Abstract
Our understanding of the cardiovascular disease (CVD) benefits of α-linolenic acid (ALA, 18:3n–3) has advanced markedly during the past decade. It is now evident that ALA benefits CVD risk. The expansion of the ALA evidence base has occurred in parallel with ongoing research on eicosapentaenoic acid (EPA, 20:5n–3) and docosahexaenoic acid (DHA, 22:6n–3) and CVD. The available evidence enables comparisons to be made for ALA vs. EPA + DHA for CVD risk reduction. The epidemiologic evidence suggests comparable benefits of plant-based and marine-derived n–3 (omega-3) PUFAs. The clinical trial evidence for ALA is not as extensive; however, there have been CVD event benefits reported. Those that have been reported for EPA + DHA are stronger because only EPA + DHA differed between the treatment and control groups, whereas in the ALA studies there were diet differences beyond ALA between the treatment and control groups. Despite this, the evidence suggests many comparable CVD benefits of ALA vs. EPA + DHA. Thus, we believe that it is time to revisit what the contemporary dietary recommendation should be for ALA to decrease the risk of CVD. Our perspective is that increasing dietary ALA will decrease CVD risk; however, randomized controlled clinical trials are necessary to confirm this and to determine what the recommendation should be. With a stronger evidence base, the nutrition community will be better positioned to revise the dietary recommendation for ALA for CVD risk reduction.
Introduction
Many studies have shown that dietary lipids modulate the progression of coronary artery disease, heart failure, and cardiac arrhythmias (1–4). PUFAs are biologically active lipid molecules that are precursors for prostaglandins and leukotrienes. Differences in chain length and the number and position of double bonds have a significant impact on the biologic function of PUFAs (5).
A large body of evidence demonstrates that marine or algae-derived very-long-chain n–3 PUFAs, particularly EPA (20:5n–3) and DHA (22:6n–3), exert a cardioprotective effect, whereas the role of α-linolenic acid (ALA3, 18:3n–3), an essential n–3 PUFAs derived from plants and vegetable oils, is not as extensive. In part, this reflects the challenges associated with studying dietary ALA because food sources also provide other nutrients, some of which have cardiovascular benefits, whereas EPA + DHA can be evaluated experimentally as isolated FAs. ALA is found in flaxseed and flaxseed oil, vegetable oils (i.e., canola oil), and some nuts (i.e., walnuts). Compared with marine n–3 PUFAs, ALA from plant sources costs less and has greater global availability. The purpose of this review is to discuss the cardiovascular benefits of ALA and to compare them with EPA + DHA. A question that is ever present is whether ALA has comparable potency to EPA + DHA for cardiovascular disease (CVD) risk reduction? Whereas some research suggests similar benefits of plant- and marine-derived n–3 PUFAs, a recent review reported no significant effects in biomarker studies for ALA and also unexplained heterogeneity in the study results (6). Moreover, the ALA clinical studies are inherently constrained by design issues that relate in part to changes in the treatment diet(s) beyond ALA. The present review discusses the ALA research and summarizes the comparative cardiovascular benefits of ALA vs. EPA + DHA.
Pathway for Conversion of ALA to Longer-Chain n–3 PUFAs
There are 2 series of PUFAs that are essential: the n−6 and n−3 series. Although plants can synthesize both the n−6 and n−3 series, humans cannot, and therefore must obtain them from the diet. A deficiency in the n−6 PUFA linoleic acid (LA, 18:2n−6) leads to poor growth, fatty liver, skin lesions, and reproductive failure (7). ALA deficiency has been largely observed in patients who received parenteral nutrition. Diagnosis of ALA deficiency typically requires clinical testing. Inadequate dietary ALA leads to reductions in both visual acuity thresholds and electroretinogram A- and B-wave responses (i.e., a measure of the electrical response of the light-sensitive cells in the eye) (8–12), which is accompanied by a decrease in brain and retina DHA and an increase in docosapentenoic acid (DPA, 22:5n–6). The decrease in DHA reflects the limited conversion of ALA to DHA. The DHA content of neural membrane phospholipids modulates the activities of several signaling pathways in the brain (13) and is critical for normal retinal function (14, 15). Thus, these findings suggest that an inadequate ALA intake decreases the availability of DHA for incorporation into neural membranes (16).
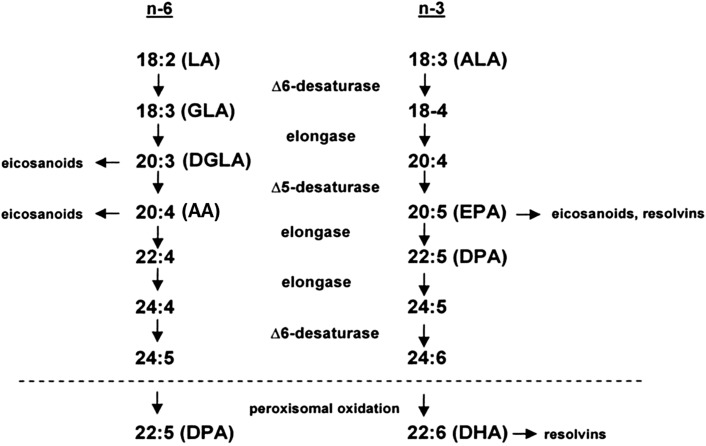
Biosynthesis of Longer-Chain n–3 PUFAs from ALA
ALA is a substrate for the synthesis of the very-long-chain n–3 PUFAs EPA and DHA; however, as noted by Burdge and Calder (17), the conversion is low (0.2% to EPA, 0.13% to DPA, and 0.05% to DHA). Compared with the amount of ALA that is elongated, a much larger proportion is oxidized [15–33%; reviewed in Burdge and Calder (17)]. The conversion of ALA to EPA and DHA, and of LA to γ-linolenic acid (18:3n–6) and arachidonic acid (AA, 20:4n–6), occurs via shared enzymatic pathways that involve elongase and desaturase enzymes (Fig. 1). The rate of conversion is affected by a number of factors, including the relative abundance of each substrate. When n–6 PUFAs are abundant, the conversion of long-chain to very-long-chain n–3 PUFAs is decreased. Interestingly, conversion is greater in women than in men and is higher in younger women than in postmenopausal women (17).
FIGURE 1.
Enzymatic conversion of LA to longer-chain n–6 PUFAs and ALA to longer-chain n–3 PUFAs. ALA, α-linolenic acid; AA, arachidonic acid; DGLA, dihomo-γ-linolenic acid; DPA, docosapentaenoic acid; GLA, γ-linolenic acid; LA, linoleic acid.
Role of n–3 PUFAs on Cardiovascular Events
Epidemiologic evidence
Although clinical benefits have not been observed consistently in all studies, most prospective observational studies suggest that ALA intake is associated with a reduced incidence of coronary heart disease (CHD). The evidence demonstrates that consumption of 2–3 g/d of ALA reduces the risk of CHD in primary and secondary prevention studies (18). Consistent with these findings, a large case-control study in 3638 men and women in Costa Rica (19) reported a strong inverse association of ALA status, measured via adipose tissue concentrations, and corresponding intakes of 1.8 g ALA/d with nonfatal myocardial infarction (MI). In addition, in a Dutch population of men (n = 20,069; aged 20–65 y), individuals consuming >1 g ALA/d had a 35–50% lower risk of incident stroke after 8 to 13 y of follow-up (20).
Atrial fibrillation (AF) is the most common chronic arrhythmia in the United States. The risk of AF increases with age, and the burden of disease is particularly high in individuals ≥65 y of age. Although several studies reported beneficial effects of EPA + DHA, little is known about the association of ALA with incident AF. In a recent prospective cohort study in older adults (≥65 y), Fretts et al. (21) observed no association of plasma phospholipid or dietary ALA with incident AF. These findings confirm those of Virtanen et al. (22), who conducted the only other study that has examined the association of circulating concentrations of ALA and incident AF in humans (22). In this study in Finnish men (mean age at baseline was 53 y), Virtanen et al. (22) observed no association between serum ALA and AF during 18 y of follow-up. Furthermore, despite previous reports of an association between dietary ALA intake and reduced CHD risk in those with low EPA + DHA intake (23), this study found no evidence that high serum ALA concentrations were associated with risk of AF, even in men with low serum very-long-chain n–3 PUFAs. Despite different populations (middle-aged adults vs. older adults), the findings by Fretts et al. (21) and Virtanen et al. (22) similarly suggest that there is no association of ALA with AF in humans.
In 2006, Wang et al. (24) published a systematic review of 14 randomized controlled trials (RCTs), 25 prospective cohort studies, and 17 case-control studies. The authors concluded that increased consumption of n–3 PUFAs from fish or fish-oil supplements, but not of ALA, reduced the rates of all-cause mortality, cardiac and sudden death, and possibly stroke (24). However, the authors recognized several limitations of the evidence available on the role of n−3 PUFAs on CVD outcomes, which makes definitive conclusions difficult. Specifically, it was difficult to accurately assess n−3 PUFA intake by using FFQs. In an attempt to remove the possibility of bias and inaccuracy in reporting, Harris et al. (25) conducted a meta-analysis of studies using FA biomarkers instead of dietary recalls to assess FA intake and risk of CHD. They observed that ALA, as measured in adipose tissue or phospholipids, was inversely associated with nonfatal CHD events, but the association did not reach significance for fatal CHD events. In contrast, very-long-chain n–3 FAs, especially DHA, were significantly reduced in patients who had experienced a CHD event.
Recently, Pan et al. (6) conducted a systematic review and meta-analysis of evidence for the association between ALA exposure, measured via dietary intake and biomarker concentrations, with risk of incident CVD. They concluded that ALA exposure was associated with a moderately lower risk of CVD. A subtype of studies analysis revealed a significantly lower pooled risk estimate of CVD in 13 comparisons that used dietary ALA, with similar but nonsignificant trends in 17 comparisons in which ALA biomarkers were used. These findings support previous results for ALA intake, providing evidence to show that each 1-g/d increment of ALA intake is associated with a 10% lower risk of CHD death. This epidemiologic evidence needs to be corroborated with clinical studies that evaluate the effects of ALA on CVD morbidity and mortality (6).
Clinical trial evidence
Effect of n–3 PUFAs on biomarkers of CVD risk.
Several of the early ALA intervention studies evaluated the effects of consuming different quantities of ALA (4–20 g/d) and reported beneficial effects on cardiovascular risk factors including total cholesterol, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), TGs, and inflammation. However, some of the studies reported decreases in HDL-C and increases in TGs. The variable results reported in these studies discussed below could be explained by the following factors: lack of control of the background diet and how ALA was provided (flaxseed oil, ground flaxseed, capsules, or spreads), which could affect the composition of the diet consumed by the subjects; the gender of the subjects studied (i.e., the majority of studies were conducted in men with only a few including both men and women); and study duration, which varied from weeks to months.
Six recent RCTs conducted between 2008 and 2010 assessed the effects of ALA on CVD risk markers (26–31). Of these, 3 compared ALA with EPA + DHA and found similar beneficial effects on CVD risk markers (LDL-C, HDL-C, TGs, or inflammatory markers) compared with baseline (26–28). In 2 (26, 28) of the 3 studies participants were randomly assigned to groups receiving 1.2, 2.0, 2.4, or 3.6 g ALA/d or 0.6, 1.2, or 2 g of EPA + DHA/d via flax or fish-oil capsules for 12 wk. There was no effect of ALA or EPA + DHA at any dose on plasma lipids or inflammatory markers. In the third study (27), participants were randomly assigned to 1 of 3 intervention groups (ALA, EPA, or DHA) and asked to consume a specified margarine daily for 6 wk. The margarines contained 4.4 g ALA, 2.2 g EPA, or 2.3 g DHA, respectively. There was no effect on total cholesterol and LDL-C; however TGs significantly decreased in each of the groups. There was no difference between groups. In addition, DHA significantly increased HDL-C, whereas no changes were found for ALA or EPA.
In the 3 other studies conducted between 2008 and 2010 (29–31), ALA was provided in the form of flaxseed oil, ALA-enriched margarines, or other ALA-enriched foods and compared with a placebo control (i.e., wheat bran). These studies showed mixed results, with modest increases or reductions in HDL-C, LDL-C, and TGs, and modest beneficial effects or neutral effects on inflammatory markers. In these 3 studies (29–31), the composition of the ALA treatment and control diets varied considerably, which could account for the variable results reported.
Relative to the simplicity of conducting EPA + DHA supplement studies, ALA studies are more complicated because ALA is incorporated via food sources, which changes the composition of the test diet. For example, ground flaxseed is often used to provide ALA; however, it is difficult to differentiate the effects of the components in flaxseed from those of ALA as well as other changes in the nutrient profile of the diet. Similarly, when provided as a spread, the inclusion of other FAs most likely plays a role in altering the diet effects. Thus, when ALA is added to a test diet via different food sources, it is challenging to control the background diet for just ALA. In contrast, in EPA + DHA supplement studies, the background diet is unchanged.
Effect of n–3 PUFAs on CVD events.
As noted in a recent review by Harris et al. (32), there have been many RCTs that evaluated the cardioprotective effects of very-long-chain n–3 FAs. Several earlier trials reported benefits for patients treated with very-long-chain n–3 FAs (33–35). More recent studies have not demonstrated beneficial effects, which may be due to the relatively low dose studied, short-term patient follow-up, and major improvements in clinical care of coronary patients (32). In contrast, the clinical trial evidence for ALA is not as robust, mainly because fewer studies have been conducted and, as noted, there are multiple dietary variables in the treatment diet beyond just ALA. Nonetheless, the clinical evidence is suggestive of a cardioprotective effect of ALA.
The Lyon Diet Heart Study, an RCT, was designed to evaluate the effectiveness of a Mediterranean-type diet (consistent with American Heart Association Diet and Lifestyle Recommendations) high in ALA on composite measures of coronary recurrence rate after a first MI. Men and women (n = 605; mean age = 53.5 y), who survived an MI within 6 mo of enrollment, were randomly assigned to a control diet (n = 303) or to an experimental diet (n = 303). Patients in the experimental group were advised by the research cardiologist and dietitian during a 1-h session to adopt a Mediterranean-type diet: more bread, root vegetables, green vegetables, and fish; less meat; fruit every day; and replace butter and cream with margarine supplied by the study. Because participants were not accustomed to using olive oil as the predominant fat in their diet, a canola oil–based margarine was provided. Compared with olive oil, this margarine contained a similar quantity of SFA (15%), less oleic acid (48% vs. 75%), and slightly greater amounts of LA (16.4% vs. 8.6%) and ALA (4.8% vs. 0.6%). After a follow-up of 27 mo, the risk of cardiac death and nonfatal MI decreased by >60% (36) in the intervention group. The event rate diverged within 2 mo after the intervention began. Although there were no differences in lipids and lipoproteins between the experimental and control groups, patients adhering to the Mediterranean-style diet had a 50–70% lower risk of recurrent heart disease after 46 mo (37). The effect of traditional risk factors on recurrence was analyzed with the multivariate Cox proportional hazards model. ALA was the only plasma FA significantly associated with a lower risk of the composite primary endpoint, MI plus cardiovascular death (RR: 0.20; 95% CI: 0.05, 0.84). Interestingly, EPA and DHA were not associated with a lower risk of CHD, which suggests that ALA was responsible for the effects observed (37).
Limitations have been raised about the effect of ALA on the risk of recurrent heart disease in the Lyon Heart Study. Notably, in addition to a difference in ALA (0.29% vs. 0.84% of calories; P < 0.01), there also were significant differences in total fat (33.6% vs. 30.4%) and SFA (11.7% vs. 8%) in the control and experimental groups, respectively. Moreover, participants in the experimental group were encouraged to consume more fruits, vegetables, breads and cereals, and fish. Thus, because of the diet differences between the 2 groups, the specific contribution of ALA has been questioned.
In 2010, a larger, double-blind, placebo-controlled trial, the Alpha Omega Trial, was conducted in a similar population of post-MI patients (n = 4837) to differentiate the effects of ALA (2 g/d) and EPA + DHA (400 mg/d) on cardiovascular events (38). Participants received 1 of 4 test margarines: margarine supplemented with a combination of EPA + DHA (with a targeted additional daily intake of 400 mg of EPA + DHA), margarine supplemented with ALA (with a targeted additional daily intake of 2 g of ALA), margarine supplemented with EPA + DHA and ALA, or a placebo margarine. After a 40-mo follow-up period, the 2 groups who received margarines containing ALA (alone or with EPA + DHA) had a rate of major cardiovascular events that was 9% lower than the rate in the groups who received placebo or EPA + DHA only, a difference that was not significant. Subgroup analyses showed no difference in cardiovascular events between the 2 groups who received EPA + DHA (alone or with ALA) and the 2 groups who received no EPA + DHA (placebo and ALA only). Likewise, there was no difference between the 2 groups who received ALA and those who did not. However, of importance was a 27% reduction in major cardiovascular events observed among women in the ALA-only group compared with the placebo and EPA + DHA–only groups, which approached significance (P = 0.07). The investigators discussed possible reasons for a lack of greater effect in this trial, including the patient population, which was mainly men who had an MI an average of 4 y before enrollment, and dose of n–3 PUFAs given (e.g., 400 mg/d EPA + DHA). In a landmark study [GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico)] in which a higher dose of EPA + DHA (1 g/d) was given to patients who were enrolled within 3 mo of an MI, there was a marked reduction in risk of sudden death after 4 mo (RR: 0.47; 95% CI: 0.22, 0.99; P < 0.05) (34).
In a follow-up analysis of participants in the Alpha Omega Trial, patients were categorized into a consistent statin user group (n = 3740) or a consistent statin nonuser group (n = 413) to assess how statin use modifies the effects of n–3 PUFAs on major adverse cardiovascular events in patients with a history of MI (39). In statin users, n–3 PUFAs had no effect on major cardiovascular events (adjusted HR: 1.02; 95% CI: 0.80, 1.31; P = 0.88). In statin nonusers, reductions in major cardiovascular events due to EPA + DHA alone or ALA alone were 18% and 10%, respectively, although this was not statistically significant. However, when the effects of EPA + DHA plus ALA were combined, only 9% experienced an event compared with 18% in the placebo group (adjusted HR: 0.46; 95% CI: 0.21, 1.01; P = 0.05). This suggests that statin treatment modifies the effects of n–3 PUFAs on the incidence of major cardiovascular events (39). Although low-dose treatment with n–3 PUFAs had no effect on major cardiovascular events in statin users, 400 mg of EPA + DHA and 2 g/d of ALA had beneficial effects on cardiovascular events in patients not taking statins. On the basis of the Lyon Diet Heart Study and the Alpha Omega Trial, there is evidence of a beneficial effect of ALA on cardiovascular events, albeit the strength of the evidence is not robust.
In addition to the 2 clinical trials that evaluated the effects of ALA on CVD, there is an interesting perspective emerging from a re-evaluation of clinical studies conducted decades ago that examined the effects of lowering SFAs and increasing n–6 PUFAs with and without n–3 PUFAs on CVD events. In 2013, Ramsden et al. (40) conducted a meta-analysis to evaluate the effectiveness of replacing SFAs with n–6 PUFAs with or without n–3 PUFAs on CHD. There was no beneficial effect on CHD events in 2 controlled trials that evaluated the specific effects of increasing n–6 PUFAs without the inclusion of n–3 PUFAs (41, 42). In contrast, in the St. Thomas Atherosclerosis Study (43), the Los Angeles Veterans Study (44), and the Medical Research Council Soy Study (45), when ALA was increased along with LA (and SFA was decreased) there was a reduction in cardiovascular mortality (40). Ramsden et al. (40) also presented evidence that when n–6 PUFAs were given with sources of EPA + DHA, CHD events decreased. The findings reported by Ramsden et al. (40) provide support on the cardiovascular benefits of ALA as well as EPA + DHA in the context of a diet that is lower in SFAs and higher in n–6 PUFAs.
Role of n–3 PUFAs on Inflammation
Epidemiologic studies.
In an epidemiologic study in subgroups of both the Physicians’ Health Study and the Nurses’ Health Study, ALA intake was not associated with markers of inflammation [i.e., C-reactive protein (CRP), IL-6, or soluble TNF receptor (sTNFR) 1 or 2] (46). In another study in a subgroup of the Nurses’ Health Study, dietary ALA was not associated with CRP, soluble intercellular adhesion molecule 1 (sICAM-1), soluble E-selectin (sE-selectin), or sTNFR2; however, it was associated with lower IL-6 and soluble vascular adhesion molecule 1 (sVCAM-1) (47). Data from a study conducted in 1123 participants aged 20–98 y reported no association between plasma ALA and IL-6 and TNF-α however, ALA was inversely associated with CRP (48). On the basis of these epidemiologic studies, ALA has a modest anti-inflammatory properties. These same observational studies showed inverse associations between dietary intake of EPA + DHA and markers of inflammation, including CRP, sTNFR1, sTNFR2, sICAM-1, and sVCAM-1, suggesting that both EPA + DHA are anti-inflammatory.
Clinical trial evidence.
It has been suggested that substituting n–6 with n–3 PUFAs may improve the synthesis of eicosanoids that have fewer inflammatory properties. Several anti-inflammatory effects have been demonstrated with n–3 PUFAs, particularly for EPA + DHA. Increased intake of very-long-chain n–3 PUFAs results in increased proportions of these FAs in inflammatory cell phospholipids (49). EPA and DHA are incorporated and in part substituted for AA, resulting in less substrate for the synthesis of inflammatory eicosanoids. Thus, n–3 PUFAs affect inflammatory processes as well as non–eicosanoid-mediated actions such as intracellular signaling and gene expression. The anti-inflammatory effects of very-long-chain n–3 PUFAs were examined in many human and animal studies (50, 51). Many supplementation studies of very-long-chain n–3 PUFAs showed that EPA + DHA decrease circulating concentrations of CRP, IL-6, TNF-α, IL-18, sICAM-1, sVCAM-1, and sE-selectin (see Table 1), although some studies did not replicate these findings (Table 1). In general, >1 g/d of EPA + DHA has anti-inflammatory effects.
TABLE 1.
Intervention studies investigating the effect of marine n–3 PUFA intake on circulating markers of low-grade inflammation1
| Authors, year (ref) | Subjects | Intake (source; duration) | Effect on low-grade inflammation |
| Blok et al., 1997 (84) | Healthy (n = 58; aged 21–87 y) | 0, 1.06, 2.13, 3.19 g/d EPA + DHA (FO capsules; up to 52 wk) | No effect: TNF-α, IL-1β, IL-1RA |
| Abe et al., 1998 (85) | Healthy and hyperlipidemic (n = 20; mean age = 51) | 0, 3.6 g/d EPA + DHA (EE capsules; up to 6 wk) | No effect: sICAM-1, sVCAM-1 |
| ↓ sE-selectin | |||
| Seljeflot et al., 1998 (86) | Hyperlipidemic smokers (n = 41; aged 41–57 y) | 0, 4.8 g/d EPA + DHA (EE capsules; 6 wk) | ↑ sVCAM-1, sE-selectin |
| No effect: sP-selectin, tissue plasminogen activator antigen | |||
| ↓ von Willibrand factor, thrombomodulin | |||
| Johansen et al., 1999 (87) | Patients with CHD (n = 54; aged 43–73 y) | 0, 5.1 g/d EPA + DHA (EE capsules; 24 wk) | ↑ sVCAM-1, sE-selectin |
| No effect: sP-selectin, tissue plasminogen activator antigen | |||
| ↓ von Willibrand factor, thrombomodulin | |||
| Sampson et al., 2001 (88) | Healthy (n = 21) and type 2 diabetics (n = 29); mean age = 55 y | 2.0 g/d EPA + DHA (FO capsules; 3 wk) | No effect: sICAM-1, sVCAM-1, sE-selectin, PAI-1 activity, PAI-1 antigen |
| Thies et al., 2001 (89) | Healthy (n = 24; aged 55–75 y) | 0, 0.7 g/d DHA (DHA-rich algal oil capsules; 12 wk), 1 g/d EPA + DHA (FO capsules; 12 wk) | EPA + DHA: No effect: sICAM-1, sE-selectin |
| ↓ sVCAM-1 | |||
| DHA: no effect: sICAM-1, sVCAM-1, sE-selectin | |||
| Chan et al., 2002 (90) | Obese (n = 48; mean age = 53 y) | 0, 3.5 g/d EPA + DHA (EE capsules; 6 wk) | No effect: CRP, IL-6, TNF-α |
| Berstad et al., 2003 (91) | Elderly at risk of CHD (n = 171; mean age = 70 y) | 0, 2.4 g/d EPA + DHA (FO capsules; 18 mo) | No effect: sICAM-1, sVCAM-1, sE-selectin, tissue plasminogen activator antigen |
| ↓ von Willibrand factor, thrombomodulin | |||
| Ciubotaru et al., 2003 (92) | Healthy on HRT (n = 30; mean age = 60 y) | 0, 1.09, 2.18 g/d EPA + DHA (FO capsules; 5 wk) | ↓ CRP, IL-6 |
| Madsen et al., 2003 (93) | Healthy (n = 60; ageds 21–57 y) | 0, 2.0, 6.6 g/d EPA + DHA (FO capsules; 12 wk) | No effect: CRP |
| Grundt et al., 2003 (94) | MI survivors (n = 300; aged 28–87 y) | 0, 3.5 g/d EPA + DHA (EE capsules; 12 mo) | No effect: sICAM-1, sVCAM-1, sE-selectin |
| Mori et al., 2003 (95), and Woodman et al., 2003 (96) | Type 2 diabetics (n = 59; aged 40–65 y) | 0, 4 g/d EPA, 4 g/d DHA (EPA EE or DHA EE capsules; 6 wk) | No effect: CRP, IL-6, TNF-α, von Willibrand factor, tissue plasminogen activator antigen, PAI-1 antigen, sP-selectin |
| Eschen et al., 2004 (97) | Healthy (n = 60; mean age = 38 y) | 0, 1.6, 5.8 g/d EPA + DHA (concentrated FO capsules; 3 y) | Low-dose: no effect: sVCAM-1, sP-selectin |
| ↓ sICAM-1 (especially in women) | |||
| High-dose: no effect: sICAM-1, sVCAM-1 | |||
| ↓ sP-selectin | |||
| Jellema et al., 2004 (98) | Obese (n = 11; age not given) | 1.1 g/d EPA + DHA (FO capsules; 6 wk) | No effect: CRP, IL-6, sTNFR1, sTNFR2, PAI-1 |
| Hjerkinn et al., 2005 (99) | Hyperlipidemic (n = 563; aged 64–76 y) | 0, 2.4 g/d EPA + DHA (FO capsules; 3 y) | No effect: sVCAM-1, sE-selectin, von Willibrand factor, tissue plasminogen activator antigen |
| ↓ sICAM-1, thrombomodulin | |||
| Cazzola et al., 2007 (100) | Healthy young (n = 93; mean age = 24 y); healthy older (n = 62; mean age = 61 y) | 0, 1.35, 2.7, 4.05 g/d EPA + DHA (EPA-rich oil; 12 wk) | No effect: sICAM-1, sVCAM-1 |
| ↑sE-selectin (young only) | |||
| Fujioka et al., 2006 (101) | Healthy (n = 141; mean age = 47 y) | 0, 0.96 g/d EPA + DHA (FO in soya milk; 12 wk) | No effect: CRP, sTNFR1, sTNFR2 |
| Krebs et al., 2006 (102) | Overweight and insulin resistant (n = 116; aged 21–69 y) | 0, 0 + weight-loss program, 4.2 g/d EPA + DHA + weight-loss program (concentrated FO capsules; 24 wk) | ↑Adiponectin |
| No effect: CRP, TNF-α, IL-6 | |||
| Sanders et al., 2006 (103) | Healthy (n = 80; mean age = 30 y) | 0, 1.5 g/d DHA (DHA-rich algal oil; 4 wk) | No effect: CRP, fibrinogen, PAI-1 activity, von Willibrand factor |
| Kabir et al., 2007 (104) | Overweight type 2 diabetics (n = 27; mean age = 55 y) | 0, 1.8 g/d EPA + DHA (FO capsules; 8 wk) | No effect:IL-6, TNF-α, SAA, adiponectin |
| ↓ PAI-1 activity, inflammatory gene expression in adipose tissue | |||
| Browning et al., 2007 (105) | Overweight and obese (n = 30; age not given) | 0, 4.2 g/d EPA + DHA (DHA-rich TAG capsules; 12 wk; crossover) | No effect: sialic acid, fibrinogen, PAI-1 activity |
| ↓ CRP, IL-6 | |||
| Rasic-Milutinovic et al., 2007 (106) | Insulin resistant with chronic renal failure (n = 35; mean age = 55 y) | 2.4 g/d EPA + DHA (FO capsules; 8 wk) | ↓ CRP, IL-6, TNF-α |
| Madsen et al., 2007 (107) | MI survivors (n = 41; mean age = 63 y) | 0, 5.2 g/d EPA + DHA (EPA-rich TAG capsules; 12 wk) | No effect: CRP |
| Murphy et al., 2007 (108) | Overweight (n = 86; age not given) | 0, 1 g/d EPA + DHA (enriched foods; 6 mo) | No effect: CRP |
| Yamada et al., 2008 (109) | Metabolic syndrome (n = 23; mean age = 50 y) | 0, 1.8 g/d EPA (EPA capsules; 12 wk) | ↓ sICAM-1, sVCAM-1 |
| Yusof et al., 2008 (110) | Healthy (n = 20; aged 35–60 y) | 0, 1.8 g/d EPA + DHA (EPA-rich oil; 8 wk) | No effect: CRP, IL-6, sVCAM-1, sE-selectin, sP-selectin |
| ↓ sICAM-1 | |||
| Kelley et al., 2009 (111) | Moderately hyperlipidemic (n = 34; aged 39–66 y) | 0, 3 g/d DHA (DHA-rich algal oil; 90 d) | No effect: SAA, TNF-α, IL-1β, IL-8, IL-10, sVCAM-1, sICAM-1, sE-selectin |
| ↓ Leukocytes, CRP, IL-6, granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor | |||
| Pot et al., 2009 (112) | Healthy (n = 77; aged 50–70 y) | 0, 1.5 g/d EPA + DHA (FO capsules; 12 wk) | No effect: 19 cytokines, chemokines, and adhesion molecules |
| Trøseid et al., 2009 (113) | Elderly at risk of CHD (n = 563; mean age = 70 y) | 0, 2.4 g/d EPA + DHA (FO capsules; 3 y) | No effect: CRP, IL-6, TNF-α, adiponectin |
| ↓ IL-18 |
Adapted from reference 51 with permission. CHD, coronary heart disease; CRP, C-reactive protein; EE, ethyl ester; FO, fish oil; HRT, hormone replacement therapy; IL-1RA, IL-1 receptor antagonist; MI, myocardial infarction; PAI-1, plasminogen activator inhibitor 1; ref, reference; SAA, serum amyloid A; sE-selectin, soluble E-selectin; sICAM-1, soluble intercellular adhesion molecule 1; sP-selectin, soluble P-selectin; sTNFR, soluble TNF receptor; sVCAM-1, soluble vascular cell adhesion molecule 1; TAG, triacylglycerol; ↓, decreased; ↑, increased.
In addition to the potential benefits of EPA + DHA, there also is substantial evidence that ALA from plant sources such as from walnuts, flaxseed, or canola oil has anti-inflammatory effects similar to marine n–3 PUFAs. In a study using a human leukemic cell line (THP-1), PUFAs were shown to have an inhibitory effect on LPS-stimulated inflammatory response, with ALA and DHA being more beneficial than LA (52). Furthermore, the individual n–3 PUFA and total n–3 PUFAs were inversely associated with some plasma inflammatory and endothelial markers in participants in the Nurses’ Health Study (47).
Several intervention studies also support the anti-inflammatory effect of ALA in humans (see Table 2). In dyslipidemic patients, a decrease in serum concentrations of inflammatory proteins was observed after dietary supplementation with ALA from walnuts, walnut oil, and flaxseed oil (53). This anti-inflammatory effect of ALA seems to be higher when the background diet is high in SFAs and low in MUFAs (54). Zhao et al. (53) observed that ALA reduces CVD risk beyond its lipid-lowering effects by inhibiting vascular inflammation and endothelial activation. In a subsequent in vitro analysis using cultured peripheral blood mononuclear cells from subjects fed the experimental diets, Zhao (55) evaluated the effects of a diet high in ALA on serum proinflammatory cytokine concentrations and cytokine production. The authors concluded that the increase in intake of ALA from walnuts, walnut oil, and flaxseed oil elicited anti-inflammatory effects by inhibiting IL-6, IL-1β, and TNF-α production in peripheral blood mononuclear cells (55).
TABLE 2.
Intervention studies investigating the effect of ALA intake on markers of low-grade inflammation1
| Authors, year (ref) | Subjects | Age | Intake (source; duration) | Effect on low-grade inflammation |
| y | ||||
| Junker et al., 2001 (114) | Healthy (n = 58) | 27 | 3 diets including 1 with foods rich in ALA (4 wk) | No effect: CRP, sL-selectin, sP-selectin, fibrinogen, PAI-1 activity, tissue plasminogen activator activity |
| Thies et al., 2001 (89) | Healthy (n = 16) | 56–74 | 0, 2 g/d ALA (flaxseed oil capsules; 12 wk) | No effect: sICAM-1 |
| ↓ sVCAM-1, sE-selectin | ||||
| Rallidis et al., 2003 (57), and 2004 (58) | Hyperlipidemic (n = 76) | Mean: 51 | 0, 8 g/d ALA (flaxseed oil; 12 wk) | No effect: sICAM-1, sE-selectin |
| ↓ CRP, IL-6, SAA, sVCAM-1 | ||||
| Bemelmans et al., 2004 (115) | High cardiovascular risk (n = 103) | Mean: 55 | 0, 6.3 g/d ALA (spread; up to 2 y) | No effect: IL-6, sICAM-1, IL-10 |
| ↓ CRP | ||||
| Zhao et al., 2004 (53) | Hyperlipidemic (n = 23) | 36–65 | 0.8%, 3.6%, 6.5% of energy from ALA (1.5, 8, 15 g/d ALA) (walnuts + walnut oil + flaxseed oil; 6 wk; crossover) | ↓ CRP, sVCAM-1, sICAM-1, sE-selectin |
| Paschos et al., 2004 (54) | Hyperlipidemic (n = 40) | 35–67 | Mediterranean diet + 8.1 g/d ALA, westernized Greek diet + 8.1 g/d ALA (flaxseed oil; 12 wk) | Mediterranean diet background: |
| No effect: CRP, IL-6, SAA, sICAM-1, sE-selectin | ||||
| ↓ sVCAM-1, MCSF | ||||
| Westernized Greek diet background: | ||||
| No effect: sICAM-1, sE-selectin | ||||
| ↓CRP, IL-6, SAA, sVCAM-1, MCSF | ||||
| Paschos et al., 2007 (59) | Hyperlipidemic (n = 40) | 38–71 | 0, 8.1 g/d ALA (flaxseed oil; 12 wk) | No effect: TNF-α, adiponectin |
Adapted from reference 51 with permission. ALA, α-linolenic acid; CRP, C-reactive protein; MCSF, macrophage colony-stimulating factor; PAI-1, plasminogen activator inhibitor 1; ref, reference; SAA, serum amyloid A; sE-selectin, soluble E-selectin; sICAM-1, soluble intercellular adhesion molecule 1; sL-selectin, soluble L-selectin; sP-selectin, soluble P-selectin; sVCAM-1, soluble vascular cell adhesion molecule 1.
There is some evidence to support a beneficial role for dietary ALA, EPA, and DHA on many factors associated with metabolic syndrome. A review by Poudyal et al. (56) suggested that ALA may exhibit anti-inflammatory properties independent of the conversion to EPA and/or DHA through the reduction in proinflammatory cytokines and cyclooxygenase and lipoxygenase enzyme metabolites of AA (56). However, findings remain inconsistent. These studies included a group with a high intake of LA and a comparison group in whom LA was replaced with ALA. Evidence supports similar effects of LA and ALA on low-grade inflammation (57, 58). The earlier intervention studies used very high intakes of ALA (>8 g/d) relative to typical intakes (<2 g/d) (54, 57–59). Paschos et al. (54) reported that ALA (8.1 g/d for 12 wk) was less effective when studied in the context of a healthy diet vs. a control diet. This indicates that dose, length of study, and background diet account for the different findings reported in ALA studies. Although there is evidence that ALA can attenuate inflammation, there are reports to the contrary.
In a longer intervention study (4 wk; n = 62) that provided 40 g/d of flaxseed or wheat bran control as part of a low-fat, low-cholesterol background diet in overweight men, there was no change in inflammatory markers (IL-6 or CRP) (30). Another study conducted in 100 adults with metabolic syndrome found no differences in monocyte chemoattractant protein 1, IL-6, or sICAM-1 between those who received a low (2.2 g/d) or high (6.6 g/d) dose of ALA over the 8-wk supplementation period (60). Overall, ALA has been less studied than EPA + DHA, and the existing data are inconclusive with respect to the role of ALA in inflammation.
Comparison of the Evidence for ALA and EPA + DHA
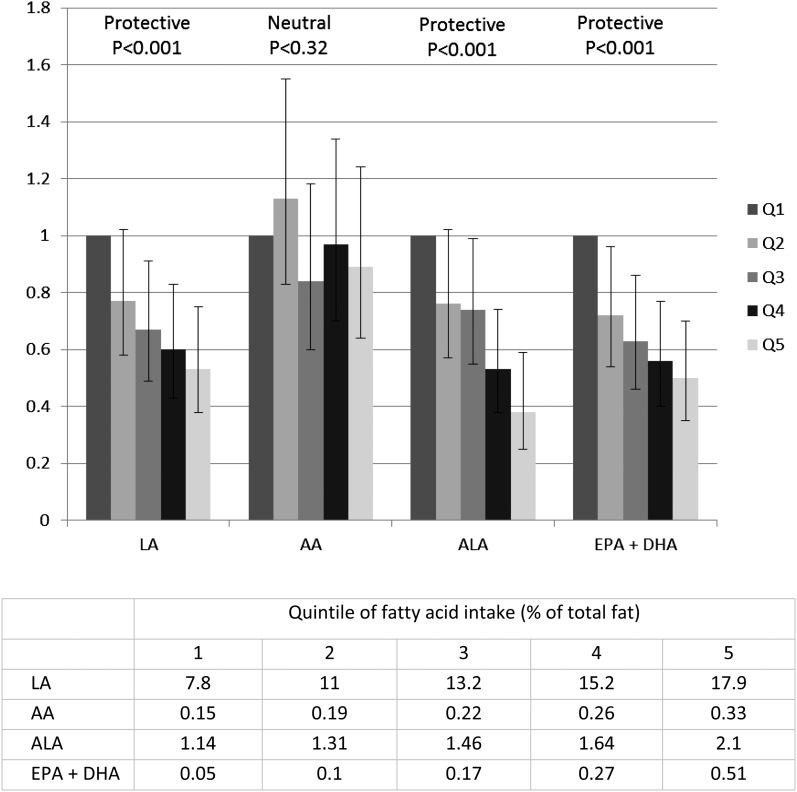
Sudden cardiac death (SCD) claims >300,000 lives in the United States annually and accounts for 50% of cardiovascular and 15–20% of total mortality. Epidemiologic evidence suggests that dietary fat quality modulates the risk of SCD. In a recent prospective cohort study in women (n = 91,981) from the Nurses’ Health Study, Chiuve et al. (61) observed an inverse and protective association of ALA, EPA + DHA, and LA on SCD risk. No effect was observed for AA. Dietary data for this analysis were collected via FFQ. As illustrated in Figure 2, women with a higher intake of FAs from both n–6 and n–3 classes of PUFAs (LA, ALA, EPA + DHA) had a significantly lower (and comparable) risk of SCD than did women in the lowest quintiles. An earlier analysis in 76,763 women participating in the Nurses’ Health Study compared the FFQ dietary intake data of women in the lowest quintile of ALA intake (0.37% of energy) with those in the highest 2 quintiles (0.6%–0.74% of energy) and observed a 38%–40% lower SCD risk. This inverse relation with SCD risk was linear and remained significant even among women with high intakes of long-chain n–3 PUFAs (62). The median intake of ALA was 0.66 g/d in the lowest and 1.39 g/d in the highest quintile; at an intake of 1.16 g/d, reductions in risk became significant. Doubling ALA intake (from the first to the fifth quintile of intake) conferred an impressive cardioprotective benefit (see Fig. 2). Also, increasing EPA + DHA intake by 2-fold (from the first to the second quintile of intake) decreased the risk significantly, but additional increases in intake (from the third to the fifth quintile of intake) lowered the risk further.
FIGURE 2.
(Top) Multivariable risk ratios of sudden cardiac death by dietary intake of n–3 and n–6 PUFAs among women in the Nurses’ Health Study (bottom) quintile of fatty acid intake as % of total fat. AA, arachidonic acid; ALA, α-linolenic acid; LA, linoleic acid; Q, quintile. Adapted from reference 61 with permission.
In contrast to previous studies, the MESA (Multi-Ethnic Study of Atherosclerosis), a large prospective cohort study in multiethnic Americans, reported no association between plasma ALA and CVD incidence (63). The investigators suggested that the inconsistencies with previous studies may have been due to food sources of ALA. In the United States, some of the major sources of ALA in the diet include refined-grain breads, desserts, pizza, potato chips, burgers, and processed meats. Most clinical studies used vegetable oils as the source of ALA and evaluated the replacement of food sources of SFAs with vegetable oils or other high-ALA food sources (i.e., walnuts, flaxseed) (64, 65). Consistent with other observational studies, in the MESA study circulating EPA + DHA were inversely associated with CVD incidence.
Other investigations that evaluated the effects of plant-based and marine-derived n–3 PUFAs reported similar outcomes. Harris et al. (25) reviewed 25 trials that evaluated the risk of coronary artery disease events as a function of n–3 and n–6 PUFA concentrations in tissue. Nine prospective cohort studies and 16 case-control cross-sectional studies were evaluated. The authors categorized studies on the basis of study design and reported that relative to the relation between tissue FA concentrations and risk of CHD events, all studies yielded similar significant results for EPA + DHA vs. ALA. Similar significant findings for the nested case-control/cohort studies were reported for both EPA + DHA and ALA. In addition, when studies were subdivided by fatal and nonfatal endpoints, EPA + DHA and ALA were significant (P = 0.08 and 0.04, respectively) for relations between tissue FA concentrations and risk of nonfatal CHD endpoints. Relative to fatal endpoints, the results were significant for EPA + DHA but not for ALA.
We compared the results from a review and meta-analysis of ALA with a review on EPA + DHA from a Cochrane review on CVD endpoints. Tables 3–6 present risk ratio analyses from the Pan et al. (6) review and meta-analyses on plant-derived n-3 PUFA exposure and risk of incident CVD and compare them with the results from the Cochrane review on marine-derived n–3 PUFAs and prevention and treatment of CVD (66). The comparisons demonstrate that similar risk reduction ratios were observed for both marine-derived (EPA + DHA) and plant-derived (ALA) n–3 PUFAs. However, the evidence for ALA is not as strong because fewer ALA studies have been conducted.
TABLE 3.
Risk ratio comparison between ALA and DHA + EPA on nonfatal MI1
| Nonfatal MI cohort studies | Study location | No. of studies | No. of participants | Effect size (95% CI) |
| ALA | ||||
| Albert et al., 2005 (62) | NHS, United States | — | 76,763 | 1.07 (0.94, 1.22) |
| Lopes et al., 2007 (116) | Portugal | — | 607 | 0.66 (0.42, 1.04) |
| Campos et al., 2008 (19) | Costa Rica | — | 3638 | 0.74 (0.58, 0.93) |
| n–3 FAs (EPA + DHA) | — | 4 | 59,475 | 0.93 (0.69,1.26) |
ALA, α-linolenic acid; MI, myocardial infarction; NHS, Nurses’ Health Study.
TABLE 6.
Association between tissue FAs and risk of CAD events, estimated by effect sizes in 25 studies1
| All studies |
||||
| FA | g1 | P | 95% CI | n |
| EPA + DHA | −0.19 | <0.01 | −0.06, −0.33 | 19 |
| DHA | −0.34 | <0.01 | −0.12, −0.59 | 19 |
| EPA | −0.10 | 0.08 | 0.01, −0.21 | 15 |
| ALA | −0.21 | 0.03 | − 0.02, −0.39 | 16 |
| LA | −0.28 | 0.02 | −0.04, −0.53 | 22 |
| AA | 0.03 | 0.81 | 0.25, −0.19 | 20 |
g:Hedges g (effect size), in which the case-control difference is expressed as a fraction of the pooled SD; negative values indicate that FA values were lower in cases relative to controls. Adapted from reference 25 with permission. AA, arachidonic acid; ALA, α-linolenic acid; CAD, coronary artery disease; LA, linoleic acid.
TABLE 4.
Risk ratio comparison between ALA and EPA + DHA on stroke1
| Stroke cohort studies | Study, location | No. of studies | No. of participants | Effect size |
| ALA | ||||
| He et al., 2002 (117) | HPFS, United States | — | 43,671 | 0.98 (0.74, 1.29) |
| de Goede et al., 2011 (20) | MORGEN study, The Netherlands | — | 20,069 | 0.71 (0.50, 1.03) |
| Larsson et al., 2012 (118) | Swedish Mammography Cohort, Sweden | — | 34,670 | 1.07 (0.92, 1.25) |
| n–3 FAs (EPA + DHA) | — | 4 | 52,026 | 0.87 (0.72, 1.04) |
ALA, α-linolenic acid; HPFS, Health Professionals Follow-Up Study; MORGEN, Monitoring Project on Risk Factors for Chronic Diseases.
TABLE 5.
Risk ratio comparison between ALA and EPA + DHA on cardiovascular death1
| Cardiovascular death cohort studies | Study, location | No. of studies | No. of participants | Effect size |
| ALA | ||||
| Dolecek, 1992 (119) | MRFIT, United States | — | 6250 | 0.71 (0.48, 1.03) |
| Hu et al., 1999 (120) | NHS, United States | — | 76,283 | 0.63 (0.41, 0.95) |
| Laaksonen et al., 2005 (121) | KIHD study, Finland | — | 1551 | 0.63 (0.33, 1.21) |
| n–3 FAs (EPA + DHA) | — | 11 | 107,303 | 0.79 (0.63,0.99) |
Cardiovascular death includes fatal coronary heart disease, fatal ischemic heart disease, and fatal cardiovascular disease. ALA, α-linolenic acid; KIHD, Kuopio Ischemic Heart Disease Risk Factor; MRFIT, Multiple Risk Factor Intervention Trial; NHS, Nurses’ Health Study.
It is important to point out that the 2004 Cochrane review by Hooper et al. (66) concluded that n–3 PUFAs do not lower mortality or cardiovascular risk. The negative results, however, were driven by the inclusion of a single secondary CHD prevention study [Diet and Reinfarction Trial 2 (DART 2)], which tested the effect of fish (2 servings/wk) or fish oil (3 g of EPA/wk) in men with angina. When the study, which was judged to be of low quality, was excluded, heterogeneity among the RCTs was eliminated and the results were similar to those of other studies. Even with the inclusion of the DART 2 study results, the authors found modest (albeit nonsignificant) decreases in cardiovascular events and total mortality with high intakes of n–3 PUFAs. Also of note, the authors excluded the Lyon Diet Heart Study, an important secondary CHD prevention trial that showed marked reductions in recurrent CHD events that were ascribed to ALA. The study was excluded because Hooper et al. (66) were not able to separate the effects of ALA from those of other dietary and lifestyle interventions, even though the Lyon Diet Heart Study investigators (37) reported that ALA was the only plasma FA that was significantly associated with MI plus cardiovascular death. Thus, the results of meta-analyses that have been conducted to evaluate the cardioprotective benefits of ALA are dependent on the studies that are included or excluded in the analysis. There is some evidence for ALA benefits on CVD; however, this is dependent on what studies are included in the meta-analyses.
Although the marine-derived n–3 PUFA secondary prevention studies were supported by a number of RCTs but not others (34, 38, 67–69), likely due to the low doses administered, short follow-up, high background n–3 PUFA intake, use of modern pharmacotherapy, relatively low-risk patient populations, and/or small sample sizes (70), they have nonetheless beneficially affected CVD risk factors such as TGs, arrhythmias, and blood pressure (71). Although similar beneficial cardiovascular outcomes of ALA intake were observed in the Pan et al. (6) meta-analysis, previous reviews found only nonsignificant trends. These inconsistencies have maintained the focus on marine-derived n–3 PUFAs, with less attention devoted to plant-derived sources of ALA. The Pan et al. (6) review is important because it evaluated both dietary and biomarker studies, which was not done to any extent in previous reviews. Despite these interesting findings, assessing dietary ALA intake in free-living populations is challenging because of the multitude of food sources and limitations with diet assessment methodologies. As a result, the study results are more confounded than those of other FAs, such as EPA + DHA. Consequently, there still remains a need to conduct well-designed, randomized, placebo-controlled clinical trials that rigorously evaluate the effects of dietary ALA on CVD risk.
Intake and Recommendations
According to the 2010 Dietary Guidelines Advisory Committee report, an ALA intake of 0.6–1.2% of total calories is sufficient to meet current recommendations and may lower CVD risk; however, there is not sufficient evidence to recommend a higher intake (72). There also is limited but supportive evidence to suggest that higher intakes of n–3 PUFAs from plant sources may reduce mortality among individuals with existing CVD (69). The 2010 Dietary Guidelines Advisory Committee recommendation for ALA is consistent with the Acceptable Macronutrient Distribution Range (AMDR) for n–3 PUFAs. The AMDR for n–3 PUFAs is based on the Adequate Intake for ALA, which is 1.6 g/d for men and 1.1 g/d for women and reflects the average intake of ALA in the United States where ALA deficiency is nonexistent in healthy individuals (73). The Adequate Intake is comparable to the intakes in numerous European countries, Australia, and Canada, which range from 0.5 to 2.2 g/d (74–78). Based on the AMDR for n–3 PUFAs, up to 10% can be provided by EPA and/or DHA. Current intake of EPA + DHA in the United States is ∼100 mg/d (79). Importantly, the evidence reviewed herein supports a higher recommended intake for both ALA as well as EPA + DHA for cardiovascular health than that issued by the National Academies (73).
Due to the many health benefits associated with very-long-chain n–3 PUFAs, dietary recommendations for adults have been issued by many organizations in the United States. On the basis of the 2010 Dietary Guidelines Advisory Committee report (72), moderate evidence shows that consumption of 2 servings of seafood/wk (4 ounces per serving; 112 g), which provide an average of 250 mg/d of very-long-chain n–3 PUFAs, is associated with reduced cardiac mortality from CHD or sudden death in persons with and without CVD (72). The American Heart Association and the American Diabetes Association also recommend 2 servings of fish/wk, preferably fatty fish (80), whereas the Academy of Nutrition and Dietetics advises 500 mg/d of EPA + DHA (81). In addition, the American Heart Association recommends that individuals with CHD should consume 1 g/d of EPA + DHA, preferably from oily fish, but supplements are acceptable (under a physician’s supervision). As is apparent, current intakes of EPA + DHA in the United States are much lower than these recommendations. The average fish consumption in the United States is ∼1 fish meal/wk, with the most popular seafood being shrimp, which is low in EPA + DHA (82). Although tuna and salmon remain among the most popular seafood (ranked second and third, respectively), per capita consumption of these fatty fish has decreased by 3.7% and 2%, respectively (82). Other popular seafood includes nonfatty sources such as tilapia, crab, and cod (83), which are relatively low in EPA + DHA.
Current dietary guidance recommends substituting unsaturated FAs for SFAs (72). Replacing SFAs with unsaturated FAs decreases CVD risk. This recommendation can be implemented in many ways where certain unsaturated FAs are featured, even exclusively. On the basis of the evidence reviewed herein, in addition to increasing EPA + DHA to 250–500 mg/d, we propose that ALA also be a candidate FA to replace SFAs. Being mindful of the need to include ALA, as well as EPA + DHA, in the mixture of unsaturated FAs that replace SFAs is important to ensure the greatest cardiovascular benefits.
Conclusions
On the basis of the current review, there is evidence demonstrating a beneficial role of ALA for the primary and secondary prevention of CVD. A similar conclusion was reached by Mozaffarian (18) who recommended that ALA intake be increased to 2–3 g/d to reduce risk of CVD. As discussed in this review, on the basis of the epidemiologic data, there may be comparable CVD benefits for EPA + DHA vs. ALA. However, there is a lack of sufficient evidence from well-controlled clinical trials on the effects of ALA on CVD risk, and consequently, establishing a recommended amount will require additional research. Thus, we propose that clinical trials be conducted to evaluate the quantitative effects of ALA intake on CVD risk. In conjunction with this, and given that a dietary recommendation exists for EPA + DHA, there also is a pressing need to further assess the collective benefits of all (both plant- and marine-derived) n–3 PUFAs on CVD risk. It is obvious that the collective cardioprotective effect of dietary n–3 PUFAs is dependent on establishing the recommended amount of ALA. This is important because it is probable that the greatest CVD benefit will be dependent on the “optimal” quantity of all dietary n–3 PUFAs, both plant- and marine-derived sources.
Acknowledgments
Both authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; AF, atrial fibrillation; ALA, α-linolenic acid; AMDR, acceptable macronutrient distribution range; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; DART 2, Diet and Reinfarction Trial 2; DPA, docosapentenoic acid; HDL-C, HDL cholesterol; LA, linoleic acid; LDL-C, LDL cholesterol; MI, myocardial infarction; RCT, randomized controlled trial; SCD, sudden cardiac death; sE-selectin, soluble E-selectin; sICAM-1, soluble intercellular adhesion molecule 1; sTNFR, soluble tumor necrosis factor receptor; sVCAM-1, soluble vascular adhesion molecule 1.
References
- 1.Skeaff CM, Miller J. Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab 2009;55:173–201. [DOI] [PubMed] [Google Scholar]
- 2.Erkkilä A, de Mello VD, Riserus U, Laaksonen DE. Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res 2008;47:172–87. [DOI] [PubMed] [Google Scholar]
- 3.Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, Whelan J, Ramsden CE, Block RC. Fatty acids in cardiovascular health and disease: a comprehensive update. J Clin Lipidol 2012;6:216–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willett WC. Dietary fats and coronary heart disease. J Intern Med 2012;272:13–24. [DOI] [PubMed] [Google Scholar]
- 5.Rennison JH, Van Wagoner DR. Impact of dietary fatty acids on cardiac arrhythmogenesis. Circ Arrhythm Electrophysiol 2009;2:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 2012;96:1262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor SL, Gustafson JR, Sexton G, Becker N, Artaud-Wild S, Connor WE. The Diet Habit Survey: a new method of dietary assessment that relates to plasma cholesterol changes. J Am Diet Assoc 1992;92:41–7. [PubMed] [Google Scholar]
- 8.Benolken RM, Anderson RE, Wheeler TG. Membrane fatty acids associated with the electrical response in visual excitation. Science 1973;182:1253–4. [DOI] [PubMed] [Google Scholar]
- 9.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr 1989;119:1880–92. [DOI] [PubMed] [Google Scholar]
- 10.Neuringer M, Connor WE, Van Petten C, Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest 1984;73:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA 1986;83:4021–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler TG, Benolken RM, Anderson RE. Visual membranes: specificity of fatty acid precursors for the electrical response to illumination. Science 1975;188:1312–4. [DOI] [PubMed] [Google Scholar]
- 13.Astorg P, Arnault N, Czernichow S, Noisette N, Galan P, Hercberg S. Dietary intakes and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids 2004;39:527–35. [DOI] [PubMed] [Google Scholar]
- 14.Jeffrey BG, Weisinger HS, Neuringer M, Mitchell DC. The role of docosahexaenoic acid in retinal function. Lipids 2001;36:859–71. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DC, Niu SL, Litman BJ. Enhancement of G protein-coupled signaling by DHA phospholipids. Lipids 2003;38:437–43. [DOI] [PubMed] [Google Scholar]
- 16.Vinton NE, Heckenlively JR, Laidlaw SA, Martin DA, Foxman SR, Ament ME, Kopple JD. Visual function in patients undergoing long-term total parenteral nutrition. Am J Clin Nutr 1990;52:895–902. [DOI] [PubMed] [Google Scholar]
- 17.Burdge GC, Calder PC. Dietary alpha-linolenic acid and health-related outcomes: a metabolic perspective. Nutr Res Rev 2006;19:26–52. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med 2005;11(3):24–30; quiz 1, 79. [PubMed] [Google Scholar]
- 19.Campos H, Baylin A, Willett WC. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation 2008;118:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Goede J, Verschuren WM, Boer JM, Kromhout D, Geleijnse JM. Alpha-linolenic acid intake and 10-year incidence of coronary heart disease and stroke in 20,000 middle-aged men and women in the Netherlands. PLoS ONE 2011;6:e17967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fretts AM, Mozaffarian D, Siscovick DS, Heckbert SR, McKnight B, King IB, Rimm EB, Psaty BM, Sacks FM, Song X, et al. Associations of plasma phospholipid and dietary alpha linolenic acid with incident atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Heart Assoc 2013;2:e003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation 2009;120:2315–21. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005;111:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 2006;84:5–17. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007;193:1–10. [DOI] [PubMed] [Google Scholar]
- 26.Kaul N, Kreml R, Austria JA, Richard MN, Edel AL, Dibrov E, Hirono S, Zettler ME, Pierce GN. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J Am Coll Nutr 2008;27:51–8. [DOI] [PubMed] [Google Scholar]
- 27.Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr 2009;139:861–8. [DOI] [PubMed] [Google Scholar]
- 28.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n–3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n–3 fatty acid. Am J Clin Nutr 2008;88:801–9. [DOI] [PubMed] [Google Scholar]
- 29.Sioen I, Hacquebard M, Hick G, Maindiaux V, Larondelle Y, Carpentier YA, De Henauw S. Effect of ALA-enriched food supply on cardiovascular risk factors in males. Lipids 2009;44:603–11. [DOI] [PubMed] [Google Scholar]
- 30.Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, Szapary PO. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr 2008;27:65–74. [DOI] [PubMed] [Google Scholar]
- 31.Dodin S, Cunnane SC, Masse B, Lemay A, Jacques H, Asselin G, Tremblay-Mercier J, Marc I, Lamarche B, Legare F, et al. Flaxseed on cardiovascular disease markers in healthy menopausal women: a randomized, double-blind, placebo-controlled trial. Nutrition 2008;24:23–30. [DOI] [PubMed] [Google Scholar]
- 32.Harris WS, Dayspring TD, Moran TJ. Omega-3 fatty acids and cardiovascular disease: new developments and applications. Postgrad Med 2013;125:100–13. [DOI] [PubMed] [Google Scholar]
- 33.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and Reinfarction Trial (DART). Lancet 1989;2:757–61. [DOI] [PubMed] [Google Scholar]
- 34.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897–903. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- 36.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994;343:1454–9. [DOI] [PubMed] [Google Scholar]
- 37.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99:779–85. [DOI] [PubMed] [Google Scholar]
- 38.Kromhout D, Giltay EJ, Geleijnse JM. n-3 Fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26. [DOI] [PubMed] [Google Scholar]
- 39.Eussen SR, Geleijnse JM, Giltay EJ, Rompelberg CJ, Klungel OH, Kromhout D. Effects of n-3 fatty acids on major cardiovascular events in statin users and non-users with a history of myocardial infarction. Eur Heart J 2012;33:1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsden CE, Zamora D, Leelarthaepin B, Majchrzak-Hong SF, Faurot KR, Suchindran CM, Ringel A, Davis JM, Hibbeln JR. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013;346:e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose GA, Thomson WB, Williams RT. Corn oil in treatment of ischaemic heart disease. BMJ 1965;1:1531–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frantz ID, Jr, Dawson EA, Ashman PL, Gatewood LC, Bartsch GE, Kuba K, Brewer ER. Test of effect of lipid lowering by diet on cardiovascular risk: the Minnesota Coronary Survey. Arteriosclerosis 1989;9:129–35. [DOI] [PubMed] [Google Scholar]
- 43.Watts GF, Lewis B, Brunt JN, Lewis ES, Coltart DJ, Smith LD, Mann JI, Swan AV. Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas’ Atherosclerosis Regression Study (STARS). Lancet 1992;339:563–9. [DOI] [PubMed] [Google Scholar]
- 44.Dayton S, Pearce ML. Trial of unsaturated-fat diet. Lancet 1968;2:1296–7. [DOI] [PubMed] [Google Scholar]
- 45.Controlled trial of soya-bean oil in myocardial infarction. Medical Research Council Soy Study Report. Lancet 1968;2:693–9. [PubMed] [Google Scholar]
- 46.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003;108:155–60. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 2004;134:1806–11. [DOI] [PubMed] [Google Scholar]
- 48.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006;91:439–46. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 2008;52:885–97. [DOI] [PubMed] [Google Scholar]
- 50.Calder PC. n-3 Polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 2003;38:343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jonsson LS, Kolb H, Lansink M, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106 Suppl 3:S5–78. [DOI] [PubMed] [Google Scholar]
- 52.Zhao G, Etherton TD, Martin KR, Vanden Heuvel JP, Gillies PJ, West SG, Kris-Etherton PM. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun 2005;336:909–17. [DOI] [PubMed] [Google Scholar]
- 53.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr 2004;134:2991–7. [DOI] [PubMed] [Google Scholar]
- 54.Paschos GK, Rallidis LS, Liakos GK, Panagiotakos D, Anastasiadis G, Votteas V, Zampelas A. Background diet influences the anti-inflammatory effect of alpha-linolenic acid in dyslipidaemic subjects. Br J Nutr 2004;92:649–55. [DOI] [PubMed] [Google Scholar]
- 55.Zhao G. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr 2007;85:385–91. [DOI] [PubMed] [Google Scholar]
- 56.Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog Lipid Res 2011;50:372–87. [DOI] [PubMed] [Google Scholar]
- 57.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis 2003;167:237–42. [DOI] [PubMed] [Google Scholar]
- 58.Rallidis LS, Paschos G, Papaioannou ML, Liakos GK, Panagiotakos DB, Anastasiadis G, Zampelas A. The effect of diet enriched with alpha-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis 2004;174:127–32. [DOI] [PubMed] [Google Scholar]
- 59.Paschos GK, Zampelas A, Panagiotakos DB, Katsiougiannis S, Griffin BA, Votteas V, Skopouli FN. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur J Nutr 2007;46:315–20. [DOI] [PubMed] [Google Scholar]
- 60.Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr 2011;141:2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiuve SE, Rimm EB, Sandhu RK, Bernstein AM, Rexrode KM, Manson JE, Willett WC, Albert CM. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr 2012;96:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albert CM, Oh K, Whang W, Manson JE, Chae CU, Stampfer MJ, Willett WC, Hu FB. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation 2005;112:3232–8. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Jr, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2013;2:e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabaté J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med 1993;328:603–7. [DOI] [PubMed] [Google Scholar]
- 65.Bassett CM, Rodriguez-Leyva D, Pierce GN. Experimental and clinical research findings on the cardiovascular benefits of consuming flaxseed. Appl Physiol Nutr Metab 2009;34(5):965–74. [DOI] [PubMed] [Google Scholar]
- 66.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey Smith G, et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev 2004;(4):CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–30. [DOI] [PubMed] [Google Scholar]
- 68.Jacobson TA. Beyond lipids: the role of omega-3 fatty acids from fish oil in the prevention of coronary heart disease. Curr Atheroscler Rep. 2007;9(2):145–53. [DOI] [PubMed] [Google Scholar]
- 69.Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, et al. n-3 Fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–18. [DOI] [PubMed] [Google Scholar]
- 70.Harris WS. Are n-3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care 2013;16:141–9. [DOI] [PubMed] [Google Scholar]
- 71.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010;376:540–50. [DOI] [PubMed] [Google Scholar]
- 72. Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. Washington, D.C.: USDA; 2010.
- 73.Panel on Dietary Reference Intakes for Macronutrients. Dietary References Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington: The National Academies Press; 2005. [Google Scholar]
- 74.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 2005;45:581–97. [DOI] [PubMed] [Google Scholar]
- 75.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 2000;71(1 Suppl):179S–88S. [DOI] [PubMed] [Google Scholar]
- 76.Hulshof KF, van Erp-Baart MA, Anttolainen M, Becker W, Church SM, Couet C, Hermann-Kunz E, Kesteloot H, Leth T, Martins I, et al. Intake of fatty acids in western Europe with emphasis on trans fatty acids: the TRANSFAIR Study. Eur J Clin Nutr 1999;53:143–57. [DOI] [PubMed] [Google Scholar]
- 77.Ollis TE, Meyer BJ, Howe PR. Australian food sources and intakes of omega-6 and omega-3 polyunsaturated fatty acids. Ann Nutr Metab 1999;43:346–55. [DOI] [PubMed] [Google Scholar]
- 78.Innis SM, Elias SL. Intakes of essential n-6 and n-3 polyunsaturated fatty acids among pregnant Canadian women. Am J Clin Nutr 2003;77:473–8. [DOI] [PubMed] [Google Scholar]
- 79.USDA, Agricultural Research Service. What we eat in America. [cited 2010 Jul 24]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=13793.
- 80.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 81.Vannice G, Rasmussen H. Position of the Academy of Nutrition and Dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet 2014;114:136–53. [DOI] [PubMed] [Google Scholar]
- 82.Undercurrent News. Shrimp still US’ favorite food [cited 2014 Jun 10]. Available from: http://www.undercurrentnews.com/2012/09/25/shrimp-still-us-favorite-seafood/.
- 83.National Fisheries Institute. Top 10 consumed seafoods [cited 2014 Jun 10]. Available from: http://www.aboutseafood.com/about/about-seafood/top-10-consumed-seafoods.
- 84.Blok WL, Deslypere JP, Demacker PN, van der Ven-Jongekrijg J, Hectors MP, van der Meer JW, Katan MB. Pro- and anti-inflammatory cytokines in healthy volunteers fed various doses of fish oil for 1 year. Eur J Clin Invest 1997;27:1003–8. [DOI] [PubMed] [Google Scholar]
- 85.Abe Y, El-Masri B, Kimball KT, Pownall H, Reilly CF, Osmundsen K, Smith CW, Ballantyne CM. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol 1998;18:723–31. [DOI] [PubMed] [Google Scholar]
- 86.Seljeflot I, Arnesen H, Brude IR, Nenseter MS, Drevon CA, Hjermann I. Effects of omega-3 fatty acids and/or antioxidants on endothelial cell markers. Eur J Clin Invest 1998;28:629–35. [DOI] [PubMed] [Google Scholar]
- 87.Johansen O, Seljeflot I, Hostmark AT, Arnesen H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 1999;19:1681–6. [DOI] [PubMed] [Google Scholar]
- 88.Sampson MJ, Davies IR, Brown JC, Morgan V, Richardson T, James AJ, Sampson AP, Hughes DA. n-3 Polyunsaturated fatty acid supplementation, monocyte adhesion molecule expression and pro-inflammatory mediators in type 2 diabetes mellitus. Diabet Med 2001;18:51–8. [DOI] [PubMed] [Google Scholar]
- 89.Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 2001;36:1183–93. [DOI] [PubMed] [Google Scholar]
- 90.Chan DC, Watts GF, Mori TA, Barrett PH, Beilin LJ, Redgrave TG. Factorial study of the effects of atorvastatin and fish oil on dyslipidaemia in visceral obesity. Eur J Clin Invest 2002;32:429–36. [DOI] [PubMed] [Google Scholar]
- 91.Berstad P, Seljeflot I, Veierod MB, Hjerkinn EM, Arnesen H, Pedersen JI. Supplementation with fish oil affects the association between very long-chain n-3 polyunsaturated fatty acids in serum non-esterified fatty acids and soluble vascular cell adhesion molecule-1. Clin Sci (Lond) 2003;105:13–20. [DOI] [PubMed] [Google Scholar]
- 92.Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem 2003;14:513–21. [DOI] [PubMed] [Google Scholar]
- 93.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr 2003;89:517–22. [DOI] [PubMed] [Google Scholar]
- 94.Grundt H, Nilsen DW, Mansoor MA, Hetland O, Nordoy A. Reduction in homocysteine by n-3 polyunsaturated fatty acids after 1 year in a randomised double-blind study following an acute myocardial infarction: no effect on endothelial adhesion properties. Pathophysiol Haemost Thromb 2003;33:88–95. [DOI] [PubMed] [Google Scholar]
- 95.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med 2003;35:772–81. [DOI] [PubMed] [Google Scholar]
- 96.Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2003;166:85–93. [DOI] [PubMed] [Google Scholar]
- 97.Eschen O, Christensen JH, De Caterina R, Schmidt EB. Soluble adhesion molecules in healthy subjects: a dose-response study using n-3 fatty acids. Nutr Metab Cardiovasc Dis 2004;14:180–5. [DOI] [PubMed] [Google Scholar]
- 98.Jellema A, Plat J, Mensink RP. Weight reduction, but not a moderate intake of fish oil, lowers concentrations of inflammatory markers and PAI-1 antigen in obese men during the fasting and postprandial state. Eur J Clin Invest 2004;34:766–73. [DOI] [PubMed] [Google Scholar]
- 99.Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, Arnesen H. Influence of long-term intervention with dietary counseling, long-chain n–3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am J Clin Nutr 2005;81:583–9. [DOI] [PubMed] [Google Scholar]
- 100.Cazzola R, Russo-Volpe S, Miles EA, Rees D, Banerjee T, Roynette CE, Wells SJ, Goua M, Wahle KW, Calder PC, et al. Age- and dose-dependent effects of an eicosapentaenoic acid-rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis 2007;193:159–67. [DOI] [PubMed] [Google Scholar]
- 101.Fujioka S, Hamazaki K, Itomura M, Huan M, Nishizawa H, Sawazaki S, Kitajima I, Hamazaki T. The effects of eicosapentaenoic acid-fortified food on inflammatory markers in healthy subjects—a randomized, placebo-controlled, double-blind study. J Nutr Sci Vitaminol (Tokyo) 2006;52:261–5. [DOI] [PubMed] [Google Scholar]
- 102.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes (Lond) 2006;30:1535–44. [DOI] [PubMed] [Google Scholar]
- 103.Sanders TA, Lewis F, Slaughter S, Griffin BA, Griffin M, Davies I, Millward DJ, Cooper JA, Miller GJ. Effect of varying the ratio of n-6 to n-3 fatty acids by increasing the dietary intake of alpha-linolenic acid, eicosapentaenoic and docosahexaenoic acid, or both on fibrinogen and clotting factors VII and XII in persons aged 45–70 y: the OPTILIP study. Am J Clin Nutr 2006;84:513–22. [DOI] [PubMed] [Google Scholar]
- 104.Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, et al. Treatment for 2 mo with n–3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9. [DOI] [PubMed] [Google Scholar]
- 105.Browning LM, Krebs JD, Moore CS, Mishra GD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab 2007;9:70–80. [DOI] [PubMed] [Google Scholar]
- 106.Rasic-Milutinovic Z, Perunicic G, Pljesa S, Gluvic Z, Sobajic S, Djuric I, Ristic D. Effects of n-3 PUFAs supplementation on insulin resistance and inflammatory biomarkers in hemodialysis patients. Ren Fail 2007;29:321–9. [DOI] [PubMed] [Google Scholar]
- 107.Madsen T, Christensen JH, Schmidt EB. C-reactive protein and n-3 fatty acids in patients with a previous myocardial infarction: a placebo-controlled randomized study. Eur J Nutr 2007;46:428–30. [DOI] [PubMed] [Google Scholar]
- 108.Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS, Tapsell LC, Noakes M, Clifton PA, Barden A, et al. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr 2007;97:749–57. [DOI] [PubMed] [Google Scholar]
- 109.Yamada H, Yoshida M, Nakano Y, Suganami T, Satoh N, Mita T, Azuma K, Itoh M, Yamamoto Y, Kamei Y, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2008;28:2173–9. [DOI] [PubMed] [Google Scholar]
- 110.Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids 2008;78:219–28. [DOI] [PubMed] [Google Scholar]
- 111.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr 2009;139:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pot GK, Brouwer IA, Enneman A, Rijkers GT, Kampman E, Geelen A. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: a randomized controlled trial in healthy, middle-aged individuals. Eur J Clin Nutr 2009;63:1353–9. [DOI] [PubMed] [Google Scholar]
- 113.Trøseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism 2009;58:1543–9. [DOI] [PubMed] [Google Scholar]
- 114.Junker R, Pieke B, Schulte H, Nofer R, Neufeld M, Assmann G, Wahrburg U. Changes in hemostasis during treatment of hypertriglyceridemia with a diet rich in monounsaturated and n-3 polyunsaturated fatty acids in comparison with a low-fat diet. Thromb Res 2001;101:355–66. [DOI] [PubMed] [Google Scholar]
- 115.Bemelmans WJ, Lefrandt JD, Feskens EJ, van Haelst PL, Broer J, Meyboom-de Jong B, May JF, Tervaert JW, Smit AJ. Increased alpha-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr 2004;58:1083–9. [DOI] [PubMed] [Google Scholar]
- 116.Lopes C, Aro A, Azevedo A, Ramos E, Barros H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J Am Diet Assoc 2007;107:276–86. [DOI] [PubMed] [Google Scholar]
- 117.He K, Rimm EB, Merchant A, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Fish consumption and risk of stroke in men. JAMA 2002;288:3130–6. [DOI] [PubMed] [Google Scholar]
- 118.Larsson SC, Virtamo J, Wolk A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis 2012;221:282–6. [DOI] [PubMed] [Google Scholar]
- 119.Dolecek TA. Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial. Proc Soc Exp Biol Med 1992;200:177–82. [DOI] [PubMed] [Google Scholar]
- 120.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, Hennekens CH, Willett WC. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr 1999;69:890–7. [DOI] [PubMed] [Google Scholar]
- 121.Laaksonen DE, Nyyssonen K, Niskanen L, Rissanen TH, Salonen JT. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med 2005;165:193–9. [DOI] [PubMed] [Google Scholar]