Abstract
Objectives
Pregnancy loss in women suffering from hyperthyroidism has been described in case reports, but the risk of pregnancy loss caused by maternal hyperthyroidism in a population is unknown. We aimed to evaluate the association between maternal hyperthyroidism and pregnancy loss in a population-based cohort study.
Study Design
All pregnancies in Denmark from 1997 to 2008 leading to hospital visits (n = 1,062,862) were identified in nationwide registers together with information on maternal hyperthyroidism for up to 2 years after the pregnancy [hospital diagnosis/prescription of antithyroid drug (ATD)]. The Cox proportional hazards model was used to estimate adjusted hazard ratio (aHR) with 95% confidence interval (CI) for spontaneous abortion (gestational age <22 weeks) and stillbirth (≥22 weeks), reference: no maternal thyroid dysfunction.
Results
When maternal hyperthyroidism was diagnosed before/during the pregnancy (n = 5,229), spontaneous abortion occurred more often both in women treated before the pregnancy alone [aHR 1.28 (95% CI 1.18-1.40)] and in women treated with ATD in early pregnancy [1.18 (1.07-1.31)]. When maternal hyperthyroidism was diagnosed and treated for the first time in the 2-year period after the pregnancy (n = 2,361), there was a high risk that the pregnancy under study had terminated with a stillbirth [2.12 (1.30-3.47)].
Conclusions
Both early (spontaneous abortion) and late (stillbirth) pregnancy loss were more common in women suffering from hyperthyroidism. Inadequately treated hyperthyroidism in early pregnancy may have been involved in spontaneous abortion, and undetected high maternal thyroid hormone levels present in late pregnancy may have attributed to an increased risk of stillbirth.
Key Words: Hyperthyroidism, Graves' disease, Antithyroid drug, Pregnancy, Pregnancy loss, Spontaneous abortion, Stillbirth
Introduction
Pregnancy loss is an adverse outcome of pregnancy in which conception does not result in a live-born child [1]. Early pregnancy loss (spontaneous abortion) is in Denmark defined as the spontaneous termination of pregnancy before gestational week 22 [2]. Of clinically recognized pregnancies, 10-15% terminate with spontaneous abortion [1]. Late pregnancy loss (stillbirth) is in Denmark defined as the birth of a child with no signs of life in or after gestational week 22 [2]. This adverse outcome of pregnancy is less common than the early pregnancy loss but frequent enough to be of major concern [1].
Hyperthyroidism in women of reproductive age is most often caused by Graves' disease with autoimmunity against the thyroid-stimulating hormone (TSH) receptor [3,4]. About 1% of pregnant women have been treated before conception or are being treated during pregnancy for Graves' hyperthyroidism [3,4]. Untreated or inadequately treated hyperthyroidism in pregnancy may lead to both maternal and fetal complications [5,6,7], and it is generally agreed that the disease should be carefully controlled and adequately treated in pregnancy [8,9,10].
Pregnancy loss in women suffering from hyperthyroidism has mainly been described in case series [5,11,12,13]. In the present study, we aimed to evaluate the risk of early (spontaneous abortion) and late (stillbirth) pregnancy loss in women suffering from hyperthyroidism in a population-based design. Using Danish nationwide registers, we identified all clinically recognized pregnancies in the years 1997-2008 and information on the outcome of pregnancy as well as maternal hyperthyroidism diagnosed before/during and in the 2-year period after the pregnancy.
We a priori speculated on possible mechanisms which could cause an association between maternal hyperthyroidism and pregnancy loss including (a) inadequate treatment of known hyperthyroidism in pregnancy (an association with hyperthyroidism diagnosed before/during the pregnancy would be expected), (b) undetected and untreated hyperthyroidism in pregnancy (an association with hyperthyroidism diagnosed for the first time after the pregnancy would be expected), or (c) thyroid autoimmunity/genetics (an association with hyperthyroidism diagnosed before/during and also after the pregnancy would be expected).
The treatment of choice for hyperthyroidism in pregnancy is antithyroid drugs (ATDs) [8,9,10]. We recently reported that use of methimazole (MMI)/carbimazole (CMZ) and also propylthiouracil (PTU) in early pregnancy were associated with an increased risk of birth defects in live-born children [14,15]. Considering this, it can be speculated whether ATD treatment in early pregnancy could be associated with an increased risk of pregnancy loss, e.g. severe birth defects not leading to the birth of a live-born child. Thus, in the group of pregnancies where maternal hyperthyroidism was diagnosed before/during the pregnancy, we subsequently identified pregnancies (a) exposed to ATD in early pregnancy and (b) pregnancies in which the mother had been treated for hyperthyroidism before the pregnancy alone, and we compared the risk of spontaneous abortion in these groups.
Materials and Methods
Study Population and Design
We conducted a population-based cohort study using Danish nationwide registers. All Danish citizens are assigned a unique ten-digit personal identification number which is used in all the nationwide registers. All data were linked in Statistic Denmark and were made available only in encrypted form. The study was approved by the Danish Data Protection Agency.
We identified all clinically recognized pregnancies in Denmark from January 1, 1997 to December 31, 2008, leading to in- or outpatient hospital visit (n = 1,062,862) with a diagnosis of spontaneous or induced abortion, molar or ectopic pregnancy, stillbirth or live birth.
Definition of Pregnancy Outcomes
The Danish National Hospital Register (DNHR) [16] holds data on all inpatients since 1977 and all in- and outpatient visits since 1995. Diagnoses were coded according to the 8th revision of the International Classification of Disease (ICD-8) from 1977 to 1993 and ICD-10 from 1994 and onwards. We identified all hospital visits with a diagnosis of spontaneous abortion (O02.0-O03.9), induced abortion (O04.0-O05.2, O05.5-O06.9), induced abortion due to fetal disease (O05.3-O05.4), molar and ectopic pregnancy (O00.0-O01.9), and births (080.0-084.9) including information on whether it was a singleton (Z37.0-Z37.1) or multiple (Z37.2-Z37.7) stillbirth or live birth.
Information on gestational age (completed weeks plus days calculated from the first day of the last menstrual period) was obtained from the DNHR. Ultrasound verification of gestational age was increasingly used during the years of the study and was offered to all pregnant women in Denmark from the year 2004 [17,18]. We included all pregnancies with a registered gestational age in the range from 2 to 45 completed weeks at the time of pregnancy termination. We used both the registered hospital diagnosis and the gestational age to define the outcomes under study. Spontaneous abortion was defined as a hospital diagnosis of spontaneous abortion with a registered gestational age <22 completed weeks [2]. Stillbirth was defined as a hospital diagnosis of stillbirth with a registered gestational age ≥22 completed weeks [2]. Live births were included when the hospital diagnosis was live birth and the registered gestational age was ≥22 completed weeks. Pregnancies with missing or inconsistent registration of gestational age were excluded from the main analyses (1.4%).
In Denmark, the definition of stillbirth was changed from ≥28 to ≥22 gestational weeks in the year 2004 [19]. Thus, the birth of a child with no signs of life in gestational weeks 22-27 would have been registered as a spontaneous abortion in the period from 1997 to 2003. These pregnancies were excluded from the main analyses because information on variables used for the analyses of stillbirth was not available. Information on the type of pregnancy (singleton/multiple) and maternal smoking status in pregnancy was only registered when the pregnancy terminated with a stillbirth or a live birth and not when the pregnancy was diagnosed as a spontaneous abortion.
Definition of Maternal Hyperthyroidism
Information on maternal hyperthyroidism was obtained from the DNHR [16]. In- and outpatient visits in the period from January 1, 1977, up to 2 years after the date of pregnancy termination were included. Hyperthyroidism was defined as ICD-8 (1977-1993): 242.00-242.29 and ICD-10 (1994-2010): E05.0-E05.9 (excluding thyrotoxicosis factitia (E05.4), overproduction of TSH (E05.8A) and thyrotoxic heart disease (E05.9A)).
The Danish National Prescription Register (DNPR) [20] holds data on all prescription drugs redeemed from Danish pharmacies since 1995 including the type of drug prescribed according to the Anatomical Therapeutic Chemical classification system (ATC) and the date of sale. Thyroid hormones (ATC H03A) and ATDs (ATC H03B) are sold solely as prescription drugs in Denmark, and we identified all prescriptions redeemed between January 1, 1995, and up to 2 years after the date of pregnancy termination.
Hyperthyroidism was defined by (1) a hospital diagnosis of hyperthyroidism and at least one prescription of ATD or (2) if no hospital diagnosis was registered, at least two prescriptions of ATD. Women with a hospital diagnosis before January 1, 1995, and no prescription of thyroid medication registered were included as treatment may have ended before the prescription registration was initiated (n = 549). Pregnancies with inconsistent registration of maternal hyperthyroidism (0.4%) were excluded from the main analyses.
Exposure Groups
The ‘onset’ of maternal hyperthyroidism was defined as the day the first prescription of ATD was redeemed. If a hospital diagnosis was registered before the year 1996, the day of first admission to hospital defined the onset of disease. The non-exposed group was defined as pregnancies with no registration of maternal thyroid dysfunction up to 2 years after the pregnancy. Thus, pregnancies with registration of maternal hypothyroidism were excluded from the study (1.0%).
In the main analyses, two exposure groups were defined: (1) maternal hyperthyroidism diagnosed and treated before the date of pregnancy termination (before/during the pregnancy) and (2) maternal hyperthyroidism diagnosed and treated for the first time in the 2-year period after the pregnancy.
To evaluate the possible association between ATD treatment in early pregnancy and spontaneous abortion, we subsequently focused on the group of pregnancies in which maternal hyperthyroidism was diagnosed before/during the pregnancy. In this group, we identified (a) pregnancies where the pregnant woman had only redeemed prescriptions more than 12 months prior to pregnancy start considered ‘previous ATD treatment’ and (b) pregnancies where the pregnant woman redeemed one or more prescriptions of ATD in the period from 6 months prior to pregnancy start to the end of gestational week 10 considered ‘exposure to ATD in early pregnancy’. If prescriptions of both MMI/CMZ and PTU were redeemed during the 6-month period prior to pregnancy start, the last prescription prior to pregnancy start defined the type of ATD exposure in early pregnancy.
Covariates
From Statistic Denmark we obtained information on maternal age, cohabitation, income, origin and geographical residence at the time of pregnancy termination. Pregnancies with missing values on these covariates were excluded from the main analyses (1.4%). Information on maternal diagnosis of hyperemesis gravidarum and preeclampsia/eclampsia as well as diagnosis and medical treatment of diabetes and psychiatric disease before, during and up to 2 years after the pregnancy was obtained from the DNHR, the Danish Psychiatric Central Register and the DNPR.
Statistical Analyses
Altogether 1,018,261 pregnancies (95.8%) were available for the main analyses. In the analyses of spontaneous abortion (follow-up from pregnancy start to the end of gestational week 21), pregnancies terminated with induced abortion, and molar or ectopic pregnancy were excluded. In the analyses of stillbirth (follow-up from pregnancy start to the day of birth) only pregnancies terminating with a singleton stillbirth or live birth were included, thus pregnancies terminated with induced or spontaneous abortion, molar or ectopic pregnancy were excluded as were multiple births.
The Cox proportional hazard model with gestational age as the underlying time scale was used to estimate crude and adjusted hazard ratio (aHR) with 95% confidence interval (95% CI) for spontaneous abortion and stillbirth in pregnancies exposed to maternal hyperthyroidism versus the reference group with no maternal thyroid dysfunction registered up to 2 years after the pregnancy. In all analyses, robust standard error was used to account for dependency between maternal multiple pregnancies. The adjusted model included: maternal age (<25, 25-29, 30-34, 35-39, and ≥40 years), parity (1, 2, 3, ≥4), income (1st, 2nd, 3rd, 4th quartile), cohabitation (married/not married), origin (born in Denmark/not born in Denmark), residence (East/West Denmark), and the year of pregnancy termination (3-year intervals). Information on maternal smoking in pregnancy (yes/no) was available for the analysis of stillbirth. Maternal diabetes, preeclampsia and psychiatric disease were a priori considered possible intermediates and included in the model in a subsequent analysis.
In a sensitivity analysis, the study population was restricted to first-time pregnancies in the study period. In other sensitivity analyses, pregnancies excluded from the main analyses due to missing or inconsistent registration were included (gestational age, maternal hyperthyroidism and maternal covariates).
Statistical analyses were performed using Stata version 11 (Stata Corp., College Station, Tex., USA). A 5% level of significance was chosen.
Results
Study Population
Altogether 836,905 pregnancies terminated with spontaneous abortion, stillbirth or live birth (table 1) and in 0.9% of these pregnancies the pregnant woman had hyperthyroidism diagnosed and treated before/during (n = 5,229) or in the 2-year period after the pregnancy (n = 2,361). In pregnancies where maternal hyperthyroidism was diagnosed before/during the pregnancy, the pregnant women were older with a higher parity (table 1), whereas in pregnancies where maternal hyperthyroidism was diagnosed for the first time and treated in the 2-year period after the pregnancy, characteristics of the pregnant women were more comparable with the large group of unexposed pregnancies.
Table 1.
Maternal characteristics at the time of pregnancy by exposure groups
| Hyperthyroidism before/during the pregnancy |
Hyperthyroidism 0 – 2 years after the pregnancy |
No hyper- or hypothyroidism up to 2 years after the pregnancy |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Pregnancies | 5,229 | – | 2,361 | – | 829,315 | – |
| Age | ||||||
| <25 years | 297 | 5.7 | 289 | 12.2 | 113,069 | 13.6 |
| 25 – 29 years | 1,373 | 26.3 | 745 | 31.6 | 281,633 | 34.0 |
| 30 – 34 years | 2,014 | 38.5 | 839 | 35.5 | 288,663 | 34.8 |
| 35 – 39 years | 1,265 | 24.2 | 408 | 17.3 | 121,622 | 14.7 |
| ≥40 years | 280 | 5.3 | 80 | 3.4 | 24,328 | 2.9 |
| Paritya | ||||||
| 1 | 2,211 | 42.3 | 1,373 | 58.2 | 449,232 | 54.2 |
| 2 | 1,798 | 34.4 | 631 | 26.7 | 250,609 | 30.2 |
| 3 | 796 | 15.2 | 243 | 10.3 | 91,043 | 11.0 |
| ≥4 | 424 | 8.1 | 114 | 4.8 | 38,431 | 4.6 |
| Cohabitation | ||||||
| Married | 3,173 | 60.7 | 1,282 | 54.3 | 470,246 | 56.7 |
| Not married | 2,056 | 39.3 | 1,079 | 45.7 | 359,069 | 43.3 |
| Income, quartiles | ||||||
| 1st (lowest) | 254 | 4.9 | 139 | 5.9 | 49,474 | 6.0 |
| 2nd | 1,760 | 33.6 | 810 | 34.3 | 267,525 | 32.3 |
| 3rd | 2,347 | 44.9 | 1,057 | 44.8 | 375,147 | 45.2 |
| 4th | 868 | 16.6 | 355 | 15.0 | 137,169 | 16.5 |
| Origin | ||||||
| Born in Denmark | 4,544 | 86.9 | 1,998 | 84.6 | 732,396 | 88.3 |
| Not born in Denmark | 685 | 13.1 | 363 | 15.4 | 96,919 | 11.7 |
| Residence | ||||||
| West Denmark | 2,800 | 53.5 | 1,297 | 54.9 | 448,465 | 54.1 |
| East Denmark | 2,429 | 46.5 | 1,064 | 45.1 | 380,850 | 45.9 |
Previous pregnancies in the study period including index pregnancy.
Table 2 provides the distribution of pregnancy outcomes by exposure groups. The unadjusted data suggested that spontaneous abortion was more frequent when maternal hyperthyroidism was diagnosed before/during the pregnancy, and stillbirth was more frequent when maternal hyperthyroidism was diagnosed for the first time and treated in the 2-year period after the pregnancy.
Table 2.
Pregnancy outcomes by exposure groups
| All pregnancies |
Hyperthyroidism before/during the pregnancy |
Hyperthyroidism 0 – 2 years after the pregnancy |
No hyper- or hypothyroidism up to 2 years after the pregnancy | pb | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | n | %a | ||
| Pregnancies | 836,905 | – | 5,229 | – | 2,361 | – | 829,315 | – | – |
| Spontaneous | |||||||||
| abortion | 110,744 | 13.2 | 838 | 16.0 | 300 | 12.7 | 109,606 | 13.2 | <0.001 |
| Stillbirth | 2,899 | 0.35 | 14 | 0.27 | 17 | 0.72 | 2,868 | 0.35 | 0.005 |
| Singleton | 2,630 | 0.31 | 13 | 0.25 | 16 | 0.68 | 2,601 | 0.31 | 0.005 |
| Multiplec | 269 | 0.03 | 1 | 0.02 | 1 | 0.04 | 267 | 0.03 | 0.7 |
| Live birth | 723,262 | 86.4 | 4,377 | 83.7 | 2,044 | 86.6 | 716,841 | 86.4 | <0.001 |
| Singleton | 709,134 | 84.7 | 4,241 | 81.1 | 2,006 | 85.0 | 702,887 | 84.7 | <0.001 |
| Multiple | 14,128 | 1.7 | 136 | 2.6 | 38 | 1.6 | 13,954 | 1.7 | <0.001 |
Percentages are the percentage of all within the column.
p values are results of the χ2 test or Fisher's exact test as appropriate: hyperthyroidism before/during the pregnancy vs. hyperthyroidism the first time 0 – 2 years after the pregnancy vs. no hyper- or hypothyroidism up to 2 years after the pregnancy.
Minimum 1 stillbirth.
Spontaneous Abortion
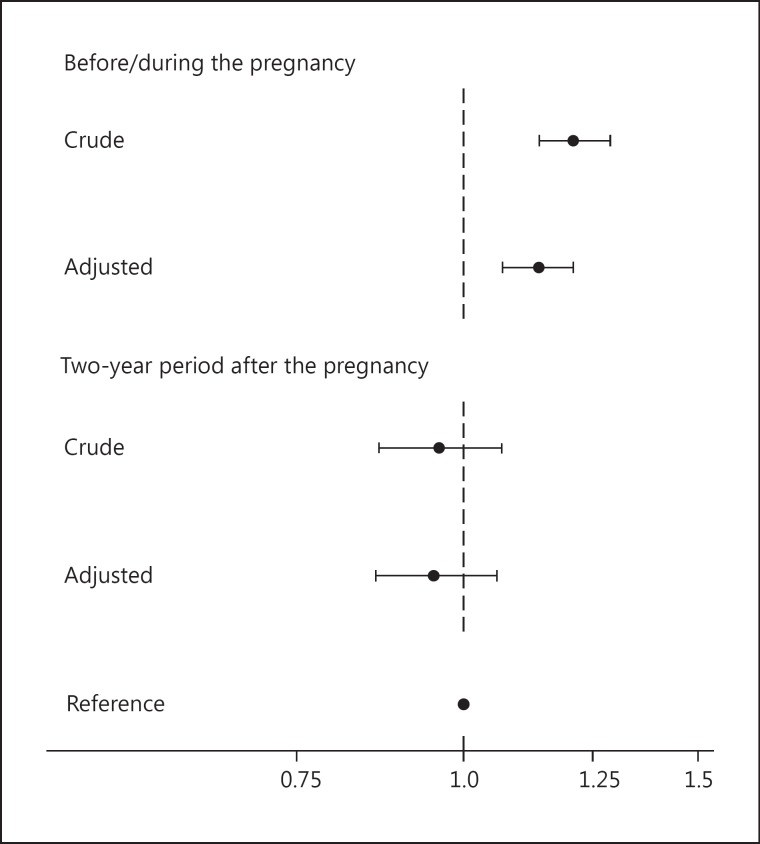
After adjustment for a number of possible confounders, an increased risk of spontaneous abortion in pregnancies with maternal hyperthyroidism before/during the pregnancy remained (fig. 1). On the other hand, no association was seen when maternal hyperthyroidism was diagnosed for the first time and treated after the pregnancy (fig. 1). Additional adjustment for maternal preeclampsia/eclampsia, diabetes and psychiatric disease did not change the results.
Fig. 1.
Crude and adjusted HR with 95% CI for spontaneous abortion in pregnancies with maternal hyperthyroidism diagnosed and treated before/during the pregnancy (top) and in pregnancies with maternal hyperthyroidism diagnosed for the first time and treated in the 2-year period after the pregnancy (bottom). The reference is pregnancies with no maternal thyroid dysfunction before, during and up to 2 years after the pregnancy. The adjusted model included the year of pregnancy termination and maternal age, parity, cohabitation, income, origin and residence (see text for details).
To evaluate the possible role of ATD treatment in early pregnancy, we subsequently focused on the group of pregnancies where maternal hyperthyroidism was diagnosed before/during the pregnancy. In this group, we were able to identify pregnancies where the pregnant woman had redeemed prescriptions of ATD before the pregnancy alone (n = 2,186) ‘previous ATD treatment’ and pregnancies where the pregnant woman redeemed prescriptions of ATD in early pregnancy (n = 1,899) ‘early pregnancy ATD treatment’. As depicted in figure 2, the aHR for spontaneous abortion was similar in the two groups suggesting that the association observed was not predominantly explained by early pregnancy ATD treatment. Results were considerably the same when stratified by the type of ATD used in early pregnancy: MMI/CMZ alone (n = 1,169): aHR 1.22 (95% CI 1.06-1.41), PTU alone (n = 576): 1.12 (0.91-1.38), both MMI/CMZ and PTU (n = 154): 1.11 (0.73-1.68), and when the window of ATD exposure in early pregnancy was restricted to redeemed prescriptions 3 and 1 month prior to pregnancy start or to the weeks after pregnancy start alone (data not shown).
Fig. 2.
Crude and adjusted HR with 95% CI for spontaneous abortion in pregnancies with maternal hyperthyroidism diagnosed and treated more than 12 months before the pregnancy alone (top) and in pregnancies where the pregnant woman redeemed prescriptions of ATD in the period from 6 months before the pregnancy start to the end of gestational week 10 (bottom). The reference is pregnancies with no maternal thyroid dysfunction before, during and up to 2 years after the pregnancy. The adjusted model included the same variables as in figure 1.
Stillbirth
Whereas the increased risk of early pregnancy loss (spontaneous abortion) was mainly seen in pregnancies with maternal hyperthyroidism diagnosed before/during the pregnancy (fig. 1), an increased risk of later pregnancy loss (stillbirth) was observed in pregnancies where maternal hyperthyroidism was diagnosed for the first time and treated in the 2-year period after the pregnancy (fig. 3). Additional adjustment for maternal smoking in the pregnancy (pregnancies with missing value (3%) excluded) revealed similar results (hyperthyroidism before/during: aHR 0.78 (95% CI 0.43-1.40), hyperthyroidism in the 2-year period after the pregnancy: 2.21 (1.35-3.61)). Adjustment for maternal preeclampsia/eclampsia, diabetes and psychiatric disease did not change the results.
Fig. 3.
Crude and adjusted HR with 95% CI for singleton stillbirth in pregnancies with maternal hyperthyroidism diagnosed and treated before/during the pregnancy (top) and in pregnancies with maternal hyperthyroidism diagnosed for the first time and treated in the 2-year period after the pregnancy (bottom). The reference is singleton births with no maternal thyroid dysfunction before, during and up to 2 years after the pregnancy. The adjusted model included the same variables as in figure 1.
Table 3 gives characteristics of the 16 exposed cases of stillbirth. The time of the first ATD treatment postpartum was fairly evenly distributed between the first and the second year after the pregnancy and an increased risk of stillbirth was observed (model including maternal smoking) both when maternal ATD treatment was initiated in the first year (aHR 2.10 (95% CI 1.13-3.92)) and in the second year postpartum (2.41 (1.08-5.37)). The median time of pregnancy termination was not significantly different between the exposed cases and the larger group of unexposed cases (median gestational week 34.5 vs. week 36, p = 0.46).
Table 3.
Characteristics of the 16 cases of singleton stillbirth in pregnancies where maternal hyperthyroidism was diagnosed the first time and treated with ATDs in the 2-year period after the pregnancy
| Birth year | Gestational week at birth | Maternal age, years | Maternal origin | Maternal smokinga | First ATH treatment (post partum month)b |
|---|---|---|---|---|---|
| ATD treatment initiated in the first year postpartum | |||||
| 1998 | 39 | 31 | Denmark | yes | 6 |
| 1998 | 28 | 29 | Denmark | no | 7 |
| 1999 | 31 | 25 | Denmark | no | 5 |
| 2001 | 34 | 28 | Denmark | yes | 9 |
| 2003 | 36 | 27 | Denmark | no | 5 |
| 2005 | 41 | 26 | Denmark | no | 7 |
| 2006 | 22 | 39 | Yugoslavia | no | 12 |
| 2007 | 35 | 23 | Denmark | no | 3 |
| 2007 | 37 | 32 | Denmark | no | 11 |
| ATD treatment initiated in the second year postpartum | |||||
| 1998 | 31 | 41 | Denmark | no | 22 |
| 2001 | 38 | 29 | Thailand | yes | 13 |
| 2002 | 37 | 36 | Denmark | no | 22 |
| 2003 | 29 | 29 | Lebanon | yes | 24 |
| 2004 | 31 | 37 | Denmark | no | 15 |
| 2006 | 32 | 33 | Denmark | no | 23 |
| 2007 | 41 | 27 | Denmark | no | 22 |
Cases are grouped according to the time (first or second year postpartum) when maternal ATD treatment was initiated and ranged by birth year of the child in each group.
Smoking or smoking cessation during the pregnancy.
The month when the first prescription of ATD was dispensed from a Danish pharmacy.
Sensitivity Analyses
When restricting analyses to first-time pregnancies in the study period (n = 452,816), an increased risk of spontaneous abortion (aHR 1.19 (95% CI 1.07-1.32)) in pregnancies with maternal hyperthyroidism diagnosed before/during the pregnancy and an increased risk of stillbirth in pregnancies with maternal hyperthyroidism diagnosed for the first time and treated after the pregnancy (2.77 (1.60-4.78)) were also observed in this group. A hospital diagnosis of hyperemesis gravidarum was more common in women identified with hyperthyroidism (2.1% vs. no thyroid dysfunction 0.9%, p < 0.001), but associations remained the same when these women were excluded (hyperthyroidism before/during the pregnancy and spontaneous abortion (aHR 1.15 (95% CI 1.07-1.23)), hyperthyroidism after the pregnancy and stillbirth (2.16 (1.32-3.53)). Moreover, an increased risk of spontaneous abortion was still observed when women were diagnosed for the first time with hyperthyroidism during the pregnancy were excluded (1.21 (1.12-1.30)). When pregnancies excluded from the main analyses due to missing or inconsistent registration of gestational age, maternal hyperthyroidism or maternal covariates were included, results were similar to the main analyses (data not shown).
Discussion
Principal Findings
In a Danish population-based study, pregnancy loss was more common in women suffering from hyperthyroidism. Early pregnancy loss (spontaneous abortion) occurred more often in women diagnosed with hyperthyroidism before/during the pregnancy, and we speculate whether inadequate treatment of maternal hyperthyroidism in early pregnancy may have been involved. Late pregnancy loss (stillbirth) occurred more often in women diagnosed for the first time with hyperthyroidism in the 2-year period after the pregnancy, and we speculate whether undetected high maternal thyroid hormone levels in late pregnancy may have contributed to an increased risk of stillbirth.
Pregnancy Loss in Hyperthyroidism
In clinical reviews and guidelines on maternal hyperthyroidism in pregnancy it is rather consistently described that maternal hyperthyroidism in pregnancy is associated with adverse pregnancy outcomes including pregnancy loss [8,9,10,21]. The evidence of pregnancy loss is mainly based on case series, and it appears that especially the untreated or inadequately treated hyperthyroidism may complicate pregnancy [5,11,12,13]. In a study published in 1989, Davis et al. [5] described 60 pregnancies complicated by maternal hyperthyroidism (women diagnosed prior to pregnancy who were euthyroid throughout pregnancy were not included). Among the 60 women included, 8 women were untreated in pregnancy, whereas 36 women were treated with PTU to euthyroidism at delivery and 16 women were inadequately treated with PTU and were hyperthyroid at the time of delivery. In total, 1 case of abortion and 5 cases of stillbirth were observed and these adverse outcomes of pregnancy all occurred in pregnancies were the mother had untreated hyperthyroidism (abortion n = 1, stillbirth n = 4) or suffered from inadequately treated hyperthyroidism (stillbirth n = 2) at the time of delivery.
Early Pregnancy Loss
Spontaneous abortion is the most common adverse outcome of pregnancy [1]. Chromosomal abnormality is the single most common cause involved in approximately half of all cases of early spontaneous abortion, but several other risk factors have been examined [22]. In our study, an increased risk of spontaneous abortion was observed in pregnancies with maternal diagnosis of hyperthyroidism before/during the pregnancy. The lack of an association with maternal hyperthyroidism diagnosed after the pregnancy contradicts an inherited genetic association to some extent. Since we had no results of thyroid function tests, we were not able to distinguish between the possible role of inadequately treated hyperthyroidism in early pregnancy and ATD treatment in early pregnancy per se. However, the finding that women treated with ATD more than 1 year before the pregnancy alone had a similar high risk of spontaneous abortion may suggest that inadequately treated hyperthyroidism in early pregnancy was the main factor involved.
Late Pregnancy Loss
Stillbirth might also be caused by genetic factors although chromosomal abnormalities are less frequently involved than in spontaneous abortion [1]. In our study, an increased risk of stillbirth was only observed in the group of pregnancies where maternal hyperthyroidism was diagnosed for the first time and treated in the 2-year period after the pregnancy. The lack of an association with maternal hyperthyroidism diagnosed before/during the pregnancy contradicts a familiar association to some extent. In Denmark, no systematic testing of thyroid function in pregnancy is implemented. We speculate whether mothers diagnosed with hyperthyroidism in the years after the pregnancy may have had undetected high thyroid hormone levels already in the late pregnancy which could have had a direct effect on the fetus. Alternatively to this, maternal co-morbidities to hyperthyroidism, e.g. preeclampsia [23], diabetes or psychiatric disease, might have been intermediate factors; however, results were similar when we adjusted for these factors in the analyses.
Thyroid Hormones and the Maternal-Fetal Unit
Thyroid hormones are essential during fetal development, especially fetal brain development [24]. Considering the role of thyroid hormones in the maintenance of pregnancy, studies in rats have shown that the uterus contains thyroid hormone (T3) receptors [25] and high activity of the enzyme type 3 iodothyronine deiodinase (D3), which inactivates thyroid hormones [26,27]. Studies in humans have demonstrated that thyroid hormones are present in the embryonic cavities (coelomic and amniotic fluid) in early pregnancy in concentrations depending on the maternal levels of thyroid hormones [28,29]. Thus, it can be speculated whether the local regulation and levels of maternal thyroid hormones in the uteroplacental unit may play a role in determining the level of maternal thyroid hormones that the fetus is exposed to, and if high maternal thyroid hormone levels can have a direct toxic effect on the uteroplacental unit and/or the fetus. In a study of Azorean families with resistance to thyroid hormone [30], the authors were able to study unaffected fetuses exposed to high maternal thyroid hormone levels in euthyroid-resistant mothers. In this group, there was a high rate of miscarriage and live-born infants had lower birth weight and suppressed levels of TSH which suggested that the high maternal thyroid hormone levels produced fetal thyrotoxicosis.
Methodological Comments
This study included clinically recognized pregnancies leading to hospital visit. For spontaneous abortion to be clinically recognized, it matters how early the pregnancy is identified. If the time of pregnancy testing is different among exposed and non-exposed groups, e.g. women with hyperthyroidism being more aware of early signs of pregnancy, it may induce bias. Similarly, we are aware that some pregnancies terminating with spontaneous abortion do not lead to hospital visit. If the propensity for hospital visit is associated with the exposure, it might induce bias. However, the median gestational age at the time of spontaneous abortion was similar in exposed and non-exposed pregnancies.
In women with recurrent spontaneous abortions, the referral pattern to hospitals may be influenced by the number of previous reproductive events, which could cause bias. However, we found similar associations when analyses were restricted to the first pregnancy in the study period.
As pointed out in the Materials and Methods section, the definition of stillbirth changed in Denmark in the year 2004 [19]. In sensitivity analyses, results did not change when we restricted analyses to the cohort of pregnancies terminated in the years 2005-2008 (data not shown).
Induced abortions may also challenge the interpretation of results (incomplete follow-up as the pregnancy could potentially have ended in a spontaneous abortion) [31], but we obtained similar results when all pregnancies were included in the analyses, and the pregnancy was censored at the time of the induced abortion, molar or ectopic pregnancy (data not shown). Information on a number of important confounders was available, but residual or uncontrolled confounding might still exist, and information on maternal smoking was only available for the analyses of stillbirth.
Perspective
It remains debatable whether routine thyroid function testing should be performed in pregnancy [32]. Low maternal serum TSH in early pregnancy has not been associated with adverse pregnancy outcomes [33]. The criteria used to define hyperthyroidism in the present study imply an attempt to identify women suffering from regular thyroid disease. Results do not advocate the detection of small aberrations in thyroid function during early pregnancy which may be secondary to placental dysfunction [32]. More studies are needed, and studies with actual measurement of maternal thyroid hormones in pregnancy are warranted. If the findings are corroborated in further studies, they might be advocated for considering routine thyroid function testing in early pregnancy to detect overt hyperthyroidism.
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
Chun Sen Wu is supported by the individual postdoctoral grants from the Danish Medical Research Council (FSS: 12-132232).
References
- 1.Simpson JL, Jauniaux ER. Pregnancy loss. In: Gabbe SG, Niebyl JR, Simpson JL, Landon MB, Galan HL, Jauniaux ER, Driscoll DA, editors. Obstetrics: Normal and Problem Pregnancies. ed 6. Philadelphia: Saunders/Elsevier; 2012. pp. 592–608. [Google Scholar]
- 2.Statens Serum Institut Fællesindhold for basisregistrering af sygehuspatienter. Vejledningsdel. 2014;23:99–116. [Google Scholar]
- 3.Laurberg P, Bournaud C, Karmisholt J, Orgiazzi J. Management of Graves' hyperthyroidism in pregnancy: focus on both maternal and foetal thyroid function, and caution against surgical thyroidectomy in pregnancy. Eur J Endocrinol. 2009;160:1–8. doi: 10.1530/EJE-08-0663. [DOI] [PubMed] [Google Scholar]
- 4.Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Laurberg P. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. Eur J Endocrinol. 2011;164:801–809. doi: 10.1530/EJE-10-1155. [DOI] [PubMed] [Google Scholar]
- 5.Davis LE, Lucas MJ, Hankins GD, Roark ML, Cunningham FG. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989;160:63–70. doi: 10.1016/0002-9378(89)90088-4. [DOI] [PubMed] [Google Scholar]
- 6.Sheffield JS, Cunningham FG. Thyrotoxicosis and heart failure that complicate pregnancy. Am J Obstet Gynecol. 2004;190:211–217. doi: 10.1016/s0002-9378(03)00944-x. [DOI] [PubMed] [Google Scholar]
- 7.Andersen SL, Olsen J, Wu CS, Laurberg P. Low birth weight in children born to mothers with hyperthyroidism and high birth weight in hypothyroidism, whereas preterm birth is common in both conditions: a Danish National Hospital Register study. Eur Thyroid J. 2013;2:135–144. doi: 10.1159/000350513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W. American Thyroid Association Taskforce on Thyroid Disease during Pregnancy and Postpartum: Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, Ross DS, Sosa JA, Stan MN. American Thyroid Association, American Association of Clinical Endocrinologists: Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 10.De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Eastman CJ, Lazarus JH, Luton D, Mandel SJ, Mestman J, Rovet J, Sullivan S. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 11.Sugrue D, Drury MI. Hyperthyroidism complicating pregnancy: results of treatment by antithyroid drugs in 77 pregnancies. Br J Obstet Gynaecol. 1980;87:970–975. doi: 10.1111/j.1471-0528.1980.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 12.Momotani N, Ito K. Treatment of pregnant patients with Basedow's disease. Exp Clin Endocrinol. 1991;97:268–274. doi: 10.1055/s-0029-1211077. [DOI] [PubMed] [Google Scholar]
- 13.Hamburger JI. Diagnosis and management of Graves' disease in pregnancy. Thyroid. 1992;2:219–224. doi: 10.1089/thy.1992.2.219. [DOI] [PubMed] [Google Scholar]
- 14.Andersen SL, Olsen J, Wu CS, Laurberg P. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J Clin Endocrinol Metab. 2013;98:4373–4381. doi: 10.1210/jc.2013-2831. [DOI] [PubMed] [Google Scholar]
- 15.Laurberg P, Andersen SL. Antithyroid drug use in early pregnancy and birth defects. Time windows of relative safety and high risk? Eur J Endocrinol. 2014;171:R13–R20. doi: 10.1530/EJE-14-0135. [DOI] [PubMed] [Google Scholar]
- 16.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46:263–268. [PubMed] [Google Scholar]
- 17.Jorgensen FS. Organization of obstetric ultrasound in Denmark 2000. Description of the development since 1990. Ugeskr Laeger. 2003;165:4404–4409. [PubMed] [Google Scholar]
- 18.Sundhedsstyrelsen Retningslinjer for fosterdiagnostik – prænatal information, risikovurdering, rådgivning og diagnostik 2004;1. http://sundhedsstyrelsen.dk/publ/Publ2004/Informeret_valg.pdf.
- 19.Sundhedsstyrelsen. Anbefalinger for Svangreomsorgen. 2013. p. 1. http://sundhedsstyrelsen.dk/publ/Publ2013/10okt/Svangreomsorg2013.pdf.
- 20.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 21.Cooper DS, Laurberg P. Hyperthyroidism in pregnancy. Lancet Diabetes Endocrinol. 2013;1:238–249. doi: 10.1016/S2213-8587(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 22.Feodor Nilsson S, Andersen P, Strandberg-Larsen K, Nybo Andersen AM. Risk factors for miscarriage from a prevention perspective: a nationwide follow-up study. BJOG. 2014. Epub ahead of print. [DOI] [PubMed]
- 23.Millar LK, Wing DA, Leung AS, Koonings PP, Montoro MN, Mestman JH. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet Gynecol. 1994;84:946–949. [PubMed] [Google Scholar]
- 24.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151:U25–U37. doi: 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- 25.Evans RW, Farwell AP, Braverman LE. Nuclear thyroid hormone receptor in the rat uterus. Endocrinology. 1983;113:1459–1463. doi: 10.1210/endo-113-4-1459. [DOI] [PubMed] [Google Scholar]
- 26.Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest. 1999;103:979–987. doi: 10.1172/JCI6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88:1384–1388. doi: 10.1210/jc.2002-021291. [DOI] [PubMed] [Google Scholar]
- 28.Contempre B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab. 1993;77:1719–1722. doi: 10.1210/jcem.77.6.8263162. [DOI] [PubMed] [Google Scholar]
- 29.Calvo RM, Jauniaux E, Gulbis B, Asuncion M, Gervy C, Contempre B, Morreale de Escobar G. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab. 2002;87:1768–1777. doi: 10.1210/jcem.87.4.8434. [DOI] [PubMed] [Google Scholar]
- 30.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292:691–695. doi: 10.1001/jama.292.6.691. [DOI] [PubMed] [Google Scholar]
- 31.Olsen J. Calculating risk ratios for spontaneous abortions: the problem of induced abortions. Int J Epidemiol. 1984;13:347–350. doi: 10.1093/ije/13.3.347. [DOI] [PubMed] [Google Scholar]
- 32.Laurberg P, Andersen SL, Pedersen IB, Andersen S, Carle A. Screening for overt thyroid disease in early pregnancy may be preferable to searching for small aberrations in thyroid function tests. Clin Endocrinol (Oxf) 2013;79:297–304. doi: 10.1111/cen.12232. [DOI] [PubMed] [Google Scholar]
- 33.Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–341. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]