Abstract
The role of dietary energy density (ED) in the regulation of energy intake (EI) is controversial. Methodologically, there is also debate about whether beverages should be included in dietary ED calculations. To address these issues, studies examining the effects of ED on EI or body weight in nonelderly adults were reviewed. Different approaches to calculating dietary ED do not appear to alter the direction of reported relations between ED and body weight. Evidence that lowering dietary ED reduces EI in short-term studies is convincing, but there are currently insufficient data to determine long-term effectiveness for weight loss. The review also identified key barriers to progress in understanding the role of ED in energy regulation, in particular the absence of a standard definition of ED, and the lack of data from multiple long-term clinical trials examining the effectiveness of low-ED diet recommendations for preventing both primary weight gain and weight regain in nonobese individuals. Long-term clinical trials designed to examine the impact of dietary ED on energy regulation, and including multiple ED calculation methods within the same study, are still needed to determine the importance of ED in the regulation of EI and body weight.
Introduction
Obesity is one of the major health crises of our time. The majority of adult Americans are now either overweight or obese (1), and recent research indicates that obesity is approaching smoking as the major cause of disability and premature death (2, 3). National improvements in dietary intake, and in particular a reversal of the documented increase in energy intake (EI)6 (4–6), are clearly an important key to preventing unwanted weight gain and associated comorbidities. However, there is no general consensus on how to achieve this important goal.
Of the many dietary factors suggested to play an important role in the regulation of EI, energy density (ED) has received particular attention (7–13) because small changes in the ED of the diet, if uncompensated for by alterations in the quantity of food consumed, could lead to large cumulative changes in EI. The ED of a food can be defined as the metabolizable energy content per unit weight of a food (kJ/g or kcal/g) (11) and is determined by the macronutrient and moisture content of the food. As the most- and least-energy-dense nutrients, fat [2.15 kJ/g (9 kcal/g)] and water (0 kJ/g), are the primary determinants of ED.
Dietary ED can be defined as the ED of the total diet. At present, no consensus has been reached on the appropriate method for calculating dietary ED, with debate centering on the inclusion of beverages in the calculation (8). Studies have used different definitions of dietary ED that vary predominantly by whether some or all beverages are included in the calculation and, if so, what types. For example, studies have used ED values based only on food, whereas others have included both food and energy-containing beverages, and some have included food and all beverages (8, 14, 15).
The goals of this review are to summarize information relevant to standardizing a definition of dietary ED, provide a review of primary research publications examining the effects of ED on EI and body weight in adults, and suggest future research directions for elucidating the role of ED in body weight regulation.
Critical Review of the Literature
Literature search, selection of studies for inclusion, and data extraction.
A literature search conducted by using PubMed identified English-language clinical and observational studies examining relations between ED and EI, appetite, and body weight and/or BMI. Studies in which ED was an explicit independent variable as well as studies in which ED was not an explicit variable or outcome but could be calculated from reported results were considered. Reference lists of these publications and relevant review articles were searched to identify additional germane studies.
The eligibility criteria for the studies reviewed herein are outlined in Table 1. In particular, we focused on studies in nonelderly adults (age 18–60 y) because regulation of EI is impaired in the elderly (16–18). We also generally included studies regardless of the method used to alter ED. As a result, resolving independent effects of ED on EI and body weight from effects of dietary factor(s) that also change when ED is altered (e.g., dietary fat and fiber content, water content of foods, palatability) becomes difficult. This is especially true in studies long enough to demonstrate changes in body weight. However, independent effects of ED are less relevant when one considers that, in free-living individuals, changing dietary ED inevitably alters multiple dietary components. Therefore, for this review we chose to consider ED as 1 dietary factor among many rather than as an independent dietary determinant of EI and body weight. The 1 exception is that studies aiming to determine the effects of adding fiber to meals were not selected for review. Although interventions using added fiber may reduce dietary ED, the reduction is small and any effects on appetite are likely outweighed by the established physiologic effects of fiber (19, 20). Readers are referred to a recent comprehensive review summarizing the evidence for effects of fiber on EI and body weight (21).
TABLE 1.
Criteria and rationale for study exclusion1
| Exclusion criteria | Rationale |
| Applied across all study designs | |
| Mean age of study population <18 or >60 y | Energy regulation dysregulated in older adults; physiologic differences between children/adolescents and adults |
| Studies in clinical or unique populations (e.g., pregnant, binge-eating disorder, etc.) (51, 122, 123) | Not reflective of general population or typical physiologic conditions |
| Studies reporting data that had been presented as part of an earlier report (124–128) | Likely to report similar findings |
| Observational studies | |
| Studies using an FFQ to measure dietary intake and not excluding nonplausible reporters (129) | Measurement error associated with FFQ may confound associations |
| Small sample size (130) | High probability of type I error |
| Clinical studies | |
| No comparison group (131) | Effect of ED modification cannot be determined |
| ED not reported and cannot be determined with confidence if change in ED differed between groups (includes studies that did not measure EI) (132–140) | Unclear if ED was altered |
| Duration of ED manipulation differed between subjects (141) | Unable to be grouped with other studies |
| Studies <1 mo duration in which the majority of the diet was not provided by study investigators (e.g., EI was measured by food record or diet recall) (132, 142–148) | High measurement error |
ED, energy density; EI, energy intake.
A total of 92 relevant studies were identified that met all of the eligibility criteria (15, 22–112). These studies were then classified by study design. Observational studies were recognized to be potentially confounded by bias in reported EI and dietary ED but, with this qualification, were included to explore the effect of inclusion or exclusion of beverages on relations between BMI and ED. Shorter-duration clinical studies (duration of <1 mo) were deemed relevant for providing mechanistic evidence of a role of ED in energy balance regulation. Only those trials in which all or most food was provided to and consumed by subjects in a laboratory setting were selected rather than studies that used self-reported EI as the key outcome because of well-recognized inaccuracies in self-reported EI (113, 114). Longer-duration clinical studies (duration of ≥1 mo) reporting change in body weight, BMI, or body fat as a primary outcome were deemed to provide the most conclusive evidence on relations between ED and body weight.
To facilitate interpretation of the evidence, clinical studies in which ED was the independent variable and EI or body weight the dependent variable were categorized according to study duration as follows: 1) preload and single-meal studies, 2) interventions of 1–3 d in duration, 3) interventions of 3 d to 4 wk in duration in which change in EI from the provided food was the outcome, and 4) interventions ≥1 mo in duration in which change in body weight was the outcome. Together, these divisions permitted an examination of the evidence for acute, short-, and longer-term effects of ED modification on appetite and energy regulation.
In addition to information on study population and design, the following information was extracted from each study to facilitate comparisons of clinical studies: 1) preload and single-meal studies [ED of the manipulated meal or preload and EI during the manipulated meal (single-meal studies) or the sum of preload EI and EI at the subsequent meal (preload studies)], 2) interventions of 1 d to 4 wk in duration [dietary ED and total daily EI (TDEI)], and 3) long-term studies (dietary ED and body weight change). Only main effects of ED were considered, although several studies examined interactions between ED and an additional factor (e.g., portion size, fat proportion, sex, dietary restraint, eating rate). Any relevant data reported in graphical rather than text format was estimated from figures.
Observational studies.
Twenty cross-sectional studies (15, 22–37, 91, 92, 94) and 7 prospective cohort studies with follow-up ranging from 6 mo to 8 y (26, 38–42, 90) examining associations between dietary ED and either body weight or BMI were identified (Tables 2 and 3). Twelve of 17 cross-sectional studies reported a positive association between ED and BMI or overweight/obesity (24, 27–29, 32–36, 91, 92, 94). An additional 3 studies noted an association between ED and BMI that was modified by sex: 2 studies reported a positive relation in women and no relation in men (25, 30) and the third reported a positive relation in certain age groups of men but no relation in women (31). Eight studies compared mean dietary ED between obese (BMI ≥30 kg/m2) and normal-weight (BMI <25 kg/m2) subjects. Mean dietary ED was significantly higher in the obese individuals in only 3 studies (30, 33, 34), with all studies observing an estimated difference of <0.06 kJ/g (0.25 kcal/g).
TABLE 2.
Cross-sectional studies examining associations between dietary ED calculated with and without the inclusion of beverages and weight status1
| Dietary ED |
Main findings |
|||||
| Authors, year (reference) | Population and age | Diet assessment method | Normal-weight(BMI <25 kg/m2) | ObeseBMI ≥30 kg/m2) | Food only | With beverages |
| kcal/g | ||||||
| Cox et al., 2000 (15) | 75 M/F; 43 y | FD | 1.36 | 1.40 | No differences in dietary ED between normal-weight and OB | No differences in dietary ED between normal-weight and OB2,3 |
| 0.762 | 0.832 | |||||
| 1.173 | 1.143 | |||||
| Cuco et al., 2001 (22) | 572 M/F; 25–65 y | FR | — | — | — | Dietary ED not associated with BMI3 |
| de Castro, 2004 (23) | 952 M/F; 35 y | FD | — | — | Dietary ED not associated with BMI | Dietary ED not associated with BMI2 |
| Esmaillzadeh et al., 2011 (91) | 486 F; 40–60 y | FFQ | — | — | BMI 2.5 kg/m2 higher and odds of high WC 4 times greater in highest vs. lowest ED tertile (>2.1 vs. < 1.6 kcal/g) | — |
| Hartline-Grafton et al., 2009 (24) | 348 F; 47 y | FR | 1.80 | 1.93 | Dietary ED (kJ/g) associated with BMI (β = 0.39 kg/m2) | — |
| Howarth et al., 2005 (25) | 1932 M/F; 20–60 y | FR | — | — | — | Dietary ED (kJ/g) associated with BMI in women (β = 0.33 kg/m2); no association in men4 |
| Howarth et al., 2006 (92) | 191,023 M/F; 45–75 | FFQ | — | — | — | Dietary ED [kJ/g] associated with BMI (β ∼ 1 kg/m2) and 4–34% increased risk of overweight depending on ethnicity |
| Kant and Graubard, 2006 (29) | 37,530 M/F; 25–74 y | FR | — | — | — | Dietary ED (kcal/g) associated with increased risk of obesity (β = 0.24)2,3 |
| Kant and Graubard, 2005 (28) | 13,017 M/F; ≥20 y | FR | 1.88 | 1.97 | Dietary ED (kJ/g) associated with BMI in men (β = 0.40 kg/m2) and women (β = 0.37 kg/m2) | Dietary ED not associated with BMI2 |
| 0.932 | 0.912 | Dietary ED (kJ/g) associated with BMI in men (β = 0.42 kg/m2) and women (β = 0.47 kg/m2)3 | ||||
| 1.283 | 1.343 | |||||
| Kant et al., 2008 (27) | 8265 M/F; ≥20 y | FR | — | — | Nonbreakfast dietary ED (kcal/g) associated with BMI in women (β = 0.95 kg/m2); trend for association in men (β = 0.30 kg/m2) | Nonbreakfast dietary ED (kcal/g) associated with BMI in women (β = 1.14 kg/m2); trend for association in men (β = 0.41 kg/m2)3 |
| Ledikwe et al., 2006 (30) | 7356 M/F; ≥20 y | FR | 1.87 | 1.95* | BMI 0.8 kg/m2 higher in highest vs. lowest dietary ED tertile (<1.6 vs. >2.0 kcal/g) in women; no association in men | — |
| Martí-Henneberg et al., 1999 (31) | 649 M/F; 21–65 y | FR | — | — | — | Positive correlation between BMI and dietary ED within some age groups in men (r = 0.29–0.44); no association in women2 |
| Mendoza et al., 2007 (32) | 9688 M/F; ≥20 y | FR | — | — | Standardized dietary ED (kcal/g) associated with BMI in men (β = 0.37 kg/m2) and women (β = 0.44 kg/m2) | — |
| Murakami et al., 2007 (94) | 1136 F; 18–22 y | FFQ | — | — | BMI 0.6 kg/m2 higher and WC 1.7 cm greater in highest vs. lowest ED tertile (1.2 vs. 1.7 kcal/g) | — |
| Raynor et al., 2011 (33) | 287 M/F; 18–65 y | FR | 1.60 | 1.83* | Dietary ED 0.2 kcal/g lower in weight-loss maintainers compared with individuals who had never been overweight and 0.4 kcal/g lower compared with overweight individuals | Dietary ED 0.1 kcal/g lower in weight-loss maintainers compared with individuals who had never been overweight and ∼0.2 kcal/g lower compared with overweight individuals2,3 |
| 0.912 | 1.012* | |||||
| 1.153 | 1.293* | |||||
| Stookey, 2001 (35) | 5783 M/F; 20–59 y | FR | 2.553,5 | 2.573,5,6 | — | Dietary ED (kJ/g) associated with increased odds of overweight (OR = 1.06)3,5 |
| Westerterp-Plantenga et al., 1996 (36) | 68 F; 33 y | FD | — | — | — | OB women consumed a greater proportion of total energy from high-ED foods (>3.6 kcal/g) (24% vs. 13%) and a lower proportion of energy from low-ED foods (<1.8 kcal/g) (24 vs. 38%) compared with NO women2 |
| Yao et al., 2003 (37) | 130 M/F; 35–49 y | FD | — | — | Dietary ED not associated with body fat percentage | — |
| Iqbal et al., 2006 (26) | 1762 (862 M/900 F); 45 y | FD | 0.932 | 0.872* | — | Dietary ED 0.06 kcal/g higher in normal-weight compared with OB men and women2 |
| Saquib et al., 2008 (34) | 2718 F; 53 y | FR | 1.41 | 1.57* | Dietary ED 0.16 kcal/g lower in normal-weight compared to overweight and OB women | — |
*Significantly different from normal-weight, P ≤ 0.05. ED, energy density; FD, food diary; FR, food recall; NO, nonobese (BMI <30 kg/m2); OB, obese; WC, waist circumference.
All foods and beverages were included.
All foods and energy-containing beverages were included.
All foods and beverages except water were included.
Only limited energy-containing beverages were included.
BMI ≥25 kg/m2.
TABLE 3.
Longitudinal associations between dietary ED calculated with and without the inclusion of beverages and weight change1
| Normal-weight (BMI <25 kg/m2) |
Overweight and obese (BMI ≥25 kg/m2) |
|||||
| Authors, year (reference) | Population, age, and follow-up | Diet assessment method | Food only | Beverages included | Food only | Beverages included |
| Bes-Rastrollo et al., 2008 (90) | 50,026 F | FFQ | Weight gain associated with increase in dietary ED; 1.0 kg greater weight gain in quintile of largest ED increase compared with ED decrease (1.0 vs. −0.1 kcal/g) | Weight gain associated with increase in dietary ED; 1.6 kg2 and 0.7 kg3 greater weight gain in quintile of largest ED increase compared with ED decrease (1.0 vs. −0.1 kcal/g) in nonobese and obese subjects | Weight gain associated with increase in dietary ED; 3.7 kg (overweight) and 3.9 kg (obese) greater weight gain in quintile of largest ED increase compared with no change in ED (∼1.0 vs. ∼0 kcal/g) | — |
| 24–44 y | ||||||
| 8 y | ||||||
| Du et al., 2009 (38) | 89,432 M/F | FFQ | BL dietary ED (kcal/g) associated with ∆weight (β = 0.03 kg/y) | — | BL dietary ED (kcal/g) associated with ∆weight (β = −0.1 kg/y) | — |
| 20–78 y | ||||||
| 6.5 y | ||||||
| Greene et al., 2006 (39) | 74 M/F | FD | — | — | BL dietary ED correlated with ∆weight (r = 0.24) | — |
| 50 y | ||||||
| 2 y | ||||||
| Iqbal et al., 2006 (26) | 1762 M/F | FD | — | BL dietary ED (kJ/g) associated with ∆weight in women (β = −0.12 kg/y); no association in men2 | — | BL dietary ED (kJ/g) associated with ∆weight in overweight (β = 0.14 kg/y) and obese women (β = 0.28 kg/y); no effect modification by BMI in men2 |
| 30–60 y | ||||||
| 5 y | ||||||
| Romaguera et al., 2010 (40) | 48,631 M/F | FFQ | BL dietary ED (kcal/g) associated with ∆waist circumference for a given BMI in nonobese and obese adults (β = 0.12 cm) | — | No effect modification by BMI | — |
| 50 y | ||||||
| 5.5 y | ||||||
| Savage et al., 2008 (41) | 168 F | FD | ∆Weight 0.65 kg/y greater in highest compared with lowest tertile of dietary ED (≥1.85 vs. ≤1.5 kcal/g) in nonobese and obese adults | — | No effect modification by BMI | — |
| 24–47 y | ||||||
| 6 y | ||||||
| Vergnaud et al., 2009 (42) | 2707 M/F | FD | ∆Weight not associated with BL dietary ED or ∆ED | ∆Weight not associated with BL dietary ED or ∆ED2 | ∆Weight 0.16 kg/y greater in highest vs. lowest tertile of BL dietary ED (1.6 vs. 1.2 kcal/g) | ∆Weight not associated with BL dietary ED2 |
| 35–60 y | ∆Weight 0.43 kg/y lower in tertile of largest decrease in dietary ED compared with tertile of increased dietary ED (−0.26 vs. 0.11 kcal/g) | ∆Weight 0.42 kg/y lower in tertile of largest decrease in dietary ED compared with tertile of increased dietary ED2 | ||||
| 6 y | ||||||
| Lindström et al., 2006 (44)5 | 500 M/F | FD | — | — | — | Weight loss 0.73 kg/y greater in lowest vs. highest quartile of mean dietary ED during follow-up (<0.8 vs. >1.0 kcal/g)4 |
| 40–64 y | ||||||
| 3 y | ||||||
| Ledikwe et al., 2007 (43)5 | 657 M/F | FR | Weight loss associated with decrease in dietary ED (kcal/g) (β = 2.2 kg); 3.5 kg greater weight loss in tertile of largest ED decrease compared with tertile of greatest ED increase (−0.9 vs. 0.2 kcal/g) in nonobese and obese subjects | — | — | — |
| ≥25 y | ||||||
| 6 mo | ||||||
BL, baseline; ED, energy density; FD, food diary; FR, food recall; ∆ED, EDpost − EDpre; ∆Weight, weightpost − weightpre.
All foods and beverages were included.
All foods and energy-containing beverages were included.
All foods and beverages except water were included.
Longitudinal analysis combining all groups within a randomized trial.
Differences in dietary intake assessment methods may underlie inconsistent findings. Food diaries were used in 5 studies, FFQs in 3, and 24-h food recalls in the other 12 (Table 2). Of the studies that used food diaries, 4 (80%) failed to observe a positive association between EI and BMI, whereas only 1 study (8%) that used 24-h recalls and no studies that used FFQs failed to find any evidence of a positive association.
Results from prospective cohort studies appeared to support a positive association between dietary ED and BMI or overweight/obesity but suggested that relations between ED and prospective weight change may be modified by weight status. Six studies explored whether associations between dietary ED and prospective weight change differed between normal-weight and overweight subjects. A positive association between ED and weight gain (41, 90) or change in waist circumference (38, 40) was reported in 4 studies. One study confirmed this association in a normal-weight cohort but also reported that higher dietary ED at baseline was associated with weight loss in an overweight cohort (38). In contrast, 2 studies documented positive relations between ED and weight gain in overweight cohorts but no association (42) or an inverse association (26) in a normal-weight cohort. Finally, 2 randomized trials examined associations between dietary ED and weight change during intentional weight loss by combining data from all subjects participating in the respective trials (43, 44). Lindström et al. (44) reported greater weight loss in individuals in the lowest compared with highest dietary ED quartile during the intervention. Similarly, Ledikwe et al. (43) reported that individuals having the largest reduction in dietary ED while enrolled in the PREMIER trial achieved a 3.5-kg greater weight loss over 6 mo compared with individuals with a slight increase in dietary ED during the trial.
Taken together, findings from observational studies are somewhat inconsistent but generally support a positive association between self-reported ED and both BMI status and weight gain. Nonetheless, causality cannot be determined from observational studies, and given the known biases in dietary reporting and the inconsistencies noted in cross-sectional studies, the possibility that the results were confounded by limitations in dietary assessment methodology cannot be ruled out.
Inclusion of beverages in ED calculations.
Several observational studies used multiple methods of calculating dietary ED by including or not including beverages and/or varying the types of beverages in the calculation. Although numerous methods have been examined [e.g., (14, 15)], the most common methods used for calculating dietary ED included food only, food and energy-containing beverages, food and all beverages except for water, and food and all beverages in the calculation. Associations between ED calculated by using any of these 4 methods and indicators of weight status were extracted from study reports allowing an examination of whether including beverages in the calculation of ED alters conclusions regarding associations between dietary ED and body weight.
As summarized in Tables 2 and 3, 5 cross-sectional (15, 23, 27, 28, 33) and 2 cohort (42, 90) studies reported associations between dietary ED and body weight when ED was calculated both with and without the inclusion of beverages. The direction and statistical significance of the associations reported in these studies were generally not altered by the method used to calculate ED.
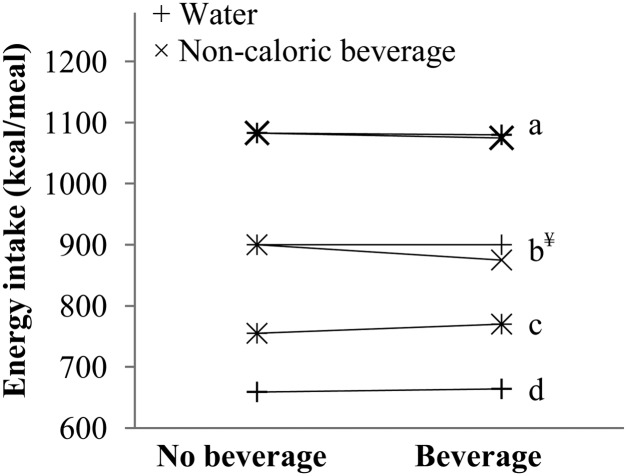
Single-meal crossover studies examining the effect of including noncaloric beverages with a meal on ad libitum EI are also relevant (56, 115–117). As recently reviewed by Daniels and Popkin (118), and summarized in Figure 1, these studies demonstrated that, relative to a no-beverage control condition, lowering the ED of an ad libitum meal by including noncaloric beverages had no effect on EI. Furthermore, decreasing the ED of a meal by including caloric beverages with the meal resulted in increases in EI that approximated the energy content of the beverage consumed (115, 117), suggesting that individuals do not eat less food to compensate for energy added by beverages. These findings are in contrast to clinical studies in which ED is manipulated by altering food composition (discussed below) and suggest that any influence of ED on EI may only occur when the change is within the nonbeverage components of a meal. Therefore, including beverage consumption in ED calculations may potentially bias associations between ED and weight status toward the null, and based on evidence to date, the effects of ED on EI (discussed below) are most readily seen in studies in which ED is calculated without the inclusion of beverages. An approach that was recently suggested is to analyze beverages separately or as a covariate for effects on EI (8).
FIGURE 1.
Plot of crossover studies examining the effect of reducing energy density through the addition of noncaloric beverages to meals on ad libitum energy intake. ¥Small beverage portion compared with large beverage portion. Data from references a115, b116, c117, and d56.
Effects of ED manipulation on EI in clinical studies.
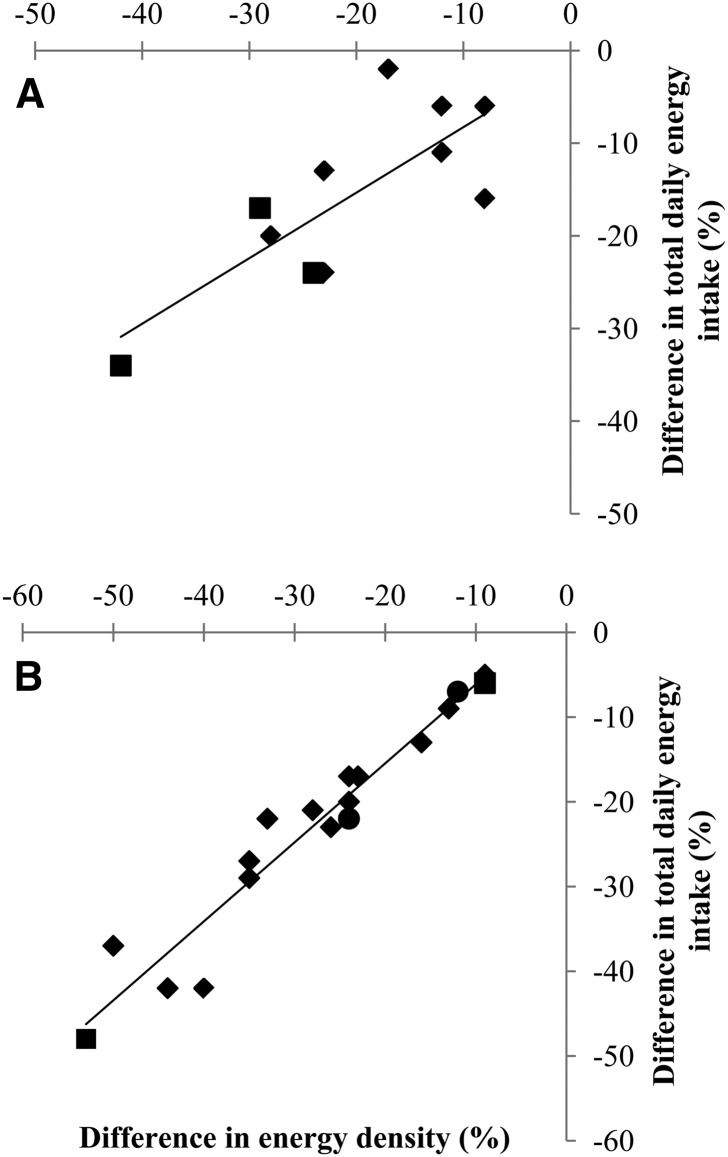
Twenty-eight preload and single-meal studies were included in this review (45–65, 95, 97–102). These studies typically provided volume, mass, or energy-matched preloads or meals and measured ad libitum EI at subsequent meals or EI during a single ad libitum meal. Nine clinical studies ranging in duration from 1 to 3 d (66–73, 103) and 14 studies ranging from 3 d to 3 wk (74–82, 107, 108, 110–112) in duration were also identified and are summarized in Figure 2. The 1-d to 3-wk studies typically followed a crossover design, most implemented a washout period between interventions, and dietary ED was manipulated by altering the ED of a portion of the diet or of all foods provided to participants. Whereas preload and single-meal designs were deemed useful for evaluating effects of ED on satiation and satiety, provided-food short-term studies were deemed useful for determining the efficacy of manipulating ED to alter TDEI. The methods used to vary ED included manipulation of fat proportion, incorporation of water into food products, addition of water-rich foods, and/or use of artificial sweeteners or fat mimetics. Most, but not all, studies controlled for food palatability.
FIGURE 2.
Plots of crossover studies examining the effect of reducing dietary ED on TDEI over 1–3 d (A) and 3 d to 3 wk (B). TDEI and dietary ED were extracted for each study. ΔTDEI were calculated by subtracting TDEI during TDEIHED from TDEI during the lowest ED condition or from TDEI during an intermediate ED condition. The percentage difference in TDEI was calculated as ΔTDEI/TDEIHED × 100. Differences in dietary ED were calculated by subtracting the ED of the total diet during EDHED from the ED of the total diet during the lowest or intermediate ED condition. The percentage difference in ED was calculated as ΔED/EDHED × 100. ♦, nonobese; ●, obese; ▪, nonobese and obese. ED, energy density; ΔED, difference in energy density; EDHED, ED of the highest ED condition; TDEI, total daily energy intake; ΔTDEI, differences in total daily energy intake; TDEIHED, TDEI of the highest energy density condition.
In 23 of the 28 included preload and single-meal studies, EI was less in the lowest-ED condition relative to the highest-ED condition irrespective of whether the preloads were volume-matched (i.e., energy content differed between treatments) or were isoenergetic (i.e., energy content was the same between treatments). There was no apparent difference in the responses of nonobese and obese subjects to ED manipulation. In no study did EI during the low-ED condition exceed EI during the high-ED condition.
There was notable consistency in the results from the 1- to 3-d and the 3-d to3-wk studies (Fig. 2). Lower ED interventions consistently resulted in decreased ad libitum TDEI in both nonobese and obese individuals. It is noteworthy that linear relations between the percentage difference in ED (%∆ED) and the percentage difference in TDEI (%∆TDEI) were observed. Moreover, the magnitude of the change in TDEI was also substantial. For example, results from the 3-d to 3-wk studies indicate that a 25% reduction in dietary ED can be expected to result in ∼20% reduction in TDEI (Fig. 2B), an amount that would have a substantial impact on body weight if sustained over time (119).
A number of conclusions can be drawn on the basis of the body of evidence from the short-term clinical trials reviewed. First, in controlled laboratory environments, low-ED foods are more satiating than comparable high-ED foods. In other words, per unit of energy, lower-ED foods acutely suppress appetite to a greater extent than higher-ED foods. Second, when provided in controlled environments, the appetite-suppressing effects of lower-ED relative to higher-ED foods persist for at least 3 wk. This effect results in lower TDEI when lower-ED foods are substituted for higher-ED foods. Third, the consistent magnitude and direction of the relation between ED and TDEI (Fig. 2) suggest that these effects are independent of the methods used to alter ED.
The relevance of the short-term controlled studies reviewed herein to long-term energy balance regulation has been questioned (10, 11). It has been argued that, over time, individuals learn to restrict intake of higher-ED foods, thereby mitigating any impact of ED on body weight (10, 11). Providing covertly manipulated and unfamiliar foods to study participants is hypothesized to uncouple learned sensory cues from the nutritional properties of foods and thereby increase the influence of food weight and volume on EI (120). Over time, individuals may learn to compensate for reductions in ED by eating more food and/or seeking higher ED foods. The time period of the studies reviewed above may have been insufficient for individuals to learn to compensate for ED manipulation. Furthermore, in free-living environments, individuals can adjust EI by selecting from foods varying widely in ED. However, the range of the ED of foods in provided-food studies is commonly less than what is available to free-living individuals, which may prevent study participants from fully compensating for reduced dietary ED (120). As such, the controlled clinical trials reviewed herein establish the efficacy of ED manipulation for altering EI. However, these studies do not themselves address the sustainability of changes in dietary ED in free-living subjects, and therefore cannot demonstrate the effectiveness of low ED diets for weight control.
Long-term clinical studies of dietary ED reduction and body weight.
A third and most important category of studies examined effects of interventions that altered dietary ED on body weight change (Table 4). Thirteen clinical studies of >1 mo duration were identified in which a dietary intervention was conducted, a change in body weight measured, and the intervention was determined to result in a quantifiable change in dietary ED (34, 43, 83, 85–89, 93, 96, 104–106).
TABLE 4.
Randomized controlled trials of ≥1 mo in duration examining effects of reducing dietary ED on body weight change in adults1
| Authors, year (reference) | Population | Duration | Intervention | ∆ED | ∆Weight |
| kcal/g | kg | ||||
| Energy restriction recommended | |||||
| de Oliveira et al., 2008 (96) | n = 49 nonobese and obese F | 7 wk | Random assignment to 1 of 3 groups: | ||
| Apple: Add 3 apples/d to usual diet | Apple: −0.2a | Apple: −1.3a | |||
| Pear: Add 3 pears/d to usual diet | Pear: −0.3a | Pear: −2.2a | |||
| Oat: Add 3 oat cookies/d to usual diet | Oat: +1.0b | Oat: −0.7b | |||
| Ledikwe et al., 2007 (43) | n = 658 nonobese and obese M/F | 6 mo | Random assignment to 1 of 2 weight-loss interventions: | ||
| Control: One weight-loss education session | Control: −0.2a | Control: −1.1a | |||
| RF: Extensive reduced-fat diet counseling | RF: −0.3a | RF: −5.1b | |||
| RF+D: Extensive reduced-fat diet and DASH diet counseling | RF+D: −0.6b | RF+D: −6.1b | |||
| Raynor et al., 2012 (89) | n = 29 nonobese & obese M/F | 3 mo | Random assignment to 1 of 2 interventions: | ||
| LE+LF: Low-kcal + low-fat diet education | LE+LF: −0.7 | LE+LF: −7.7a | |||
| LED+LE+LF: Low-ED + low-kcal + low-fat diet intervention | LED+LE+LF: −0.7* | LED+LE+LF: −5.7b | |||
| Rolls et al., 2005 (86) | n = 200 nonobese and obese M/F | 12 mo | Random assignment to 1 of 4 interventions during weight loss (6 mo) and weight-loss maintenance (6 mo) | 6 mo | 6 mo |
| Control: No additional intervention | C: −0.5a | C: −9.0a | |||
| S1: Eat 1 serving/d of low-ED soup | S1: −0.6b | S1: −7.9ab | |||
| S2: Eat 2 servings/d of low-ED soup | S2: −0.8c | S2: −7.6ab | |||
| HS: Eat 2 servings/d of high-ED snacks | HS: −0.2d | HS: −6.1b | |||
| 12 mo | 12 mo | ||||
| C: −0.3a | C: −8.1a | ||||
| S1: −0.5b | S1: −6.1ab | ||||
| S2: −0.6b | S2: −7.2a | ||||
| HS: −0.1c | HS: −4.8b | ||||
| Weight-loss maintenance | |||||
| Due et al., 2008 (104) | n = 106 nonobese and obese M/F | 6 mo | Random assignment to 1 of 3 weight-loss maintenance interventions: | ||
| Control: 35% of energy from fat | Control: 02,a | Control: +3.8 | |||
| LF: 20–30% of energy from fat | LF: −0.7b | LF: +2.2 | |||
| HF: 35–45% of energy from fat | HF: +0.3a | HF: +2.5 | |||
| Lowe et al., 2008 (93) | n = 103 nonobese and obese M/F | 14 wk | Random assignment to 1 of 3 weight-loss maintenance interventions: | ||
| CB: received cognitive behavior therapy | CB: −0.13,a | CB: −2.9% | |||
| SM: received enhanced self-monitoring training | SM: −0.1a | SM: −2.4% | |||
| SM+ED: received ESM + low-ED diet education | SM+ED: −0.4b,* | SM+ED: −2.7% | |||
| No recommendation to restrict energy intake | |||||
| Bray et al., 2002 (106) | n = 36; nonobese and obese M | 9 mo | Random assignment to 1 of 3 interventions; all food provided throughout: | ||
| Control: 33% of energy from fat | Control: 0 | Control: −3.8 | |||
| FR: Fat-reduced diet, 25% of energy from fat | FR: −0.1 | FR: −1.8 | |||
| FS: Fat-substituted diet, 25% of energy from fat + 33 g/d olestra | FS: −0.2 | FS: −6.3 | |||
| Ello-Martin et al., 2007 (83) | n = 71 obese F | 12 mo | Random assignment to 1 of 2 groups counseled to reduce dietary ED by: | ||
| LF: Reducing dietary fat intake | LF: −0.36a | LF: −6.4a | |||
| LF+FV: Reducing dietary fat intake + increasing fruit and vegetable intake | LF+FV: −0.41b | LF+FV: −7.9b | |||
| Raben et al., 2002 (85) | n = 41 nonobese M/F | 10 wk | Random assignment to 1 of 2 groups incorporating, into habitual diets, beverages and foods containing: | ||
| Asp: Aspartame | Asp: −0.23,a | Asp: −1.0a | |||
| Suc: Sucrose | Suc: 0.0b | Suc: +1.6b | |||
| Raynor et al., 2012 (89) | n = 29; nonobese and obese M/F | 3 mo | Random assignment to 1 of 2 interventions: | ||
| LED: Low-ED education (no energy restriction recommendation) | LED: −0.9 | LED: −9.3a | |||
| LE+LF: Low kcal + low fat diet education | LE+LF: −0.7* | LE+LF: −7.7a | |||
| Roy et al., 2002 (105) | n = 15 nonobese and obese F | 10–12 wk | Random assignment to 1 of 3 interventions; all food provided throughout: | ||
| Control: 40% of energy from fat | Control: 0a | Control: −2.5a | |||
| FR: Fat-reduced diet, 31% of energy from fat | FR: −0.3b | FR: −3.0a | |||
| FS: Fat-substituted diet, 31% of energy from fat + 30 g/d olestra | FS: −0.7b | FS: −5.0b | |||
| Saquib et al., 2008 (34) | n = 2718 nonobese and obese F | 4 y | Random assignment to 1 of 2 groups: | ||
| Control: Received print education materials | C: +0.05a | C: +1.4 | |||
| FV: Counseled to follow a high-fiber, fruit and vegetable, and low-fat diet | FV: −0.2b | FV: +1.8 | |||
| Saris et al., 2000 (87) | n = 398 obese M/F | 6 mo | Random assignment to 1 of 3 groups: | ||
| Control: Diet of typical macronutrient content | C: −0.06a | C: +0.8a | |||
| LF+SC: Low-fat + high simple-CHO diet | LF+SC: −0.10b | LF+SC: −0.9b | |||
| LF+CC: Low-fat + high complex-CHO diet | LF+CC: −0.18c | LF+CC: −1.8b | |||
| Westerterp- Plantenga et al., 1998 (88) | n = 40 nonobese M/F | 6 mo | Restrained (R) and unrestrained (UR) eaters randomly assigned to: | RF-R: −0.1 | RF-R: −1.5 |
| RF: Reduced-fat diet | RF-UR: −0.1 | RF-UR: −0.2 | |||
| FF: Full-fat diet | FF-R: +0.1 | FF-R: +0.2 | |||
| FF-UR: +0.1 | FF-UR: +1.8 |
CHO, carbohydrate; DASH, Dietary Approaches to Stop Hypertension; ED, energy density; ∆, change. Within a column, means without a common superscript letter are significantly different. *Significance not altered by inclusion or exclusion of energy-containing beverages in ED calculation.
Baseline ED not provided.
Energy-containing beverages included in ED calculation.
Study designs and interventions were heterogeneous. Few interventions focused specifically on dietary ED (83, 89, 93). Rather, the majority of interventions were designed to decrease (e.g., dietary fat) or increase (e.g., fruits and vegetables) consumption of diet components that influence ED. Nine studies explicitly recommended dietary fat reduction (34, 43, 83, 87–89, 104–106), with 3 of those also emphasizing increased consumption of dietary fiber and low-ED fruits and vegetables (34, 43, 83). Eight studies provided food to participants: 2 used fat mimetics and provided all food to study participants (105, 106), 3 provided a selection of reduced-fat or full-fat foods (87, 88, 104), 2 provided single low-ED or high-ED foods (86, 96, 104), and 1 provided sucrose-containing or artificially sweetened foods and beverages (85). Three studies included recommendations to restrict EI (43, 86, 104), 2 were designed to examine maintenance of weight loss (93, 104), 7 made no EI recommendations (34, 83, 85, 87, 88, 105, 106), and 1 included groups receiving separate instructions with respect to EI (89). Self-reported dietary intake was used to calculate ED in all but the 2 studies in which all food was provided.
Study results were inconsistent, with those that implemented interventions focused specifically on reducing dietary ED reporting favorable (83, 89), attenuated (89), or no (93) effects on body weight. Furthermore, findings from long-term studies appeared to be associated less with the method used to alter dietary ED and more with recommendations regarding EI restriction. When reductions in dietary ED were not coupled with recommendations to restrict EI, a modest reduction in body weight of ∼2 kg more than the comparison group over 10 wk to 1 y was observed in 6 of 8 studies (83, 85, 87, 88, 105, 106), although differences reached statistical significance in only 4 studies (83, 87, 88, 105). Findings from studies in which recommendations to reduce ED were coupled with recommendations to restrict EI were less consistent (43, 86, 96). Ledikwe et al. (43) documented a 1.7-kJ/g (0.4-kcal/g) greater reduction in dietary ED and 5-kg greater weight loss over 6 mo in individuals receiving extensive counseling on both weight loss and the Dietary Approaches to Stop Hypertension (DASH) diet compared with a control group who received only 1 diet education session. However, when compared with a third group who received the weight-loss counseling but not the DASH diet counseling, the weight loss + DASH group did not lose more weight despite a 1.3-kJ/g (0.3-kcal/g) greater reduction in dietary ED (43). The absence of an effect suggests that the more involved counseling rather than the reduction in dietary ED underpinned differences in weight loss between the DASH and control groups. In separate studies, de Oliveira et al. (96) and Rolls et al. (86) provided evidence that adding high-ED foods to energy-restricted diets may attenuate weight loss. However, Rolls et al. failed to demonstrate an added weight-loss benefit of reducing dietary ED (by adding soups to the daily diet) relative to a control condition (86). In a pilot study, Raynor et al. (89) reported that adding low-ED diet education to recommendations to restrict energy and reduce fat intake attenuated weight loss and did not impact dietary ED. Two studies examining diet modification for maintaining weight loss did not show any effect of altering dietary ED on weight-loss maintenance (93, 104).
Taken together, the long-term trials reviewed herein provide some evidence that lowering dietary ED may promote small spontaneous reductions in body weight when ad libitum consumption is recommended. The studies do not provide consistent support for the hypothesis that lower-ED diets are more effective for weight loss than EI restriction alone but suggest that adding high-ED foods to energy-restricted diets could attenuate weight loss. None of the studies suggest that reducing dietary ED promotes weight gain. However, the number of studies is small, their interventions heterogeneous, and further work in this area is needed.
Summary of the evidence.
Different approaches to calculating dietary ED that vary according to beverage inclusion criteria do not appear to alter the direction of reported relations between ED and body weight status. Nevertheless, given that EI is influenced by both food and beverage consumption, but that beverages appear to have little effect on appetite (121), it is recommended that future studies of ED–energy balance interrelations routinely analyze results with calculations of ED both for food-only and food + all beverages (caloric and noncaloric) so that the effects of beverages in the calculation of dietary ED can be further evaluated. Standardization of semisolid products such as milkshakes and drinkable yogurts as a food or beverage is needed to ensure consistency across studies.
A substantial number of short-term studies providing food and manipulating ED have been conducted. A considerable portion of this work (56–62, 66–68, 71, 72) was produced by a single research laboratory. Nonetheless, similar studies have been completed by other groups, and when the total body of evidence is considered, a strong positive association between ED and ad libitum EI is consistently observed. This association provides evidence that lowering dietary ED is efficacious for reducing TDEI and provides indirect evidence that lower-ED diets may be efficacious for weight management. This relation appears to be independent of the method used to alter ED.
Prospective cohort studies suggest a positive association between ED and weight change. However, there are relatively few long-term interventions that have implemented interventions focused specifically on reducing dietary ED. Studies reporting changes in dietary ED and body weight resulting from interventions that aim to increase or decrease consumption of dietary determinants of ED have provided some evidence relevant to determining the effectiveness of reducing dietary ED for healthy weight management. In long-term studies that recommend ad libitum consumption of low-ED diets, a modest reduction in body weight is generally observed. In long-term studies that recommend energy restriction for weight loss, no consistent benefit of consuming a lower-ED diet beyond energy restriction alone is observed. These modest effects of dietary ED reduction on body weight are surprising considering the consistent, robust effects of ED manipulation on EI observed in shorter trials. One potential interpretation of these data are that less-energy-dense diets consumed ad libitum may be effective for prevention of weight gain but may not confer advantage for weight loss. Others have suggested that, over time, individuals learn to eat smaller portions of high-ED foods or a greater total amount of food in response to dietary ED reduction, thereby mitigating long-term effects of ED manipulation on body weight (10, 120). Possibly, more substantial reductions in dietary ED need to be achieved to demonstrate weight-loss benefit. However, the lack of an adequate number of long-term interventions specifically focused on ED manipulation and the inconsistencies between prospective and short-term studies relative to longer-term studies suggest that more research is needed before definitive conclusions regarding the effectiveness of low-ED diets can be made.
Future Directions
Substantial effort has been devoted to evaluating the short-term effects of ED on EI. However, a key barrier to progress in understanding the role of ED in weight regulation is the lack of data from multiple, long-term clinical trials examining the effectiveness of low-ED diet education for the prevention of weight gain and weight regain in nonobese individuals (a separate topic) and for promoting weight loss in obese and overweight individuals. Although not included in this review, a lack of long-term trials conducted in children and adolescents is also evident (13). Moreover, there are few data from long-term randomized controlled trials providing food so that the results are not confounded by inaccurate self-reports of EI and so that the efficacy of ED manipulation for long-term weight management can be definitively determined. Prioritization of funding for clinical trials that provide food is needed to understand the efficacy of ED for healthy weight management. Such studies should monitor all beverage consumption by study participants so that the question of whether beverage intakes should be included in calculations of ED can be resolved. In lieu of such trials, interventions targeting ED specifically rather than dietary components related to ED (e.g., reducing fat intake) would inform on the effectiveness of low-ED diet education for weight management. It would also be useful for all long-term dietary intervention trials examining weight loss or weight maintenance to report dietary ED. Ideally, these trials should assess both food and total beverage intake.
Studies are also needed to examine psychological and physiologic mechanisms underpinning relations between ED and EI, and how these relations change when attempting to reduce dietary ED, to provide support for or against the postulated effectiveness of low-ED diets for healthy weight management (13). In addition, studies examining barriers to adoption of low-ED diets in free-living subjects would help provide necessary information for adapting low-ED eating plans to community interventions should randomized controlled trials confirm that such diets are efficacious for healthy weight management.
Acknowledgments
Both of the authors contributed to the writing, editing, and revision of the manuscript.
Footnotes
Abbreviations used: DASH, Dietary Approaches to Stop Hypertension; ED, energy density; EI, energy intake; TDEI, total daily energy intake.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among us adults, 1999–2008. JAMA 2010;303:235–41. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815–25. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004;291:1238–45. [DOI] [PubMed] [Google Scholar]
- 4.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr 2011;93:836–43. [DOI] [PubMed] [Google Scholar]
- 5.Briefel RR, Johnson CL. Secular trends in dietary intake in the united states. Annu Rev Nutr 2004;24:401–31. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res 2002;10:370–8. [DOI] [PubMed] [Google Scholar]
- 7.Drewnowski A, Almiron-Roig E, Marmonier C, Lluch A. Dietary energy density and body weight: is there a relationship? Nutr Rev 2004;62:403–13. [DOI] [PubMed] [Google Scholar]
- 8.Johnson L, Wilks DC, Lindroos AK, Jebb SA. Reflections from a systematic review of dietary energy density and weight gain: is the inclusion of drinks valid? Obes Rev 2009;10:681–92. [DOI] [PubMed] [Google Scholar]
- 9.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav 2009;97:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stubbs RJ, Whybrow S. Energy density, diet composition and palatability: influences on overall food energy intake in humans. Physiol Behav 2004;81:755–64. [DOI] [PubMed] [Google Scholar]
- 11.Westerterp-Plantenga MS. Effects of energy density of daily food intake on long-term energy intake. Physiol Behav 2004;81:765–71. [DOI] [PubMed] [Google Scholar]
- 12.Yao M, Roberts SB. Dietary energy density and weight regulation. Nutr Rev 2001;59:247–58. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Escamilla R, Obbagy JE, Altman JM, Essery EV, McGrane MM, Wong YP, Spahn JM, Williams CL. Dietary energy density and body weight in adults and children: a systematic review. J Acad Nutr Diet. 2012;112:671–84. [DOI] [PubMed] [Google Scholar]
- 14.Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr 2005;135:273–8. [DOI] [PubMed] [Google Scholar]
- 15.Cox DN, Mela DJ. Determination of energy density of freely selected diets: methodological issues and implications. Int J Obes Relat Metab Disord 2000;24:49–54. [DOI] [PubMed] [Google Scholar]
- 16.Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev 2006;86:651–67. [DOI] [PubMed] [Google Scholar]
- 17.Roberts SB, Fuss P, Heyman MB, Evans WJ, Tsay R, Rasmussen H, Fiatarone M, Cortiella J, Dallal GE, Young VR. Control of food intake in older men. JAMA 1994;272:1601–6. [DOI] [PubMed] [Google Scholar]
- 18.Roberts SB. Effects of aging on energy requirements and the control of food intake in men. J Gerontol A Biol Sci Med Sci 1995;50:101–6. [DOI] [PubMed] [Google Scholar]
- 19.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev 2001;59:129–39. [DOI] [PubMed] [Google Scholar]
- 20.Slavin J, Green H. Dietary fibre and satiety. Nutr Bull 2007;32:32–42. [Google Scholar]
- 21.Wanders AJ, van den Borne JJ, de Graaf C, Hulshof T, Jonathan MC, Kristensen M, Mars M, Schols HA, Feskens EJ. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev 2011;12:724–39. [DOI] [PubMed] [Google Scholar]
- 22.Cucó G, Arija V, Marti-Henneberg C, Fernandez-Ballart J. Food and nutritional profile of high energy density consumers in an adult Mediterranean population. Eur J Clin Nutr 2001;55:192–9. [DOI] [PubMed] [Google Scholar]
- 23.de Castro JM. Dietary energy density is associated with increased intake in free-living humans. J Nutr 2004;134:335–41. [DOI] [PubMed] [Google Scholar]
- 24.Hartline-Grafton HL, Rose D, Johnson CC, Rice JC, Webber LS. Energy density of foods, but not beverages, is positively associated with body mass index in adult women. Eur J Clin Nutr 2009;63:1411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howarth NC, Huang TT, Roberts SB, McCrory MA. Dietary fiber and fat are associated with excess weight in young and middle-aged US adults. J Am Diet Assoc 2005;105:1365–72. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal SI, Helge JW, Heitmann BL. Do energy density and dietary fiber influence subsequent 5-year weight changes in adult men and women? Obesity (Silver Spring) 2006;14:106–14. [DOI] [PubMed] [Google Scholar]
- 27.Kant AK, Andon MB, Angelopoulos TJ, Rippe JM. Association of breakfast energy density with diet quality and body mass index in American adults: National Health and Nutrition Examination Surveys, 1999–2004. Am J Clin Nutr 2008;88:1396–404. [DOI] [PubMed] [Google Scholar]
- 28.Kant AK, Graubard BI. Energy density of diets reported by American adults: association with food group intake, nutrient intake, and body weight. Int J Obes (Lond) 2005;29:950–6. [DOI] [PubMed] [Google Scholar]
- 29.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr 2006;84:1215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledikwe JH, Blanck HM, Kettel Khan L, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr 2006;83:1362–8. [DOI] [PubMed] [Google Scholar]
- 31.Martí-Henneberg C, Capdevila F, Arija V, Perez S, Cuco G, Vizmanos B, Fernandez-Ballart J. Energy density of the diet, food volume and energy intake by age and sex in a healthy population. Eur J Clin Nutr 1999;53:421–8. [DOI] [PubMed] [Google Scholar]
- 32.Mendoza JA, Drewnowski A, Christakis DA. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 2007;30:974–9. [DOI] [PubMed] [Google Scholar]
- 33.Raynor HA, Van Walleghen EL, Bachman JL, Looney SM, Phelan S, Wing RR. Dietary energy density and successful weight loss maintenance. Eat Behav 2011;12:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saquib N, Natarajan L, Rock CL, Flatt SW, Madlensky L, Kealey S, Pierce JP. The impact of a long-term reduction in dietary energy density on body weight within a randomized diet trial. Nutr Cancer 2008;60:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stookey JD. Energy density, energy intake and weight status in a large free-living sample of Chinese adults: exploring the underlying roles of fat, protein, carbohydrate, fiber and water intakes. Eur J Clin Nutr 2001;55:349–59. [DOI] [PubMed] [Google Scholar]
- 36.Westerterp-Plantenga MS, Pasman WJ, Yedema MJ, Wijckmans-Duijsens NE. Energy intake adaptation of food intake to extreme energy densities of food by obese and non-obese women. Eur J Clin Nutr 1996;50:401–7. [PubMed] [Google Scholar]
- 37.Yao M, McCrory MA, Ma G, Tucker KL, Gao S, Fuss P, Roberts SB. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr 2003;77:1409–16. [DOI] [PubMed] [Google Scholar]
- 38.Du H, van der A DL, Ginder V, Jebb SA, Forouhi NG, Wareham NJ, Halkjaer J, Tjonneland A, Overvad K, Jakobsen MU, et al. Dietary energy density in relation to subsequent changes of weight and waist circumference in European men and women. PLoS ONE 2009;4:e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene LF, Malpede CZ, Henson CS, Hubbert KA, Heimburger DC, Ard JD. Weight maintenance 2 years after participation in a weight loss program promoting low-energy density foods. Obesity (Silver Spring) 2006;14:1795–801. [DOI] [PubMed] [Google Scholar]
- 40.Romaguera D, Angquist L, Du H, Jakobsen MU, Forouhi NG, Halkjaer J, Feskens EJ, van der A DL, Masala G, Steffen A, et al. Dietary determinants of changes in waist circumference adjusted for body mass index—a proxy measure of visceral adiposity. PLoS ONE 2010;5:e11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savage JS, Marini M, Birch LL. Dietary energy density predicts women's weight change over 6 y. Am J Clin Nutr 2008;88:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vergnaud AC, Estaquio C, Czernichow S, Peneau S, Hercberg S, Galan P, Bertrais S. Energy density and 6-year anthropometric changes in a middle-aged adult cohort. Br J Nutr 2009;102:302–9. [DOI] [PubMed] [Google Scholar]
- 43.Ledikwe JH, Rolls BJ, Smiciklas-Wright H, Mitchell DC, Ard JD, Champagne C, Karanja N, Lin PH, Stevens VJ, Appel LJ. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr 2007;85:1212–21. [DOI] [PubMed] [Google Scholar]
- 44.Lindström J, Peltonen M, Eriksson JG, Louheranta A, Fogelholm M, Uusitupa M, Tuomilehto J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 2006;49:912–20. [DOI] [PubMed] [Google Scholar]
- 45.Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, Williamson DA. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite 2010;55:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang UJ, Hong YH, Suh HJ, Jung EY. Lowering the energy density of parboiled rice by adding water-rich vegetables can decrease total energy intake in a parboiled rice-based diet without reducing satiety on healthy women. Appetite 2010;55:338–42. [DOI] [PubMed] [Google Scholar]
- 47.de Graaf C, Hulshof T. Effects of weight and energy content of preloads on subsequent appetite and food intake. Appetite 1996;26:139–51. [DOI] [PubMed] [Google Scholar]
- 48.Drewnowski A, Massien C, Louis-Sylvestre J, Fricker J, Chapelot D, Apfelbaum M. Comparing the effects of aspartame and sucrose on motivational ratings, taste preferences, and energy intakes in humans. Am J Clin Nutr 1994;59:338–45. [DOI] [PubMed] [Google Scholar]
- 49.Gray R, French S, Robinson T, Yeomans M. Dissociation of the effects of preload volume and energy content on subjective appetite and food intake. Physiol Behav 2002;76:57–64. [DOI] [PubMed] [Google Scholar]
- 50.Hogenkamp PS, Mars M, Stafleu A, de Graaf C. Intake during repeated exposure to low- and high-energy-dense yogurts by different means of consumption. Am J Clin Nutr 2010;91:841–7. [DOI] [PubMed] [Google Scholar]
- 51.Latner JD, Rosewall JK, Chisholm AM. Energy density effects on food intake, appetite ratings, and loss of control in women with binge eating disorder and weight-matched controls. Eat Behav 2008;9:257–66. [DOI] [PubMed] [Google Scholar]
- 52.Louis-Sylvestre J, Tournier A, Chapelot D, Chabert M. Effect of a fat-reduced dish in a meal on 24-h energy and macronutrient intake. Appetite 1994;22:165–72. [DOI] [PubMed] [Google Scholar]
- 53.Mars M, Hogenkamp PS, Gosses AM, Stafleu A, De Graaf C. Effect of viscosity on learned satiation. Physiol Behav 2009;98:60–6. [DOI] [PubMed] [Google Scholar]
- 54.Mazlan N, Horgan G, Stubbs RJ. Energy density and weight of food effect short-term caloric compensation in men. Physiol Behav 2006;87:679–86. [DOI] [PubMed] [Google Scholar]
- 55.Norton GN, Anderson AS, Hetherington MM. Volume and variety: relative effects on food intake. Physiol Behav 2006;87:714–22. [DOI] [PubMed] [Google Scholar]
- 56.Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. Am J Clin Nutr 1999;70:448–55. [DOI] [PubMed] [Google Scholar]
- 57.Rolls BJ, Castellanos VH, Halford JC, Kilara A, Panyam D, Pelkman CL, Smith GP, Thorwart ML. Volume of food consumed affects satiety in men. Am J Clin Nutr 1998;67:1170–7. [DOI] [PubMed] [Google Scholar]
- 58.Rolls BJ, Kim-Harris S, Fischman MW, Foltin RW, Moran TH, Stoner SA. Satiety after preloads with different amounts of fat and carbohydrate: implications for obesity. Am J Clin Nutr 1994;60:476–87. [DOI] [PubMed] [Google Scholar]
- 59.Rolls BJ, Laster LJ, Summerfelt A. Hunger and food intake following consumption of low-calorie foods. Appetite 1989;13:115–27. [DOI] [PubMed] [Google Scholar]
- 60.Rolls BJ, Roe LS. Effect of the volume of liquid food infused intragastrically on satiety in women. Physiol Behav 2002;76:623–31. [DOI] [PubMed] [Google Scholar]
- 61.Rolls BJ, Roe LS, Meengs JS. Salad and satiety: energy density and portion size of a first-course salad affect energy intake at lunch. J Am Diet Assoc 2004;104:1570–6. [DOI] [PubMed] [Google Scholar]
- 62.Rolls BJ, Roe LS, Meengs JS. Portion size can be used strategically to increase vegetable consumption in adults. Am J Clin Nutr 2010;91:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Specter SE, Bellisle F, Hemery-Veron S, Fiquet P, Bornet FR, Slama G. Reducing ice cream energy density does not condition decreased acceptance or engender compensation following repeated exposure. Eur J Clin Nutr 1998;52:703–10. [DOI] [PubMed] [Google Scholar]
- 64.Yeomans MR, Gould NJ, Leitch M, Mobini S. Effects of energy density and portion size on development of acquired flavour liking and learned satiety. Appetite 2009;52:469–78. [DOI] [PubMed] [Google Scholar]
- 65.Yeomans MR, Weinberg L, James S. Effects of palatability and learned satiety on energy density influences on breakfast intake in humans. Physiol Behav 2005;86:487–99. [DOI] [PubMed] [Google Scholar]
- 66.Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr 1998;67:412–20. [DOI] [PubMed] [Google Scholar]
- 67.Bell EA, Rolls BJ. Energy density of foods affects energy intake across multiple levels of fat content in lean and obese women. Am J Clin Nutr 2001;73:1010–8. [DOI] [PubMed] [Google Scholar]
- 68.Blatt AD, Roe LS, Rolls BJ. Hidden vegetables: an effective strategy to reduce energy intake and increase vegetable intake in adults. Am J Clin Nutr 2011;93:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devitt AA, Mattes RD. Effects of food unit size and energy density on intake in humans. Appetite 2004;42:213–20. [DOI] [PubMed] [Google Scholar]
- 70.Durrant ML, Royston JP, Wloch RT, Garrow JS. The effect of covert changes in energy density of preloads on subsequent ad libitum energy intake in lean and obese human subjects. Hum Nutr Clin Nutr 1982;36:297–306. [PubMed] [Google Scholar]
- 71.Kral TV, Roe LS, Rolls BJ. Does nutrition information about the energy density of meals affect food intake in normal-weight women? Appetite 2002;39:137–45. [DOI] [PubMed] [Google Scholar]
- 72.Rolls BJ, Roe LS, Meengs JS. Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. Am J Clin Nutr 2006;83:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tremblay A, Lavallee N, Almeras N, Allard L, Despres JP, Bouchard C. Nutritional determinants of the increase in energy intake associated with a high-fat diet. Am J Clin Nutr 1991;53:1134–7. [DOI] [PubMed] [Google Scholar]
- 74.Donahoo W, Wyatt HR, Kriehn J, Stuht J, Dong F, Hosokawa P, Grunwald GK, Johnson SL, Peters JC, Hill JO. Dietary fat increases energy intake across the range of typical consumption in the united states. Obesity (Silver Spring) 2008;16:64–9. [DOI] [PubMed] [Google Scholar]
- 75.Foltin RW, Fischman MW, Emurian CS, Rachlinski JJ. Compensation for caloric dilution in humans given unrestricted access to food in a residential laboratory. Appetite 1988;10:13–24. [DOI] [PubMed] [Google Scholar]
- 76.Lissner L, Levitsky DA, Strupp BJ, Kalkwarf HJ, Roe DA. Dietary fat and the regulation of energy intake in human subjects. Am J Clin Nutr 1987;46:886–92. [DOI] [PubMed] [Google Scholar]
- 77.Poppitt SD, Swann DL. Dietary manipulation and energy compensation: does the intermittent use of low-fat items in the diet reduce total energy intake in free-feeding lean men? Int J Obes Relat Metab Disord 1998;22:1024–31. [DOI] [PubMed] [Google Scholar]
- 78.Porikos KP, Booth G, Van Itallie TB. Effect of covert nutritive dilution on the spontaneous food intake of obese individuals: a pilot study. Am J Clin Nutr 1977;30:1638–44. [DOI] [PubMed] [Google Scholar]
- 79.Porikos KP, Hesser MF, van Itallie TB. Caloric regulation in normal-weight men maintained on a palatable diet of conventional foods. Physiol Behav 1982;29:293–300. [DOI] [PubMed] [Google Scholar]
- 80.Stubbs RJ, Harbron CG, Murgatroyd PR, Prentice AM. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am J Clin Nutr 1995;62:316–29. [DOI] [PubMed] [Google Scholar]
- 81.Stubbs RJ, Ritz P, Coward WA, Prentice AM. Covert manipulation of the ratio of dietary fat to carbohydrate and energy density: effect on food intake and energy balance in free-living men eating ad libitum. Am J Clin Nutr 1995;62:330–7. [DOI] [PubMed] [Google Scholar]
- 82.Thomas CD, Peters JC, Reed GW, Abumrad NN, Sun M, Hill JO. Nutrient balance and energy expenditure during ad libitum feeding of high-fat and high-carbohydrate diets in humans. Am J Clin Nutr 1992;55:934–42. [DOI] [PubMed] [Google Scholar]
- 83.Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr 2007;85:1465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kendall A, Levitsky DA, Strupp BJ, Lissner L. Weight loss on a low-fat diet: consequence of the imprecision of the control of food intake in humans. Am J Clin Nutr 1991;53:1124–9. [DOI] [PubMed] [Google Scholar]
- 85.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 86.Rolls BJ, Roe LS, Beach AM, Kris-Etherton PM. Provision of foods differing in energy density affects long-term weight loss. Obes Res 2005;13:1052–60. [DOI] [PubMed] [Google Scholar]
- 87.Saris WH, Astrup A, Prentice AM, Zunft HJ, Formiguera X, Verboeket-van de Venne WP, Raben A, Poppitt SD, Seppelt B, Johnston S, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. Int J Obes Relat Metab Disord 2000;24:1310–8. [DOI] [PubMed] [Google Scholar]
- 88.Westerterp-Plantenga MS, Wijckmans-Duijsens NE, Verboeket-van de Venne WP, de Graaf K, van het Hof KH, Weststrate JA. Energy intake and body weight effects of six months reduced or full fat diets, as a function of dietary restraint. Int J Obes Relat Metab Disord 1998;22:14–22. [DOI] [PubMed] [Google Scholar]
- 89.Raynor HA, Looney SM, Steeves EA, Spence M, Gorin AA. The effects of an energy density prescription on diet quality and weight loss: a pilot randomized controlled trial. J Acad Nutr Diet. 2012;112:1397–402. [DOI] [PubMed] [Google Scholar]
- 90.Bes-Rastrollo M, van Dam RM, Martinez-Gonzalez MA, Li TY, Sampson LL, Hu FB. Prospective study of dietary energy density and weight gain in women. Am J Clin Nutr 2008;88:769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Esmaillzadeh A, Azadbakht L. Dietary energy density and the metabolic syndrome among Iranian women. Eur J Clin Nutr 2011;65:598–605. [DOI] [PubMed] [Google Scholar]
- 92.Howarth NC, Murphy SP, Wilkens LR, Hankin JH, Kolonel LN. Dietary energy density is associated with overweight status among 5 ethnic groups in the Multiethnic Cohort Study. J Nutr 2006;136:2243–8. [DOI] [PubMed] [Google Scholar]
- 93.Lowe MR, Tappe KA, Annunziato RA, Riddell LJ, Coletta MC, Crerand CE, Didie ER, Ochner CN, McKinney S. The effect of training in reduced energy density eating and food self-monitoring accuracy on weight loss maintenance. Obesity (Silver Spring) 2008;16:2016–23. [DOI] [PubMed] [Google Scholar]
- 94.Murakami K, Sasaki S, Takahashi Y, Uenishi K; Japan Dietetic Students’ Study for Nutrition and Biomarkers Group. Dietary energy density is associated with body mass index and waist circumference, but not with other metabolic risk factors, in free-living young Japanese women. Nutrition 2007;23:798–806. [DOI] [PubMed] [Google Scholar]
- 95.Karl JP, Young AJ, Rood J, Montain SJ. Independent and combined effects of eating rate and energy density on energy intake, appetite and gut hormones. Obesity (Silver Spring) 2013;21:E244–52. [DOI] [PubMed] [Google Scholar]
- 96.de Oliveira MC, Sichieri R, Venturim Mozzer R. A low-energy-dense diet adding fruit reduces weight and energy intake in women. Appetite 2008;51:291–5. [DOI] [PubMed] [Google Scholar]
- 97.Spiegel TA. Caloric regulation of food intake in man. J Comp Physiol Psychol 1973;84:24–37. [DOI] [PubMed] [Google Scholar]
- 98.Cotton JR, Burley VJ, Weststrate JA, Blundell JE. Fat substitution and food intake: effect of replacing fat with sucrose polyester at lunch or evening meals. Br J Nutr 1996;75:545–56. [DOI] [PubMed] [Google Scholar]
- 99.Hulshof T, de Graaf C, Weststrate JA. Short-term satiating effect of the fat replacer sucrose polyester (SPE) in man. Br J Nutr 1995;74:569–85. [DOI] [PubMed] [Google Scholar]
- 100.Rolls BJ, Pirraglia PA, Jones MB, Peters JC. Effects of Olestra, a noncaloric fat substitute, on daily energy and fat intakes in lean men. Am J Clin Nutr 1992;56:84–92. [DOI] [PubMed] [Google Scholar]
- 101.Marmonier C, Chapelot D, Louis-Sylvestre J. Effects of macronutrient content and energy density of snacks consumed in a satiety state on the onset of the next meal. Appetite 2000;34:161–8. [DOI] [PubMed] [Google Scholar]
- 102.Himaya A, Fantino M, Antoine JM, Brondel L, Louis-Sylvestre J. Satiety power of dietary fat: a new appraisal. Am J Clin Nutr 1997;65:1410–8. [DOI] [PubMed] [Google Scholar]
- 103.Cotton JR, Weststrate JA, Blundell JE. Replacement of dietary fat with sucrose polyester: effects on energy intake and appetite control in nonobese males. Am J Clin Nutr 1996;63:891–6. [DOI] [PubMed] [Google Scholar]
- 104.Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr 2008;88:1232–41. [DOI] [PubMed] [Google Scholar]
- 105.Roy HJ, Most MM, Sparti A, Lovejoy JC, Volaufova J, Peters JC, Bray GA. Effect on body weight of replacing dietary fat with Olestra for two or ten weeks in healthy men and women. J Am Coll Nutr 2002;21:259–67. [DOI] [PubMed] [Google Scholar]
- 106.Bray GA, Lovejoy JC, Most-Windhauser M, Smith SR, Volaufova J, Denkins Y, de Jonge L, Rood J, Lefevre M, Eldridge AL, et al. A 9-mo randomized clinical trial comparing fat-substituted and fat-reduced diets in healthy obese men: the Ole study. Am J Clin Nutr 2002;76:928–34. [DOI] [PubMed] [Google Scholar]
- 107.Hill JO, Seagle HM, Johnson SL, Smith S, Reed GW, Tran ZV, Cooper D, Stone M, Peters JC. Effects of 14 d of covert substitution of Olestra for conventional fat on spontaneous food intake. Am J Clin Nutr 1998;67:1178–85. [DOI] [PubMed] [Google Scholar]
- 108.Glueck CJ, Hastings MM, Allen C, Hogg E, Baehler L, Gartside PS, Phillips D, Jones M, Hollenbach EJ, Braun B, et al. Sucrose polyester and covert caloric dilution. Am J Clin Nutr 1982;35:1352–9. [DOI] [PubMed] [Google Scholar]
- 109.Stubbs RJ, Johnstone AM, O'Reilly LM, Barton K, Reid C. The effect of covertly manipulating the energy density of mixed diets on ad libitum food intake in “pseudo free-living” humans. Int J Obes Relat Metab Disord 1998;22:980–7. [DOI] [PubMed] [Google Scholar]
- 110.Stubbs RJ, Johnstone AM, Harbron CG, Reid C. Covert manipulation of energy density of high carbohydrate diets in “pseudo free-living” humans. Int J Obes Relat Metab Disord 1998;22:885–92. [DOI] [PubMed] [Google Scholar]
- 111.Duncan KH, Bacon JA, Weinsier RL. The effects of high and low energy density diets on satiety, energy intake, and eating time of obese and nonobese subjects. Am J Clin Nutr 1983;37:763–7. [DOI] [PubMed] [Google Scholar]
- 112.Rolls BJ, Bell EA, Castellanos VH, Chow M, Pelkman CL, Thorwart ML. Energy density but not fat content of foods affected energy intake in lean and obese women. Am J Clin Nutr 1999;69:863–71. [DOI] [PubMed] [Google Scholar]
- 113.Hill RJ, Davies PS. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br J Nutr 2001;85:415–30. [DOI] [PubMed] [Google Scholar]
- 114.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr 2003;133:895S–920S. [DOI] [PubMed] [Google Scholar]
- 115.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav 1990;48:19–26. [DOI] [PubMed] [Google Scholar]
- 116.Flood JE, Roe LS, Rolls BJ. The effect of increased beverage portion size on energy intake at a meal. J Am Diet Assoc 2006;106:1984–90. [DOI] [PubMed] [Google Scholar]
- 117.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite 2005;44:187–93. [DOI] [PubMed] [Google Scholar]
- 118.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev 2010;68:505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stubbs J, Ferres S, Horgan G. Energy density of foods: effects on energy intake. Crit Rev Food Sci Nutr 2000;40:481–515. [DOI] [PubMed] [Google Scholar]
- 121.Wolf A, Bray GA, Popkin BM. A short history of beverages and how our body treats them. Obes Rev 2008;9:151–64. [DOI] [PubMed] [Google Scholar]
- 122.Deierlein AL, Siega-Riz AM, Herring A. Dietary energy density but not glycemic load is associated with gestational weight gain. Am J Clin Nutr 2008;88:693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olin AO, Osterberg P, Hadell K, Armyr I, Jerstrom S, Ljungqvist O. Energy-enriched hospital food to improve energy intake in elderly patients. JPEN J Parenter Enteral Nutr 1996;20:93–7. [DOI] [PubMed] [Google Scholar]
- 124.Cox TL, Malpede CZ, Desmond RA, Faulk LE, Myer RA, Henson CS, Heimburger DC, Ard JD. Physical activity patterns during weight maintenance following a low-energy density dietary intervention. Obesity (Silver Spring) 2007;15:1226–32. [DOI] [PubMed] [Google Scholar]
- 125.de Castro JM. Macronutrient and dietary energy density influences on the intake of free-living humans. Appetite 2006;46:1–5. [DOI] [PubMed] [Google Scholar]
- 126.de Castro JM. Stomach filling may mediate the influence of dietary energy density on the food intake of free-living humans. Physiol Behav 2005;86:32–45. [DOI] [PubMed] [Google Scholar]
- 127.Howarth NC, Huang TT, Roberts SB, Lin BH, McCrory MA. Eating patterns and dietary composition in relation to BMI in younger and older adults. Int J Obes (Lond) 2007;31:675–84. [DOI] [PubMed] [Google Scholar]
- 128.Esmaillzadeh A, Boroujeni HK, Azadbakht L. Consumption of energy-dense diets in relation to cardiometabolic abnormalities among iranian women. Public Health Nutr 2012;15:868–75. [DOI] [PubMed] [Google Scholar]
- 129.Flood A, Mitchell N, Jaeb M, Finch EA, Laqua PS, Welsh EM, Hotop A, Langer SL, Levy RL, Jeffery RW. Energy density and weight change in a long-term weight-loss trial. Int J Behav Nutr Phys Act 2009;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang CL, Hunt JN. The relation between freely chosen meals and body habitus. Am J Clin Nutr 1983;38:32–40. [DOI] [PubMed] [Google Scholar]
- 131.Schusdziarra V, Hausmann M, Wiedemann C, Hess J, Barth C, Wagenpfeil S, Erdmann J. Successful weight loss and maintenance in everyday clinical practice with an individually tailored change of eating habits on the basis of food energy density. Eur J Nutr 2011;50:351–61. [DOI] [PubMed] [Google Scholar]
- 132.Cheskin LJ, Davis LM, Lipsky LM, Mitola AH, Lycan T, Mitchell V, Mickle B, Adkins E. Lack of energy compensation over 4 days when white button mushrooms are substituted for beef. Appetite 2008;51:50–7. [DOI] [PubMed] [Google Scholar]
- 133.Foltin RW, Fischman MW, Moran TH, Rolls BJ, Kelly TH. Caloric compensation for lunches varying in fat and carbohydrate content by humans in a residential laboratory. Am J Clin Nutr 1990;52:969–80. [DOI] [PubMed] [Google Scholar]
- 134.Song SW, Bae YJ, Lee DT. Effects of caloric restriction with varying energy density and aerobic exercise on weight change and satiety in young female adults. Nutr Res Pract 2010;4:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tremblay A, Plourde G, Despres JP, Bouchard C. Impact of dietary fat content and fat oxidation on energy intake in humans. Am J Clin Nutr 1989;49:799–805. [DOI] [PubMed] [Google Scholar]
- 136.Viskaal-van Dongen M, Kok FJ, de Graaf C. Effects of snack consumption for 8 weeks on energy intake and body weight. Int J Obes (Lond) 2010;34:319–26. [DOI] [PubMed] [Google Scholar]
- 137.Durrant M, Royston P. Short-term effects of energy density on salivation, hunger and appetite in obese subjects. Int J Obes 1979;3:335–47. [PubMed] [Google Scholar]
- 138.Hogenkamp PS, Stafleu A, Mars M, de Graaf C. Learning about the energy density of liquid and semi-solid foods. Int J Obes (Lond) 2012;36:1229–35. [DOI] [PubMed] [Google Scholar]
- 139.Kelly SM, Shorthouse M, Cotterell JC, Riordan AM, Lee AJ, Thurnham DI, Hanka R, Hunter JO. A 3-month, double-blind, controlled trial of feeding with sucrose polyester in human volunteers. Br J Nutr 1998;80:41–9. [DOI] [PubMed] [Google Scholar]
- 140.Koonsvitsky BP, Berry DA, Jones MB, Lin PY, Cooper DA, Jones DY, Jackson JE. Olestra affects serum concentrations of alpha-tocopherol and carotenoids but not vitamin D or vitamin K status in free-living subjects. J Nutr 1997;127 Suppl:1636S–45S. [PubMed] [Google Scholar]
- 141.Campbell RG, Hashim SA, Van Itallie TB. Studies of food-intake regulation in man. Responses to variations in nutritive density in lean and obese subjects. N Engl J Med 1971;285:1402–7. [DOI] [PubMed] [Google Scholar]
- 142.Mattes R. Soup and satiety. Physiol Behav 2005;83:739–47. [DOI] [PubMed] [Google Scholar]
- 143.Mattes RD, Pierce CB, Friedman MI. Daily caloric intake of normal-weight adults: response to changes in dietary energy density of a luncheon meal. Am J Clin Nutr 1988;48:214–9. [DOI] [PubMed] [Google Scholar]
- 144.Lappalainen R, Mennen L, van Weert L, Mykkanen H. Drinking water with a meal: a simple method of coping with feelings of hunger, satiety and desire to eat. Eur J Clin Nutr 1993;47:815–9. [PubMed] [Google Scholar]
- 145.De Graaf C, Hulshof T, Weststrate JA, Hautvast JG. Nonabsorbable fat (sucrose polyester) and the regulation of energy intake and body weight. Am J Physiol 1996;270:R1386–93. [DOI] [PubMed] [Google Scholar]
- 146.Hulshof T, de Graaf C, Weststrate JA. Short-term effects of high-fat and low-fat/high-SPE croissants on appetite and energy intake at three deprivation periods. Physiol Behav 1995;57:377–83. [DOI] [PubMed] [Google Scholar]
- 147.Westerterp-Plantenga MS, Wijckmans-Duijsens NE, ten Hoor F, Weststrate JA. Effect of replacement of fat by nonabsorbable fat (sucrose polyester) in meals or snacks as a function of dietary restraint. Physiol Behav 1997;61:939–47. [DOI] [PubMed] [Google Scholar]
- 148.Miller DL, Castellanos VH, Shide DJ, Peters JC, Rolls BJ. Effect of fat-free potato chips with and without nutrition labels on fat and energy intakes. Am J Clin Nutr 1998;68:282–90. [DOI] [PubMed] [Google Scholar]