resistant Staphylococcus aureus colonization of oral cavities in newborns. Clin Infect Dis 2001;32:1408–13. [DOI] [PubMed] [Google Scholar]
- 36.Nickerson SC, Boddie RL. Effect of naturally occurring coagulase-negative staphylococcal infections on experimental challenge with major mastitis pathogens. J Dairy Sci 1994;77:2526–36. [DOI] [PubMed] [Google Scholar]
- 37.Gueimonde M, Laitinen K, Salminen S, Isolauri E. Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatology 2007;92:64–6. [DOI] [PubMed] [Google Scholar]
- 38.Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 2007;119:e724–32. [DOI] [PubMed] [Google Scholar]
- 39.Kulagina EV, Shkoporov AN, Kafarskaia LI, Khokhlova EV, Volodin NN, Donskikh EE, Korshunova OV, Efimov BA. Molecular genetic study of species and strain variability in bifidobacteria population in intestinal microflora of breastfed infants and their mothers. Bull Exp Biol Med 2010;150:61–4. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi H, Mikami K, Nishino R, Matsuoka T, Kimura M, Koga Y. Comparative analysis of the properties of bifidobacterial isolates from fecal samples of mother-infant pairs. J Pediatr Gastroenterol Nutr 2010;51:653–60. [DOI] [PubMed] [Google Scholar]
- 41.Makino H, Kushiro A, Ishikawa E, Muylaert D, Kubota H, Sakai T, Oishi K, Martin R, Ben Amor K, Oozeer R, et al. Transmission of intestinal Bifidobacterium longum subsp. longum strains from mother to infant, determined by multilocus sequencing typing and amplified fragment length polymorphism. Appl Environ Microbiol 2011;77:6788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiménez E, Fernández L, Maldonado A, Martín R, Olivares M, Xaus J, Rodríguez JM. Oral administration of lactobacilli strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl Environ Microbiol 2008;74:4650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Björkstén B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr 2009;49:349–54. [DOI] [PubMed] [Google Scholar]
- 44.Arroyo R, Martín V, Maldonado A, Jiménez E, Fernández L, Rodríguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of lactobacilli isolated from breast milk. Clin Infect Dis 2010;50:1551–8. [DOI] [PubMed] [Google Scholar]
- 45.Ouwehand AC, Isolauri E, He F, Hashimoto H, Benno Y, Salminen S. Differences in Bifidobacterium flora composition in allergic and healthy infants. J Allergy Clin Immunol 2001;108:144–5. [DOI] [PubMed] [Google Scholar]
- 46.Vankerckhoven VV, Autgaerden TA, Huys G, Vancanneyt M, Swings J, Goossens H. Establishment of the PROSAFE collection of probiotic and human lactic acid bacteria. Microb Ecol Health Dis 2004;16:131–6. [Google Scholar]
- 47.Begier EM, Barrett NL, Mshar PA, Johnson DG, Hadler JL; Connecticut Bioterrorism Field Epidemiology Response Team. Gram-positive rod surveillance for early anthrax detection Emerg Infect Dis 2005;11:1483–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasanayake AP, Li Y, Wiener H, Ruby JD, Lee MJ. Salivary Actinomyces naeslundii genospecies 2 and Lactobacillus casei levels predict pregnancy outcomes. J Periodontol 2005;76:171–7. [DOI] [PubMed] [Google Scholar]
- 49.Rescigno M, Urbano M, Valzasina B, Francolín M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–7. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks WT, Fang FC. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 1999;401:804–8. [DOI] [PubMed] [Google Scholar]
- 51.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 2004;303:1662–5. [DOI] [PubMed] [Google Scholar]
- 52.Roitt I. Essential immunology. Oxford (UK): Blackwell Scientific Publications; 2001. [Google Scholar]
- 53.Bertotto A, Gerli R, Castellucci G, Scalise F, Vaccaro R. Human milk lymphocytes bearing the gamma/delta T-cell receptor are mostly delta TCS1-positive cells. Immunology 1991;74:360–1. [PMC free article] [PubMed] [Google Scholar]
- 54.Newburg DS. Innate immunity and human milk. J Nutr 2005;135:1308–12. [DOI] [PubMed] [Google Scholar]
- 55.Langa S. Interactions between lactic acid bacteria, intestinal epithelial cells and immune cells: development of in vitro models [dissertation]. Madrid (Spain): Complutense University of Madrid; 2006.
- 56.Langa S, Maldonado A, Delgado S, Martín R, Martín V, Jiménez E, Ruíz-Barba JL, Mayo B, Connor RI, Suárez E, et al. Characterization of Lactobacillus salivarius CECT 5713, a strain isolated from human milk: from genotype to phenotype. Appl Microbiol Biotechnol 2012;94:1279–87. [DOI] [PubMed] [Google Scholar]
- 57.Schweinburg FB, Seligman AM, Fine J. Transmural migration of intestinal bacteria: study based on use of radioactive Escherichia coli. N Engl J Med 1950;242:747–51. [DOI] [PubMed] [Google Scholar]
- 58.Wolochow H, Hildebrand GJ, Lammanna C. Translocation of microorganism across the intestinal wall in rats: effect of microbial size and concentration. J Infect Dis 1966;116:523–8. [DOI] [PubMed] [Google Scholar]
- 59.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979;23:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lichtman SM. Bacterial translocation in humans. J Pediatr Gastroenterol Nutr 2001;33:1–10. [DOI] [PubMed] [Google Scholar]
- 61.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol 1995;3:149–54. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez AV, Baigorí MD, Alvarez S, Castro GR, Oliver G. Phosphatidylinositol-specific phospholipase C activity in Lactobacillus rhamnosus with capacity to translocate. FEMS Microbiol Lett 2001;204:33–8. [DOI] [PubMed] [Google Scholar]
- 63.Moore FA, Moore EE, Poggetti RS, Read RA. Postinjury shock and early bacteremia: a lethal combination. Arch Surg 1992;127:893–7. [DOI] [PubMed] [Google Scholar]
- 64.Kernéis S, Caliot E, Stubbe H, Bogdanova A, Kraehenbuhl J, Pringault E. Molecular studies of the intestinal mucosal barrier physiopathology using cocultures of epithelial and immune cells: a technical update. Microbes Infect 2000;2:1119–24. [DOI] [PubMed] [Google Scholar]
- 65.Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect 2001;3:1183–90. [DOI] [PubMed] [Google Scholar]
- 66.Bengmark S, Jeppsson B. Gastrointestinal surface protection and mucosa reconditioning. JPEN J Parenter Enteral Nutr. 1995;19:410–5. [DOI] [PubMed] [Google Scholar]
- 67.MacFie J. Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull 2004;71:1–11. [DOI] [PubMed] [Google Scholar]
- 68.Hammond KA. Adaptation of the maternal intestine during lactation. J Mammary Gland Biol Neoplasia 1997;2:243–52. [DOI] [PubMed] [Google Scholar]
- 69.Beischer NA, Mackay EV, Colditz PB. Obstetrics and the newborn. Philadelphia: W.B. Saunders Company; 1997. [Google Scholar]
- 70.Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–76. [DOI] [PubMed] [Google Scholar]